Identification and Characterisation of New Microbial Antagonists for Biocontrol of Monilinia laxa, the Causal Agent of Brown Rot on Stone Fruit
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro and in Vivo Screening of Microbes against M. laxa
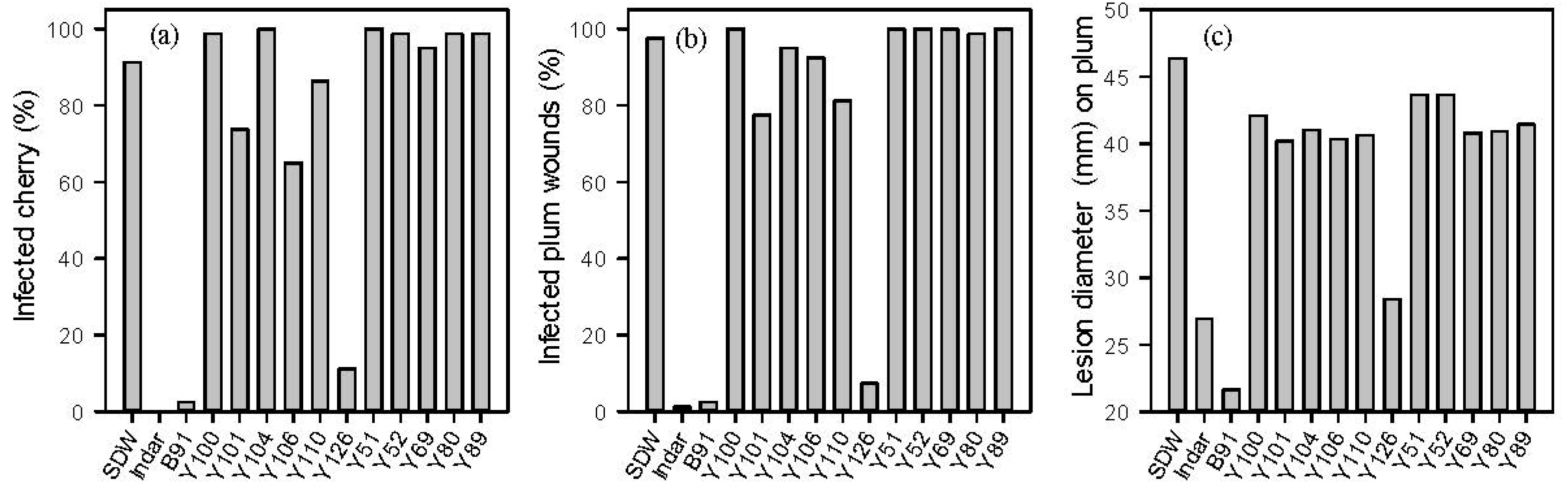
2.2. Identification
2.3. Effect of Temperature on Antagonists B91 and Y126
2.3.1. Growth of B91 and Y126
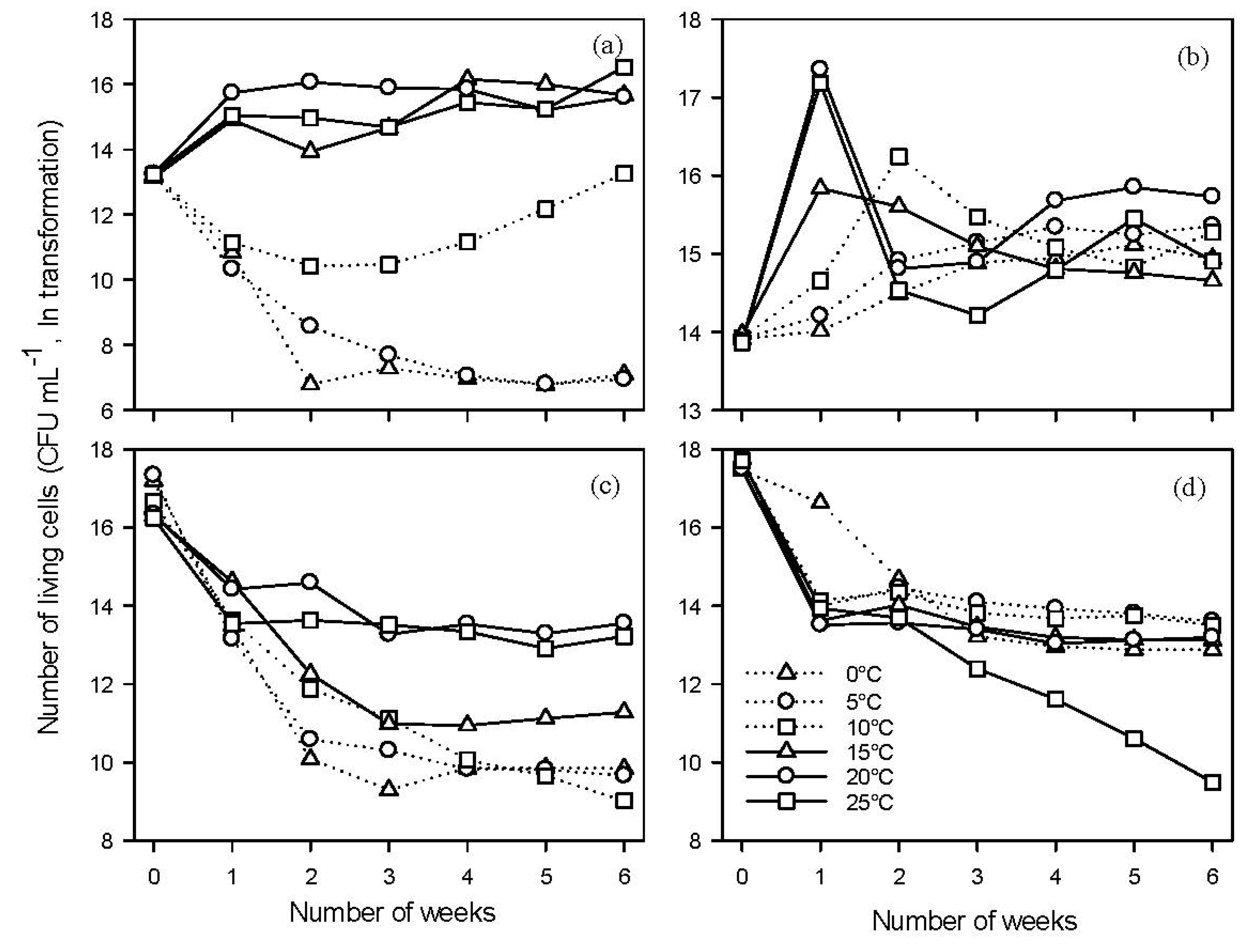
2.3.2. Mortality of Antagonists B91 and Y126
2.4. Possible Modes of Action
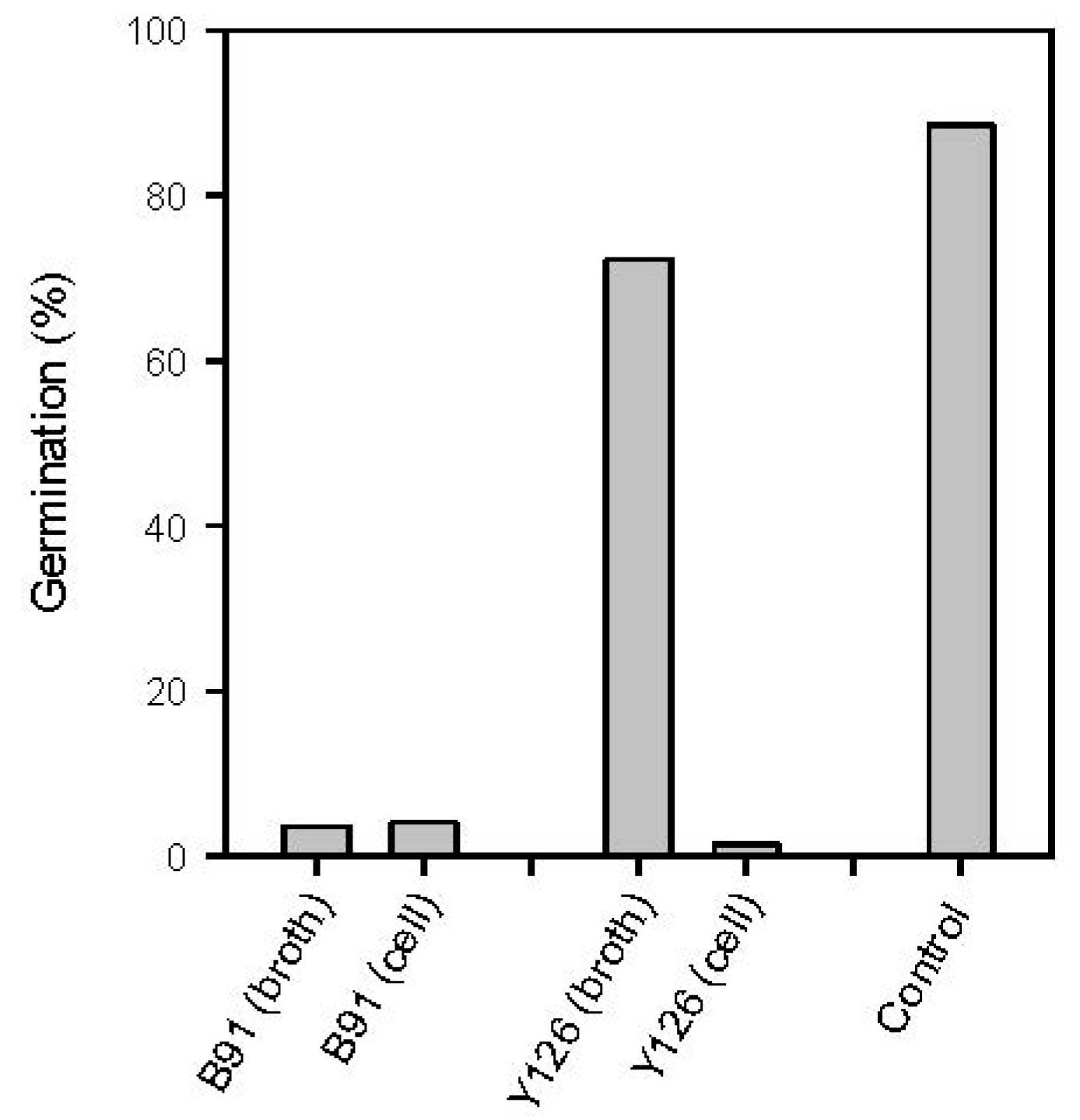
2.4.1. Inhibition of Spore Germination via Antibiosis and Competition for Nutrients
2.4.2. Inhibition of Mycelium Growth by Microbial VOCs
3. Experimental Section
3.1. In Vitro and In Vivo Screening
3.1.1. Microbial Isolation
3.1.2. Dual Culture Screening
3.1.3. Production of M. laxa Inoculum
3.1.4. In Vivo Tests on Detached Fruits
3.2. Identification
3.2.1. Molecular Identification
3.2.2. Conventional Identification
3.3. Temperature Effects on Growth and Mortality of B91 and Y126
3.3.1. Growth
3.3.2. Survival
3.4. Modes of Action
3.4.1. Inhibition of Spore Germination
3.4.2. Production of Volatile Organic Compounds (VOCs)
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Borve, J.; Stensvand, A. Use of a plastic rain shield reduces fruit decay and need for fungicides in sweet cherry. Plant Dis. 2003, 87, 523–528. [Google Scholar] [CrossRef]
- Snowdon, A.L. A Colour Atlas of Post Harvest Diseases and Disorders of Fruits and Vegetables, Volume 1: General Introduction & Fruits; Wolfe Scientific Limited: Barcelona, Spain, 1990; Volume 1, pp. 218–236. [Google Scholar]
- Wherrett, A.D.; Sivasithamparam, K.; Kumar, S. Detection of possible systemic fungicide resistance in Western Australian Monilinia populations. Phytopathology 2001, 91, S95. [Google Scholar]
- Chen, F.; Liu, X.; Schnabel, G. First report of brown rot caused by Monilinia fructicola in sweet cherry in Maryland. Plant Dis. 2012, 97, 145. [Google Scholar]
- European and Mediterranean Plant Protection Organization (EPPO). Diagnostics: Monilinia fructicola. EPPO Bull. 2009, 39, 337–343. [CrossRef]
- Adaskaveg, J.E.; Forster, H.; Gubler, W.D.; Teviotdale, B.L.; Thompson, D.F. Reduced-risk fungicides help manage brown rot and other fungal diseases of stone fruit. Calif. Agric. 2005, 59, 109–114. [Google Scholar] [CrossRef]
- Malandrakis, A.; Koukiasas, N.; Veloukas, T.; Karaoglanidis, G.; Markoglou, A. Baseline sensitivity of Monilinia laxa from Greece to fenhexamid and analysis of fenhexamid-resistant mutants. Crop Prot. 2013, 46, 13–17. [Google Scholar] [CrossRef]
- Thomidis, T.; Michailides, T.; Exadaktylou, E. Contribution of pathogens to peach fruit rot in northern Greece and their sensitivity to iprodione, carbendazim, thiophanate-methyl and tebuconazole fungicides. J. Phytopathol. 2009, 157, 194–200. [Google Scholar] [CrossRef]
- Pimenta, R.S.; da Silva, J.F.M.; Buyer, J.S.; Janisiewicz, W.J. Endophytic fungi from plums (Prunus domestica) and their antifungal activity against Monilinia fructicola. J. Food Prot. 2012, 75, 1883–1889. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Jurick Ii, W.M.; Vico, I.; Peter, K.A.; Buyer, J.S. Culturable bacteria from plum fruit surfaces and their potential for controlling brown rot after harvest. Postharvest Biol. Technol. 2013, 76, 145–151. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Baykal, N. Biological control of postharvest diseases of peaches and nectarines by yeasts. J. Phytopathol. 2003, 151, 130–134. [Google Scholar] [CrossRef]
- Zhou, T.; Schneider, K.E.; Li, X. Development of biocontrol agents from food microbial isolates for controlling post-harvest peach brown rot caused by Monilinia fructicola. Int. J. Food Microbiol. 2008, 126, 180–185. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Zhao, X.; Liu, Z.; Yang, H.Q.; Zhao, X.M.; Zhao, X.Y.; Liu, Z.P. Antifungal mechanism against monilinia fruticola and stability of the antagonistic substance from bacterium strain CE. J. Fruit Sci. 2011, 28, 204–208. [Google Scholar]
- Bonaterra, A.; Mari, M.; Casalini, L.; Montesinos, E. Biological control of Monilinia laxa and Rhizopus stolonifer in postharvest of stone fruit by Pantoea agglomerans EPS125 and putative mechanisms of antagonism. Int. J. Food Microbiol. 2003, 84, 93–104. [Google Scholar] [CrossRef]
- Mari, M.; Torres, R.; Casalini, L.; Lamarca, N.; Mandrin, J.F.; Lichou, J.; Larena, I.; de Cal, M.A.; Melgarejo, P.; Usall, J. Control of post-harvest brown rot on nectarine by Epicoccum nigrum and physico-chemical treatments. J. Sci. Food Agric. 2007, 87, 1271–1277. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Baykal, N. Evaluation of the use of microwave power for the control of postharvest diseases of peaches. Postharvest Biol. Technol. 2002, 26, 237–240. [Google Scholar] [CrossRef]
- Casals, C.; Teixido, N.; Vinas, I.; Silvera, E.; Lamarca, N.; Usall, J. Combination of hot water, Bacillus subtilis CPA-8 and sodium bicarbonate treatments to control postharvest brown rot on peaches and nectarines. Eur. J. Plant Pathol. 2010, 128, 51–63. [Google Scholar] [CrossRef]
- Guijarro, B.; Melgarejo, P.; Torres, R.; Lamarca, N.; Usall, J.; de Cal, A. Penicillium frequentans population dynamics on peach fruits after its applications against brown rot in orchards. J. Appl. Microbiol. 2008, 104, 659–671. [Google Scholar] [CrossRef]
- Larena, I.; de Cal, A.; Melgarejo, P. Enhancing the adhesion of Epicoccum nigrum conidia to peach surfaces and its relationship to the biocontrol of brown rot caused by Monilinia laxa. J. Appl. Microbiol. 2010, 109, 583–593. [Google Scholar]
- Zhang, D.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Selection and evaluation of new antagonists for their efficacy against postharvest brown rot of peaches. Postharvest Biol. Technol. 2010, 55, 174–181. [Google Scholar] [CrossRef]
- European Union (EU). Regulation (EC) No 1007/2009 of the European Parliament and of the Council of 16 September 2009. Off. J. Eur. Community 2009, L309, 1–50.
- European Union (EU). Council Directive 91/414/EEC. Off. J. Eur. Community 1991, L230, 1–32.
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A Review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Gueldner, R.C.; Reilly, C.C.; Pusey, P.L.; Costello, C.E.; Arrendale, R.F.; Cox, R.H.; Himmelsbach, D.S.; Crumley, F.G.; Cutler, H.G. Isolation and identification of iturins as antifungal peptides in biological control of peach brown rot with Bacillus subtilis. J. Agric. Food Chem. 1988, 36, 366–370. [Google Scholar] [CrossRef]
- Pusey, P.L.; Wilson, C.L. Postharvest biological control of stone fruit brown rot by Bacillus subtilis. Plant Dis. 1984, 68, 753–756. [Google Scholar]
- Pusey, P.L.; Wilson, C.L.; Hotchkiss, M.W.; Fraklin, J.D. Compatibility of Bacillus subtilis for postharvest control of peach brown rot with commercial fruit waxes, dicloran and cold storage conditions. Plant Dis. 1986, 70, 587–590. [Google Scholar] [CrossRef]
- CRD Chemicals Regulation Directorate: Biopesticides. Available online: http://www.pesticides.gov.uk/guidance/industries/pesticides/user-areas/biopesticides-home (accessed on 19th October 2013).
- Wang, X.; Li, G.; Jiang, D.; Huang, H.-C. Screening of plant epiphytic yeasts for biocontrol of bacterial fruit blotch (Acidovorax avenae subsp. citrulli) of hami melon. Biol. Control 2009, 50, 164–171. [Google Scholar] [CrossRef]
- Raspor, P.; Miklic-Milek, D.; Avbelj, M.; Cadez, N. Biocontrol of grey mould disease on grape caused by Botrytis cinerea with autochthonous wine yeasts. Food Technol. Biotechnol. 2010, 48, 336–343. [Google Scholar]
- Whipps, J.M. Effect of media on growth and interactions between a range of soil-borne glasshouse pathogens and antagonistic fungi. New Phytol. 1987, 107, 127–142. [Google Scholar] [CrossRef]
- Magan, N. Ecophysiology of Biocontrol Agents for Improved Completence in the Phyllophere. In Microbial Ecology of Aerial Plant Surfaces; Bailey, M.J., Lilley, A.K., Timms-Wilson, T.M., Spencer-Phillips, P.T.N., Eds.; CAB International: Wallingford, UK, 2006; pp. 149–164. [Google Scholar]
- Janisiewicz, W.J.; Kurtzman, C.P.; Buyer, J.S. Yeasts associated with nectarines and their potential for biological control of brown rot. Yeast 2010, 27, 389–398. [Google Scholar] [CrossRef]
- Mignard, S.; Flandrois, J.P. 16S rRNA sequencing in routine bacterial identification: A 30-month experiment. J. Microbiol. Methods 2006, 67, 574–581. [Google Scholar] [CrossRef]
- Alvarez, F.; Castro, M.; Principe, A.; Borioli, G.; Fischer, S.; Mori, G.; Jofre, E. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J. Appl. Microbiol. 2012, 112, 159–174. [Google Scholar] [CrossRef]
- Chun, J.; Bae, K.S. Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek 2000, 78, 123–127. [Google Scholar] [CrossRef]
- O’Donnell, A.G.; Norris, J.R.; Berkeley, R.C.W.; Claus, D.; Kaneko, T.; Logan, N.A.; Nozaki, R. Characterization of Bacillus subtilis, Bacillus pumilus, Bacillus licheniformis, and Bacillus amyloliquefaciens by pyrolysis gas-liquid chromatography, deoxyribonucleic acid-deoxyribonucleic acid hybridization, biochemical tests, and API systems. Int. J. Syst. Bacteriol. 1980, 30, 448–459. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1978; p. 252. [Google Scholar]
- Klein, W.; Weber, M.H.W.; Marahiel, M.A. Cold shock response of Bacillus subtilis: Isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 1999, 181, 5341–5349. [Google Scholar]
- D’Amico, S.; Collins, T.; Marx, J.-C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef]
- Warth, A.D. Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species. J. Bacteriol. 1978, 134, 699–705. [Google Scholar]
- Tokuda, Y.; Ano, T.; Shoda, M. Survival of Bacillus subtilis NB22 and its transformant in soil. Appl. Soil Ecol. 1995, 2, 85–94. [Google Scholar] [CrossRef]
- Budde, I.; Steil, L.; Scharf, C.; Volker, U.; Bremer, E. Adaptation of Bacillus subtilis to growth at low temperature: A combined transcriptomic and proteomic appraisal. Microbiology 2006, 152, 831–853. [Google Scholar] [CrossRef]
- Sugumaran, K.R.; Gowthami, E.; Swathi, B.; Elakkiya, S.; Srivastava, S.N.; Ravikumar, R.; Gowdhaman, D.; Ponnusami, V. Production of pullulan by Aureobasidium pullulans from Asian palm kernel: A novel substrate. Carbohydr. Polym. 2013, 92, 697–703. [Google Scholar] [CrossRef]
- Zalar, P.; Gostincar, C.; de Hoog, G.S.; Ursic, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef]
- Yanez-Mendizabal, V.; Zeriouh, H.; Vinas, I.; Torres, R.; Usall, J.; de Vicente, A.; Perez-Garcia, A.; Teixido, N. Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. Eur. J. Plant Pathol. 2012, 132, 609–619. [Google Scholar] [CrossRef]
- Zeriouh, H.; Romero, D.; Garcia-Gutierrez, L.; Cazorla, F.M.; de Vicente, A.; Perez-Garcia, A. The iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol. Plant Microbe Interact. 2011, 24, 1540–1552. [Google Scholar] [CrossRef]
- Zhang, D.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Efficacy of the antagonist Aureobasidium pullulans PL5 against postharvest pathogens of peach, apple and plum and its modes of action. Biol. Control 2010, 54, 172–180. [Google Scholar] [CrossRef]
- Castoria, R.; de Curtis, F.; Lima, G.; Caputo, L.; Pacifico, S.; de Cicco, V. Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: Study on its modes of action. Postharvest Biol. Technol. 2001, 22, 7–17. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; Belen Gonzalez, M.; Fernanda Garat, M.; Wisniewski, M. Aureobasidium pullulans as a biocontrol agent of post-harvest pathogens of apples in Uruguay. Biocontrol Sci. Technol. 2009, 19, 1033–1049. [Google Scholar] [CrossRef]
- Bencheqroun, S.K.; Bajji, M.; Massart, S.; Bentata, F.; Labhilili, M.; Achbani, H.; El Jaafari, S.; Jijakli, M.H. Biocontrol of blue mold on apple fruits by Aureobasidium pullulans (strain Ach 1–1): In vitro and in situ evidence for the possible involvement of competition for nutrients. Commun. Agric. Appl. Biol. Sci. 2006, 71, 1151–1157. [Google Scholar]
- Swadling, I.R.; Jeffries, P. Isolation of microbial antagonists for biocontrol of grey mould disease of strawberries. Biocontrol Sci. Technol. 1996, 6, 125–136. [Google Scholar] [CrossRef]
- Xu, X.-M.; Bertone, C.; Berrie, A. Effects of wounding, fruit age and wetness duration on the development of cherry brown rot in the UK. Plant Pathol. 2007, 56, 114–119. [Google Scholar]
- Fang, H.; Hedin, G. Rapid screening and identification of methicillin-resistant Staphylococcus aureus from clinical samples by selective-broth and real-time PCR assay. J. Clin. Microbiol. 2003, 41, 2894–2899. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; Wiley: New York, NY, USA, 1991; pp. 115–147. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cyptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar]
- White, T.J.; Bruns, T.L.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogentics. In A Guide to Molecular Methods and Applications; Innis, M.A., Gelfand, D.H., Snisky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Martini, M.; Musetti, R.; Grisan, S.; Polizzotto, R.; Borselli, S.; Pavan, F.; Osler, R. DNA-Dependent detection of the grapevine fungal endophytes Aureobasidium pullulans and Epicoccum nigrum. Plant Dis. 2009, 93, 993–998. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Logan, N.A.; de Vos, P. Bacillus. In Bergey’s Manual of Systematic Bacteriology: The Firmicutes, 2nd ed.; De Vos, P., Garrity, G., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.H., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 21–128. [Google Scholar]
- De Hoog, G.S.; Guarro, J.; Gene, J.; Figueras, M.J. Atlas of Clinical Fungi; Centraalbureau voor Schimmecultures: Utrecht, The Netherlands, 2000; p. 1126. [Google Scholar]
- Swadling, I. Biological Control of Botrytis cinerea in Strawberries. Ph.D. Thesis, University of Kent, Canterbury, UK, July 1994. [Google Scholar]
- Payne, R.; Soutar, D.; Baird, D.; Thompson, R.; Gilmour, A.R.; Todd, A.D.; Harding, S.A.; Wilson, G.T.; Lane, P.W.; Webster, R.; Murray, D.; Welham, S. The Guide to GenStat, Part 2: Statistics; VSN International Ltd.: Hemel Hempstead, UK, 2000; p. 782. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rungjindamai, N.; Xu, X.-M.; Jeffries, P. Identification and Characterisation of New Microbial Antagonists for Biocontrol of Monilinia laxa, the Causal Agent of Brown Rot on Stone Fruit. Agronomy 2013, 3, 685-703. https://doi.org/10.3390/agronomy3040685
Rungjindamai N, Xu X-M, Jeffries P. Identification and Characterisation of New Microbial Antagonists for Biocontrol of Monilinia laxa, the Causal Agent of Brown Rot on Stone Fruit. Agronomy. 2013; 3(4):685-703. https://doi.org/10.3390/agronomy3040685
Chicago/Turabian StyleRungjindamai, Nattawut, Xiang-Ming Xu, and Peter Jeffries. 2013. "Identification and Characterisation of New Microbial Antagonists for Biocontrol of Monilinia laxa, the Causal Agent of Brown Rot on Stone Fruit" Agronomy 3, no. 4: 685-703. https://doi.org/10.3390/agronomy3040685
APA StyleRungjindamai, N., Xu, X.-M., & Jeffries, P. (2013). Identification and Characterisation of New Microbial Antagonists for Biocontrol of Monilinia laxa, the Causal Agent of Brown Rot on Stone Fruit. Agronomy, 3(4), 685-703. https://doi.org/10.3390/agronomy3040685




