Increasing Food Production in Africa by Boosting the Productivity of Understudied Crops
Abstract
:Abbreviations and Acronyms
| AATF | African Agricultural Technology Foundation |
| ABNETA | Agricultural Biotechnology Network in Africa |
| ABSPII | Agricultural Biotechnology Support Project II |
| AFLP | Amplified Fragment Length Polymorphisms |
| AGRA | Alliance for a Green Revolution in Africa |
| ASARECA | Association for Strengthening Agricultural Research in Eastern and Central Africa |
| BecA | Biosciences eastern and central Africa |
| BioInnovate | Bio-resource Innovations Network for Eastern Africa Development |
| CAADP | Comprehensive Africa Agriculture Development Program |
| CGA | Candidate Gene Approach |
| CGIAR | Consultative Group on International Agricultural Research |
| CIAT | International Center for Tropical Agriculture |
| CIMMYT | International Maize and Wheat Improvement Center |
| CIP | International Potato Center |
| CIRAD | Agricultural Research Centre for International Development |
| CORAF/WECARD | West and Central African Council for Agric. Research and Development |
| CTA | Technical Centre for Agricultural and Rural Cooperation |
| DZARC | Debre Zeit Agricultural Research Center |
| EIAR | Ethiopian Institute of Agricultural Research |
| FAO | Food and Agriculture Organization of the United Nations |
| FAOSTAT | FAO statistical database |
| FARA | Forum for Agricultural Research in Africa |
| GA | Gibberellic acid |
| GBS | Genotyping-by-sequencing |
| GCP | Generation Challenge Programme |
| GFAR | Global Forum on Agricultural Research |
| GFU | Global Facilitation Unit for Underutilized Species |
| IAA | indole acetic acid |
| IAEA | International Atomic Energy Agency |
| IARCs | International agricultural research centers |
| ICARDA | International Center for Agricultural Research in the Dry Areas |
| ICRISAT | International Crops Research Institute for the Semi-Arid Tropics |
| ICUC | International Centre for Underutilized-Crops |
| IFAD | International Fund for Agricultural Development |
| IFPRI | International Food Policy Research Institute |
| IITA | International Institute of Tropical Agriculture |
| ILRI | International Livestock Research Institute |
| INDEL | Insertions and Deletions |
| IPBO | Institute of Plant Biotechnology for developing Countries |
| IRD | Institut de recherche pour le développement |
| ISAAA | International Service for the Acquisition of Agri-biotech Applications |
| MAS | marker-assisted selection |
| MoA | Ministry of Agriculture |
| NARS | National Agricultural Research Systems |
| NEPAD | New Partnership for Africa’s Development |
| NERICA | New Rice for Africa |
| NGO | non-governmental organization |
| NUE | nitrogen use efficiency |
| ODAP | β-N-Oxalyl-L-α, β-diaminopropanoic acid |
| PAEPARD | Platform for African–European Partnerships on Agric. Research for Development |
| PPB | participatory plant breeding |
| PVS | participatory variety selection |
| QTL | quantitative trait locus; RIL: recombinant inbred line |
| RAD | Restriction-site Associated DNA |
| SADC/FANR | Southern African Development Community/Food, Agric. and Natural Resources |
| SNP | Single Nucleotide Polymorphisms |
| SSR | Simple Sequence Repeats, also known as microsatellites |
| TALEN | Transcription Activator-like Effector Nuclease |
| TILLING | Targeting Induced Local Lesion IN Genomes |
| TIP | Tef Improvement Project |
1. Types and Significance of African Indigenous Crops
| Type of crop | Common Name | Botanical name | Desirable property | Undesirable property | Reference |
|---|---|---|---|---|---|
| Cereals | Finger millet | Eleusine coracana | High in iron & protein, low in glycemic index | Low productivity | [2,8] |
| Fonio | Digitaria exilis | Fast maturing | Low productivity | [5,8] | |
| African rice | Oryza glaberrima | Resistance to diseases & pests | Lodging & shattering of seed | [5,9] | |
| Pearl millet | Pennisetum glaucum | Drought & heat tolerance | Insect pests & diseases | [10] | |
| Tef | Eragrostis tef | Abiotic stress tolerance, free of gluten | Low productivity & lodging | [11,12] | |
| Leguminous crops | Bambara groundnut | Vigna subterranea | Nutritious & drought tolerance | Late maturing | [3] |
| Cowpea | Vigna unguiculata | Drought tolerance & nutritious | Low productivity & insects | [3] | |
| Grass pea | Lathyrus sativus | Extreme drought tolerance & nutritious | Toxic seeds | [13] | |
| Vegetables | Amaranth | Amaranthus spp. | Fast growing & nutritious | Insect pests & diseases | [3] |
| Celosia | Celosia argentea | High productivity | Sensitivity to nematodes & water-logging | [3,8] | |
| Dika | Irvingia gabonensis, I. wombolu | Rich in oil | Difficulty of kernel removal | [3] | |
| Okra | Abelmoschus esculentus | Tolerance to biotic stresses, fast growing & nutritious | Short shelf-life | [14] | |
| Oil seeds | Ethiopian Mustard | Brassica carinata | Drought tolerance & resistance to insect pests | Poor quality oil | [15] |
| Noug | Guizotia abyssinica | High oil content | Low productivity, insect pests | [16] | |
| Sesame | Sesamum indicum | Oxidatively stable oil | Low productivity & shattering | [2] | |
| Vernonia | Vernonia galamensis | High in industrial oil | [8,17] | ||
| Root crops | Cassava | Manihot esculentum | Drought tolerance | Toxic, less nutritious & diseases | [18] |
| African yam bean | Sphenostylis stenocarpa | High protein content | Late maturing | [3] | |
| Enset | Ensete ventricosum | Drought tolerance | Less nutritious | [19] | |
| Yam | Dioscorea spp | Drought tolerance | Less nutritious | [8] | |
| Sweet potato | Ipomoea batatas | Rich in riboflavin & calcium | Diseases & insect pests | [2] | |
| Fruits | Banana | Musa spp. | Healthy & nutritious | Pests & diseases | [20] |
| Plantain | Musa spp. | Healthy & nutritious | Pests & diseases | [20] |
1.1. Cereals
1.2. Leguminous Crops
1.3. Vegetables
1.4. Oil Seeds
1.5. Root Crops
1.6. Fruits
2. Need for Improving African Crops
2.1. Africa is Largely Food Insecure
2.2. Africa Missed Green Revolution
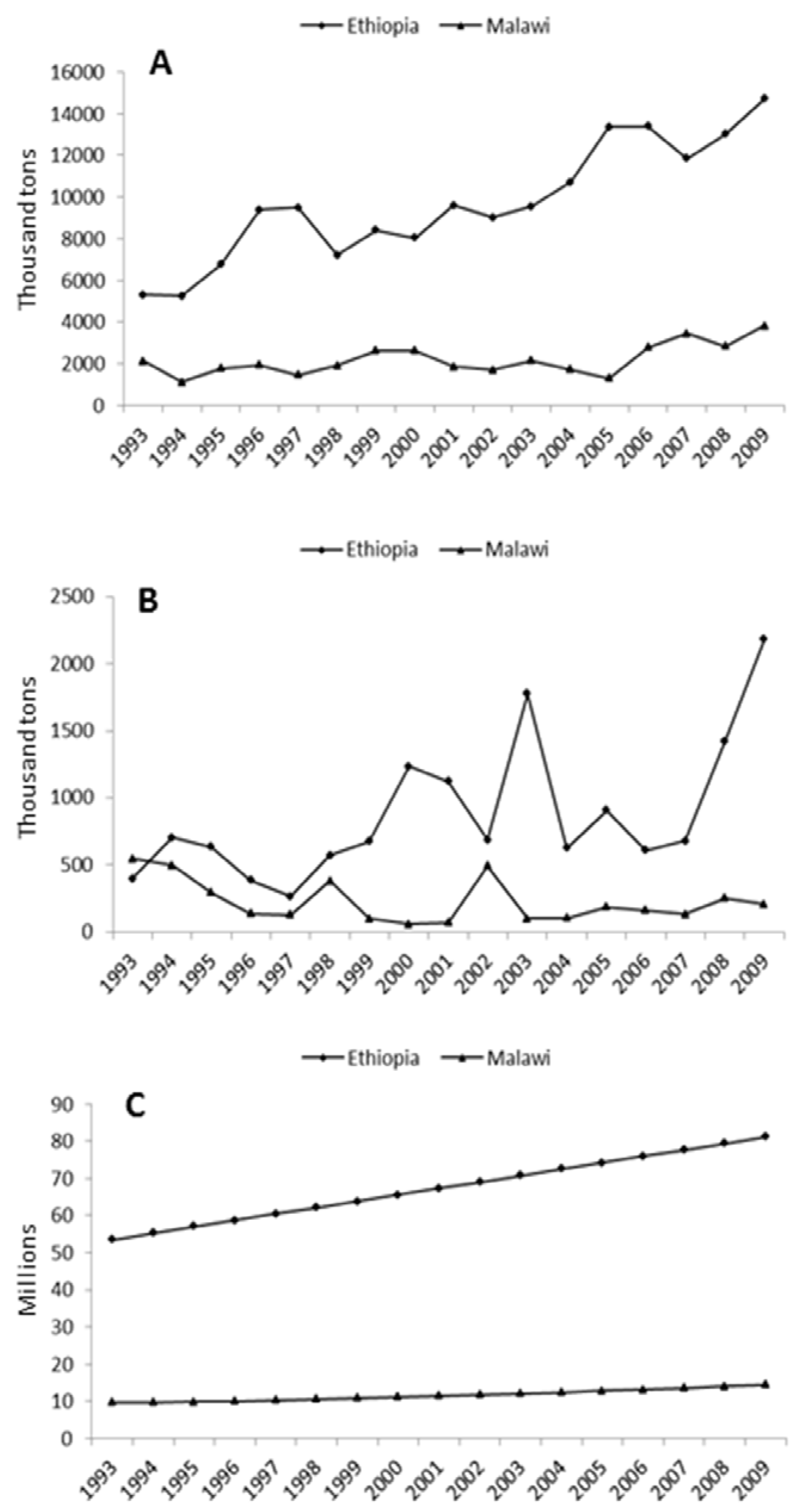
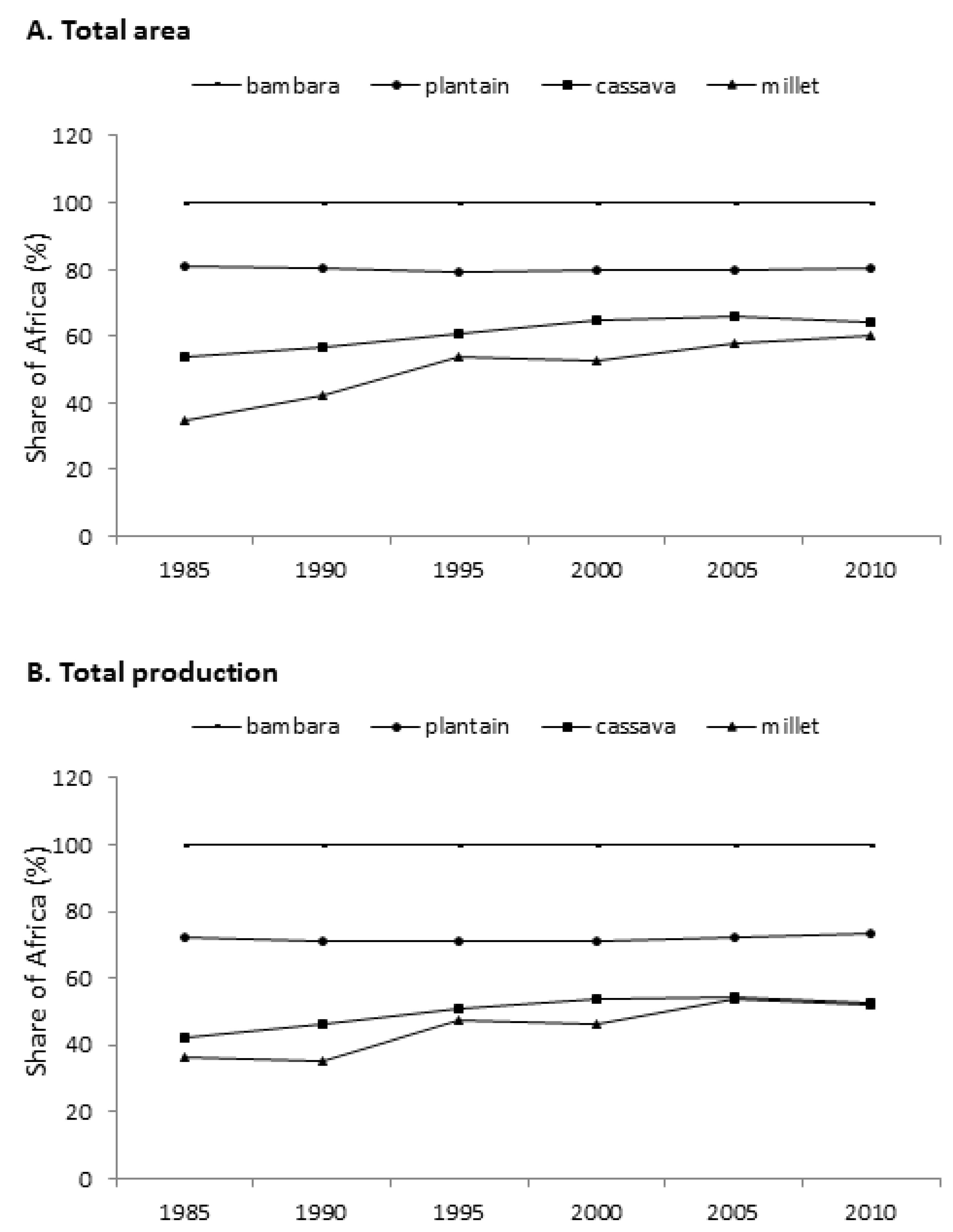
2.3. African Crops Fit the Agro-Ecology and Socio-Economic Conditions
2.4. African Crops Are Poor in Productivity
2.5. Efficient Tools and Inputs Are Not Applied in African Agriculture
2.6. Some African Crops Are Poor in Nutrition
2.7. Several African Crops Produce Toxic Substances
2.8. Prevalence of Large-Scale Biotic and Abiotic Stresses
2.9. Climate Change Adversely Affects Crop Production
3. Tools for Crop Improvement
3.1. Domestication and Selection
3.2. Hybridization
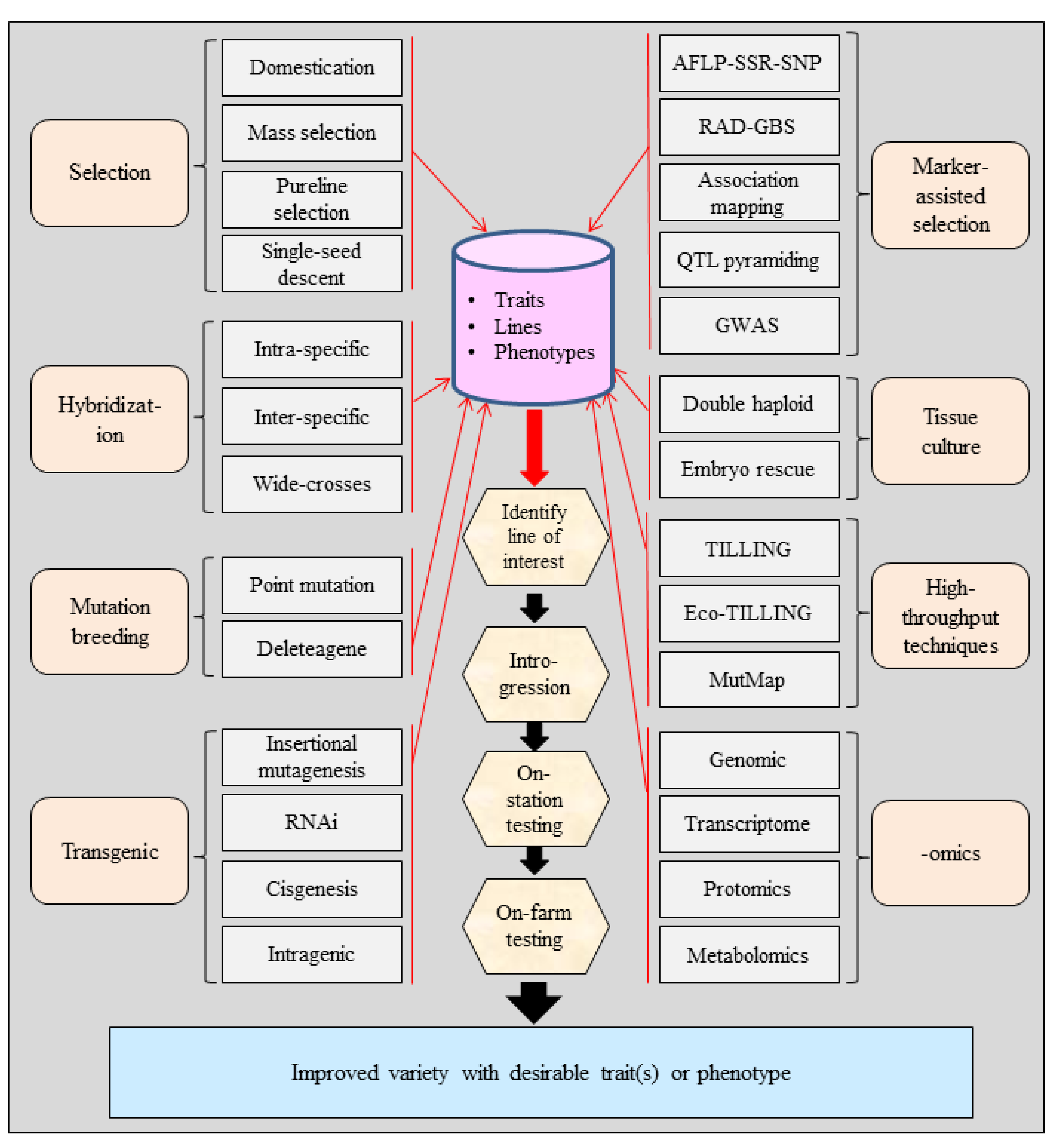
3.3. Mutation Breeding
3.4. Plant Cell and Tissue Culture
3.5. Marker-Assisted Selection
3.6. Candidate Gene Approach (CGA)
3.7. High-Throughput Mutation Detection
3.8. Genetic Engineering or Transgenics
3.9. Application of Genome and Transcriptome Sequencing
4. Agriculturally Important Traits
4.1. Yield Components
| Traits | Gene or locus identified | Reference | |||
|---|---|---|---|---|---|
| General | Specific | Name | Crop | Cloning method | |
| Plant architecture | Semi-dwarfism | Sd-1 | rice | Map-based | [135] |
| Rht-1 | wheat | Candidate gene | [136,137] | ||
| D8 | maize | Candidate gene | [136] | ||
| D1 | rice | Map-based | [138] | ||
| D2 | rice | Map-based | [139] | ||
| D11 | rice | Map-based | [140] | ||
| D35 | rice | Map-based | [141] | ||
| Unnamed | rice | Mutmap | [134] | ||
| Tillering | MOC1 | rice | Map-based | [142] | |
| TAC1 | rice | Map-based | [143] | ||
| HTD1 | rice | Map-based & Candidate Gene | [144] | ||
| Culm strength | FC1 | rice | T-DNA | [145] | |
| Lateral root | ZmHO-1 | maize | T-DNA | [146,147] | |
| Fruit size | Fw2.2 | tomato | Map-based | [148] | |
| Abiotic tolerance | Drought tolerance | Stg1 | sorghum | Map-based | [149] |
| Submergence tolerance | Sub1 | rice | Map-based | [150] | |
| Aluminum tolerance | MATE | sorghum | Map-based | [151] | |
| Salt tolerance | SKC1 | rice | Map-based | [152] | |
| Biotic tolerance | Bacterial resistance | Xa21 | rice | Map-based | [153] |
| Fungal resistance | Pi9 | rice | Map-based | [154] | |
| Nutritional quality | Starch | Waxy | rice | Sequencing | [155] |
| Consumer preference | Eating & cooking quality | Several genes | rice | Sequencing | [156] |
| Color of grain | R | wheat | Candidate gene | [157] | |
| Multiple traits | Leaf angle & grain yield | DWARF4 | rice | Tos17 Retrotransposon | [158] |
| Shoot branching & grain yield | SPL14 | rice | Map-based | [159] | |
| Branching pattern & grain yield | CKX2 | rice | Map-based | [160] | |
| Grain size & seed yield | qSW5 | rice | Map-based | [161] | |
| Grain filling & seed yield | GIF1 | rice | Map-based | [162] | |
| Panicle & grain yield | DEP1 | rice | Map-based | [163] | |
| Heading date & seed yield | Ghd7 | rice | Map-based | [164] | |
4.2. Stress Tolerance
4.3. Plant Architecture
4.4. Nutritional Quality
5. Institutions Involved in African Crops Research and Development
5.1. National Agricultural Research Systems (NARS)
| Major institute | Subsidiary institute/program | Role/involvement | Relevance to African crops | HQ or regional office | Reference |
|---|---|---|---|---|---|
| FARA | ASARECA | Strengthen NARS activity | Staple and non-staple crops | Entebbe, Uganda | [168] |
| CORAF/ | Research coordination | Staple and non-staple crops | Dakar, Senegal | [169] | |
| SADC/FANR | Research & Development | Not specified | Gaborone, Botswana | [170] | |
| Other African institutes | AATF | Technology transfer | Cassava, banana & cowpea | Kenya | [171] |
| Africa Harvest | Technology transfer | Banana & sorghum | Nairobi, Kenya | [172] | |
| ABNETA | Information provision | Not specified | Nairobi, Kenya | [173] | |
| AGRA | Capacity building | African crops | Nairobi, Kenya | [174] | |
| BeCA Hub | Research, training | Not specified | Nairobi, Kenya, | [175] | |
| BioInnovate Africa | bio-resource-based innovation systems | Millet, bean, cassava, sweet potato | Nairobi, Kenya | [176] | |
| CAADP | Research & development | Not specified | South Africa | [177] | |
| CGIAR centers | Africa Rice Center | Research & development | African rice | Contonou, Benin | [178] |
| Bioversity International | Research | Banana, plantain | Rome, Italy | [179] | |
| CIAT | Research | Beans, cassava | Cali, Colombia | [180] | |
| CIMMYT | Research | Wheat and maize | Mexico | [181] | |
| CIP | Research | Potato & sweet potato | Lima, Peru | [182] | |
| ICARDA | Research and training | lentil, barley and faba bean | Aleppo, Syria | [183] | |
| ICRISAT | Research | Pearl millet, Pigeonpea, chickpea, small millets | Patancheru, India | [184] | |
| GCP | Research & capacity building | Tropical legumes | Mexico | [185] | |
| IFPRI | Policy research | Not specified | Washington D.C. | [186] | |
| IITA | Research & capacity building | Cassava, yam, cowpea, banana, plantain | Ibadan, Nigeria | [187] | |
| Other organizations | ABSPII | Promote agricultural biotechnology | Banana | Cornell Univ. Ithaca, USA | [188] |
| CIRAD | Research & training | Banana, plantain, tree crops | Montpellier, France | [189] | |
| Crops for the Future | Training & policy issues | underutilized crops | Serdang, Malaysia | [190] | |
| CTA | Information & communication | Not specified | Wageningen, Netherlands | [191] | |
| ETH Zurich | Research & training | cassava | Zurich, Switzerland | [192] | |
| FAO | Development, Information systems | Not specified | Rome, Italy | [193] | |
| GFAR | Discussion forum | Not specified | Rome, Italy | [194] | |
| HarvestPlus | Research on biofortification | beans, cassava, maize, millet, rice, sweet potato | Washington DC, USA | [195] | |
| IFAD | Development | Not specified | Rome, Italy | [196] | |
| IPBO | Training and research | Banana, cassava, grass pea, sweet potato | Gent, Belgium | [197] | |
| IRD | Research & training | Not specified | Montpellier, France | [198] | |
| ISAAA AfriCenter | Development, & information provision | Banana | Nairobi, Kenya | [199] | |
| Joint FAO/IAEA Programme | Training, research & service provision | Not specified | Vienna, Austria | [200] | |
| Lab. Trop. Crop Improv. | Research and training | Banana and plantain | K.U. Leuven, Belgium | [201] | |
| PAEPARD | knowledge sharing | Not specified | Brussels, Belgium | [202] | |
| University of Bern | Research and training | Tef | Bern, Switzerland | [203] |
5.2. Consultative Group on International Agricultural Research (CGIAR) Centers
5.3. African Institutions
5.3.1. Comprehensive Africa Agriculture Development Program (CAADP)
5.3.2. Forum for Agricultural Research in Africa (FARA)
5.3.3. AGRA (Alliance for a Green Revolution in Africa)
5.3.4. BecA (Biosciences Eastern and Central Africa) Hub
5.3.5. AATF (African Agricultural Technology Foundation)
6. Successes in Improving African Crops: Case Examples
6.1. NERICA (New Rice for Africa): High Yielding and Stress Tolerant Rice
6.2. Quncho: A Popular Tef for Both Farmers and Consumers
7. Suggestions for Future Research and Development
7.1. Invest in Agricultural Research and Development
7.2. Germplasm Collection and Utilization
7.3. Identify the Right Breeding Tools
7.4. Define Ideotypes for Each Crop and Environment
7.5. Focus on Both Boosting Crop Productivity and Improving Ecosystem
| Crop | Average farmers’ yield | Yield potential | Yield gap | Improved system | Location/country | References |
|---|---|---|---|---|---|---|
| (kg ha−1) | ||||||
| Banana | 6,080 | 27,400 | 21,320 | Genotypes and management | West Africa | [226] |
| Cassava | 6,800 | 19,680 | 12,880 | Management, genotypes & fertilizer | Kenya | [227] |
| 10,300 | 23,333 | 13,033 | Management, genotypes & fertilizer | Uganda | [227] | |
| 9,150 | 14,000 | 4,850 | Genotypes and management | West Africa | [226] | |
| Millet | 720 | 2,430 | 1,710 | Genotypes and management | West Africa | [226] |
| Pearl millet | 1,610 | 4,200 | 2,590 | Genotype (dwarf type) | Samanko, Mali | [228] |
| 1,610 | 4,500 | 2,890 | Genotype (early maturing) | Cinzana, Mali | [228] | |
| Tef | 1,200 | 4,599 | 3,399 | Genotype (Dukem cultivar) | Debre Zeit, Ethiopia | [229] |
7.6. Select the Right Type of Strategy
7.7. Develop Crops That Adapt to Changing Climate
7.8. Invest in Innovation Agriculture
7.9. Focus on Sustainable Agriculture
7.10. Create Robust Extension System
7.11. Establish Partnership with Relevant Stakeholders
8. Conclusions
Acknowledgments
References and Notes
- Naylor, R.L.; Falcon, W.P.; Goodman, R.M.; Jahn, M.M.; Sengooba, T.; Tefera, H.; Nelson, R.J. Biotechnology in the developing world: A case for increased investments in orphan crops. Food Policy 2004, 29, 15–44. [Google Scholar] [CrossRef]
- Dawson, I.; Jaenicke, H. Underutilised Plant Species: The Role of Biotechnology; The International Centre for Underutilised Crops (ICUC): Colombo, Sri Lanka, 2006; p. 27. [Google Scholar]
- Lost Crops of Africa; National Academies Press: Washington, DC, USA, 2006; Volume ii, Vegetables.
- Lost Crops of Africa; National Academies Press: Washington, DC, USA, 2008; Volume iii, Fruits.
- Lost Crops of Africa; National Academy Press: Washington, DC, USA, 1996; Volume i, Grains.
- Bermejo, J.E.H.; León, J. Neglected Crops: 1492 from a Different Perspective; FAO: Rome, Italy, 1994. [Google Scholar]
- CFF Crops for the future. Available online: http://www.Cropsforthefuture.Org/ (accessed on 10 October 2012).
- Williams, J.T.; Haq, N. Global Research on Underutilised Crops: An. Assessment of Current Activities and Proposals for Enhanced Cooperation; International Centre for Underutilised Crops (ICUC): Southampton, UK, 2000; p. 50. [Google Scholar]
- Linares, O.F. African rice (Oryza glaberrima): History and future potential. Proc. Natl. Acad. Sci. USA 2002, 99, 16360–16365. [Google Scholar] [CrossRef]
- Wikipedia Pearl millet. Available online: http://en.wikipedia.org/wiki/Pearl_millet (accessed on 9 July 2012).
- Ketema, S. Tef, Eragrostis tef (Zucc.) Trotter; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1997; p. 52. [Google Scholar]
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; Koning, F. The Ethiopian cereal tef in celiac disease. N. Engl. J. Med. 2005, 353, 1748–1749. [Google Scholar] [CrossRef]
- Campell, C.G. Grass pea (Lathyrus sativusL.); International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- Wikipedia Okra. Available online: http://en.wikipedia.org/wiki/Okra (accessed on 9 July 2012).
- Getinet, A.; Rakow, G.; Downey, R.K. Agronomic performance and seed quality of Ethiopian mustard in Saskatchewan. Can. J. Plant Sci. 1996, 76, 387–392. [Google Scholar]
- Getinet, A.; Sharma, S.M. Niger. Guizotia abyssinica (L. F.) Cass; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1996. [Google Scholar]
- Arraiano, L.S.; Brading, P.A.; Dedryver, F.; Brown, J.K.M. Resistance of wheat to septoria tritici blotch (Mycosphaerella graminicola) and associations with plant ideotype and the 1BL-1RS translocation. Plant. Pathol. 2006, 55, 54–61. [Google Scholar] [CrossRef]
- Ceballos, H.; Iglesias, C.A.; Perez, J.C.; Dixon, A.G.O. Cassava breeding: Opportunities and challenges. Plant. Mol. Biol. 2004, 56, 503–516. [Google Scholar] [CrossRef]
- Brandt, S.A. The "Tree Against Hunger": Enset-Based Agricultural System in Ethiopia; American Association for the Advancement of Science: Washington, DC, USA, 1997. [Google Scholar]
- Heslop-Harrison, J.S.; Schwarzacher, T. Domestication, genomics and the future for banana. Ann. Bot. Lond. 2007, 100, 1073–1084. [Google Scholar]
- Léder, I. Sorghum and Millets; UNESCO, Eolss Publishers: Oxford, UK, 2004. [Google Scholar]
- Chandrasekara, A.; Shahidi, F. Antiproliferative potential and DNA scission inhibitory activity of phenolics from whole millet grains. J. Funct. Foods 2011, 3, 159–170. [Google Scholar]
- ICRISAT Pearl millet [Pennisetum glaucum (L.) r. Br.]. Available online: http://www.icrisat.org/crop-pearlmillet.htm (accessed on 17 July 2012).
- Chandrashekar, A. Finger millet Eleusine coracana. Adv. Food Nutr. Res. 2010, 59, 215–262. [Google Scholar] [CrossRef]
- CSA, Agricultural Sample Survey 2010/11; Central Statistical Agency: Addis Ababa, Ethiopia, 2011.
- Hopman, E.; Dekking, L.; Blokland, M.L.; Wuisman, M.; Zuijderduin, W.; Koning, F.; Schweizer, J. Tef in the diet of celiac patients in the Netherlands. Scand. J. Gastroenterol. 2008, 43, 277–282. [Google Scholar]
- IPGRI, Promoting Fonio Production in West and Central Africa through Germplasm Management and Improvement of Post Harvest Technology; International Plant Genetic Resources Institute: Cotonou, Benin, 2004; p. 18.
- Asiwe, J.A.N. Field Evaluation of Bambara Groundnut; University of Bern, Stampfli: Bern, Switzerland, 2009; pp. 93–98. [Google Scholar]
- Sanginga, N.; Lyasse, O.; Singh, B.B. Phosphorus use efficiency and nitrogen balance of cowpea breeding lines in a low P soil of the derived savanna zone in West Africa. Plant. Soil 2000, 220, 119–128. [Google Scholar] [CrossRef]
- Valenzuela, H.; Smith, J. Cowpea; University of Hawaii, College of Tropical Agriculture and Human Resources: Manoa, HI, USA, 2002; p. 3. [Google Scholar]
- Sautter, C.; Poletti, S.; Zhang, P.; Gruissem, W. Biofortification of essential nutritional compounds and trace elements in rice and cassava. P. Nutr. Soc. 2006, 65, 153–159. [Google Scholar]
- FAOSTAT Crop production. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor (accessed on 9 July 2012).
- Fungo, R. Potential of bananas in alleviating micronutrient eficiencies in the Great Lakes Region of East Africa. In African Crop Science Conference Proceedings; African Crop Science Society: Kampala, Uganda, 2009; 9, p. 8. [Google Scholar]
- GFS The global food security (GFS) initiative. Available online: http://www.globalfoodsec.net/modules/fast_facts (accessed on 6 Septemeber 2012).
- FAOSTAT Trade. Available online: http://faostat.fao.org/site/342/default.aspx (accessed on 6 September 2012).
- Worldbank Population. Available online: http://data.worldbank.org/indicator/SP.POP.TOTL (accessed on 6 September 2012).
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar]
- IFPRI, Green Revolution: Curse or Blessing? International Food Policy Research Institute: Washington, DC, USA, 2002; p. 4.
- Ejeta, G. African green revolution needn’t be a mirage. Science 2010, 327, 831–832. [Google Scholar]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar]
- Kebede, A.; McCann, J.C.; Kiszewski, A.E.; Ye-Ebiyo, Y. New evidence of the effects of agro-ecologic change on malaria transmission. Am. J. Trop. Med. Hyg. 2005, 73, 676–680. [Google Scholar]
- Ye-Ebiyo, Y.; Pollack, R.J.; Spielman, A. Enhanced development in nature of larval Anopheles arabiensis mosquitoes feeding on maize pollen. Am. J. Trop. Med. Hyg. 2000, 63, 90–93. [Google Scholar]
- Pollack, R.J.; Robich, R.M.; Kiszewski, A.E.; Kebede, A.; Ye-Ebiyo, Y.; Hailemariam, A.T.; Diblasi, M.; McCann, J.; Spielman, A. Influence of maize pollen on anopheles productivity and malaria transmission dynamics. Am. J. Trop. Med. Hyg. 2007, 77, 254–255. [Google Scholar]
- WHO, Malaria. Fact. Sheet N°94; World Health Organization: Geneva, Switzerland, 2012.
- Assefa, K.; Yu, J.K.; Zeid, M.; Belay, G.; Tefera, H.; Sorrells, M.E. Breeding tef [Eragrostis tef (Zucc.) Trotter]: Conventional and molecular approaches. Plant Breed. 2011, 130, 1–9. [Google Scholar] [CrossRef]
- Post harvest losses information system. Available online: http://www.aphlis.net/index.php?form=home (accessed on 7 September 2012).
- ECA Agricultural input business development in Africa: Opportunities, issues and challenges. Available online: http://www.uneca.org/sa/publications/SRO-SA-AGRI-IPUTS-BUSINESS-OPPORTUNITIES.pdf (accessed on 7 September 2012).
- Denning, G.; Kabambe, P.; Sanchez, P.; Malik, A.; Flor, R.; Harawa, R.; Nkhoma, P.; Zamba, C.; Banda, C.; Magombo, C.; Keating, M.; Wangila, J.; Sachs, J. Input subsidies to improve smallholder maize productivity in Malawi: Toward an African green revolution. PLoS Biol. 2009, 7, 2–10. [Google Scholar]
- Stephenson, K.; Amthor, R.; Mallowa, S.; Nungo, R.; Maziya-Dixon, B.; Gichuki, S.; Mbanaso, A.; Manary, M. Consuming cassava as a staple food places children 2–5 years old at risk for inadequate protein intake, an observational study in Kenya and Nigeria. Nutr. J. 2010, 9, 9. [Google Scholar]
- Gegios, A.; Amthor, R.; Maziya-Dixon, B.; Egesi, C.; Mallowa, S.; Nungo, R.; Gichuki, S.; Mbanaso, A.; Manary, M.J. Children consuming cassava as a staple food are at risk for inadequate zinc, iron, and vitamin a intake. Plant. Food Hum. Nutr. 2010, 65, 64–70. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Spencer, P.S.; Li, Z.X.; Liang, Y.M.; Wang, Y.F.; Wang, C.Y.; Li, F.M. Lathyrus sativus (grass pea) and its neurotoxin ODAP. Phytochemistry 2006, 67, 107–121. [Google Scholar]
- Getahun, H.; Lambein, F.; Vanhoorne, M.; Van der Stuyft, P. Food-aid cereals to reduce neurolathyrism related to grass-pea preparations during famine. Lancet 2003, 362, 1808–1810. [Google Scholar]
- Gale, M. Applications of Molecular Biology and Genomics to Genetic Enhancement of Crop Tolerance to Abiotic Stress: A Discussion Document; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; p. 56. [Google Scholar]
- Muller, C.; Cramer, W.; Hare, W.L.; Lotze-Campen, H. Climate change risks for African agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 4313–4315. [Google Scholar]
- Peng, S.B.; Huang, J.L.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.H.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar]
- Funk, C.; Dettinger, M.D.; Michaelsen, J.C.; Verdin, J.P.; Brown, M.E.; Barlow, M.; Hoell, A. Warming of the indian ocean threatens eastern and southern African food security but could be mitigated by agricultural development. Proc. Natl. Acad. Sci. USA 2008, 105, 11081–11086. [Google Scholar]
- Fauchereau, N.; Trzaska, S.; Rouault, M.; Richard, Y. Rainfall variability and changes in southern Africa during the 20th century in the global warming context. Nat. Hazards 2003, 29, 139–154. [Google Scholar]
- Shongwe, M.E.; van Oldenborgh, G.J.; van den Hurk, B.J.J.M.; de Boer, B.; Coelho, C.A.S.; van Aalst, M.K. Projected changes in mean and extreme precipitation in Africa under global warming. Part i: Southern Africa. J. Climate 2009, 22, 3819–3837. [Google Scholar] [CrossRef]
- Shongwe, M.E.; van Oldenborgh, G.J.; van den Hurk, B.; van Aalst, M. Projected changes in mean and extreme precipitation in Africa under global warming. Part ii: East Africa. J. Climate 2011, 24, 3718–3733. [Google Scholar] [CrossRef]
- Sarr, B. Present and future climate change in the semi-arid region of West Africa: A crucial input for practical adaptation in agriculture. Atmos. Sci. Lett. 2012, 13, 108–112. [Google Scholar] [CrossRef]
- Sang, T. Genes and mutations underlying domestication transitions in grasses. Plant. Physiol. 2009, 149, 63–70. [Google Scholar]
- Gross, B.L.; Olsen, K.M. Genetic perspectives on crop domestication. Trends Plant. Sci. 2010, 15, 529–537. [Google Scholar]
- Ghosh, A.K. Modern methods for selection of plants with better characterisitcs. Available online: http://www.biotecharticles.com/Agriculture-Article/Modern-Methods-For-Selection-of-Plants-With-Better-Characterisitcs-860.html (accessed on 6 July 2012).
- Baenziger, P.S.; Russell, W.K.; Graef, G.L.; Campbell, B.T. Improving lives: 50 years of crop breeding, genetics, and cytology (c-1). Crop. Sci. 2006, 46, 2230–2244. [Google Scholar] [CrossRef]
- Borlaug, N.E. Sixty-two years of fighting hunger: Personal recollections. Euphytica 2007, 157, 287–297. [Google Scholar]
- Lippman, Z.B.; Zamir, D. Heterosis: Revisiting the magic. Trends Genet. 2007, 23, 60–66. [Google Scholar]
- Kim, B.Y.; Baier, A.C.; Somers, D.J.; Gustafson, J.P. Aluminum tolerance in triticale, wheat, and rye. Euphytica 2001, 120, 329–337. [Google Scholar] [CrossRef]
- Sharma, H.C. How wide can a wide cross be. Euphytica 1995, 82, 43–64. [Google Scholar]
- Li, X.; Lassner, M.; Zhang, Y.L. Deleteagene: A fast neutron deletion mutagenesis-based gene knockout system for plants. Comp. Funct. Genomics 2002, 3, 158–160. [Google Scholar]
- Ahloowalia, B.S.; Maluszynski, M.; Nichterlein, K. Global impact of mutation-derived varieties. Euphytica 2004, 135, 187–204. [Google Scholar]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar]
- Jimenez, V.M. Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul. 2005, 47, 91–110. [Google Scholar] [CrossRef]
- Maqbool, S.B.; Devi, P.; Sticklen, M.B. Biotechnology: Genetic improvement of sorghum (Sorghum bicolor (L.) Moench). In Vitro Cell. Dev. Biol. Plant 2001, 37, 504–515. [Google Scholar] [CrossRef]
- Vasil, I.K. Plant-tissue culture and molecular-biology as tools in understanding plant development and in plant improvement. Curr. Opin. Biotechnol. 1991, 2, 158–163. [Google Scholar]
- Lakshmanan, P.; Taji, A. Somatic embryogenesis in leguminous plants. Plant Biol. 2000, 2, 136–148. [Google Scholar]
- Bal, U.; Abak, K. Haploidy in tomato (Lycopersicon esculentum mill.): A critical review. Euphytica 2007, 158, 1–9. [Google Scholar] [CrossRef]
- Ochoa-Alejo, N.; Ramirez-Malagon, R. Invited review: In vitro chili pepper biotechnology. In Vitro Cell. Dev. Biol. Plant 2001, 37, 701–729. [Google Scholar] [CrossRef]
- Wahid, M.B.; Abdullah, S.N.A.; Henson, I.E. Oil palm—Achievements and potential. Plant. Prod. Sci. 2005, 8, 288–297. [Google Scholar] [CrossRef]
- Uma, S.; Lakshmi, S.; Saraswathi, M.S.; Akbar, A.; Mustaffa, M.M. Embryo rescue and plant regeneration in banana (Musa spp.). Plant Cell. Tiss. Org. 2011, 105, 105–111. [Google Scholar] [CrossRef]
- Giri, C.C.; Shyamkumar, B.; Anjaneyulu, C. Progress in tissue culture, genetic transformation and applications of biotechnology to trees: An overview. Trees Struct. Funct. 2004, 18, 115–135. [Google Scholar] [CrossRef]
- Golle, D.P.; Reiniger, L.R.S.; Curti, A.R.; Bevilacqua, C.B. Forestry improvement: Emphasis on biotechnology application. Cienc. Rural. 2009, 39, 1606–1613. [Google Scholar]
- Bapat, V.A.; Yadav, S.R.; Dixit, G.B. Rescue of endangered plants through biotechnological applications. Natl. Acad. Sci. Lett. 2008, 31, 201–210. [Google Scholar]
- Germana, M.A. Gametic embryogenesis and haploid technology as valuable support to plant breeding. Plant. Cell. Rep. 2011, 30, 839–857. [Google Scholar] [CrossRef]
- Akinbo, O.; Labuschagne, M.; Fregene, M. Embryo rescue as a method to develop and multiply a backcross population of cassava (Manihot esculenta Crantz) from an interspecific cross of Manihot esculenta ssp. flabellifolia. Afr. J. Biotechnol. 2010, 9, 7058–7062. [Google Scholar]
- Clarke, H.J.; Wilson, J.G.; Kuo, I.; Lulsdorf, M.M.; Mallikarjuna, N.; Kuo, J.; Siddique, K.H.M. Embryo rescue and plant regeneration in vitro of selfed chickpea (Cicer arietinum L.) and its wild annual relatives. Plant Cell. Tiss. Org. 2006, 85, 197–204. [Google Scholar] [CrossRef]
- Price, H.J.; Hodnett, G.L.; Burson, B.L.; Dillon, S.L.; Rooney, W.L. A Sorghum bicolor × S. macrospermum hybrid recovered by embryo rescue and culture. Aust. J. Bot. 2005, 53, 579–582. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar]
- Charcosset, A.; Moreau, L. Use of molecular markers for the development of new cultivars and the evaluation of genetic diversity. Euphytica 2004, 137, 81–94. [Google Scholar]
- Collard, B.C.Y.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B 2008, 363, 557–572. [Google Scholar]
- Anonymous, Amplified Fragment Length Polymorphism (AFLP®) Analysis on Applied Biosystems Capillary Electrophoresis Systems; Applied Biosystems: San Francisco, CA, USA, 2005.
- Kloth, K.J.; Thoen, M.P.M.; Bouwmeester, H.J.; Jongsma, M.A.; Dicke, M. Association mapping of plant resistance to insects. Trends Plant Sci. 2012, 17, 311–319. [Google Scholar]
- Santra, D.K.; Chen, X.M.; Santra, M.; Campbell, K.G.; Kidwell, K.K. Identification and mapping QTL for high-temperature adult-plant resistance to stripe rust in winter wheat (Triticum aestivum L.) cultivar 'Stephens'. Theor. Appl. Genet. 2008, 117, 793–802. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Lemmens, M.; Steiner, B.; Buerstmayr, H. Advanced backcross QTL mapping of resistance to Fusarium head blight and plant morphological traits in a Triticum macha × T. aestivum population. Theor. Appl. Genet. 2011, 123, 293–306. [Google Scholar] [CrossRef]
- Brown, P.J.; Rooney, W.L.; Franks, C.; Kresovich, S. Efficient mapping of plant height quantitative trait loci in a sorghum association population with introgressed dwarfing genes. Genetics 2008, 180, 629–637. [Google Scholar]
- Richardson, K.L.; Vales, M.I.; Kling, J.G.; Mundt, C.C.; Hayes, P.M. Pyramiding and dissecting disease resistance QTL to barley stripe rust. Theor. Appl. Genet. 2006, 113, 485–495. [Google Scholar]
- Bovill, W.D.; Horne, M.; Herde, D.; Davis, M.; Wildermuth, G.B.; Sutherland, M.W. Pyramiding QTL increases seedling resistance to crown rot (Fusarium pseudograminearum) of wheat (Triticum aestivum). Theor. Appl. Genet. 2010, 121, 127–136. [Google Scholar] [CrossRef]
- Fungo, R.; Pillay, M. Beta-carotene content of selected banana genotypes from uganda. Afr. J. Biotechnol. 2011, 10, 5423–5430. [Google Scholar]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 2011, 6, e19379. [Google Scholar]
- Barchi, L.; Lanteri, S.; Portis, E.; Acquadro, A.; Vale, G.; Toppino, L.; Rotino, G.L. Identification of SNP and SSR markers in eggplant using RAD tag sequencing. BMC Genomics 2011, 12, 304. [Google Scholar]
- Pfender, W.F.; Saha, M.C.; Johnson, E.A.; Slabaugh, M.B. Mapping with rad (restriction-site associated DNA) markers to rapidly identify QTL for stem rust resistance in Lolium perenne. Theor. Appl. Genet. 2011, 122, 1467–1480. [Google Scholar] [CrossRef]
- Weng, J.F.; Xie, C.X.; Hao, Z.F.; Wang, J.J.; Liu, C.L.; Li, M.S.; Zhang, D.G.; Bai, L.; Zhang, S.H.; Li, X.H. Genome-wide association study identifies candidate genes that affect plant height in chinese elite maize (Zea mays L.) inbred lines. PLoS One 2011, 6, e29229. [Google Scholar]
- Raman, H.; Stodart, B.; Ryan, P.R.; Delhaize, E.; Emebiri, L.; Raman, R.; Coombes, N.; Milgate, A. Genome-wide association analyses of common wheat (Triticum aestivum L.) germplasm identifies multiple loci for aluminium resistance. Genome 2010, 53, 957–966. [Google Scholar] [CrossRef]
- Pflieger, S.; Lefebvre, V.; Causse, M. The candidate gene approach in plant genetics: A review. Mol. Breed. 2001, 7, 275–291. [Google Scholar] [CrossRef]
- McCallum, C.M.; Comai, L.; Greene, E.A.; Henikoff, S. Targeted screening for induced mutations. Nat. Biotechnol. 2000, 18, 455–457. [Google Scholar] [CrossRef]
- Till, B.J.; Reynolds, S.H.; Greene, E.A.; Codomo, C.A.; Enns, L.C.; Johnson, J.E.; Burtner, C.; Odden, A.R.; Young, K.; Taylor, N.E.; Henikoff, J.G.; Comai, L.; Henikoff, S. Large-scale discovery of induced point mutations with high-throughput TILLING. Genome Res. 2003, 13, 524–530. [Google Scholar] [CrossRef]
- Till, B.J.; Reynolds, S.H.; Weil, C.; Springer, N.; Burtner, C.; Young, K.; Bowers, E.; Codomo, C.A.; Enns, L.C.; Odden, A.R.; Greene, E.A.; Comai, L.; Henikoff, S. Discovery of induced point mutations in maize genes by TILLING. BMC Plant Biol. 2004, 4, 12. [Google Scholar] [CrossRef]
- Slade, A.J.; Fuerstenberg, S.I.; Loeffler, D.; Steine, M.N.; Facciotti, D. A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat. Biotechnol. 2005, 23, 75–81. [Google Scholar]
- Uauy, C.; Paraiso, F.; Colasuonno, P.; Tran, R.K.; Tsai, H.; Berardi, S.; Comai, L.; Dubcovsky, J. A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biol. 2009, 9, 115. [Google Scholar]
- Till, B.J.; Cooper, J.; Tai, T.H.; Colowit, P.; Greene, E.A.; Henikoff, S.; Comai, L. Discovery of chemically induced mutations in rice by TILLING. BMC Plant Biol. 2007, 7, 19. [Google Scholar]
- Suzuki, T.; Eiguchi, M.; Kumamaru, T.; Satoh, H.; Matsusaka, H.; Moriguchi, K.; Nagato, Y.; Kurata, N. Mnu-induced mutant pools and high performance TILLING enable finding of any gene mutation in rice. Mol. Genet. Genomics 2008, 279, 213–223. [Google Scholar]
- Caldwell, D.G.; McCallum, N.; Shaw, P.; Muehlbauer, G.J.; Marshall, D.F.; Waugh, R. A structured mutant population for forward and reverse genetics in barley (Hordeum vulgare L.). Plant J. Cell Mol. Biol. 2004, 40, 143–150. [Google Scholar] [CrossRef]
- Lababidi, S.; Mejlhede, N.; Rasmussen, S.K.; Backes, G.; Al-Said, W.; Baum, M.; Jahoor, A. Identification of barley mutants in the cultivar ‘Lux’ at the Dhn loci through TILLING. Plant Breed. 2009, 128, 332–336. [Google Scholar]
- Xin, Z.G.; Wang, M.L.; Barkley, N.A.; Burow, G.; Franks, C.; Pederson, G.; Burke, J. Applying genotyping (TILLING) and phenotyping analyses to elucidate gene function in a chemically induced sorghum mutant population. BMC Plant Biol. 2008, 8, 103. [Google Scholar] [CrossRef]
- Tadele, Z.; Mba, C.; Till, B.J. TILLING for Mutations in Model Plants and Crops. In Molecular Techniques in Crop Improvement, 2nd; Jain, S.M., Brar, S.D., Eds.; Springer: Dordrecht, the Netherlands, 2010; pp. 307–332. [Google Scholar]
- Comai, L.; Young, K.; Till, B.J.; Reynolds, S.H.; Greene, E.A.; Codomo, C.A.; Enns, L.C.; Johnson, J.E.; Burtner, C.; Odden, A.R.; Henikoff, S. Efficient discovery of DNA polymorphisms in natural populations by Ecotilling. Plant J. 2004, 37, 778–786. [Google Scholar] [CrossRef]
- Bhatnagar-Mathur, P.; Vadez, V.; Sharma, K.K. Transgenic approaches for abiotic stress tolerance in plants: Retrospect and prospects. Plant Cell. Rep. 2008, 27, 411–424. [Google Scholar]
- Small, I. RNAi for revealing and engineering plant gene functions. Curr. Opin. Biotechnol. 2007, 18, 148–153. [Google Scholar]
- Watanabe, Y. Overview of plant rnai. Methods Mol. Biol. 2011, 744, 1–11. [Google Scholar]
- Tang, G.L.; Galili, G.; Zhuang, X. RNAi and microRNA: Breakthrough technologies for the improvement of plant nutritional value and metabolic engineering. Metabolomics 2007, 3, 357–369. [Google Scholar]
- Rosso, M.N.; Jones, J.T.; Abad, P. RNAi and functional genomics in plant parasitic nematodes. Annu. Rev. Phytopathol. 2009, 47, 207–232. [Google Scholar]
- Niu, J.H.; Jian, H.; Xu, J.M.; Guo, Y.D.; Liu, Q.A. RNAi technology extends its reach: Engineering plant resistance against harmful eukaryotes. Afr. J. Biotechnol. 2010, 9, 7573–7582. [Google Scholar]
- Mentewab, A.; Stewart, C.N., Jr. Overexpression of an Arabidopsis thaliana ABC transporter confers kanamycin resistance to transgenic plants. Nat. Biotechnol. 2005, 23, 1177–1180. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Prasad, K.; Bhatnagar-Mathur, P.; Narasu, M.L.; Waliyar, F.; Sharma, K.K. An efficient method for the production of marker-free transgenic plants of peanut (Arachis hypogaea L.). Plant Cell. Rep. 2010, 29, 495–502. [Google Scholar] [CrossRef]
- Shukla, V.K.; Doyon, Y.; Miller, J.C.; DeKelver, R.C.; Moehle, E.A.; Worden, S.E.; Mitchell, J.C.; Arnold, N.L.; Gopalan, S.; Meng, X.; Choi, V.M.; Rock, J.M.; Wu, Y.Y.; Katibah, G.E.; Zhifang, G.; McCaskill, D.; Simpson, M.A.; Blakeslee, B.; Greenwalt, S.A.; Butler, H.J.; Hinkley, S.J.; Zhang, L.; Rebar, E.J.; Gregory, P.D.; Urnov, F.D. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 2009, 459, 437–441. [Google Scholar]
- Townsend, J.A.; Wright, D.A.; Winfrey, R.J.; Fu, F.; Maeder, M.L.; Joung, J.K.; Voytas, D.F. High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 2009, 459, 442–445. [Google Scholar] [CrossRef]
- Jacobsen, E.; Schouten, H.J. Cisgenesis strongly improves introgression breeding and induced translocation breeding of plants. Trends Biotechnol. 2007, 25, 219–223. [Google Scholar] [CrossRef]
- Schouten, H.J.; Krens, F.A.; Jacobsen, E. Cisgenic plants are similar to traditionally bred plants: International regulations for genetically modified organisms should be altered to exempt cisgenesis. EMBO Rep. 2006, 7, 750–753. [Google Scholar]
- Rommens, C.M. Intragenic crop improvement: Combining the benefits of traditional breeding and genetic engineering. J. Agric. Food Chem. 2007, 55, 4281–4288. [Google Scholar] [CrossRef]
- Rommens, C.M.; Haring, M.A.; Swords, K.; Davies, H.V.; Belknap, W.R. The intragenic approach as a new extension to traditional plant breeding. Trends Plant Sci. 2007, 12, 397–403. [Google Scholar]
- Cermak, T.; Doyle, E.L.; Christian, M.; Wang, L.; Zhang, Y.; Schmidt, C.; Baller, J.A.; Somia, N.V.; Bogdanove, A.J.; Voytas, D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011, 39, 7879–7879. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency talen-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef]
- Perez-de-Castro, A.M.; Vilanova, S.; Canizares, J.; Pascual, L.; Blanca, J.M.; Diez, M.J.; Prohens, J.; Pico, B. Application of genomic tools in plant breeding. Curr. Genomics 2012, 13, 179–195. [Google Scholar]
- Mutasa-Gottgens, E.S.; Joshi, A.; Holmes, H.F.; Hedden, P.; Gottgens, B. A new rnaseq-based reference transcriptome for sugar beet and its application in transcriptome-scale analysis of vernalization and gibberellin responses. BMC Genomics 2012, 13, 99. [Google Scholar]
- Abe, A.; Kosugi, S.; Yoshida, K.; Natsume, S.; Takagi, H.; Kanzaki, H.; Matsumura, H.; Mitsuoka, C.; Tamiru, M.; Innan, H.; Cano, L.; Kamoun, S.; Terauchi, R. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 2012, 30, 174–178. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef]
- Peng, J.R.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; Sudhakar, D.; Christou, P.; Snape, J.W.; Gale, M.D.; Harberd, N.P. Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar]
- Pearce, S.; Saville, R.; Vaughan, S.P.; Chandler, P.M.; Wilhelm, E.P.; Sparks, C.A.; Al-Kaff, N.; Korolev, A.; Boulton, M.I.; Phillips, A.L.; Hedden, P.; Nicholson, P.; Thomas, S.G. Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat. Plant Physiol. 2011, 157, 1820–1831. [Google Scholar] [CrossRef]
- Ashikari, M.; Wu, J.; Yano, M.; Sasaki, T.; Yoshimura, A. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 1999, 96, 10284–10289. [Google Scholar]
- Hong, Z.; Ueguchi-Tanaka, M.; Umemura, K.; Uozu, S.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome p450. Plant cell 2003, 15, 2900–2910. [Google Scholar] [CrossRef]
- Yamamuro, C.; Ihara, Y.; Wu, X.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant cell 2000, 12, 1591–1606. [Google Scholar] [Green Version]
- Itoh, H.; Tatsumi, T.; Sakamoto, T.; Otomo, K.; Toyomasu, T.; Kitano, H.; Ashikari, M.; Ichihara, S.; Matsuoka, M. A rice semi-dwarf gene, Tan-Ginbozu (d35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant. Mol. Biol. 2004, 54, 533–547. [Google Scholar] [CrossRef]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Wang, X.; Liu, X.; Teng, S.; Hiroshi, F.; Yuan, M.; Luo, D.; Han, B.; Li, J. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar]
- Yu, B.; Lin, Z.; Li, H.; Li, X.; Li, J.; Wang, Y.; Zhang, X.; Zhu, Z.; Zhai, W.; Wang, X.; Xie, D.; Sun, C. TAC1, a major quantitative trait locus controlling tiller angle in rice. Plant J. Cell Mol. Biol. 2007, 52, 891–898. [Google Scholar] [CrossRef]
- Zou, J.H.; Zhang, S.Y.; Zhang, W.P.; Li, G.; Chen, Z.X.; Zhai, W.X.; Zhao, X.F.; Pan, X.B.; Xie, Q.; Zhu, L.H. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J. 2006, 48, 687–696. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Y.; Yao, J.L.; Chen, G.X.; Li, X.H.; Zhang, Q.F.; Wu, C.Y. FLEXIBLE CULM 1 encoding a cinnamyl-alcohol dehydrogenase controls culm mechanical strength in rice. Plant Mol. Biol. 2009, 69, 685–697. [Google Scholar]
- Han, B.; Xu, S.; Xie, Y.J.; Huang, J.J.; Wang, L.J.; Yang, Z.; Zhang, C.H.; Sun, Y.; Shen, W.B.; Xie, G.S. ZmHO-1, a maize haem oxygenase-1 gene, plays a role in determining lateral root development. Plant Sci. 2012, 184, 63–74. [Google Scholar]
- Brady, S.M.; Sarkar, S.F.; Bonetta, D.; McCourt, P. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant. J. 2003, 34, 67–75. [Google Scholar] [CrossRef]
- Frary, A.; Nesbitt, T.C.; Frary, A.; Grandillo, S.; van der Knaap, E.; Cong, B.; Liu, J.P.; Meller, J.; Elber, R.; Alpert, K.B.; Tanksley, S.D. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar]
- Sajjanar, G.M.; Biradar, B.D.; Kuruvinashetty, M.S.; Biradar, D.P.; Yashoda, M.H.; Biradar, S.S. Marker assisted introgression of terminal drought resistance (stay-green) QTLS in Sorghum. Res. J. Biotechnol. 2008, 340–344. [Google Scholar]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef]
- Magalhaes, J.V.; Liu, J.; Guimaraes, C.T.; Lana, U.G.P.; Alves, V.M.C.; Wang, Y.H.; Schaffert, R.E.; Hoekenga, O.A.; Pineros, M.A.; Shaff, J.E.; Klein, P.E.; Carneiro, N.P.; Coelho, C.M.; Trick, H.N.; Kochian, L.V. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 2007, 39, 1156–1161. [Google Scholar]
- Ren, Z.H.; Gao, J.P.; Li, L.G.; Cai, X.L.; Huang, W.; Chao, D.Y.; Zhu, M.Z.; Wang, Z.Y.; Luan, S.; Lin, H.X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar]
- Song, W.Y.; Wang, G.L.; Chen, L.L.; Kim, H.S.; Pi, L.Y.; Holsten, T.; Gardner, J.; Wang, B.; Zhai, W.X.; Zhu, L.H.; Fauquet, C.; Ronald, P. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995, 270, 1804–1806. [Google Scholar]
- Qu, S.H.; Liu, G.F.; Zhou, B.; Bellizzi, M.; Zeng, L.R.; Dai, L.Y.; Han, B.; Wang, G.L. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 2006, 172, 1901–1914. [Google Scholar]
- Olsen, K.M.; Purugganan, M.D. Molecular evidence on the origin and evolution of glutinous rice. Genetics 2002, 162, 941–950. [Google Scholar]
- Tian, Z.X.; Qian, Q.; Liu, Q.Q.; Yan, M.X.; Liu, X.F.; Yan, C.J.; Liu, G.F.; Gao, Z.Y.; Tang, S.Z.; Zeng, D.L.; Wang, Y.H.; Yu, J.M.; Gu, M.H.; Li, J.Y. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar]
- Himi, E.; Noda, K. Red grain colour gene (R) of wheat is a Myb-type transcription factor. Euphytica 2005, 143, 239–242. [Google Scholar]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; Tanaka, H.; Kitano, H.; Matsuoka, M. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 2006, 24, 105–109. [Google Scholar]
- Miura, K.; Ikeda, M.; Matsubara, A.; Song, X.J.; Ito, M.; Asano, K.; Matsuoka, M.; Kitano, H.; Ashikari, M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 2010, 42, 545–549. [Google Scholar] [CrossRef]
- Ashikari, M.; Sakakibara, H.; Lin, S.Y.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
- Shomura, A.; Izawa, T.; Ebana, K.; Ebitani, T.; Kanegae, H.; Konishi, S.; Yano, M. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 2008, 40, 1023–1028. [Google Scholar]
- Wang, E.; Wang, J.; Zhu, X.D.; Hao, W.; Wang, L.Y.; Li, Q.; Zhang, L.X.; He, W.; Lu, B.R.; Lin, H.X.; Ma, H.; Zhang, G.Q.; He, Z.H. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008, 40, 1370–1374. [Google Scholar]
- Huang, X.Z.; Qian, Q.; Liu, Z.B.; Sun, H.Y.; He, S.Y.; Luo, D.; Xia, G.M.; Chu, C.C.; Li, J.Y.; Fu, X.D. Natural variation at the Dep1 locus enhances grain yield in rice. Nat. Genet. 2009, 41, 494–497. [Google Scholar] [CrossRef]
- Xue, W.Y.; Xing, Y.Z.; Weng, X.Y.; Zhao, Y.; Tang, W.J.; Wang, L.; Zhou, H.J.; Yu, S.B.; Xu, C.G.; Li, X.H.; Zhang, Q.F. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop. Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- FARA Fara offers information on organisations, projects and experts in Africa. Available online: http://fara.infosysplus.org/ (accessed on 18 September 2012).
- Ye-Ebiyo, Y.; Pollack, R.J.; Kiszewski, A.; Spielman, A. A component of maize pollen that stimulates Larval mosquitoes (Diptera: Culicidae) to feed and increases toxicity of microbial larvicides. J. Med. Entomol 2003, 40, 860–864. [Google Scholar] [CrossRef]
- ASARECA Association for strengthening agricultural research in eastern and central Africa. Available online: http://www.asareca.org/ (accessed on 18 September 2012).
- CORAF. Available online: http://www.coraf.org/English/English.html (accessed on 18 September 2012).
- FANR. Available online: http://www.sadc.int/fanr/index.php (accessed on 18 September 2012).
- AATF. Available online: http://www.aatf-Africa.org/ (accessed on 18 September 2012).
- AfricaHarvest. Available online: http://Africaharvest.org/ (accessed on 18 September 2012).
- ABNETA Agricultural biotechnology network in Africa. Available online: http://www.abneta.org/ (accessed on 18 September 2012).
- AGRA. Available online: http://www.agra-alliance.org/ (accessed on 18 September 2012).
- BecA Biosciences eastern and central Africa (BecA) Hub. Available online: http://hub.Africabiosciences.org/ (accessed on 18 September 2012).
- Bioinnovate. Available online: http://bioinnovate-Africa.org/ (accessed on 18 September 2012).
- CAADP. Available online: http://www.nepad-caadp.net (accessed on 18 September 2012).
- AfricaRice. Available online: http://www.Africarice.org (accessed on 18 September 2012).
- Bioversity Bioversity international. Available online: http://www.bioversityinternational.org/ (accessed on 18 September 2012).
- CIAT. Available online: www.ciat.cgiar.org (accessed on 18 September 2012).
- CIMMYT. Available online: http://www.cimmyt.org/ (accessed on 18 September 2012).
- CIP. Available online: http://www.cipotato.org/ (accessed on 18 September 2012).
- ICARDA. Available online: http://www.icarda.org/ (accessed on 18 September 2012).
- ICRISAT. Available online: http://www.icrisat.org/index.htm (accessed on 18 September 2012).
- GCP. Available online: http://www.generationcp.org/index.php (accessed on 18 September 2012).
- IFPRI. Available online: http://www.ifpri.org (accessed on 18 September 2012).
- IITA. Available online: http://www.iita.org/ (accessed on 18 September 2012).
- ABSPII. Available online: http://www.absp2.cornell.edu/ (accessed on 18 September 2012).
- CIRAD. Available online: http://www.cirad.fr/en (accessed on 18 September 2012).
- Crops-for-future. Available online: http://www.cropsforthefuture.org/ (accessed on 18 September 2012).
- CTA. Available online: http://knowledge.cta.int/ (accessed on 18 September 2012).
- ETH-Zurich. Available online: http://www.pb.ethz.ch/research/cassava (accessed on 18 September 2012).
- FAO. Available online: http://www.fao.org/ (accessed on 18 September 2012).
- GFAR. Available online: http://www.egfar.org/egfar/website?contentId=-1& (accessed on 18 September 2012).
- HarvestPlus. Available online: http://www.harvestplus.org/ (accessed on 18 September 2012).
- IFAD. Available online: http://www.ifad.org/ (accessed on 18 September 2012).
- IPBO. Available online: http://www.ugent.be/we/genetics/ipbo/en (accessed on 18 September 2012).
- IRD. Available online: http://www.ird.fr (accessed on 18 September 2012).
- ISAAA. Available online: http://www.isaaa.org/inbrief/regionalcenters/africenter/default.asp (accessed on 18 September 2012).
- FAO-IAEA. Available online: http://www-naweb.iaea.org/nafa/index.html (accessed on 18 September 2012).
- KU-Leuven. Available online: http://www.biw.kuleuven.be/DTP/TRO/_data/home.htm (accessed on 18 September 2012).
- PAEPARD. Available online: http://paepard.blogspot.com/2010/02/first-paepard-ii-consortium-meeting.html (accessed on 18 September 2012).
- IPS-unibe. Available online: http://www.biology.unibe.ch/botany/content/deve/tef/index_eng.html (accessed on 18 September 2012).
- CGIAR Cgiar research programs. Available online: http://www.cgiar.org/our-research/cgiar-research-programs (accessed on 18 September 2012).
- Renkow, M.; Byerlee, D. The impacts of CGIAR research: A review of recent evidence. Food Policy 2010, 35, 391–402. [Google Scholar] [CrossRef]
- Ye-Ebiyo, Y.; Pollack, R.J.; Kiszewski, A.; Spielman, A. Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. Am. J. Tropical Med. Hyg. 2003, 68, 748–752. [Google Scholar]
- SRO. Available online: http://na.infosysplus.org/ (accessed on 18 September 2012).
- Toenniessen, G.; Adesina, A.; DeVries, J. Building an alliance for a green revolution in Africa. Ann. N. Y. Acad. Sci. 2008, 1136, 233–242. [Google Scholar] [CrossRef]
- Kijima, Y.; Sserunkuuma, D.; Otsuka, K. How revolutionary is the "NERICA revolution"? Evidence from Uganda. Dev. Econ. 2006, 44, 252–267. [Google Scholar]
- Atera, E.A.; Onyango, J.C.; Azuma, T.; Asanuma, S.; Itoh, K. Field evaluation of selected NERICA rice cultivars in Western Kenya. Afr. J. Agric. Res. 2011, 6, 60–66. [Google Scholar]
- Dibba, L.; Fialor, S.C.; Diagne, A.; Nimoh, F. The impact of NERICA adoption on productivity and poverty of the small-scale rice farmers in the Gambia. Food Secur. 2012, 4, 253–265. [Google Scholar]
- Diagne, A. Diffusion and adoption of NERICA rice varieties in cote d'ivoire. Dev. Econ. 2006, 44, 208–231. [Google Scholar]
- MoA, Crop Variety Register Issue No. 13; Ministry of Agriculture: Addis Ababa, Ethiopia, 2010; p. 227.
- Assefa, K.; Aliye, S.; Belay, G.; Metaferia, G.; Tefera, H.; Sorrells, M.E. Quncho: The first popular tef variety in Ethiopia. Int. J. Agric. Sustain. 2011, 9, 25–34. [Google Scholar] [CrossRef]
- AU 10 percent national budget allocation to agriculture development: Maputo declaration on agriculture and food security. Available online: http://www.Africa-union.org/root/ua/Conferences/2008/avril/REA/01avr/Pamphlet_rev6.pdf (accessed on 17 July 2012).
- Mack, M. The ‘African Century’ Can be Real. The Wall Street Journal 2012. [Google Scholar]
- Peng, S.B.; Khush, G.S.; Virk, P.; Tang, Q.Y.; Zou, Y.B. Progress in ideotype breeding to increase rice yield potential. Field Crop Res. 2008, 108, 32–38. [Google Scholar]
- Sarlikioti, V.; de Visser, P.H.B.; Buck-Sorlin, G.H.; Marcelis, L.F.M. How plant architecture affects light absorption and photosynthesis in tomato: Towards an ideotype for plant architecture using a functional-structural plant model. Ann. Bot. Lond. 2011, 108, 1065–1073. [Google Scholar]
- Berry, P.M.; Sylvester-Bradley, R.; Berry, S. Ideotype design for lodging-resistant wheat. Euphytica 2007, 154, 165–179. [Google Scholar] [CrossRef]
- Jantaboon, J.; Siangliw, M.; Im-mark, S.; Jamboonsri, W.; Vanavichit, A.; Toojinda, T. Ideotype breeding for submergence tolerance and cooking quality by marker-assisted selection in rice. Field Crop Res. 2011, 123, 206–213. [Google Scholar]
- Mi, G.H.; Chen, F.J.; Wu, Q.P.; Lai, N.W.; Yuan, L.X.; Zhang, F.S. Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. Sci. China Life Sci. 2010, 53, 1369–1373. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Hawkesford, M.J. Food security: Increasing yield and improving resource use efficiency. P. Nutr. Soc. 2010, 69, 592–600. [Google Scholar] [CrossRef]
- Misselhorn, A.; Aggarwal, P.; Ericksen, P.; Gregory, P.; Horn-Phathanothai, L.; Ingram, J.; Wiebe, K. A vision for attaining food security. Curr. Opin. Envion. Sust. 2012, 4, 7–17. [Google Scholar] [CrossRef]
- Ahrens, T.D.; Lobell, D.B.; Ortiz-Monasterio, J.I.; Li, Y.; Matson, P.A. Narrowing the agronomic yield gap with improved nitrogen use efficiency: A modeling approach. Ecol. Appl. 2010, 20, 91–100. [Google Scholar] [CrossRef]
- Lobell, D.B.; Cassman, K.G.; Field, C.B. Crop yield gaps: Their importance, magnitudes, and causes. Annu. Rev. Envion. Resour. 2009, 34, 179–204. [Google Scholar] [CrossRef]
- Nin-Pratt, A.; Johnson, M.; Magalhaes, E.; You, L.; Diao, X.; Chamberlin, J. Yield Gaps and Potential Agricultural Growth in West and Central Africa; International Food Policy Research Institute, Research Monograph: Washington, DC, USA, 2011; p. 158. [Google Scholar]
- Fermont, A.M.; van Asten, P.J.A.; Tittonell, P.; van Wijk, M.T.; Giller, K.E. Closing the cassava yield gap: An analysis from smallholder farms in East Africa. Field Crop. Res. 2009, 112, 24–36. [Google Scholar] [CrossRef]
- PROMISO Pearl millet and sorghum yield potential in west Africa. Available online: http://www.fidafrique.net/IMG/pdf/Pearl_millet_and_sorghum_yield_potential_in_West_Africa.pdf (accessed on 7 September 2012).
- Teklu, Y.; Tefera, H. Genetic improvement in grain yield potential and associated agronomic traits of tef (Eragrostis tef). Euphytica 2005, 141, 247–254. [Google Scholar] [CrossRef]
- Thompson, C. Africa: Green Revolution or Rainbow Evolution? Foreign Policy In Focus: Washington, DC, USA, 2007. Available online: http://www.fpif.org/articles/Africa_green_revolution_or_rainbow_evolution (accessed on 20 September 2012).
- Horlings, L.G.; Marsden, T.K. Towards the real green revolution? Exploring the conceptual dimensions of a new ecological modernisation of agriculture that could ‘feed the world’. Glob. Environ. Chang. 2011, 21, 441–452. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant. Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Temple, L.; Kwa, M.; Tetang, J.; Bikoi, A. Organizational determinant of technological innovation in food agriculture and impacts on sustainable development. Agron. Sustain. Dev. 2011, 31, 745–755. [Google Scholar] [CrossRef]
- Fungo, R. Opportunties for banana (Musa) in alleviating micronutrient deficiency in the great lakes region of East Africa. Ann. Nutr. Metab. 2009, 55, 243–243. [Google Scholar]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Amudavi, D.M.; Khan, Z.R.; Wanyama, J.M.; Midega, C.A.O.; Pittchar, J.; Nyangau, I.M.; Hassanali, A.; Pickett, J.A. Assessment of technical efficiency of farmer teachers in the uptake and dissemination of push-pull technology in Western Kenya. Crop. Prot. 2009, 28, 987–996. [Google Scholar] [CrossRef]
- Hassanali, A.; Herren, H.; Khan, Z.R.; Pickett, J.A.; Woodcock, C.M. Integrated pest management: The push-pull approach for controlling insect pests and weeds of cereals, and its potential for other agricultural systems including animal husbandry. Philos. Trans. R. Soc. B 2008, 363, 611–621. [Google Scholar] [CrossRef]
- Khan, Z.R.; Midega, C.A.O.; Amudavi, D.M.; Hassanali, A.; Pickett, J.A. On-farm evaluation of the ‘push-pull’ technology for the control of stemborers and striga weed on maize in Western Kenya. Field Crop. Res. 2008, 106, 224–233. [Google Scholar] [CrossRef]
- Pretty, J.; Toulmin, C.; Williams, S. Sustainable intensification in African agriculture. Int. J. Agric. Sustain. 2011, 9, 5–24. [Google Scholar] [CrossRef]
- Ferroni, M.; Castle, P. Public-private partnerships and sustainable agricultural development. Sustainability 2011, 3, 1064–1073. [Google Scholar] [CrossRef]
- Partnering to improve tef, New Agriculturist; WRENmedia: Suffolk, UK, 2010.
- Blum, A. Plant breeding for water-limited environments epilogue. In Plant Breeding for Water-Limited Environments; Springer: City, Country, 2011; pp. 245–247. [Google Scholar]
- Diao, X.S.; Headey, D.; Johnson, M. Toward a green revolution in Africa: What would it achieve, and what would it require? Agric. Econ. Blackwell 2008, 39, 539–550. [Google Scholar]
- World Agriculture Towards 2015/2030: An FAO Perspective; Bruinsma, J. (Ed.) FAO: Rome, Italy, 2003; p. 444.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tadele, Z.; Assefa, K. Increasing Food Production in Africa by Boosting the Productivity of Understudied Crops. Agronomy 2012, 2, 240-283. https://doi.org/10.3390/agronomy2040240
Tadele Z, Assefa K. Increasing Food Production in Africa by Boosting the Productivity of Understudied Crops. Agronomy. 2012; 2(4):240-283. https://doi.org/10.3390/agronomy2040240
Chicago/Turabian StyleTadele, Zerihun, and Kebebew Assefa. 2012. "Increasing Food Production in Africa by Boosting the Productivity of Understudied Crops" Agronomy 2, no. 4: 240-283. https://doi.org/10.3390/agronomy2040240
APA StyleTadele, Z., & Assefa, K. (2012). Increasing Food Production in Africa by Boosting the Productivity of Understudied Crops. Agronomy, 2(4), 240-283. https://doi.org/10.3390/agronomy2040240





