Effects of Chloro-Organophosphate Ester on Photosynthesis, Chlorophyll Fluorescence, Antioxidation and Nutrients of Green Onion (Allium fistulosum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Cl-OPEs Preparation
2.2. Plant Material and Culture Conditions
2.3. Measurement of Morphological and Physiological Characteristics
2.4. Measurement of Photosynthetic Parameters
2.5. Measurement of Chlorophyll Fluorescence
2.6. Measurement of Antioxidant Capacity
2.7. Measurement of Nutrients
2.8. Statistical Analyses
3. Results
3.1. Effect of Cl-OPE Concentration on Green Onion Morphology and Phenotype
3.2. Effect of Cl-OPEs Concentration on Green Onion Leaf Photosynthesis
3.3. Effect of Cl-OPE Concentration on Green Onion Leaf Chlorophyll Fluorescence
3.4. Effect of Cl-OPE Concentration on Green Onion Leaf Antioxidative Capacity
3.5. Effect of Cl-OPEs Concentration on Green Onion Nutrients
3.6. Relationships Between Studied Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.; Zheng, X.; Hu, C.; Luo, Q.; Liu, X.; Liu, S.; He, P.; Chang, K.; Yang, F.; Ding, Y. A review of organophosphorus esters in soil: Pollution status, migration, risks, and transformation. Curr. Opin. Environ. Sci. Health 2025, 44, 100599. [Google Scholar] [CrossRef]
- Tian, Y.X.; Chen, H.Y.; Ma, J.; Liu, Q.Y.; Qu, Y.J.; Zhao, W.H. A critical review on sources and environmental behavior of organophosphorus flame retardants in the soil: Current knowledge and future perspectives. J. Hazard. Mater. 2023, 452, 131161. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Lin, C.; Wu, T.; Xin, M.; Gu, X.; Lu, S.; Cao, Y.; Wang, B.; Ouyang, W.; Liu, X.; et al. Occurrence, spatiotemporal distribution, and ecological risks of organophosphate esters in the water of the Yellow River to the Laizhou Bay, Bohai Sea. Sci. Total Environ. 2021, 787, 147528. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Sun, J.; Ke, Z.; Yin, H.; Yang, L.; Yen, H.; Li, X.; Xu, Y. Organophosphate esters in surface soils from a heavily urbanized region of Eastern China: Occurrence, distribution, and ecological risk assessment. Environ. Pollut. 2021, 291, 118200. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, W.; Zhang, S.; Wang, Y.; Zhang, X.; Lu, Y.; Sun, H.; Wang, L. Organophosphite Antioxidants in Mulch Films Are Important Sources of Organophosphate Pollutants in Farmlands. Environ. Sci. Technol. 2021, 55, 7398–7406. [Google Scholar] [CrossRef]
- Ali, N.; Eqani, S.; Ismail, I.M.I.; Malarvannan, G.; Kadi, M.W.; Albar, H.M.S.; Rehan, M.; Covaci, A. Brominated and organophosphate flame retardants in indoor dust of Jeddah, Kingdom of Saudi Arabia: Implications for human exposure. Sci. Total Environ. 2016, 569–570, 269–277. [Google Scholar] [CrossRef]
- Cao, D.; Guo, J.; Wang, Y.; Li, Z.; Liang, K.; Corcoran, M.B.; Hosseini, S.; Bonina, S.M.; Rockne, K.J.; Sturchio, N.C.; et al. Organophosphate Esters in Sediment of the Great Lakes. Environ. Sci. Technol. 2017, 51, 1441–1449. [Google Scholar] [CrossRef]
- Cui, K.; Wen, J.; Zeng, F.; Li, S.; Zhou, X.; Zeng, Z. Occurrence and distribution of organophosphate esters in urban soils of the subtropical city, Guangzhou, China. Chemosphere 2017, 175, 514–520. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, W.; Kannan, K.; Moon, H.B. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 2016, 103, 182–188. [Google Scholar] [CrossRef]
- Guo, Y.; Liang, C.; Zeng, M.-X.; Wei, G.-L.; Zeng, L.-X.; Liu, L.-Y.; Zeng, E.Y. An overview of organophosphate esters and their metabolites in humans: Analytical methods, occurrence, and biomonitoring. Sci. Total Environ. 2022, 848, 157669. [Google Scholar] [CrossRef]
- Wan, W.; Zhang, S.; Huang, H.; Wu, T. Occurrence and distribution of organophosphorus esters in soils and wheat plants in a plastic waste treatment area in China. Environ. Pollut. 2016, 214, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zeng, Y.; Huang, Y.-Q.; Guan, Y.-F.; Sun, Y.-X.; Chen, S.-J.; Mai, B.-X. Accumulation and translocation of traditional and novel organophosphate esters and phthalic acid esters in plants during the whole life cycle. Chemosphere 2022, 307, 135670. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhong, Y.; Zhu, X.; Wang, H.; Yang, W.; Deng, Y.; Huang, W.; Peng, P. Reductive degradation of chlorinated organophosphate esters by nanoscale zerovalent iron/cetyltrimethylammonium bromide composites: Reactivity, mechanism and new pathways. Water Res. 2021, 188, 116447. [Google Scholar] [CrossRef] [PubMed]
- Campone, L.; Piccinelli, A.L.; Östman, C.; Rastrelli, L. Determination of organophosphorous flame retardants in fish tissues by matrix solid-phase dispersion and gas chromatography. Anal. Bioanal. Chem. 2010, 397, 799–806. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, W.; Xiao, B.; Liu, Q.; Yang, L.; Covaci, A.; Zhu, L. Bioavailability and biomagnification of organophosphate esters in the food web of Taihu Lake, China: Impacts of chemical properties and metabolism. Environ. Int. 2019, 125, 25–32. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, H.; Chang, X. Effects of halogenated flame retardants and cadmium on the germination and seedling growth of rice. J. Agro-Environ. Sci. 2020, 39, 1460–1469. [Google Scholar]
- Su, Y.H.; Liu, T.; Liang, Y.C. Transport via xylem of trichloroethylene in wheat, corn, and tomato seedlings. J. Hazard. Mater. 2010, 182, 472–476. [Google Scholar] [CrossRef]
- Hyland, K.C.; Blaine, A.C.; Dickenson, E.R.; Higgins, C.P. Accumulation of contaminants of emerging concern in food crops-part 1: Edible strawberries and lettuce grown in reclaimed water. Environ. Toxicol. Chem. 2015, 34, 2213–2221. [Google Scholar] [CrossRef]
- Gao, S.; Kong, Y.; Lv, Y.; Cao, B.; Chen, Z.; Xu, K. Effect of different LED light quality combination on the content of vitamin C, soluble sugar, organic acids, amino acids, antioxidant capacity and mineral elements in green onion (Allium fistulosum L.). Food Res. Int. 2022, 156, 111329. [Google Scholar] [CrossRef]
- Holm, G. Chlorophyll Mutations in Barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Yang, X.; Xu, H.; Shao, L.; Li, T.; Wang, Y.; Wang, R. Response of photosynthetic capacity of tomato leaves to different LED light wavelength. Environ. Exp. Bot. 2018, 150, 161–171. [Google Scholar] [CrossRef]
- Riches, M.; Lee, D.; Farmer, D.K. Simultaneous leaf-level measurement of trace gas emissions and photosynthesis with a portable photosynthesis system. Atmos. Meas. Tech. 2020, 13, 4123–4139. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Y.; Gao, S.; Xu, K. Ofloxacin induces etiolation in Welsh onion leaves. Chemosphere 2021, 267, 128918. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynthesis Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zhou, Y.; Tao, Y.; Mao, W.; Shi, K.; Asami, T.; Chen, Z.; Yu, J. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Gong, B.; Miao, L.; Kong, W.; Bai, J.G.; Wang, X.; Wei, M.; Shi, Q. Nitric oxide, as a downstream signal, plays vital role in auxin induced cucumber tolerance to sodic alkaline stress. Plant Physiol. Biochem. 2014, 83, 258–266. [Google Scholar] [CrossRef]
- Yang, L.; Mai, Z.; Zhu, L.; Zhang, L. Comparison and Optimization of Determination Methods of Vitamin C Content in Fruits and Vegetables. J. Anhui Agric. Sci. 2018, 46, 232–236. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.; Gao, S.; Lv, Y.; Chen, Z.; Cao, B.; Xu, K. Different responses of two Chinese cabbage (Brassica rapa L. ssp. pekinensis) cultivars in photosynthetic characteristics and chloroplast ultrastructure to salt and alkali stress. Planta 2021, 254, 102. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, L.-Y.; Stubbings, W.A.; Wang, S. Analysis and subcellular distribution of organophosphate esters (OPEs) in rice tissues. Environ. Sci. Pollut. Res. 2023, 30, 74021–74030. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Y.; Chen, H.; Zhang, Q.; Li, C.; Zhao, L.; Guo, S.; Cheng, Z.; Wang, Y.; Wang, L.; et al. Identification of Novel Organophosphate Esters in Hydroponic Lettuces (Lactuca sativa L.): Biotransformation and Acropetal Translocation. Environ. Sci. Technol. 2022, 56, 10699–10709. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.; Vandana Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Zhan, X.; Xing, B. Phenanthrene-triggered Chlorosis is caused by elevated Chlorophyll degradation and leaf moisture. Environ. Pollut. 2017, 220, 1311–1321. [Google Scholar] [CrossRef]
- Pattanayak, G.K.; Tripathy, B.C. Overexpression of protochlorophyllide oxidoreductase C regulates oxidative stress in Arabidopsis. PLoS ONE 2011, 6, e26532. [Google Scholar] [CrossRef]
- Zhang, K.M.; Shen, Y.; Zhou, X.Q.; Fang, Y.M.; Liu, Y.; Ma, L.Q. Photosynthetic electron-transfer reactions in the gametophyte of Pteris multifida reveal the presence of allelopathic interference from the invasive plant species Bidens pilosa. J. Photochem. Photobiol. B 2016, 158, 81–88. [Google Scholar] [CrossRef]
- Zeb, B.S.; Hayat, M.T.; Zeb, T.; Khan, F.Y.; Abbasi, H.Z.; Nawaz, I.; Ebadi, A. Uptake of Organic Pollutants and the Effects on Plants. In Sustainable Plant Nutrition under Contaminated Environments; Mahmood, Q., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 209–234. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Sun, X.; Wang, L.; Du, N.; Tao, Y.; Sun, G.; Erinle, K.O.; Wang, P.; Zhou, C.; et al. Effect of dimethyl phthalate (DMP) on germination, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. Environ. Sci. Pollut. Res. Int. 2016, 23, 1183–1192. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Ren, X.; Zhang, W.; Li, Q. Organophosphate esters uptake, translocation and accumulation in rice (Oryza sativa L.): Impacts of lipid transporters and chemical properties. Environ. Sci. Process. Impacts 2024, 26, 1171–1183. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Ni, Y.; Zhang, Q.; Zhao, L. Uptake by roots and translocation to shoots of polychlorinated dibenzo-p-dioxins and dibenzofurans in typical crop plants. Chemosphere 2009, 76, 740–746. [Google Scholar] [CrossRef]
- Liu, Q.; He, Q.; Yi, X.; Zhang, J.; Gao, H.; Liu, X. Uptake, accumulation and translocation mechanisms of organophosphate esters in cucumber (Cucumis sativus) following foliar exposure. Sci. Total Environ. 2024, 912, 169462. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 49–70. [Google Scholar]
- Lichtenthaler, H.K. In Vivo Chlorophyll Fluorescence as a Tool for Stress Detection in Plants; Springer: Dordrecht, The Netherlands, 1988; pp. 129–142. [Google Scholar]
- Lv, Y.; Li, Y.; Liu, X.; Xu, K. The tolerance mechanism and accumulation characteristics of Phragmites australis to sulfamethoxazole and ofloxacin. Chemosphere 2020, 253, 126695. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, Y.; Liu, X.; Xu, K. Effect of soil sulfamethoxazole on strawberry (Fragaria ananassa): Growth, health risks and silicon mitigation. Environ. Pollut. 2021, 286, 117321. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, J.; Xu, K.; Liu, X.; Guo, X.; Lu, S.; Xi, B. Accumulation characteristics and biological response of ginger to sulfamethoxazole and ofloxacin. Environ. Pollut. 2020, 262, 114203. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Geng, Y.; Lin, B.; Xi, Z. Molecular mechanisms underlying mitochondrial damage, endoplasmic reticulum stress, and oxidative stress induced by environmental pollutants. Toxicol. Res. 2023, 12, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]
- Maryam, K.; Abolghasem, J. Challenges on Determination of Malondialdehyde in Plant Samples. Arch. Crop Sci. 2020, 4, 64–66. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Teng, M.; Wang, D.; Yan, J.; Miao, J.; Zhou, Z.; Zhu, W. Toxicity and metabolomics study of isocarbophos in adult zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 163, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Laserna, A.K.C.; Li, S.F.Y. Metabolomics reveals that tris(1,3-dichloro-2-propyl) phosphate (TDCPP) causes disruption of membrane lipids in microalga Scenedesmus obliquus. Sci. Total Environ. 2020, 708, 134498. [Google Scholar] [CrossRef]
- Atkin, O.K.; Millar, A.H.; Gardeström, P.; Day, D.A. Photosynthesis, Carbohydrate Metabolism and Respiration in Leaves of Higher Plants. In Photosynthesis: Physiology and Metabolism; Leegood, R.C., Sharkey, T.D., von Caemmerer, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 153–175. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, L.; Le, X.C. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.). Environ. Pollut. 2017, 230, 302–310. [Google Scholar] [CrossRef]
- Vallarino, J.G.; Osorio, S. Organic Acids. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 207–224. [Google Scholar] [CrossRef]
- Jurczyk, B.; Rapacz, M.; Pociecha, E.; Kościelniak, J. Changes in carbohydrates triggered by low temperature waterlogging modify photosynthetic acclimation to cold in Festuca pratensis. Environ. Exp. Bot. 2016, 122, 60–67. [Google Scholar] [CrossRef]
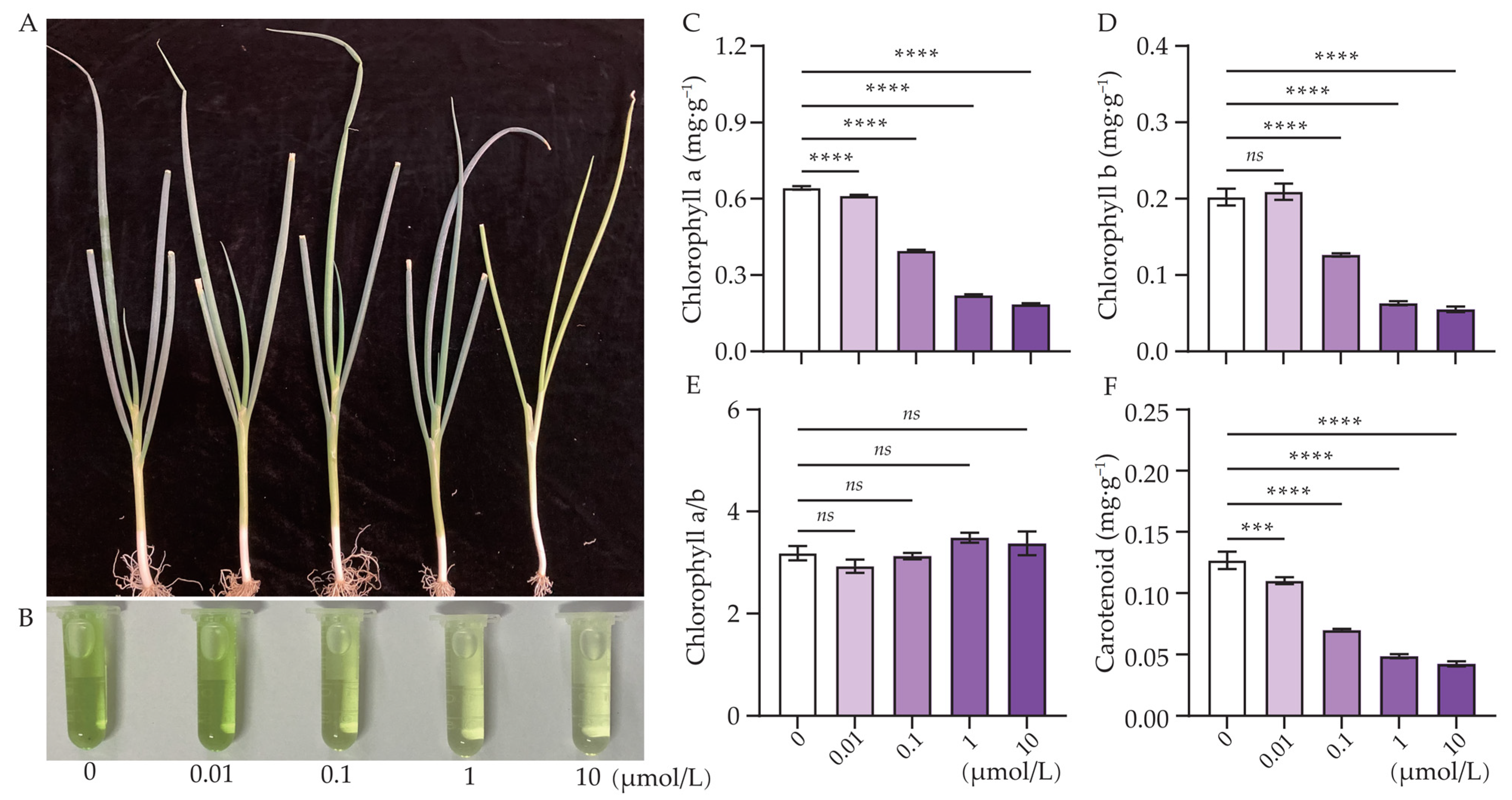
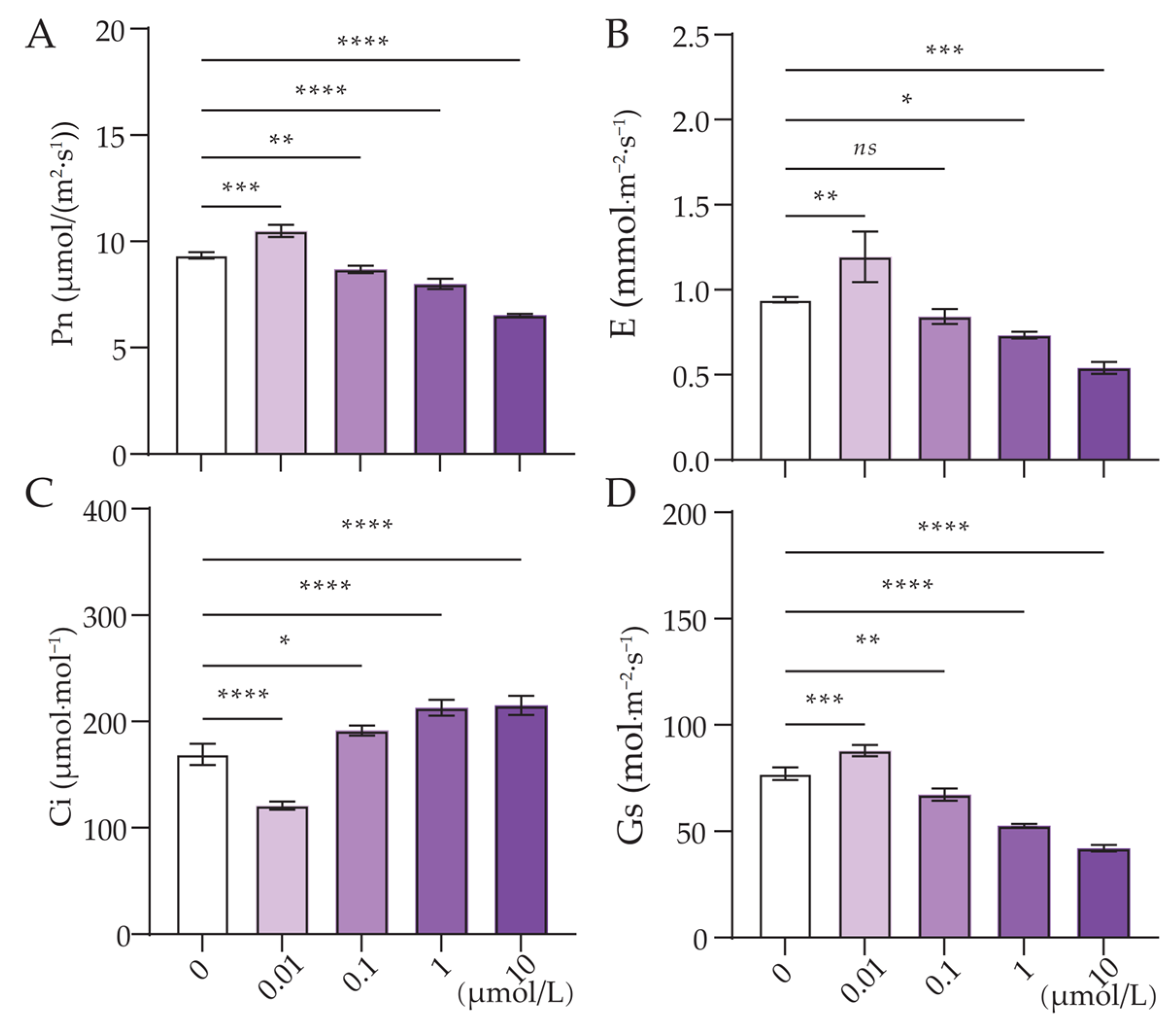




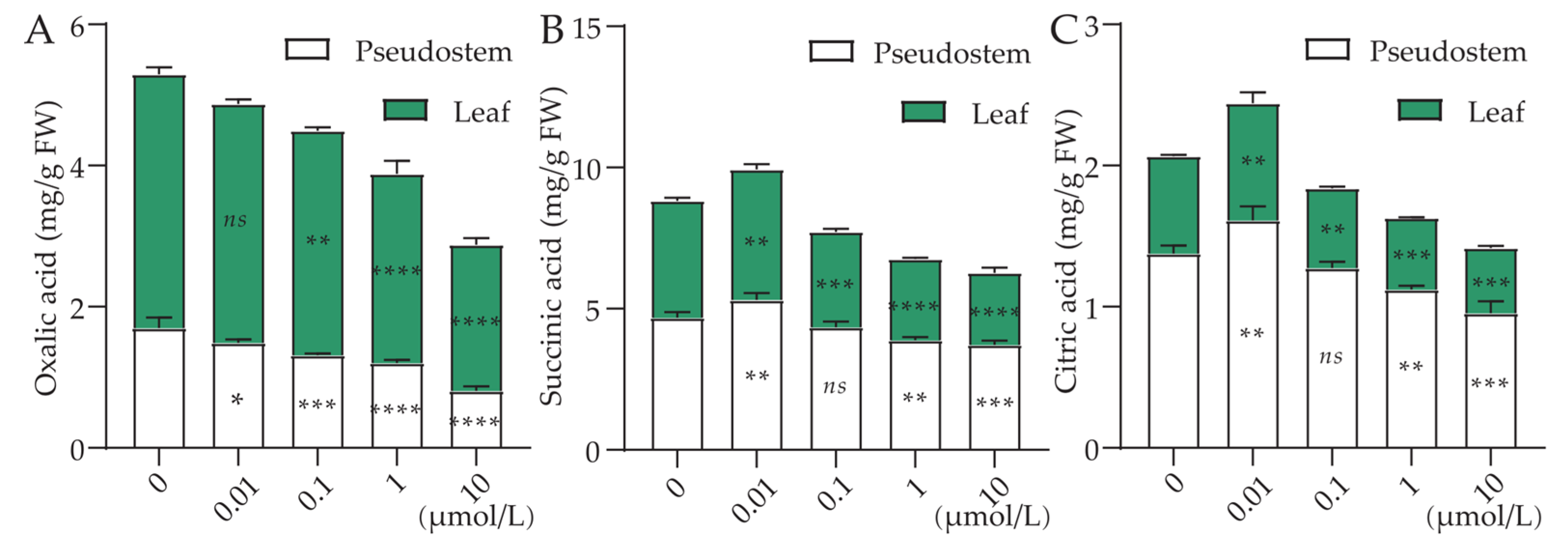


| Cl-OPEs (μmol/L) | Number of Leaves | Plant Height (cm) | Pseudostem Height (cm) | Pseudostem Diameter (mm) | Leaf FW (g/Plant) | Pseudostem FW (g/Plant) |
|---|---|---|---|---|---|---|
| 0 | 4.67 ± 0.58 | 58.77 ± 0.55 | 18.80 ± 0.20 | 0.98 ± 0.01 | 11.80 ± 0.38 | 9.65 ± 0.23 |
| 0.01 | 4.00 ± 0.00 ns | 63.30 ± 0.79 **** | 21.57 ± 0.40 **** | 0.89 ± 0.01 *** | 10.52 ± 0.18 *** | 10.24 ± 0.28 * |
| 0.1 | 4.00 ± 0.00 ns | 58.27 ± 0.40 ns | 18.47 ± 0.31 ns | 0.87 ± 0.02 **** | 9.97 ± 0.08 **** | 9.30 ± 0.20 ns |
| 1 | 3.67 ± 0.58 * | 54.57 ± 0.65 **** | 18.37 ± 0.40 ns | 0.86 ± 0.02 **** | 9.93 ± 0.16 **** | 9.10 ± 0.17 * |
| 10 | 3.00 ± 0.00 *** | 53.37 ± 0.93 **** | 18.00 ± 0.20 * | 0.58 ± 0.02 **** | 5.24 ± 0.19 **** | 5.42 ± 0.18 **** |
| Cl-OPEs (μmol/L) | Fv/Fm | ΦPSII | Y (NPQ) | Y (NO) |
|---|---|---|---|---|
| 0 | 0.79 ± 0.006 | 0.39 ± 0.007 | 0.36 ± 0.005 | 0.25 ± 0.010 |
| 0.01 | 0.80 ± 0.002 ** | 0.41 ± 0.002 ** | 0.31 ± 0.007 **** | 0.28 ± 0.005 ** |
| 0.1 | 0.77 ± 0.003 *** | 0.36 ± 0.002 ** | 0.42 ± 0.005 **** | 0.22 ± 0.006 ** |
| 1 | 0.75 ± 0.006 **** | 0.32 ± 0.004 **** | 0.50 ± 0.003 **** | 0.18 ± 0.004 **** |
| 10 | 0.75 ± 0.004 **** | 0.22 ± 0.012 **** | 0.53 ± 0.002 **** | 0.25 ± 0.011 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Gao, S.; Guo, Y.; Wang, Y.; Li, N.; Liu, T.; Guo, Y.; Xu, K. Effects of Chloro-Organophosphate Ester on Photosynthesis, Chlorophyll Fluorescence, Antioxidation and Nutrients of Green Onion (Allium fistulosum L.). Agronomy 2026, 16, 7. https://doi.org/10.3390/agronomy16010007
Gao S, Guo Y, Wang Y, Li N, Liu T, Guo Y, Xu K. Effects of Chloro-Organophosphate Ester on Photosynthesis, Chlorophyll Fluorescence, Antioxidation and Nutrients of Green Onion (Allium fistulosum L.). Agronomy. 2026; 16(1):7. https://doi.org/10.3390/agronomy16010007
Chicago/Turabian StyleGao, Song, Yuwei Guo, Yanzhou Wang, Ning Li, Touming Liu, Yuanyuan Guo, and Kun Xu. 2026. "Effects of Chloro-Organophosphate Ester on Photosynthesis, Chlorophyll Fluorescence, Antioxidation and Nutrients of Green Onion (Allium fistulosum L.)" Agronomy 16, no. 1: 7. https://doi.org/10.3390/agronomy16010007
APA StyleGao, S., Guo, Y., Wang, Y., Li, N., Liu, T., Guo, Y., & Xu, K. (2026). Effects of Chloro-Organophosphate Ester on Photosynthesis, Chlorophyll Fluorescence, Antioxidation and Nutrients of Green Onion (Allium fistulosum L.). Agronomy, 16(1), 7. https://doi.org/10.3390/agronomy16010007







