Response of Soil Organic Carbon in Citrus Orchards at Different Slope Positions to Citrus Peel Biochar and Field Snail Shell Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Material Preparation
2.3. Experimental Design
2.4. Measurement Methods
2.5. Data Analysis and Statistics
3. Results
3.1. Characterization of Citrus Peel Biochar and Snail Shell Powder
3.2. Effects of Different Amendments on Soil Physical and Chemical Properties
3.3. Effects of Different Amendments on Soil Organic Carbon Mineralization
3.4. Effects of Different Amendments on Soil Organic Carbon and Its Active Carbon Fractions
3.5. Analyses of Correlation, Random Forest Model, and Structural Equation Model
4. Discussion
4.1. Regulation of Soil SOC and CO2 Emissions by Amendments
4.2. Slope Position Modulates Amendment Efficacy
4.3. Model Integration and Practical Implications
- Lower Slopes: Apply CPB (4%) for maximal carbon sequestration.
- Middle Slopes: Use CPB + SSP (2% CPB + 2% SSP) to balance SOC storage and fertility (via DOC/ROC).
- Upper Slopes: Prioritize erosion control (e.g., terracing) before amendment use.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujisaki, K.; Chevallier, T.; Chapuis-Lardy, L.; Albrecht, A.; Razafimbelo, T.; Masse, D.; Ndour, Y.B.; Chotte, J.-L. Soil carbon stock changes in tropical croplands are mainly driven by carbon inputs: A synthesis. Agric. Ecosyst. Environ. 2018, 259, 147–158. [Google Scholar] [CrossRef]
- Oelbermann, M.; Jiang, R.W.; Mechler, M.A. Predicting changes in soil organic carbon after a low dosage and one-time addition of biochar blended with manure and nitrogen fertilizer. Front. Soil Sci. 2023, 3, 1209530. [Google Scholar] [CrossRef]
- Gerke, J. Carbon Accumulation in Arable Soils: Mechanisms and the Effect of Cultivation Practices and Organic Fertilizers. Agronomy 2021, 11, 1079. [Google Scholar] [CrossRef]
- Nazir, M.J.; Li, G.; Nazir, M.M.; Zulfiqar, F.; Siddique, K.H.M.; Iqbal, B.; Du, D. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Tillage Res. 2024, 237, 105959. [Google Scholar] [CrossRef]
- Hallman, L.M.; Fox, J.-P.; Paoli, J.P.; Hussain, K.; Wright, A.L.; Rossi, L. Soil Organic Matter Influences Citrus Growth, Nutrient Uptake, and Root Architecture. Hortscience 2024, 59, 1781–1788. [Google Scholar] [CrossRef]
- Huang, K.; Ma, Z.; Xia, P.; Lin, T.; Zhang, Z.; Jiang, X.; Wang, X.; Huang, X. Spatial pattern and controlling factors of soil organic carbon density in a typical karst province, China. Soil Tillage Res. 2024, 242, 106160. [Google Scholar] [CrossRef]
- Mei, T.; Zeng, Q.; Chen, R.; Tan, W. Soil microbial necromass carbon contributions to soil organic carbon after three decades of citrus cultivation. Front. Microbiol. 2025, 16, 1589966. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, S.; Zhang, C.; Yan, P.; Wang, H.; Xu, W.; Song, M.; Aurangzeib, M. Key factors influencing the spatial distribution of soil organic carbon and its fractions in Mollisols. Catena 2024, 247, 108522. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, M.; Guo, X.; Yang, H.; Zhang, Z.; Zhang, K. Effects of topographic factors on runoff and soil loss in Southwest China. Catena 2018, 160, 394–402. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, S.; Marathe, R.A. Organic Citrus: Soil Fertility and Plant Nutrition. J. Sustain. Agric. 2002, 19, 5–29. [Google Scholar] [CrossRef]
- Niu, Y.H.; Wang, L.; Wan, X.G.; Peng, Q.Z.; Huang, Q.; Shi, Z.H. A systematic review of soil erosion in citrus orchards worldwide. CATENA 2021, 206, 105558. [Google Scholar] [CrossRef]
- Han, L.; Lu, C.; Chen, L.; Wang, F.; Chen, Q.a.; Gao, K.; Yu, Y.; Xu, C. Carbon sequestration potential of biochar in soil from the perspective of organic carbon structural modification. Appl. Soil Ecol. 2024, 198, 105389. [Google Scholar] [CrossRef]
- Ji, C.; E, T.; Cheng, Y.; Yang, S.; Chen, L.; Wang, D.; Wang, Y.; Li, Y. Preparation of Mn modified waste dander biochar and its effect on soil carbon sequestration. Environ. Res. 2024, 247, 118147. [Google Scholar] [CrossRef] [PubMed]
- Masek, O.; Buss, W.; Brownsort, P.; Rovere, M.; Tagliaferro, A.; Zhao, L.; Cao, X.; Xu, G. Potassium doping increases biochar carbon sequestration potential by 45%, facilitating decoupling of carbon sequestration from soil improvement. Sci. Rep. 2019, 9, 5514. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hu, J.; Li, B.; Liu, Y.; Tong, W.K.; Yue, M.R.; Wang, J.; Wang, W.; Gao, M.-t.; Liu, N.; et al. Degradation-Resistant Biochar Improves Soil Organic Carbon Storage: Promoting Autotrophic Metabolism & Increasing Refractory Organic Carbon. Bioresour. Technol. 2025, 428, 132452. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, L. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622, 1391–1399. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Adani, F. Biochar contribution in greenhouse gas mitigation and crop yield considering pyrolysis conditions, utilization strategies and plant type—A meta-analysis. Field Crops Res. 2025, 333, 110040. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Sandilya, S.P.; Sarma, B.; Pandey, A.K.; Dutta, J.; Mahanta, K.; Lesueur, D.; Nath, B.C.; Borah, D.; Borgohain, D.J. Biochar as Soil Amendment in Climate-Smart Agriculture: Opportunities, Future Prospects, and Challenges. J. Soil Sci. Plant Nutr. 2024, 24, 135–158. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Y.; Zhuang, H.; Shan, S. In-situ retention of nitrogen, phosphorus in agricultural drainage and soil nutrients by biochar at different temperatures and the effects on soil microbial response. Sci. Total Environ. 2023, 904, 166292. [Google Scholar] [CrossRef]
- Saeed, M.; Kamboh, A.A.; Huayou, C. Promising future of citrus waste into fermented high-quality bio-feed in the poultry nutrition and safe environment. Poult. Sci. 2024, 103, 103549. [Google Scholar] [CrossRef]
- Sial, T.A.; Lan, Z.; Khan, M.N.; Zhao, Y.; Kumbhar, F.; Liu, J.; Zhang, A.; Hill, R.L.; Lahori, A.H.; Memon, M. Evaluation of orange peel waste and its biochar on greenhouse gas emissions and soil biochemical properties within a loess soil. Waste Manag. 2019, 87, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Z.; Zhan, Y.; Zheng, X.; Zhou, M.; Yan, G.; Wang, L.; Werner, C.; Butterbach-Bahl, K. Potential benefits of liming to acid soils on climate change mitigation and food security. Glob. Change Biol. 2021, 27, 2807–2821. [Google Scholar] [CrossRef]
- Mhanna, M.; El-Khateeb, A.I.; Hasan, H.; Zahra, B.; Al-kaady, M.; Habib, A. Selecting a new citrus rootstock for Satsuma (Citrus unshiu Marc.) in the Syrian coast. DYSONA—Appl. Sci. 2023, 4, 42–50. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Srivastava, A.K.; Raiesi, T. Citrus nutrition in Iran: Lessons from calcareous soils. J. Plant Nutr. 2024, 47, 3367–3392. [Google Scholar] [CrossRef]
- Mosharrof, M.; Uddin, M.K.; Sulaiman, M.F.; Mia, S.; Shamsuzzaman, S.M.; Haque, A.N.A. Combined Application of Biochar and Lime Increases Maize Yield and Accelerates Carbon Loss from an Acidic Soil. Agronomy 2021, 11, 1313. [Google Scholar] [CrossRef]
- Oral, B.; Cosgun, A.; Guenay, M.E.; Yildirim, R. Machine learning-based exploration of biochar for environmental management and remediation. J. Environ. Manag. 2024, 360, 121162. [Google Scholar] [CrossRef]
- Wu, J.; Teng, B.; Zhong, Y.; Duan, X.; Gong, L.; Guo, W.; Qi, P.; Haider, F.U.; Cai, L. Enhancing Soil Aggregate Stability and Organic Carbon in Northwestern China through Straw, Biochar, and Nitrogen Supplementation. Agronomy 2024, 14, 899. [Google Scholar] [CrossRef]
- Hu, L.; Qin, R.; Zhou, L.; Deng, H.; Li, K.; He, X. Effects of Orange Peel Biochar and Cipangopaludina chinensis Shell Powder on Soil Organic Carbon Transformation in Citrus Orchards. Agronomy 2023, 13, 1801. [Google Scholar] [CrossRef]
- Hu, L.; Li, S.; Li, K.; Huang, H.; Wan, W.; Huang, Q.; Li, Q.; Li, Y.; Deng, H.; He, T. Effects of Two Types of Straw Biochar on the Mineralization of Soil Organic Carbon in Farmland. Sustainability 2020, 12, 10586. [Google Scholar] [CrossRef]
- Gao, S.; Hoffman-Krull, K.; DeLuca, T.H. Soil biochemical properties and crop productivity following application of locally produced biochar at organic farms on Waldron Island, WA. Biogeochemistry 2017, 136, 31–46. [Google Scholar] [CrossRef]
- Huang, R.; Tian, D.; Liu, J.; Lu, S.; He, X.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Jien, S.-H.; Chen, W.-C.; Ok, Y.S.; Awad, Y.M.; Liao, C.-S. Short-term biochar application induced variations in C and N mineralization in a compost-amended tropical soil. Environ. Sci. Pollut. Res. 2018, 25, 25715–25725. [Google Scholar] [CrossRef]
- Sarma, B.; Borkotoki, B.; Narzari, R.; Kataki, R.; Gogoi, N. Organic amendments: Effect on carbon mineralization and crop productivity in acidic soil. J. Clean. Prod. 2017, 152, 157–166. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Li, F.; Gao, S.; Zhang, J. Effect of compost and inorganic fertilizer on organic carbon and activities of carbon cycle enzymes in aggregates of an intensively cultivated Vertisol. PLoS ONE 2020, 15, e0229644. [Google Scholar] [CrossRef]
- Xu, H.; Shao, H.; Lu, Y. Arbuscular mycorrhiza fungi and related soil microbial activity drive carbon mineralization in the maize rhizosphere. Ecotoxicol. Environ. Saf. 2019, 182, 109476. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Huang, S.; Jia, J.; Wang, C.; Hu, L.; Li, K.; Deng, H. Effects of Wildfire on Soil CO2 Emission and Bacterial Community in Plantations. Microorganisms 2024, 12, 1666. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Dermatas, D.; Xu, X.; Shen, G. Immobilization of lead in shooting range soils by means of cement, quicklime, and phosphate amendments. Environ. Sci. Pollut. Res. Int. 2008, 15, 120–127. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Duan, C.; Wang, X.; Zhang, X.; Ju, W.; Chen, H.; Yue, S.; Wang, Y.; Li, S.; et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.; Jin, Y.; Zou, J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. Glob. Change Biol. Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Cui, L.; Li, X.; Lin, J.; Guo, G.; Zhang, X.; Zeng, G. The mineralization and sequestration of soil organic carbon in relation to gully erosion. Catena 2022, 214, 106218. [Google Scholar] [CrossRef]
- Grover, S.P.; Butterly, C.R.; Wang, X.; Tang, C. The short-term effects of liming on organic carbon mineralisation in two acidic soils as affected by different rates and application depths of lime. Biol. Fertil. Soils 2017, 53, 431–443. [Google Scholar] [CrossRef]
- Jouichat, H.; Khiari, L.; Gallichand, J.; Ismail, M. Modeling temporal variation of soil acidity after the application of liming materials. Soil Tillage Res. 2024, 240, 106050. [Google Scholar] [CrossRef]
- Han, B.; Addo, F.G.; Mu, X.; Zhang, L.; Zhang, S.; Lv, X.; Li, X.; Wang, P.; Wang, C. Epiphytic bacterial community shift drives the nutrient cycle during Potamogeton malaianus decomposition. Chemosphere 2019, 236, 124253. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Baath, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, Y.; Feng, S.; Ge, Y.; Zhang, W.; He, X.; Wang, K. Soil organic carbon mineralization with fresh organic substrate and inorganic carbon additions in a red soil is controlled by fungal diversity along a pH gradient. Geoderma 2018, 321, 79–89. [Google Scholar] [CrossRef]
- Barbosa Borges, W.L.; Hipolito, J.L.; Tokuda, F.S.; Gasparino, A.C.; de Freitas, R.S.; Andreotti, M. Methodologies for the application of calcium compounds in agropastoral systems: Effects on crop yields. Agron. J. 2021, 113, 5527–5540. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, X. Structural control effects on hot springs’ hydrochemistry in the northern Red River Fault Zone: Implications for geothermal systems in fault zones. J. Hydrol. 2023, 623, 129836. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Baldock, J.A.; Butterly, C.R.; Gazey, C. Long-term effect of lime application on the chemical composition of soil organic carbon in acid soils varying in texture and liming history. Biol. Fertil. Soils 2016, 52, 295–306. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Papaioannou, A. Correlations Between Soil Exchangeable Ca2+, Mg2+, K+ and Foliar Nutrient Concentrations in Mature Biological Olive Groves (Olea europaea L., cv. ‘Chondrolia Chalkidikis’). Commun. Soil Sci. Plant Anal. 2019, 50, 492–501. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Wang, R.; Yao, L.; Guo, S. Slope sensitivity: A coefficient to represent the dependency of soil CO2 emissions to slope gradients. Catena 2020, 193, 104659. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Chen, M.; Fei, C.; Zhang, W.; Li, Y.; Ding, X. Fe-modified biochar combined with mineral fertilization promotes soil organic phosphorus mineralization by shifting the diversity of phoD-harboring bacteria within soil aggregates in saline-alkaline paddy soil. J. Soils Sediments 2023, 23, 619–633. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, R.; Sun, Q.; Du, L.; Zhao, M.; Hu, Y.; Guo, S. Soil CO2 emissions from different slope gradients and positions in the semiarid Loess Plateau of China. Ecol. Eng. 2017, 105, 231–239. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, M.; Song, X.; Han, Z.; Jia, A.; Gao, Q.; Nan, S.; Li, S.; Wu, X. Compaction shapes CO2 emission contrasts in sloping farmland: Erosion-deposition zones respond via soil structure and microbiome. Appl. Soil Ecol. 2025, 212, 106162. [Google Scholar] [CrossRef]
- Gaspar, L.; Mabit, L.; Lizaga, I.; Navas, A. Lateral mobilization of soil carbon induced by runoff along karstic slopes. J. Environ. Manag. 2020, 260, 110091. [Google Scholar] [CrossRef]
- He, L.; Zhao, J.; Yang, S.; Zhou, H.; Wang, S.; Zhao, X.; Xing, G. Successive biochar amendment improves soil productivity and aggregate microstructure of a red soil in a five-year wheat-millet rotation pot trial. Geoderma 2020, 376, 114570. [Google Scholar] [CrossRef]
- Buss, W.; Shepherd, J.G.; Heal, K.V.; Masek, O. Spatial and temporal microscale pH change at the soil-biochar interface. Geoderma 2018, 331, 50–52. [Google Scholar] [CrossRef]
- Guo, D.; Ge, Y.; Wang, X.; Liu, H.; Su, S.; Li, C.; Tao, T. Evaluating the filtration efficiency of close-coupled catalyzed gasoline particulate filter (cGPF) over the WLTC and simulated RDE cycles. Chemosphere 2022, 301, 134717. [Google Scholar] [CrossRef] [PubMed]
- Trakal, L.; Veselska, V.; Safarik, I.; Vitkova, M.; Cihalova, S.; Komarek, M. Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour. Technol. 2016, 203, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Yu, X.Y.; Huang, L.; Zhang, X.F.; Wang, D.K.; Zhao, X.; Li, Y.; He, Z.B.; Kang, L.; Li, X.T.; et al. Responses of Phaseolus calcaltus to lime and biochar application in an acid soil. Peerj 2019, 7, e6346. [Google Scholar] [CrossRef]
- Wang, L.; Butterly, C.R.; Wang, Y.; Herath, H.M.S.K.; Xi, Y.G.; Xiao, X.J. Effect of crop residue biochar on soil acidity amelioration in strongly acidic tea garden soils. Soil Use Manag. 2014, 30, 119–128. [Google Scholar] [CrossRef]
- Raza, S.; Sommer, R.; Margenot, A.J. Do soil enzyme activities explain stimulated carbon mineralization following liming? Soil Biol. Biochem. 2024, 194, 109416. [Google Scholar] [CrossRef]
- Yang, W.H.; Xu, Y.; Deng, Z.W.; Zhou, X.; Bai, M.X.; Li, W.P. Study on the adsorption characteristics of polypyrrole modified biochar to fluorine. China Environ. Sci. 2023, 43, 592–600. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Preparation of high surface area-activated carbon from lignin of papermaking black liquor by koh activation for ni(ii) adsorption. Chem. Eng. J. 2013, 217, 345–353. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Wang, H.; Lv, Q.; Xue, J. Enhanced removal of phosphate from aqueous solution using mg/fe modified biochar derived from excess activated sludge: Removal mechanism and environmental risk. Environ. Sci. Pollut. Res. 2021, 28, 16282–16297. [Google Scholar] [CrossRef] [PubMed]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (eichornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Ma, J.; Huang, W.; Zhang, X.; Li, Y.; Wang, N. The utilization of lobster shell to prepare low-cost biochar for high-efficient removal of copper and cadmium from aqueous: Sorption properties and mechanisms. J. Environ. Chem. Eng. 2021, 9, 104703. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, J.; Qi, Y.; Wang, F.; Hong, W.; Li, H.; Jiang, Y. Facile preparation of hydroxyl-rich mesoporous magnesium silicate with excellent adsorption performance. Surf. Interfaces 2020, 20, 100519. [Google Scholar] [CrossRef]
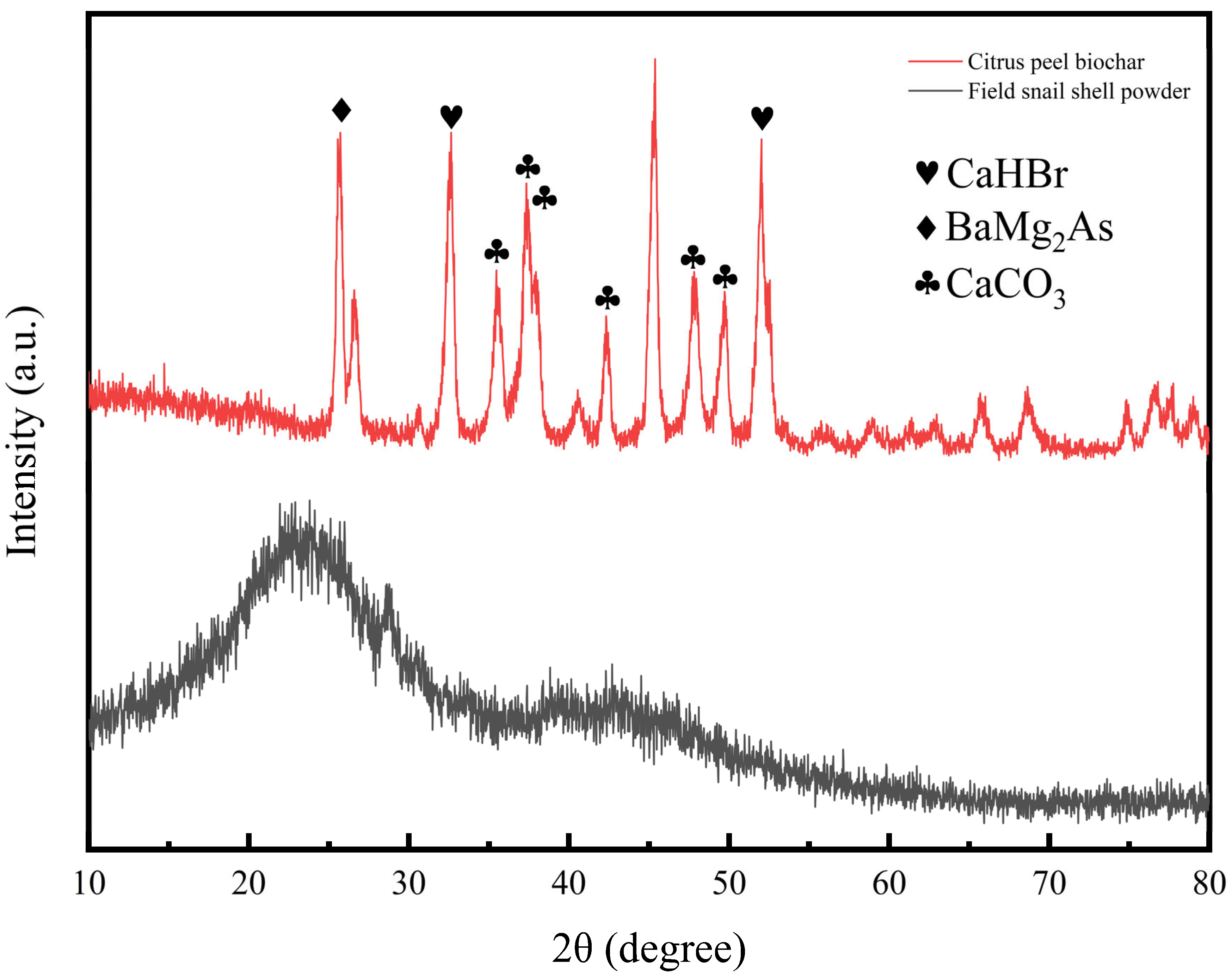
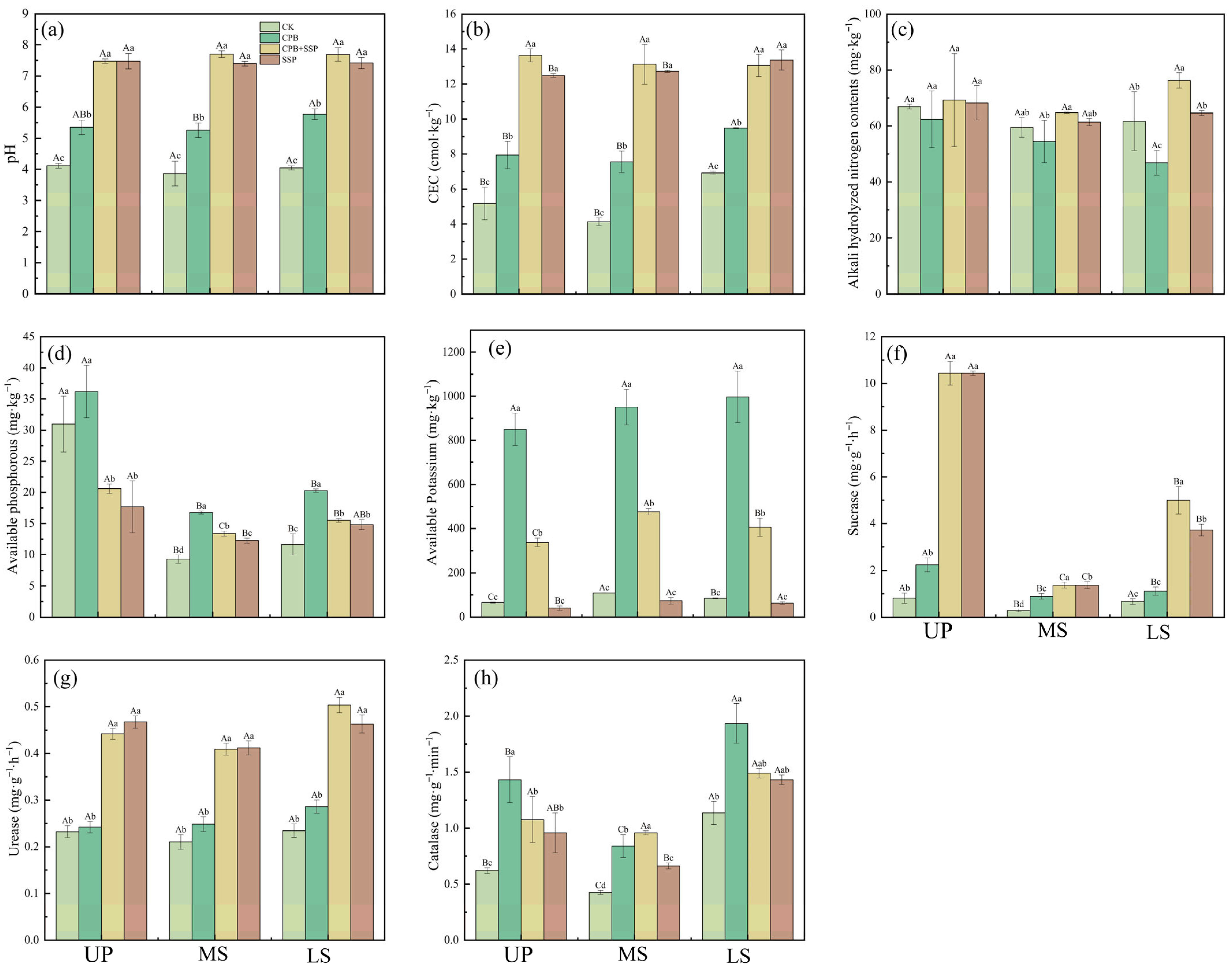
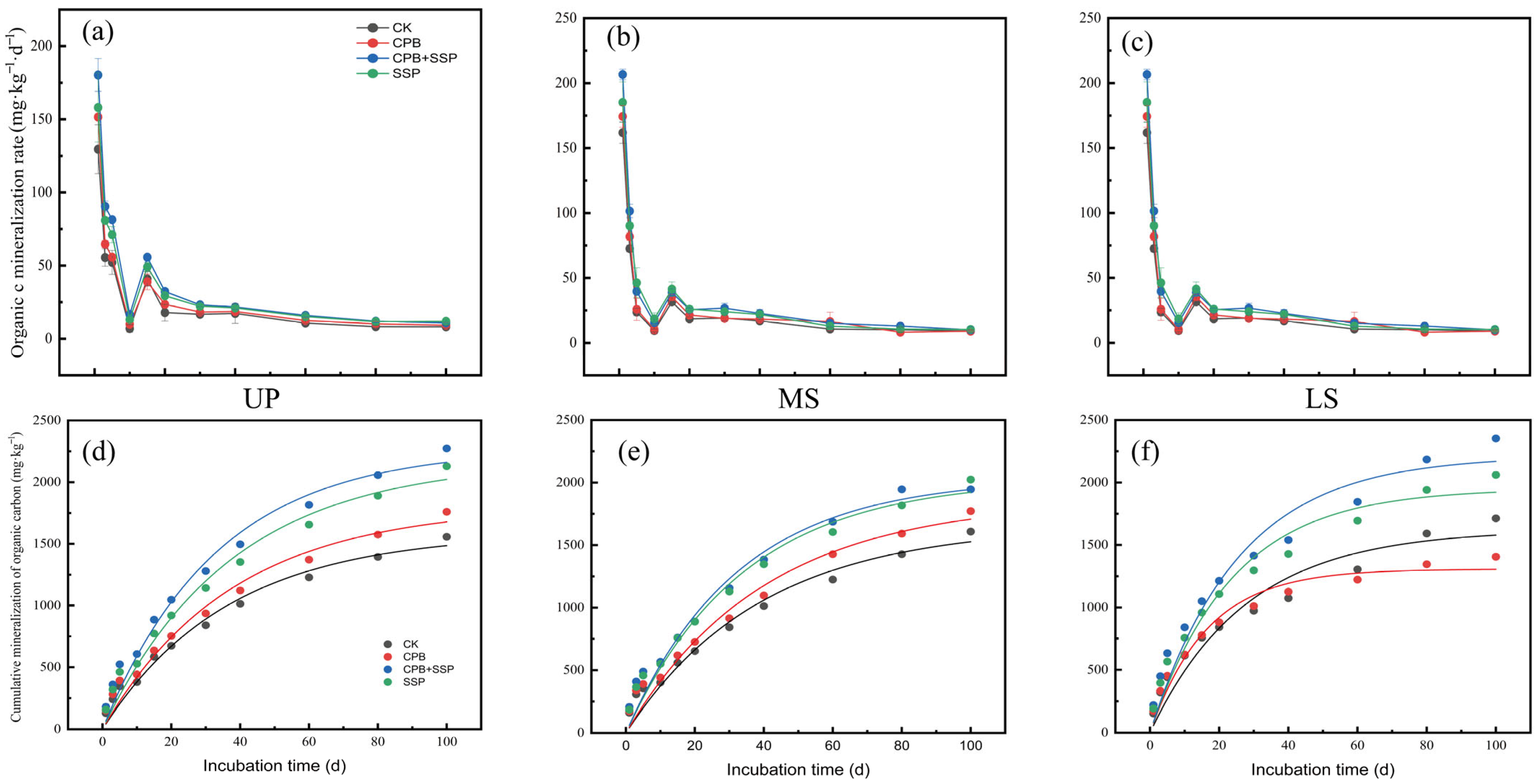
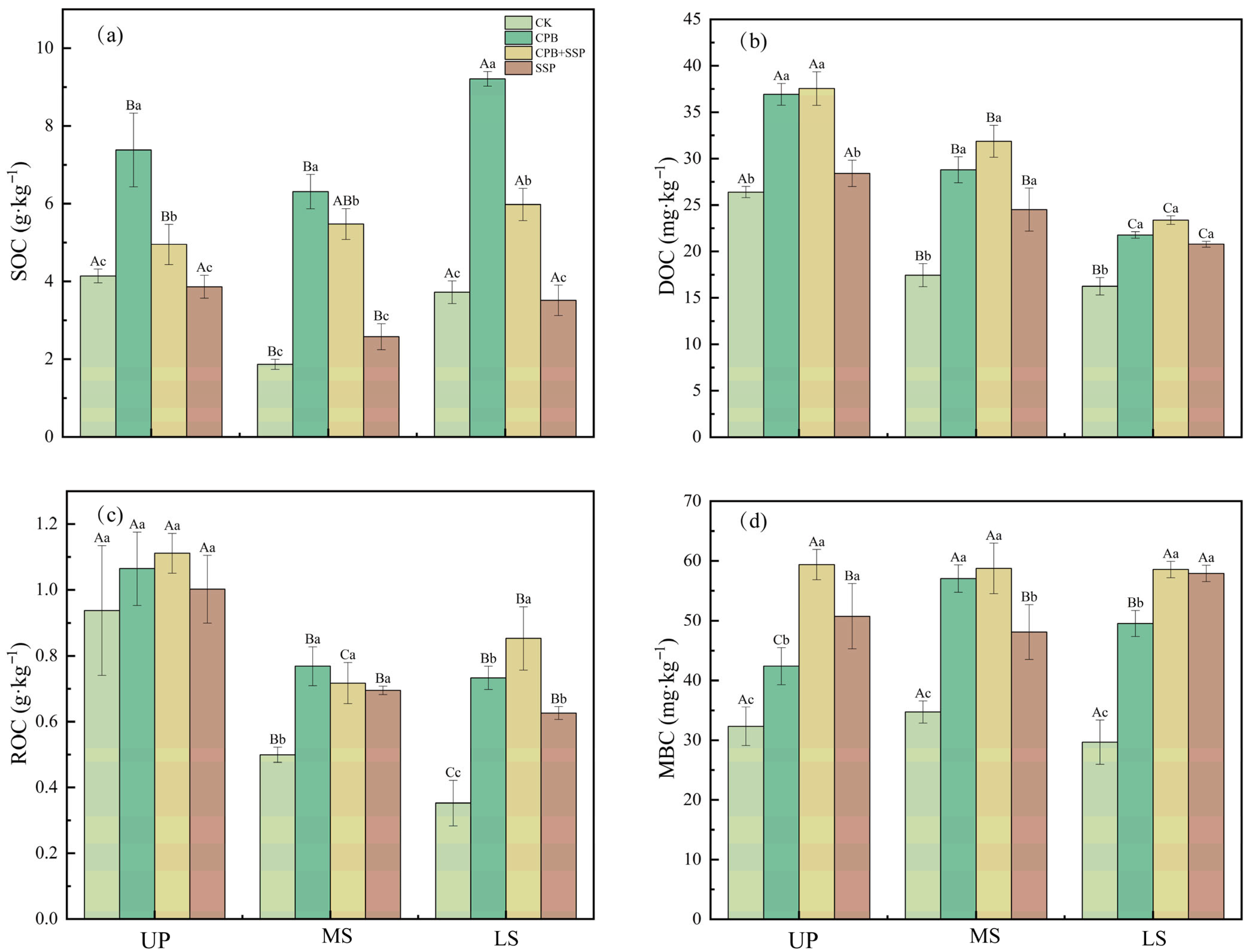

| Soil Slope Position | pH | Volume Weight of Soil | SOC | AP (Available Phosphorus) | AK (Available Potassium) | AN (Alkali-Hydrolyzable Nitrogen) (mg·kg−1) |
|---|---|---|---|---|---|---|
| (g·cm−3) | (g·kg−1) | (mg·kg−1) | (mg·kg−1) | |||
| Upper | 3.98 ± 0.07 | 1.20 ± 0.03 | 4.15 ± 0.03 | 22.70 ± 0.83 | 30.72 ± 4.09 | 47.83 ± 2.38 |
| Middle | 4.02 ± 0.04 | 0.95 ± 0.03 | 3.46 ± 0.06 | 10.98 ± 0.88 | 48.56 ± 1.64 | 40.25 ± 4.70 |
| Lower | 3.95 ± 0.05 | 0.86 ± 0.02 | 4.00 ± 0.03 | 9.07 ± 0.27 | 38.03 ± 1.1 | 34.76 ± 4.22 |
| Soil Slope Position | Treatment Group | Type of Improver | Add Proportion (w/w) |
|---|---|---|---|
| Upper | CK | Untreated soil with no amendment addition | 0% |
| CPB | citrus peel biochar | 4% | |
| CPB + SSP | citrus peel biochar + field snail shell powder | 2% + 2% | |
| SSP | field snail shell powder | 4% | |
| Middle | CK | Untreated soil with no amendment addition | 0% |
| CPB | citrus peel biochar | 4% | |
| CPB + SSP | citrus peel biochar + field snail shell powder | 2% + 2% | |
| SSP | field snail shell powder | 4% | |
| Lower | CK | Untreated soil with no amendment addition | 0% |
| CPB | citrus peel biochar | 4% | |
| CPB + SSP | citrus peel biochar + field snail shell powder | 2% + 2% | |
| SSP | field snail shell powder | 4% |
| pH | CEC | C/N | C/H | Elemental Content (%) | |||
|---|---|---|---|---|---|---|---|
| (cmol·kg−1) | C | H | N | ||||
| citrus peel biochar | 9.6 ± 0.02 | 30.96 ± 0.14 | 38.59 ± 0.15 | 14.95 ± 0.13 | 77.81 ± 5.20 | 5.2 ± 0.04 | 2.02 ± 0.01 |
| field snail shell powder | 8.53 ± 0.04 | 440.3 ± 0.22 | 31.40 ± 0.35 | 112.53 ± 0.91 | 12.65 ± 0.1 | 0.11 ± 0.0 | 0.40 ± 0.02 |
| Different Treatments | Fitting Parameters | ||||
|---|---|---|---|---|---|
| C0/mg·kg−1 | k/d−1 | R2 | C0/SOC | ||
| UP | CK | 1587.89 ± 94.33 | 0.0301 ± 0.003 | 0.973 | 0.3835 |
| CPB | 1803.42 ± 117.09 | 0.0266 ± 0.003 | 0.970 | 0.2443 | |
| CPB + SSP | 2272.68 ± 123.57 | 0.0301 ± 0.003 | 0.973 | 0.4591 | |
| SSP | 2171.39 ± 130.47 | 0.0267 ± 0.003 | 0.974 | 0.5619 | |
| MS | CK | 1655.06 ± 132.19 | 0.0255 ± 0.004 | 0.956 | 0.6961 |
| CPB | 1859.68 ± 146.01 | 0.0250 ± 0.004 | 0.960 | 0.2946 | |
| CPB + SSP | 2037.87 ± 125.76 | 0.0308 ± 0.004 | 0.962 | 0.3721 | |
| SSP | 2029.00 ± 122.56 | 0.0294 ± 0.004 | 0.967 | 0.6093 | |
| LS | CK | 1622.62 ± 117.11 | 0.0361 ± 0.006 | 0.930 | 0.4359 |
| CPB | 1308.39 ± 50.34 | 0.0608 ± 0.007 | 0.959 | 0.1420 | |
| CPB + SSP | 2212.59 ± 137.55 | 0.0392 ± 0.006 | 0.940 | 0.3701 | |
| SSP | 1950.59 ± 100.64 | 0.0422 ± 0.005 | 0.954 | 0.5551 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Ding, Z.; Qin, R.; Xiao, M.; Feng, M.; Liang, J.; Fan, Q.; Li, X.; Liu, S. Response of Soil Organic Carbon in Citrus Orchards at Different Slope Positions to Citrus Peel Biochar and Field Snail Shell Powder. Agronomy 2025, 15, 2209. https://doi.org/10.3390/agronomy15092209
Hu L, Ding Z, Qin R, Xiao M, Feng M, Liang J, Fan Q, Li X, Liu S. Response of Soil Organic Carbon in Citrus Orchards at Different Slope Positions to Citrus Peel Biochar and Field Snail Shell Powder. Agronomy. 2025; 15(9):2209. https://doi.org/10.3390/agronomy15092209
Chicago/Turabian StyleHu, Lening, Zerui Ding, Rui Qin, Meifang Xiao, Mintuan Feng, Jingxiao Liang, Qijun Fan, Xianliang Li, and Shengqiu Liu. 2025. "Response of Soil Organic Carbon in Citrus Orchards at Different Slope Positions to Citrus Peel Biochar and Field Snail Shell Powder" Agronomy 15, no. 9: 2209. https://doi.org/10.3390/agronomy15092209
APA StyleHu, L., Ding, Z., Qin, R., Xiao, M., Feng, M., Liang, J., Fan, Q., Li, X., & Liu, S. (2025). Response of Soil Organic Carbon in Citrus Orchards at Different Slope Positions to Citrus Peel Biochar and Field Snail Shell Powder. Agronomy, 15(9), 2209. https://doi.org/10.3390/agronomy15092209






