Influence of LED Light Spectra on Morphogenesis, Secondary Metabolite Production and Antioxidant Potential in Eucomis autumnalis Cultured In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and In Vitro Culture Conditions
2.2. Experiment Design, Biometric Parameters and Photosynthetic Pigments Content
2.3. Analyses of Polyphenol Content
2.4. Antioxidant Potential Assay
2.5. Statistical Analysis
3. Results
3.1. Biometric and Photosynthetic Content Response
3.2. Analyses of Polyphenol Content
3.3. Antioxidant Activity
4. Discussion
4.1. Biometric and Photosynthetic Content Response
4.2. Analyses of Polyphenol Content
4.3. Antioxidant Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS•+ | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging assay |
| B | 100% blue LED light |
| BA | 6-benzylaminopurine |
| Car | carotenoids |
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay |
| DW | dry weight |
| FRAP | ferric reducing antioxidant power assay |
| FW | fresh weight |
| LED | light-emitting diodes |
| MS | Murashige and Skoog |
| NAA | 1-naphthaleneacetic acid |
| PPFD | photosynthetic photon flux density |
| RB | 70% red + 30% blue LED light |
| RBfR | 50% RB + 50% far-red LED light |
| RBG | 50% RB + 50% green LED light |
| RBY | 50% RB + 50% yellow LED light |
| RBUV | 50% RB + 50% ultraviolet LED light |
| R | 100% red LED light |
| WLed | white LED light |
References
- Salachna, P.; Zawadzińska, A. Foliar Application of Depolymerised Chitosan Enhances the growth and Content of Polyphenols and L-Ascorbic Acid in Eucomis autumnalis, an Ornamental and Medicinal Plant. Prog. Chem. Appl. Chitin Deriv. 2024, 29, 254–262. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Mulaudzi, R.B.; Kulkarni, M.G.; Van Staden, J. Effect of Environmental Factors on Seedling Growth, Bulb Development and Pharmacological Properties of Medicinal Eucomis autumnalis. S. Afr. J. Bot. 2012, 79, 1–8. [Google Scholar] [CrossRef]
- Alaribe, F.N.; Maepa, M.J.; Mkhumbeni, N.; Motaung, S.C.K.M. Possible Roles of Eucomis autumnalis in Bone and Cartilage Regeneration: A Review. Trop. J. Pharm Res. 2018, 17, 741–749. [Google Scholar] [CrossRef]
- Salachna, P.; Zawadzińska, A. Effect of Daminozide and Flurprimidol on Growth, Flowering and Bulb Yield of Eucomis autumnalis (Mill.) Chitt. Folia Hortic. 2017, 29, 33–38. [Google Scholar] [CrossRef][Green Version]
- Salachna, P.; Zawadzińska, A.; Wierzbiński, Ł.; Senderek, W. Enhancing Growth in Eucomis autumnalis (Mill.) Chitt. Seedlings with Exogenous Application of Nitric Oxide. J. Hortic. Res. 2016, 24, 13–17. [Google Scholar] [CrossRef][Green Version]
- Mkhumbeni, N.; Pillay, M.; Mtunzi, F.; Motaung, K.S.C. Effect of Eucomis autumnalis on the Osteogenic Differentiation of Adipose-Derived Stem Cells. Tissue Eng. Part A 2022, 28, 136–149. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Rengasamy, K.R.R.; Amoo, S.O.; Gruz, J.; Bíba, O.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Doležal, K.; et al. Physiological Role of Phenolic Biostimulants Isolated from Brown Seaweed Ecklonia maxima on Plant Growth and Development. Planta 2015, 241, 1313–1324. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Gruz, J.; Doležal, K.; Van Staden, J. Potential of Smoke-Water and One of Its Active Compounds (Karrikinolide, KAR1) on the Phytochemical and Antioxidant Activity of Eucomis autumnalis. Antioxidants 2019, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Masondo, N.A.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Growth and Phytochemical Levels in Micropropagated Eucomis autumnalis Subspecies Autumnalis Using Different Gelling Agents, Explant Source, and Plant Growth Regulators. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 102–110. [Google Scholar] [CrossRef]
- Masondo, N.A.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Plant Growth Regulator Induced Phytochemical and Antioxidant Variations in Micropropagated and Acclimatized Eucomis autumnalis Subspecies Autumnalis (Asparagaceae). Acta Physiol. Plant. 2014, 36, 2467–2479. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED Light Enhances Growth, Phytochemical Contents, and Antioxidant Enzyme Activities of Rehmannia glutinosa Cultured in Vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Cioć, M.; Łopusiewicz, Ł.; Pietrak, A.; Firszt, R.; Sienkiewicz, M. Organogenesis and Antioxidant Capacity of Streptocarpus × hybridus In Vitro under Different LED Light Spectrum Composition. Agronomy 2023, 13, 3009. [Google Scholar] [CrossRef]
- Morańska, E.; Simlat, M.; Warchoł, M.; Skrzypek, E.; Waligórski, P.; Laurain-Mattar, D.; Spina, R.; Ptak, A. Phenolic Acids and Amaryllidaceae Alkaloids Profiles in Leucojum aestivum L. In Vitro Plants Grown under Different Light Conditions. Molecules 2023, 28, 1525. [Google Scholar] [CrossRef]
- Niazian, M.; Sabbatini, P. Traditional in vitro strategies for sustainable production of bioactive compounds and manipulation of metabolomic profile in medicinal, aromatic and ornamental plants. Planta 2021, 254, 111. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current Review of the Modulatory Effects of LED Lights on Photosynthesis of Secondary Metabolites and Future Perspectives of Microgreen Vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Wan Razali, W.A.; Waiho, K.; Wong, K.Y.; Foo, S.S.; Kamaruzzan, A.S.; Derek, C.J.C.; Ma, N.L.; Chang, J.S.; Dong, C.D.; et al. Light-Emitting Diodes (LEDs) for Culturing Microalgae and Cyanobacteria. Chem. Eng. J. 2024, 485, 149619. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Agarwal, A. Influence of LED Lighting on In Vitro Plant Regeneration and Associated Cellular Redox Balance. In Light Emitting Diodes for Agriculture: Smart Lighting; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 273–303. [Google Scholar]
- Gam, D.T.; Khoi, P.H.; Ngoc, P.B.; Linh, L.K.; Hung, N.K.; Anh, P.T.L.; Thu, N.T.; Hien, N.T.T.; Khanh, T.D.; Ha, C.H. Led Lights Promote Growth and Flavonoid Accumulation of Anoectochilus roxburghii and Are Linked to the Enhanced Expression of Several Related Genes. Plants 2020, 9, 1344. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Chen, L.; Zhou, C.; Tarin, M.W.K.; Yang, D.; Ren, K.; He, T.; Rong, J.; Zheng, Y. Transcriptomic and Metabolomic Profiling Reveals the Effect of LED Light Quality on Morphological Traits, and Phenylpropanoid-Derived Compounds Accumulation in Sarcandra glabra Seedlings. BMC Plant Biol. 2020, 20, 476. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Pradhan, S. Regulation of Gene Expression by LED Lighting. In Light Emitting Diodes for Agriculture: Smart Lighting; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 237–258. [Google Scholar]
- Lupo, M.; Bashir, M.A.; Silvestri, C.; Brunori, E.; Pica, A.L.; Cristofori, V. LED Lighting Effects on Plant Growth and Quality of Pyrus communis L. Propagated In Vitro. Agronomy 2022, 12, 2531. [Google Scholar] [CrossRef]
- Murasnige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pawłowska, B.; Żupnik, M.; Szewczyk-Taranek, B.; Cioć, M. Impact of LED Light Sources on Morphogenesis and Levels of Photosynthetic Pigments in Gerbera jamesonii Grown in Vitro. Hortic. Environ. Biotechnol. 2018, 59, 115–123. [Google Scholar] [CrossRef]
- Sumanta, N.; Imranul Haque, C.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Szopa, A.; Kubica, P.; Ekiert, H. Agitated Shoot Cultures of Aronia Arbutifolia and Aronia × prunifolia: Biotechnological Studies on the Accumulation of Phenolic Compounds and Biotransformation Capability. Plant Cell Tissue Organ Cult. 2018, 134, 467–479. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krośniak, M.; Marzec-Wróblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Comparative Analysis of Different Groups of Phenolic Compounds in Fruit and Leaf Extracts of Aronia Sp.: A. melanocarpa, A. arbutifolia, and A. × prunifolia and Their Antioxidant Activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, Characterization, and Bioactivity of Non-Dairy Kefir-like Fermented Beverage Based on Flaxseed Oil Cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef]
- Pietrak, A.; Łopusiewicz, Ł.; Salachna, P. Growth, Leaf Pigment Content, and Antioxidant Potential of Ferns Grown in Peat Substrate Amended with Camelina Press Cake. Agronomy 2022, 12, 3100. [Google Scholar] [CrossRef]
- Beuel, A.K.; Jablonka, N.; Heesel, J.; Severin, K.; Spiegel, H.; Rasche, S. LEDitSHAKE: A lighting system to optimize the secondary metabolite content of plant cell suspension cultures. Sci. Rep. 2021, 11, 23353. [Google Scholar] [CrossRef]
- Guo, Y.; Zhong, Y.; Mo, L.; Zhang, W.; Chen, Y.; Wang, Y.C.; Chen, H.; Wang, Z.; Song, X.; Meng, X. Different Combinations of Red and Blue LED Light Affect the Growth, Physiology and Photosynthesis of in Vitro-Cultured Dendrobium nobile ‘Zixia’. Hortic. Environ. Biotechnol. 2023, 64, 393–407. [Google Scholar] [CrossRef]
- Cioć, M.; Tokarz, K.; Dziurka, M.; Pawłowska, B. Energy-Saving Led Light Affects the Efficiency of the Photosynthetic Apparatus and Carbohydrate Content in Gerbera Jamesonii Bolus Ex Hook. F. Axillary Shoots Multiplied in Vitro. Biology 2021, 10, 1035. [Google Scholar] [CrossRef]
- Miler, N.; Kulus, D.; Woźny, A.; Rymarz, D.; Hajzer, M.; Wierzbowski, K.; Nelke, R.; Szeffs, L. Application of Wide-Spectrum Light-Emitting Diodes in Micropropagation of Popular Ornamental Plant Species: A Study on Plant Quality and Cost Reduction. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 99–108. [Google Scholar] [CrossRef]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The Effect of Light Quality on the Growth and Development of In Vitro Cultured Doritaenopsis Plants. Acta Physiol. Plant 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Mawphlang, O.; Kharshiing, E. Photoreceptor Mediated Plant Growth Responses: Implications for Photoreceptor Engineering toward Improved Performance in Crops. Front. Plant Sci. 2017, 8, 1181. [Google Scholar] [CrossRef]
- Pech, R.; Volná, A.; Hunt, L.; Bartas, M.; Červeň, J.; Pečinka, P.; Špunda, V.; Nezval, J. Regulation of Phenolic Compound Production by Light Varying in Spectral Quality and Total Irradiance. Int. J. Mol. Sci. 2022, 23, 6533. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Hnatuszko-Konka, K.; Lebelt, L.; Grzegorczyk-Karolak, I. The Protective Function and Modification of Secondary Metabolite Accumulation in Response to Light Stress in Dracocephalum forrestii Shoots. Int. J. Mol. Sci. 2021, 22, 7965. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond vegetables: Effects of indoor LED light on specialized metabolite biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J. Sci. Food Agric. 2021, 102, 472–487. [Google Scholar] [CrossRef]
- Su, P.; Ding, S.; Wang, D.; Kan, W.; Yuan, M.; Chen, X.; Tang, C.; Hou, J.; Wu, L. Plant Morphology, Secondary Metabolites and Chlorophyll Fluorescence of Artemisia argyi under Different LED. Photosynth. Res. 2024, 159, 153–164. [Google Scholar] [CrossRef]
- Pałka, P.; Muszyńska, B.; Szewczyk, A.; Pawłowska, B. Elicitation and Enhancement of Phenolics Synthesis with Zinc Oxide Nanoparticles and LED Light in Lilium candidum L. Cultures In Vitro. Agronomy 2023, 13, 1437. [Google Scholar] [CrossRef]
- Cioć, M.; Szewczyk, A.; Żupnik, M.; Kalisz, A.; Pawłowska, B. LED Lighting Affects Plant Growth, Morphogenesis and Phytochemical Contents of Myrtus communis L. in Vitro. Plant Cell Tissue Organ Cult. 2018, 132, 433–447. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kuźma, Ł.; Grzegorczyk-Karolak, I. The Effect of Different Light Treatments on Morphogenesis, Phenolic Compound Accumulation and Antioxidant Potential of Dracocephalum forrestii Transformed Shoots Cultured in Vitro. J. Photochem. Photobiol. B 2021, 224, 112329. [Google Scholar] [CrossRef] [PubMed]
- Menicucci, F.; Marino, G.; Sillo, F.; Carli, A.; dos Santos Nascimento, L.B.; Detti, C.; Centritto, M.; Brunetti, C.; Balestrini, R.M. Blue and Red LEDs Modulate Polyphenol Production in Precoce and Tardiva Cultivars of Cichorium intybus L. Front. Plant Sci. 2025, 16, 1529804. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Chen, L.l.; Wang, H.Y.; Gong, X.C.; Zeng, Z.H.; Xue, X.Z.; Hu, Y.G. Transcriptome Analysis Reveals Effects of Red and Blue Light-Emitting Diodes (LEDs) on the Growth, Chlorophyll Fluorescence and Endogenous Plant Hormones of Potato (Solanum tuberosum L.) Plantlets Cultured in Vitro. J. Integr. Agric. 2021, 20, 2914–2931. [Google Scholar] [CrossRef]
- Oliveira, T.d.R.; Aragão, V.P.M.; Moharana, K.C.; Fedosejevs, E.; do Amaral, F.P.; Sousa, K.R.; Thelen, J.J.; Venâncio, T.M.; Silveira, V.; Santa-Catarina, C. Light Spectra Affect the in Vitro Shoot Development of Cedrela fissilis Vell. (Meliaceae) by Changing the Protein Profile and Polyamine Contents. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140529. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. Physiological and Proteomic Insights Into Red and Blue Light-Mediated Enhancement of in Vitro Growth in Scrophularia kakudensis—A Potential Medicinal Plant. Front. Plant Sci. 2021, 11, 607007. [Google Scholar] [CrossRef]
- Rai, N.; Kumari, S.; Singh, S.; Saha, P.; Pandey, A.K.; Pandey-Rai, S. Modulation of Morpho-Physiological Attributes and in Situ Analysis of Secondary Metabolites Using Raman Spectroscopy in Response to Red and Blue Light Exposure in Artemisia annua. Environ. Exp. Bot. 2024, 217, 105563. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Light as an Elicitor for Enhanced of Secondary Metabolites in Plant Cell, Tissue, and Organ Cultures. Plant Growth Regul. 2024, 104, 31–49. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of Photosynthetic Processes and the Accumulation of Secondary Metabolites in Plants in Response to Monochromatic Light Environments: A Review. Biochim. Biophys. Acta–Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. Hortscience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Zhang, F.; Gu, Y.; Tian, W.; Tian, W.; Tong, Y.; Li, J. The Combination of Red and Blue Light Increases the Biomass and Steroidal Saponin Contents of Paris Polyphylla Var. Yunnanensis. Ind. Crops Prod. 2023, 194, 116311. [Google Scholar] [CrossRef]
- Da Silva, A.P.G.; Sganzerla, W.G.; John, O.D.; Marchiosi, R. A Comprehensive Review of the Classification, Sources, Biosynthesis, and Biological Properties of Hydroxybenzoic and hydroxycinnamic Acids. Phytochem. Rev. 2023, 24, 94. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Ulrih, N.P.; Cigić, B. Relevance and Standardization of in Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative Study of DPPH, ABTS and FRAP Assays for Determination of Antioxidant Activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Nazir, M.; Ullah, M.A.; Younas, M.; Siddiquah, A.; Shah, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Light-Mediated Biosynthesis of Phenylpropanoid Metabolites and Antioxidant Potential in Callus Cultures of Purple Basil (Ocimum basilicum L. Var Purpurascens). Plant Cell Tissue Organ Cult. 2020, 142, 107–120. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.; Tulio, A.; Scheerens, J.; Miller, A. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (abts) method to measure antioxidant capacity of Selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kitamura, Y.; Nagano, H.; Kawatsu, J.; Gotoh, H. DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols. Antioxidants 2024, 13, 309. [Google Scholar] [CrossRef]
- Minarti, M.; Ariani, N.; Megawati, M.; Hidayat, A.; Hendra, M.; Primahana, G.; Darmawan, A. Potential Antioxidant Activity Methods DPPH, ABTS, FRAP, Total Phenol and Total Flavonoid Levels of Macaranga hypoleuca (Reichb. f. & Zoll.) Leaves Extract and Fractions. E3S Web Conf. 2024, 503, 07005. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef]
- Chen, C.L.; Yang, J.P.; Huang, W.D.; Chen, C.C. The Effect of Far-Red Light and Nutrient Level on the Growth and Secondary Metabolites of the In Vitro Culture of Prunella vulgaris. Agronomy 2023, 13, 2250. [Google Scholar] [CrossRef]
- Hashim, M.; Ahmad, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants in Vitro Cultures. Plants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Chung, I.M.; Hwang, M.H.; Kim, S.H.; Yu, C.Y.; Ghimire, B.K. Application of Light-Emitting Diodes for Improving the Nutritional Quality and Bioactive Compound Levels of Some Crops and Medicinal Plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef] [PubMed]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of Different Spectral Lights with Biomass Accumulation and Production of Antioxidant Secondary Metabolites in Callus Cultures of Medicinally Important Prunella vulgaris L. J. Photochem. Photobiol. B 2016, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, R.; Ullah, M.A.; Tungmunnithum, D.; Drouet, S.; Shah, M.; Zaeem, A.; Hameed, S.; Hano, C.; Abbasi, B.H. Lights Triggered Differential Accumulation of Antioxidant and Antidiabetic Secondary Metabolites in Callus Culture of Eclipta alba L. PLoS ONE 2020, 15, e0233963. [Google Scholar] [CrossRef]
- Biswal, B.; Jena, B.; Giri, A.K.; Acharya, L. Monochromatic Light Elicited Biomass Accumulation, Antioxidant Activity, and Secondary Metabolite Production in Callus Culture of Operculina turpethum (L.). Plant Cell Tissue Organ Cult. 2022, 149, 123–134. [Google Scholar] [CrossRef]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of Light Quality on Growth, Secondary Metabolites Production and Antioxidant Activity in Callus Culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B 2018, 183, 258–265. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, N.; Rab, A. Light-Induced Biochemical Variations in Secondary Metabolite Production and Antioxidant Activity in Callus Cultures of Stevia rebaudiana (Bert). J. Photochem. Photobiol. B 2016, 154, 51–56. [Google Scholar] [CrossRef]
- Biswajit, J.; Bhagyashree, B.; Kumar, G.A.; Laxmikanta, A. Influence of Monochromatic Light on Growth, Secondary Metabolites and Antioxidant Activity in in Vitro Culture of Eryngium foetidum L. Res. J. Biotechnol. 2024, 19, 75–84. [Google Scholar] [CrossRef]
- Santos-Tierno, R.; Garcia, R.; Fonseca, E.; Faleiro, F.; Moreira, D.; Pacheco, G.; Mansur, E. Light Quality and Explant Type Modulate Growth, Antioxidant Properties and Bioactive Compounds Production of Calluses of Passiflora setacea Cv BRS Pérola Do Cerrado. Plant Cell Tissue Organ Cult. 2021, 147, 635–646. [Google Scholar] [CrossRef]
- Lai, C.C.; Pan, H.; Zhang, J.; Wang, Q.; Que, Q.X.; Pan, R.; Lai, Z.X.; Lai, G.T. Light Quality Modulates Growth, Triggers Differential Accumulation of Phenolic Compounds, and Changes the Total Antioxidant Capacity in the Red Callus of Vitis davidii. J. Agric. Food Chem. 2022, 70, 13264–13278. [Google Scholar] [CrossRef] [PubMed]
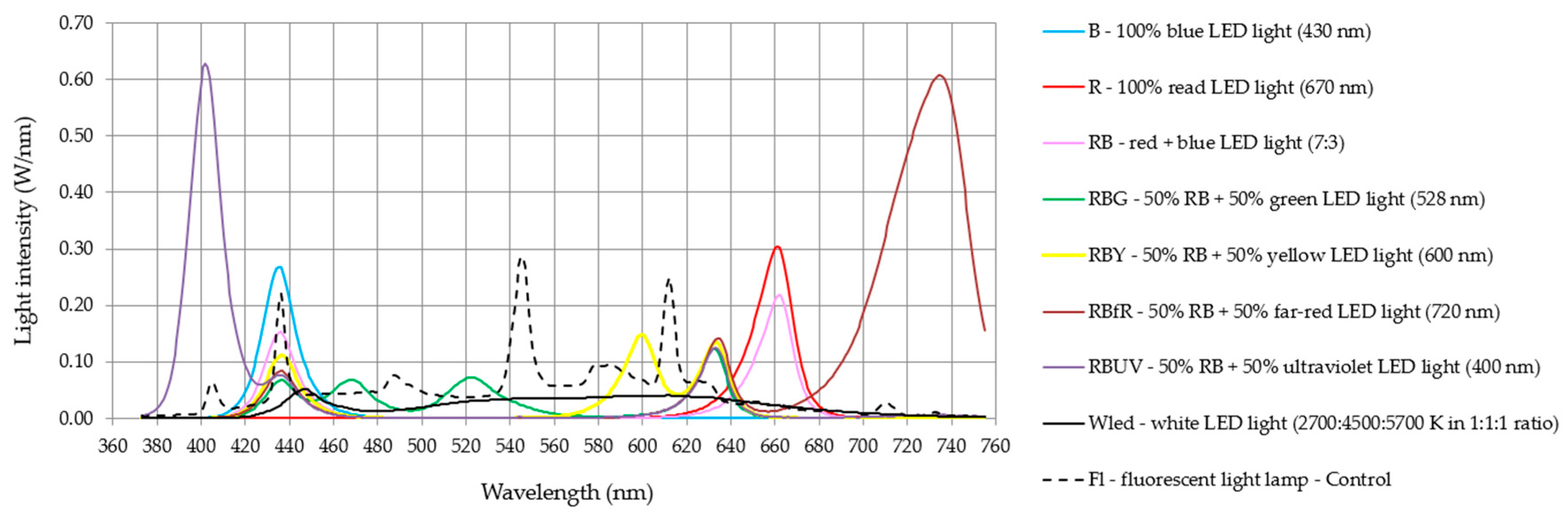

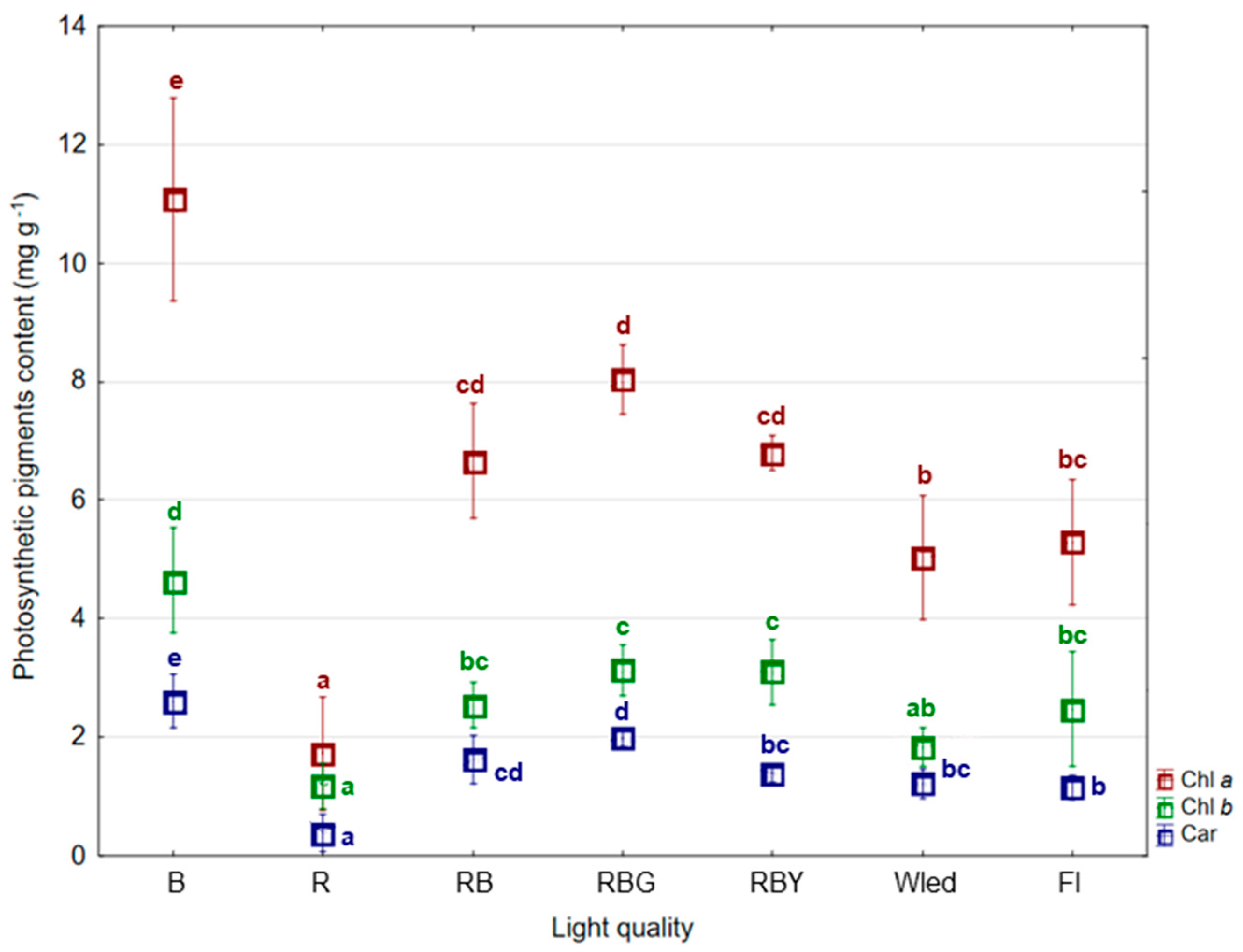
| Light Quality | No. of Shoots | Plants Height (cm) | No. of Leaves | No. of Roots | Roots Lenght (cm) | FW (g) | DW (%) |
|---|---|---|---|---|---|---|---|
| B 1 | 8.92 ± 3.60 ab 2 | 0.29 ± 0.09 a | 0.96 ± 0.12 a | 1.08 ± 1.04 b | 0.26 ± 0.25 a | 0.27 ± 0.06 a | 8.49 ± 0.24 d |
| R | 6.72 ± 2.79 a | 0.36 ± 0.23 ab | 1.07 ± 0.11 a | 0.12 ± 0.33 a | 0.19 ± 0.64 a | 0.28 ± 0.10 a | 5.80 ± 0.32 a |
| RB | 9.64 ± 2.84 b | 0.37 ± 0.14 ab | 2.52 ± 2.14 b | 0.59 ± 0.49 cd | 0.59 ± 0.49 b | 0.40 ± 0.08 ab | 7.33 ± 0.35 bc |
| RBG | 12.36 ± 3.47 c | 0.36 ± 0.12 ab | 1.30 ± 0.16 b | 2.84 ± 2.08 d | 0.66 ± 0.38 b | 0.51 ± 0.15 ab | 7.83 ± 0.43 cd |
| RBY | 15.48 ± 4.69 d | 0.57 ± 0.23 c | 1.72 ± 0.43 c | 1.68 ± 1.31 bc | 0.67 ± 0.61 b | 0.77 ± 0.30 c | 6.45 ± 0.68 ab |
| Wled | 12.32 ± 4.36 c | 0.39 ± 0.14 ab | 1.30 ± 0.13 b | 2.12 ± 1.99 cd | 0.43 ± 0.42 ab | 0.48 ± 0.14 ab | 7.30 ± 0.78 bc |
| Fl | 12.08 ± 5.47 c | 0.36 ± 0.07 ab | 1.34 ± 0.19 b | 1.76 ± 2.10 bc | 0.44 ± 0.43 ab | 0.46 ± 0.16 bc | 7.40 ± 0.95 c |
| Light Quality | Polyphenol Content (mg/100 g DW) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Acids | Flavonols | Flavanones | |||||||||||||
| Eucomic | Protocatechuic | Gentisic | Chlorogenic | p-Hydroxybenzoic | Vanillic | Caffeic | Syringic | Ferulic | o-Coumaric | Cinnamic | Catechin | Epicatechin | Hesperetin | Hesperidin | |
| B 1 | 358.7 ± 2.0 bc 2 | 5.6 ± 0.1 e | 175.9 ± 1.2 d | 14.3 ± 1.0 g | 1.0 ± 0.2 de | 3.5 ± 0.5 bc | 0.8 ± 0.1 a | 3.3 ± 0.2 a | 4.3 ± 0.4 a | 8.7 ± 0.3 bc | 6.5 ± 0.1 e | 273.2 ± 2.2 e | 162.6 ± 7.6 b | 52.7 ± 0.4 cd | 250.7 ± 2.3 e |
| R | 329.1 ± 3.4 a | 4.9 ± 0.1 bc | 214.3 ± 10.3 e | 9.4 ± 0.1 b | 0.8 ± 0.1 b | 4.9 ± 0.1 f | 2.5 ± 0.1 b | 5.3 ± 0.1 b | 5.1 ± 0.5 b | 8.9 ± 0.1 cd | 5.4 ± 0.1 b | 305.6 ± 1.4 f | 145.6 ± 1.5 a | 51.8 ± 0.7 cd | 238.3 ± 1.6 e |
| RB | 373.4 ± 3.9 d | 5.3 ± 0.1 de | 160.8 ± 1.3 c | 12.8 ± 0.3 d–f | 0.8 ± 0.1 cd | 4.0 ± 0.1 de | 2.1 ± 0.1 b | 5.8 ± 0.1 cd | 5.3 ± 0.1 b | 8.8 ± 0.1 cd | 5.7 ± 0.1 c | 235.7 ± 2.4 cd | 165.1 ± 1.7 b | 50.4 ± 0.9 c | 229.8 ± 2.2 d |
| RBG | 429.5 ± 4.2 g | 5.2 ± 0.4 c–e | 162.4 ± 7.9 c | 13.3 ± 0.3 e–g | 0.9 ± 0.3 cd | 3.7 ± 0.3 cd | 2.2 ± 0.2 b | 6.1 ± 0.1 d | 5.5 ± 0.1 bc | 8.9 ± 0.3 cd | 5.9 ± 0.1 d | 242.3 ± 13.2 d | 182.0 ± 14.7 c | 34.6 ± 0.4 a | 228.7 ± 3.4 d |
| RBY | 353.1 ± 1.0 b | 2.8 ± 0.3 a | 129.1 ± 0.9 a | 12.6 ± 0.2 de | 1.1 ± 0.1 e | 3.2 ± 0.1 ab | 5.3 ± 0.2 d | 5.0 ± 0.2 b | 4.9 ± 0.4 b | 7.0 ± 0.1 a | 5.5 ± 0.6 bc | 228.4 ± 12.4 c | 156.2 ± 0.4 ab | 43.2 ± 5.1 b | 201.4 ± 2.1 c |
| RBfR | 418.9 ± 5.7 f | 5.1 ± 0.2 b–d | 210.5 ± 5.1 e | 13.8 ± 1.4 fg | 0.8 ± 0.2 c | 4.1 ± 0.2 de | 2.1 ± 0.2 b | 5.9 ± 0.3 cd | 5.5 ± 0.5 bc | 8.4 ± 0.5 b | 6.0 ± 0.2 d | 262.4 ± 7.7 e | 181.3 ± 8.8 c | 53.0 ± 2.5 cd | 245.4 ± 5.9 |
| RBUV | 364.1 ± 6.9 c | 4.8 ± 0.1 b | 140.6 ± 0.8 b | 12.0 ± 0.2 cd | 1.1 ± 0.1 e | 3.1 ± 0.2 a | 3.6 ± 0.6 c | 6.1 ± 0.6 d | 5.1 ± 0.3 b | 7.4 ± 0.1 a | 5.6 ± 0.1 bc | 146.0 ± 0.5 a | 165.0 ± 5.5 b | 31.3 ± 0.5 a | 142.0 ± 4.7 a |
| Wled | 406.7 ± 5.0 e | 4.9 ± 0.1 bc | 182.3 ± 1.9 d | 11.1 ± 0.6 c | 0.9 ± 0.1 cd | 4.2 ± 0.1 e | 6.1 ± 0.2 e | 5.4 ± 0.1 bc | 5.3 ± 0.1 b | 9.3 ± 0.2 d | 5.2 ± 0.1 a | 192.1 ± 3.0 b | 179.9 ± 2.2 c | 34.1 ± 0.5 a | 160.1 ± 0.6 b |
| Fl | 409.7 ± 4.4 e | 5.1 ± 0.1 b–d | 229.2 ± 3.2 f | 8.1 ± 0.1 a | 0.7 ± 0.1 a | 5.0 ± 0.1 f | 9.6 ± 0.1 f | 5.7 ± 0.2 cd | 6.1 ± 0.2 c | 10.8 ± 0.2 e | 5.5 ± 0.1 b | 272.3 ± 3.6 e | 181.2 ± 2.0 c | 55.3 ± 1.5 d | 287.9 ± 3.1 f |
| Light Quality | DPPH (µg TE/g DW) | ABTS•+ (µg TE/g DW) | FRAP (µg AAE/g DW) |
|---|---|---|---|
| B 1 | 882.50 ± 76.60 ab 2 | 864.58 ± 41.25 a | 227.25 ± 0.87 de |
| R | 905.42 ± 79.55 ab | 847.92 ± 6.14 a | 175.80 ± 0.34 a |
| RB | 959.58 ± 97.23 ab | 968.75 ± 23.57 bc | 184.49 ± 1.02 b |
| RBG | 999.17 ± 100.17 b | 1112.50 ± 8.84 d | 221.45 ± 2.05 d |
| RBY | 1036.67 ± 76.60 b | 972.92 ± 35.36 bc | 224.83 ± 3.42 de |
| RBfR | 1059.58 ± 85.44 b | 1127.08 ± 11.79 d | 231.84 ± 8.54 e |
| RBUV | 799.17 ± 70.71 a | 931.25 ± 5.89 b | 205.75 ± 3.76 c |
| Wled | 922.08 ± 32.41 ab | 1002.08 ± 53.03 c | 200.19 ± 0.68 c |
| Fl | 1009.58 ± 61.87 b | 1022.92 ± 11.79 c | 205.99 ± 2.73 c |
| Identified Substances | Antioxidant Activity Analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS•+ | FRAP | |||||||||
| Phenolic acids | |||||||||||
| eucomic | 0.493395 | 0.908473 1 | 0.421057 | ||||||||
| protocatechuic | −0.308370 | 0.028803 | −0.183731 | ||||||||
| gentisic | 0.240283 | 0.106341 | −0.208743 | ||||||||
| chlorogenic | 0.062258 | 0.201267 | 0.603172 | ||||||||
| p-hydroxybenzoic | −0.402752 | −0.274433 | 0.362846 | ||||||||
| vanilic | 0.257448 | 0.039558 | −0.515750 | ||||||||
| caffeic | 0.235696 | 0.178663 | −0.090315 | ||||||||
| syryngic | 0.191007 | 0.591515 | −0.244962 | ||||||||
| ferulic | 0.462964 | 0.671658 | −0.138310 | ||||||||
| o-coumaric | 0.184765 | 0.163838 | −0.282185 | ||||||||
| cinnamic | 0.028167 | 0.046870 | 0.613751 | ||||||||
| Flavonols | |||||||||||
| catechin | 0.433175 | -0.104443 | -0.064575 | ||||||||
| epicatechin | 0.383471 | 0.835899 | 0.427261 | ||||||||
| Flavanones | |||||||||||
| hesperetin | 0.382889 | −0.158628 | −0.054075 | ||||||||
| hesperidin | 0.533925 | 0.122407 | 0.095530 | ||||||||
| r≥ | −1 | −0.8 | −0.6 | −0.4 | −0.2 | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioć, M.; Szopa, A.; Prokopiuk, B.; Pawłowska, B.; Łopusiewicz, Ł. Influence of LED Light Spectra on Morphogenesis, Secondary Metabolite Production and Antioxidant Potential in Eucomis autumnalis Cultured In Vitro. Agronomy 2025, 15, 2197. https://doi.org/10.3390/agronomy15092197
Cioć M, Szopa A, Prokopiuk B, Pawłowska B, Łopusiewicz Ł. Influence of LED Light Spectra on Morphogenesis, Secondary Metabolite Production and Antioxidant Potential in Eucomis autumnalis Cultured In Vitro. Agronomy. 2025; 15(9):2197. https://doi.org/10.3390/agronomy15092197
Chicago/Turabian StyleCioć, Monika, Agnieszka Szopa, Barbara Prokopiuk, Bożena Pawłowska, and Łukasz Łopusiewicz. 2025. "Influence of LED Light Spectra on Morphogenesis, Secondary Metabolite Production and Antioxidant Potential in Eucomis autumnalis Cultured In Vitro" Agronomy 15, no. 9: 2197. https://doi.org/10.3390/agronomy15092197
APA StyleCioć, M., Szopa, A., Prokopiuk, B., Pawłowska, B., & Łopusiewicz, Ł. (2025). Influence of LED Light Spectra on Morphogenesis, Secondary Metabolite Production and Antioxidant Potential in Eucomis autumnalis Cultured In Vitro. Agronomy, 15(9), 2197. https://doi.org/10.3390/agronomy15092197









