Transcriptome Analysis of the Regulatory Mechanism of Exogenous Manganese Sulfate Application on Wheat Grain Yield and Carotenoids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Measurement Indices and Methods
2.3.1. Wheat Yield
2.3.2. Determination of Carotenoid Content in Grains
2.3.3. Transcriptome Sequencing and Data Analysis
- padjust = p.adjust (p,method = “BH”)
- HISAT2 version 2.2.1: hisat2 -x <hisat2 index> -p 4 --dta -t --phred33 -1 sample_1.clean.fq.gz -2 sample_2.clean.fq.gz --un-conc-gz sample.unmap.fq.gz 2> sample_align.log | samtools sort -O BAM --threads 4 -o sample.bam -. These were used as default parameters.
2.4. Data Processing
3. Results
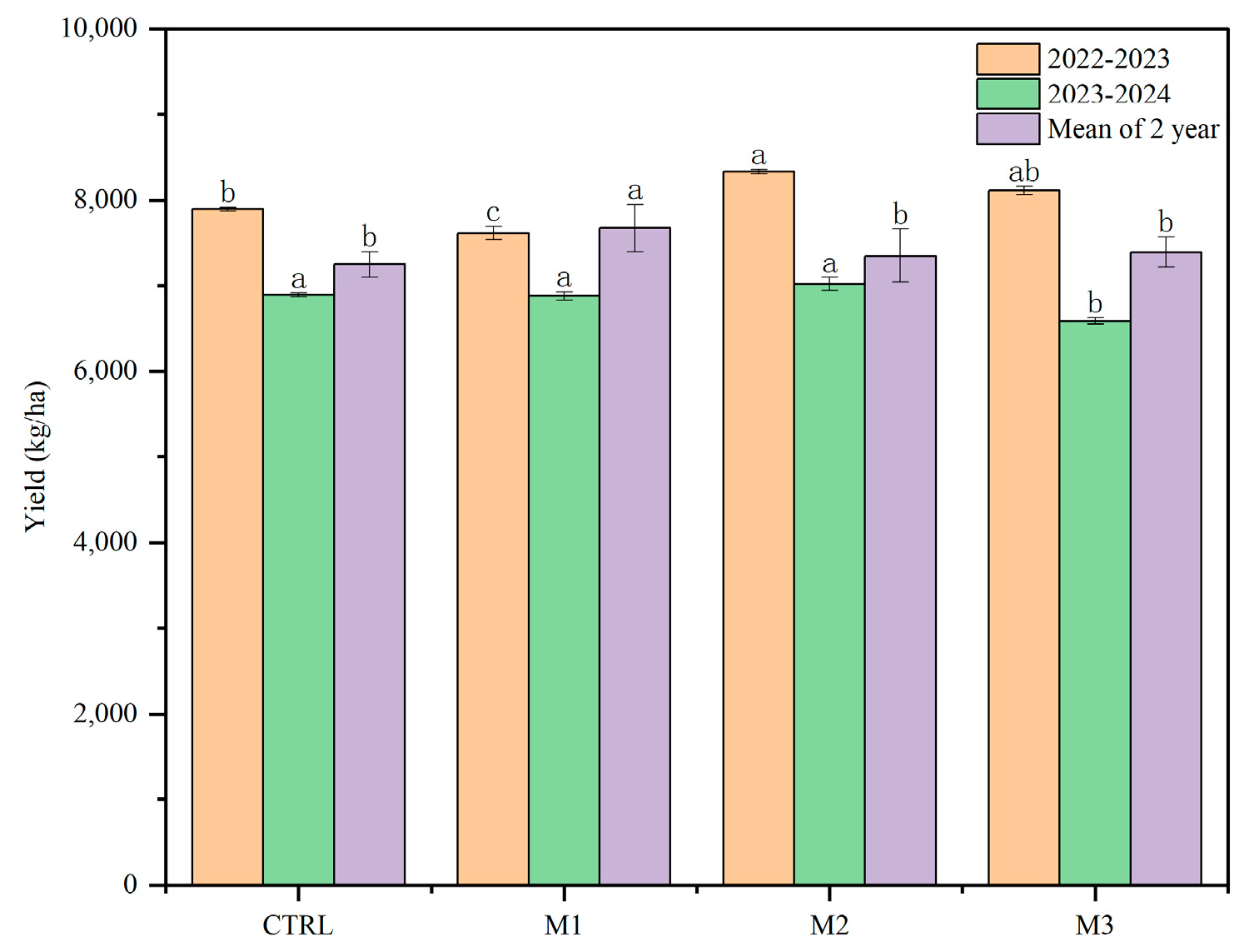
3.1. Effects of Foliar Application of Different Concentrations of Manganese Sulfate on Wheat Grain Yield
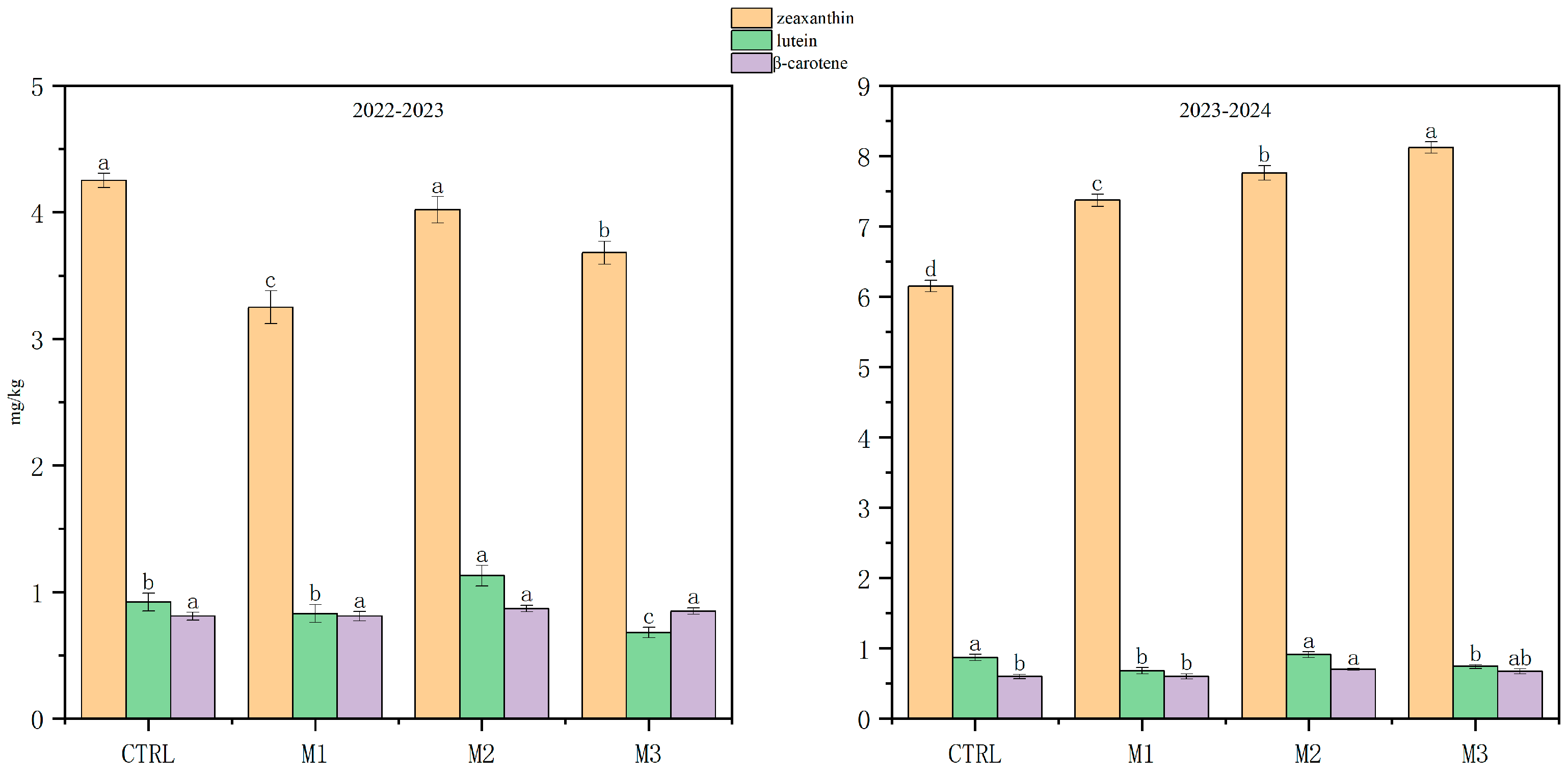
3.2. Effects of Foliar Application of Different Concentrations of Manganese Sulfate on Carotenoid Content in Wheat Grains
3.3. Transcriptome Sequencing Analysis of Wheat Grains Under Foliar Application of Exogenous Manganese Sulfate
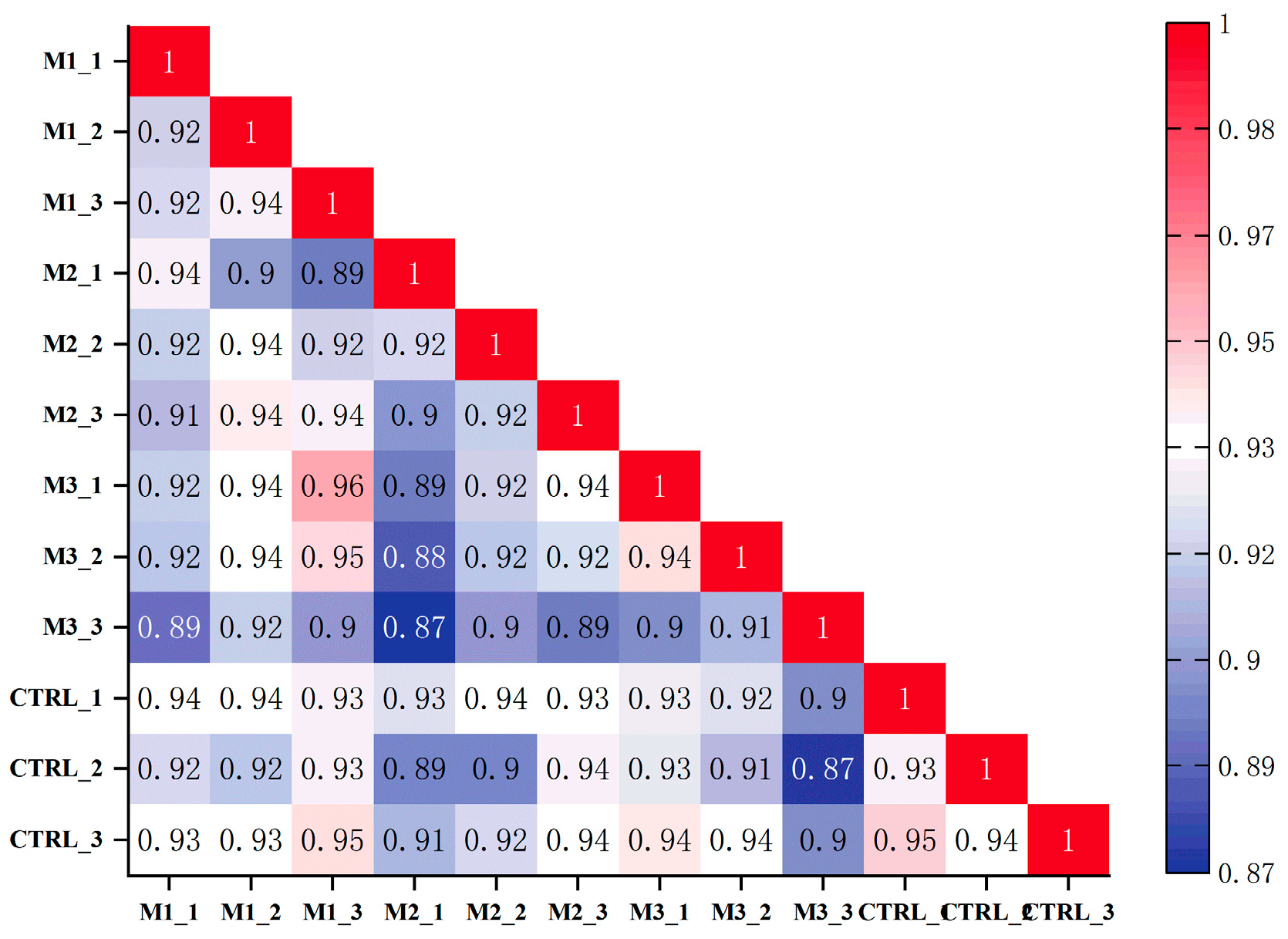
3.3.1. Quality Assessment of Sequencing Data
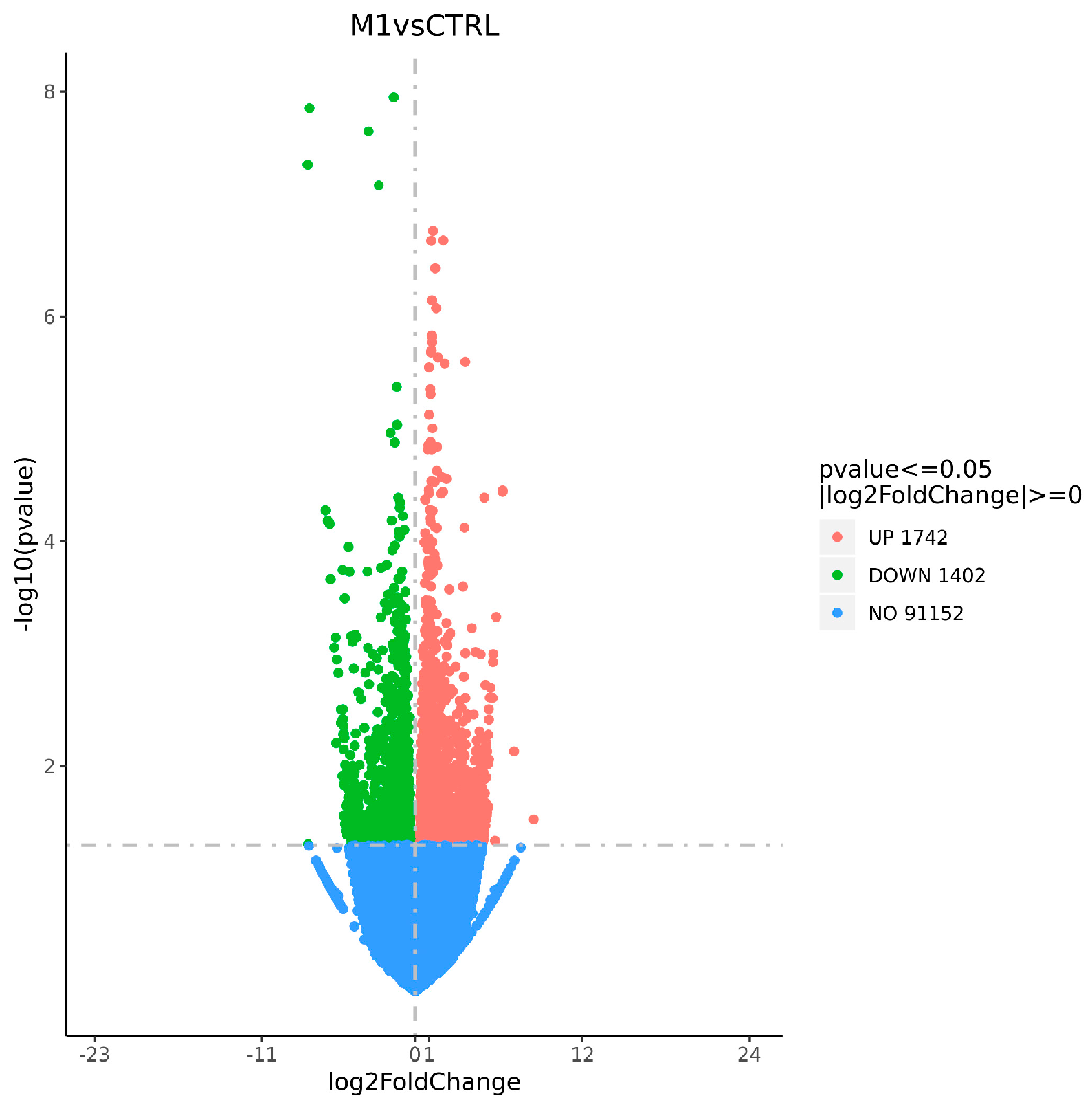
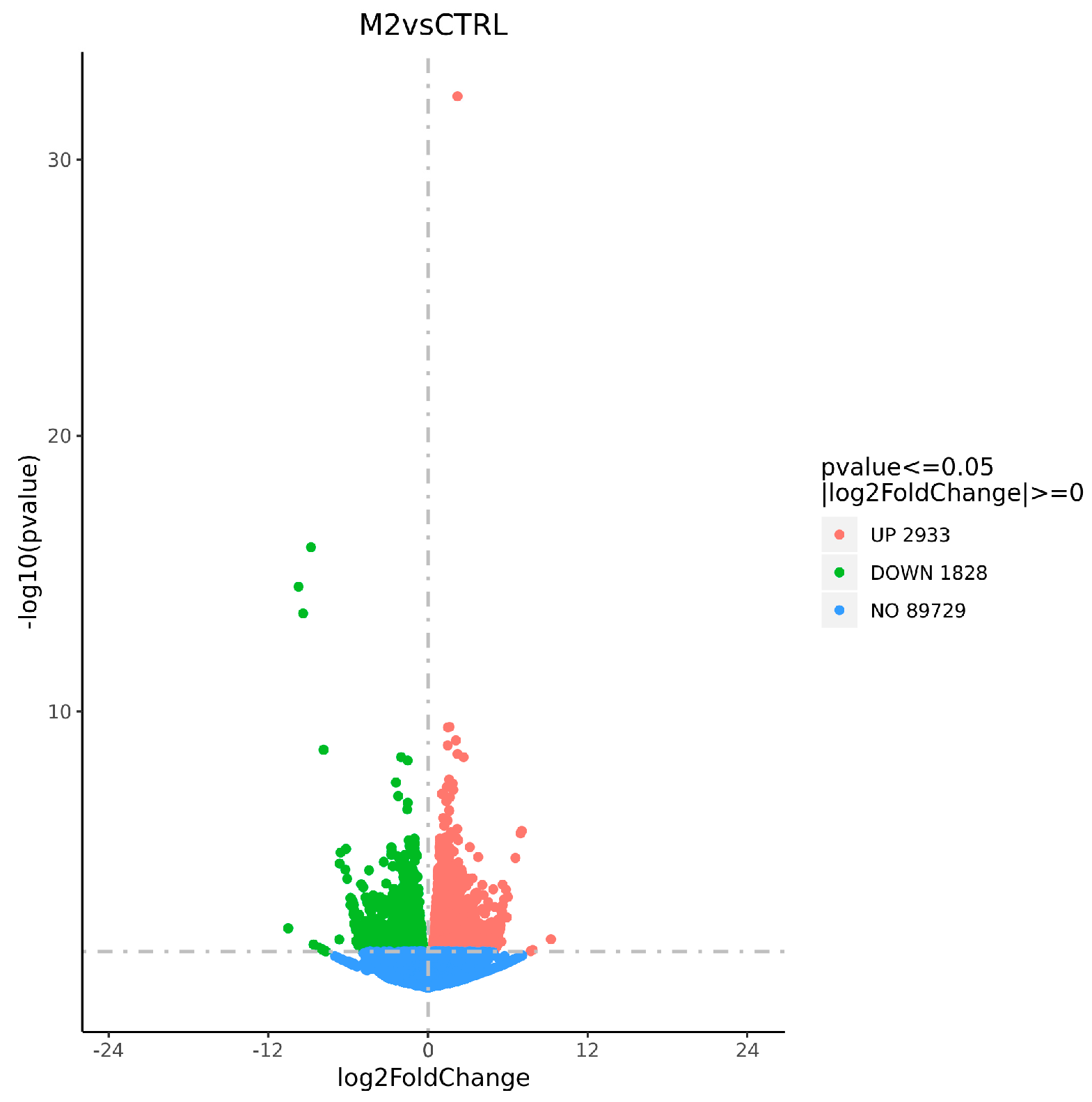
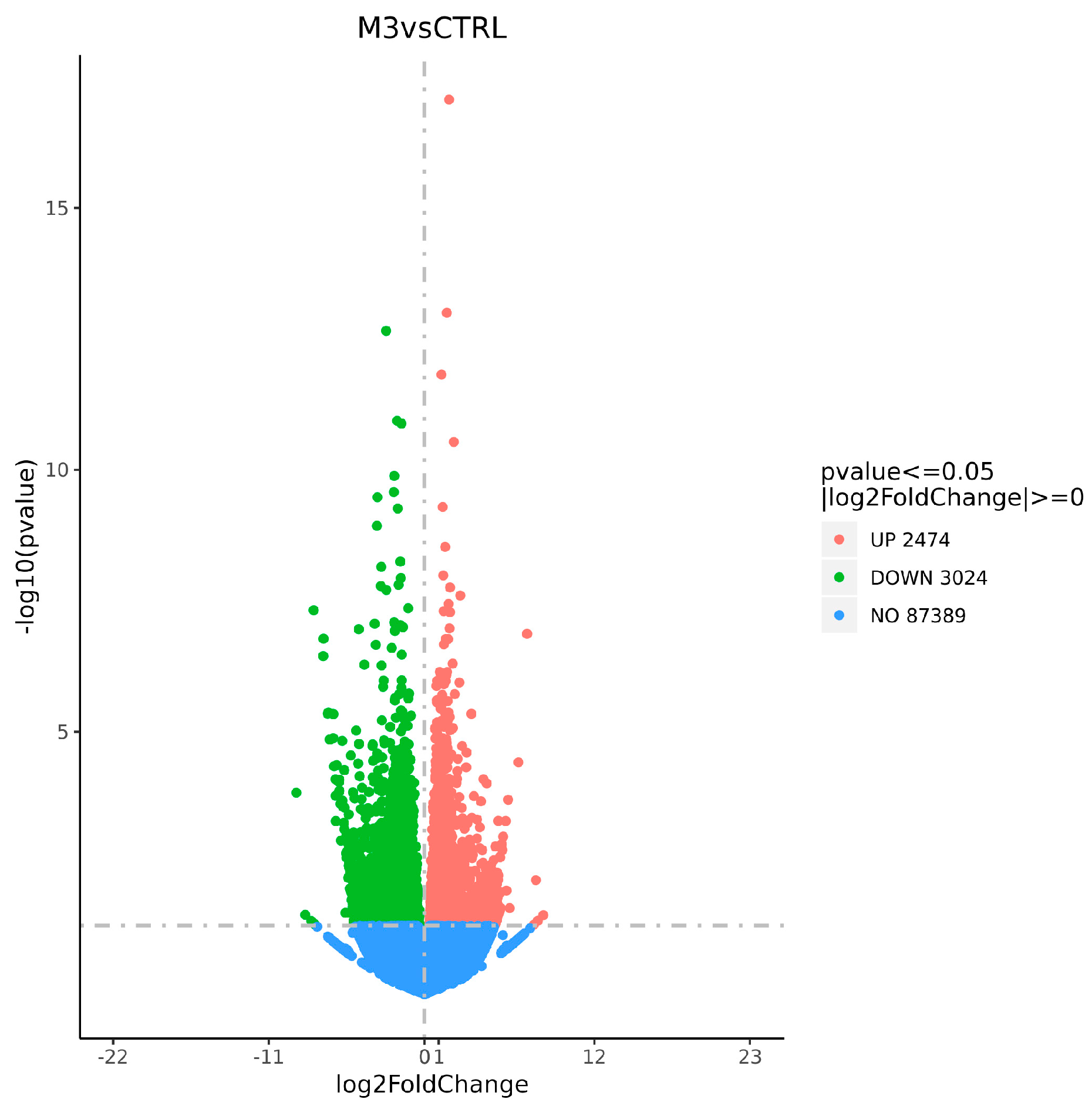
3.3.2. Statistical Analysis of DEGs
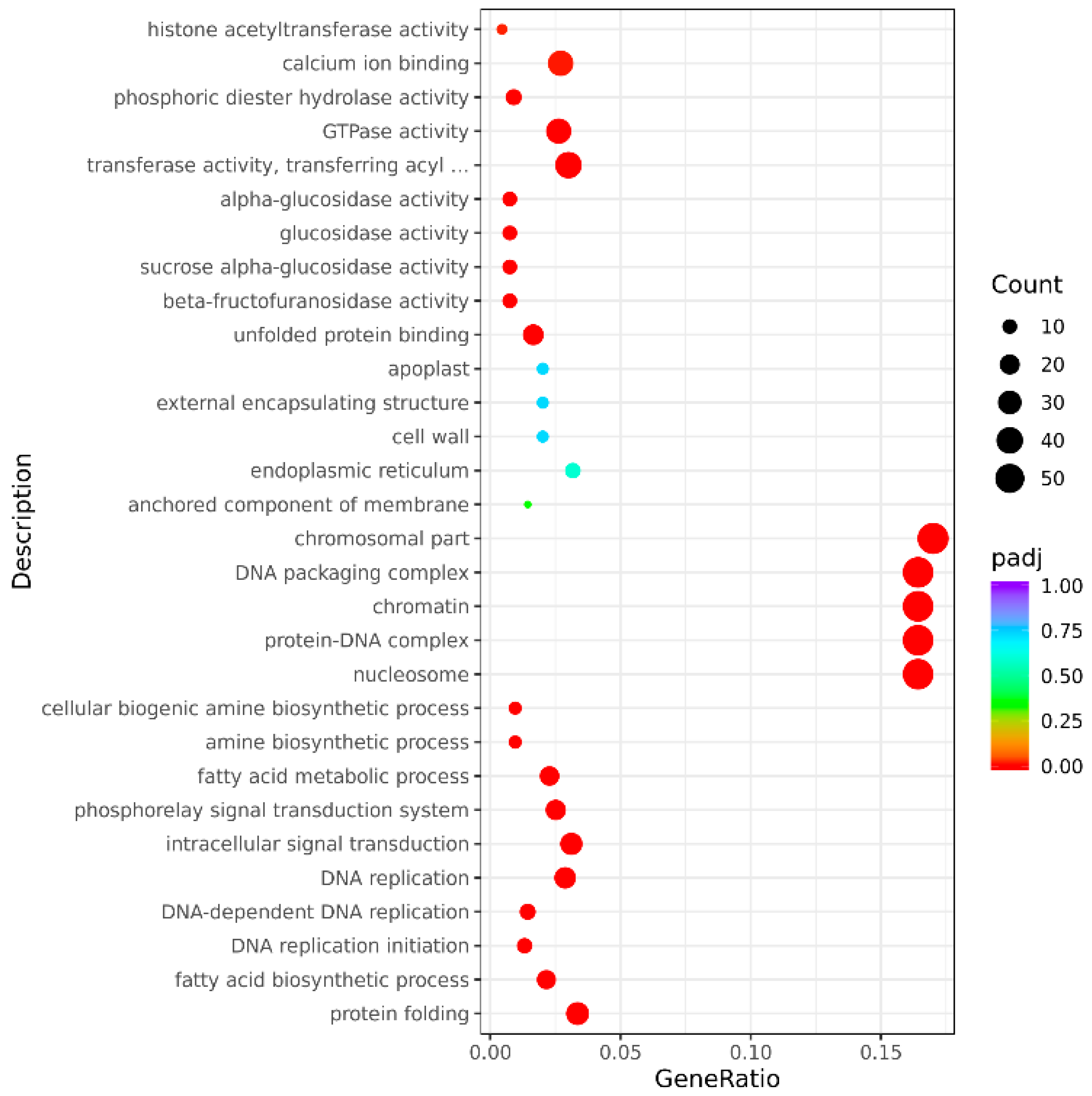
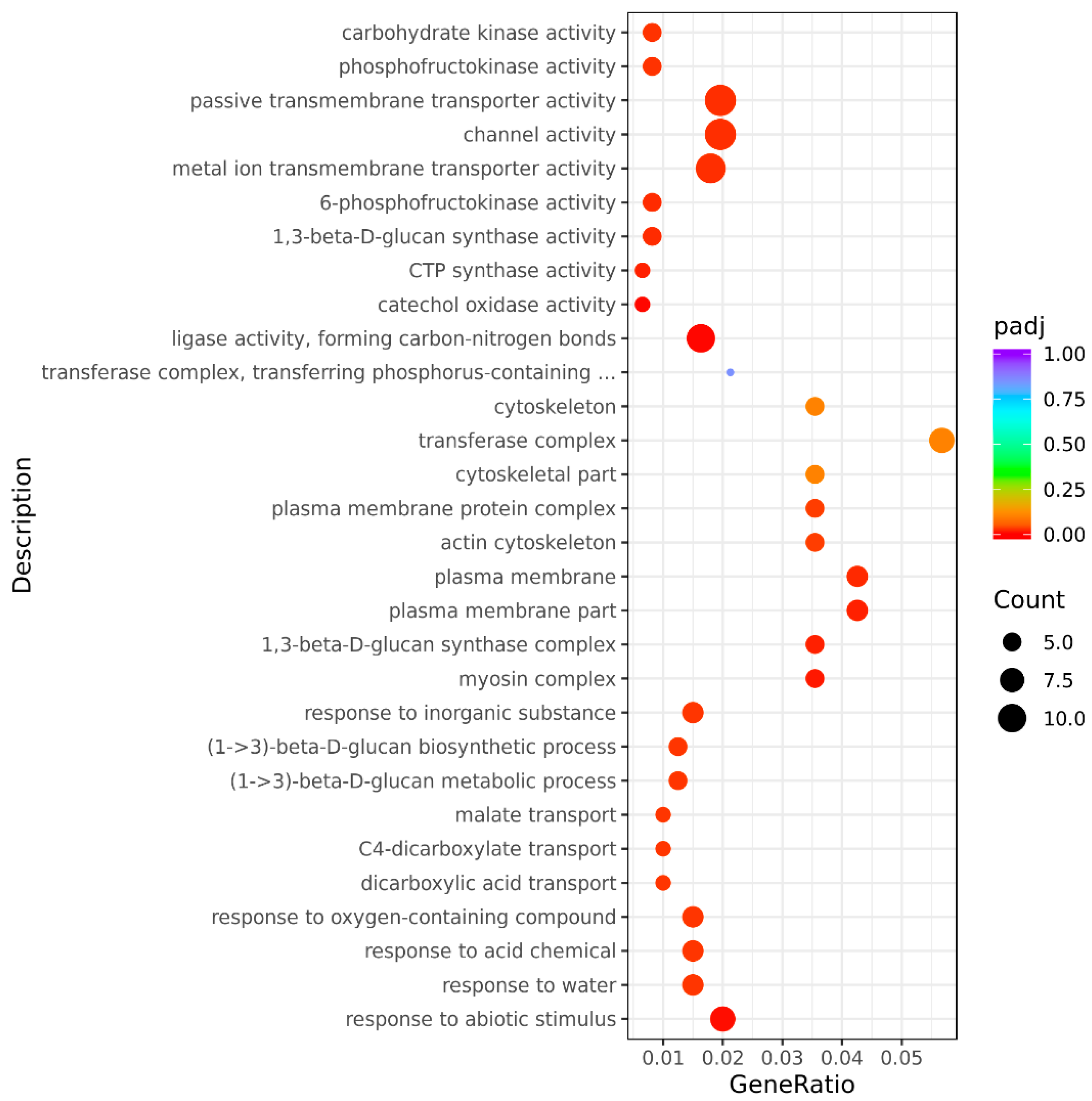
3.3.3. GO Classification and Enrichment Analysis of Differentially Expressed Genes
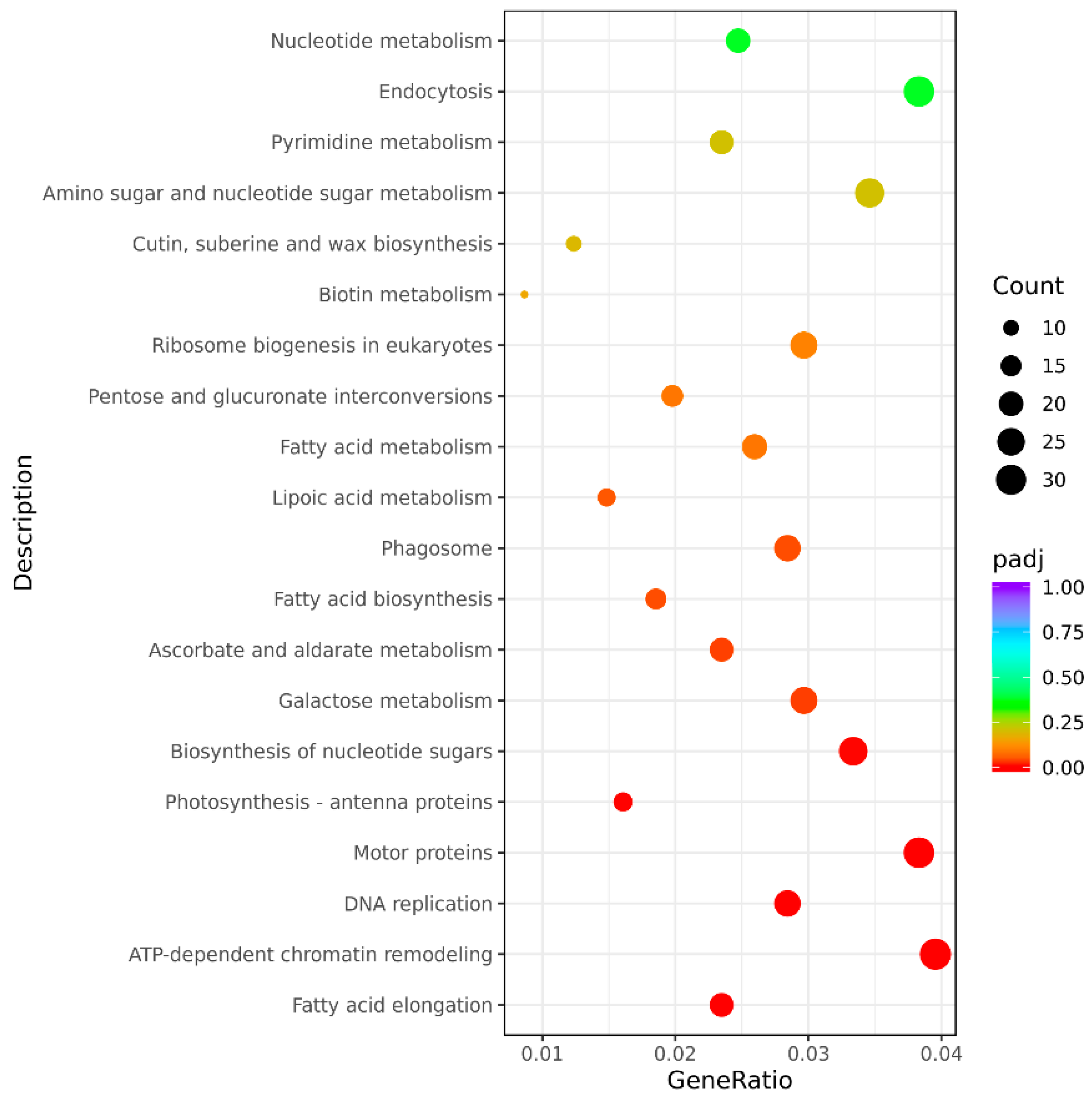
3.3.4. The KEGG Pathway Analysis of Significant Differentially Expressed Genes
3.3.5. Alternative Splicing Analysis
Analysis of Alternative Splicing Events
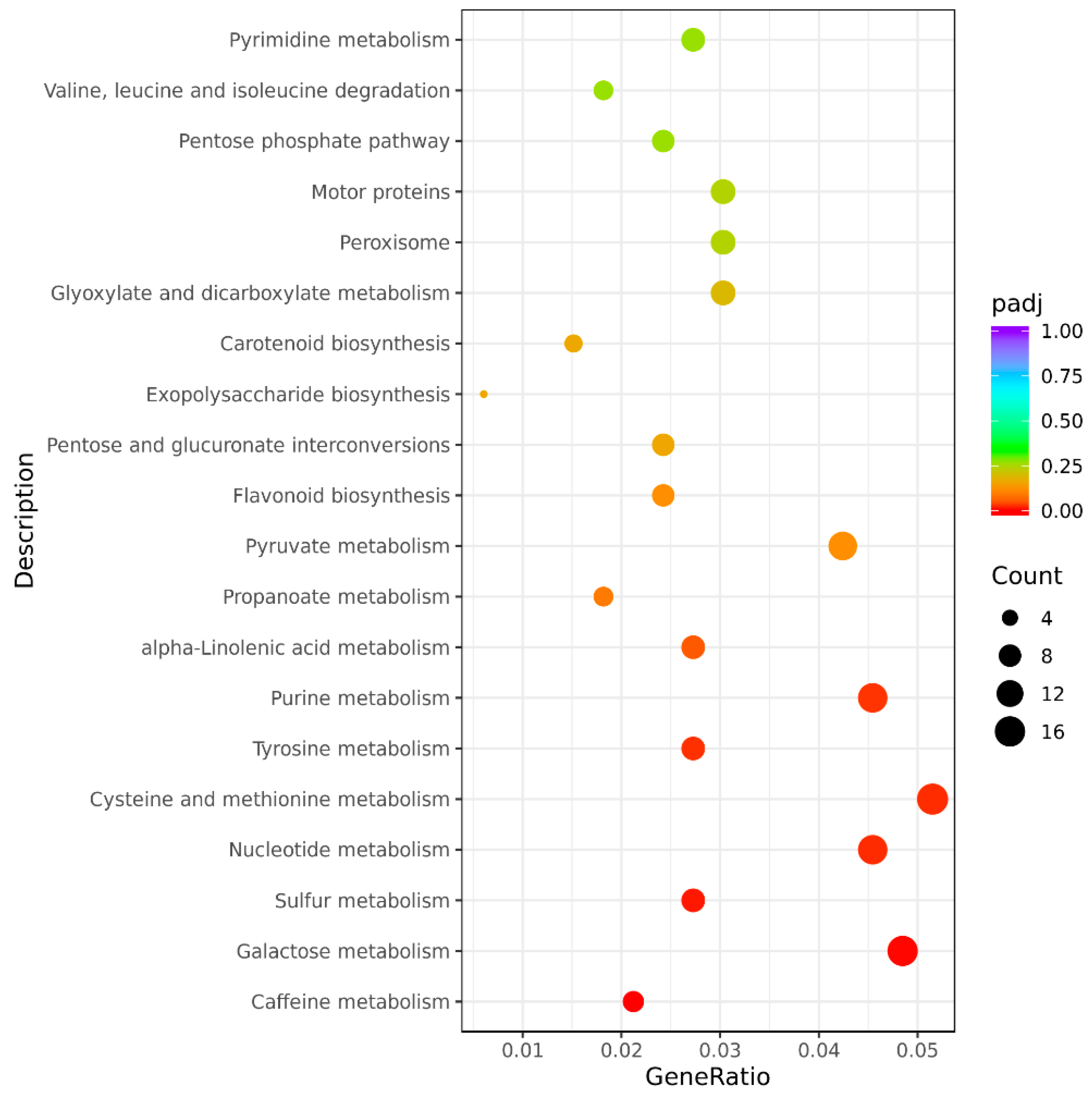
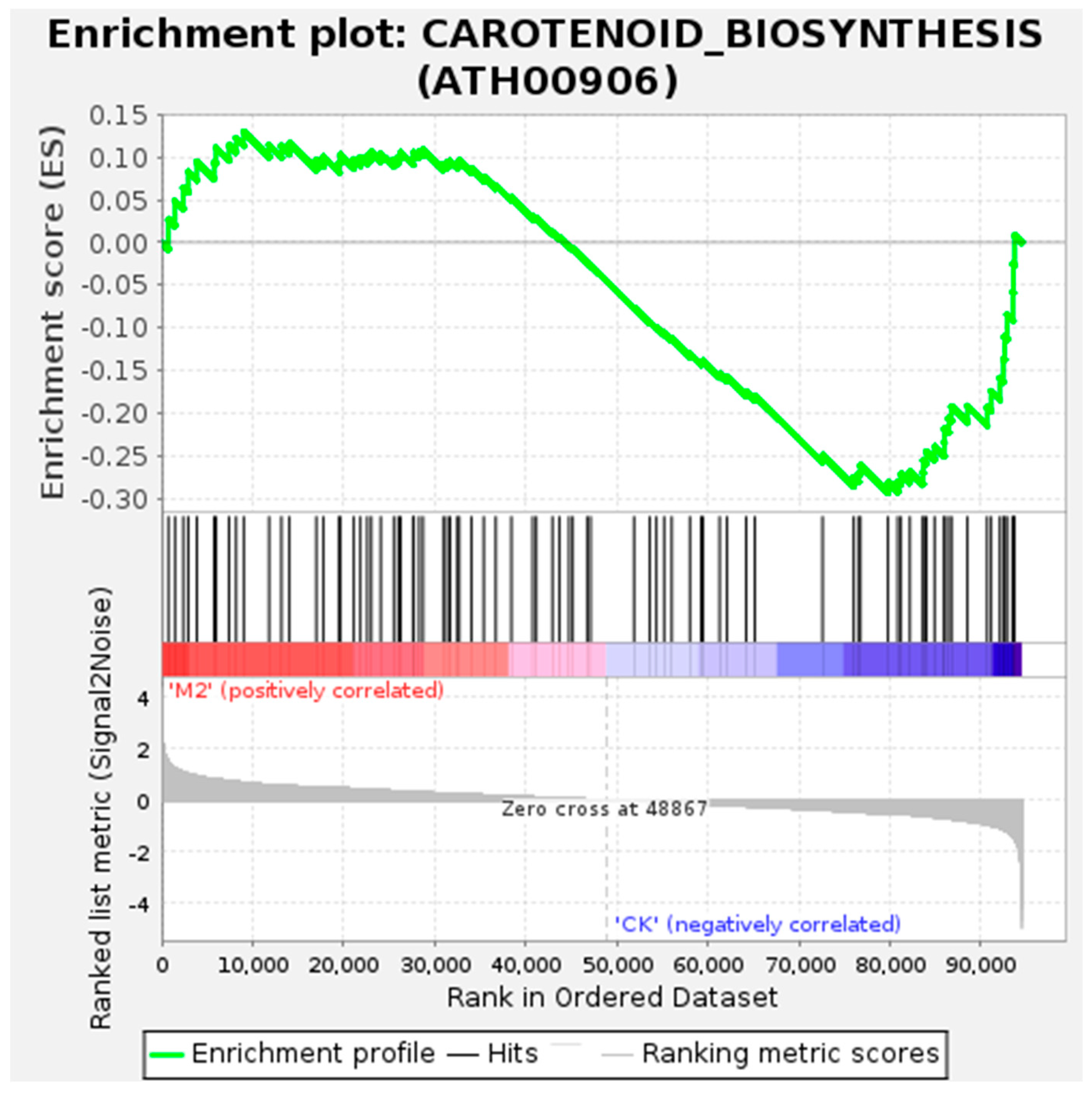
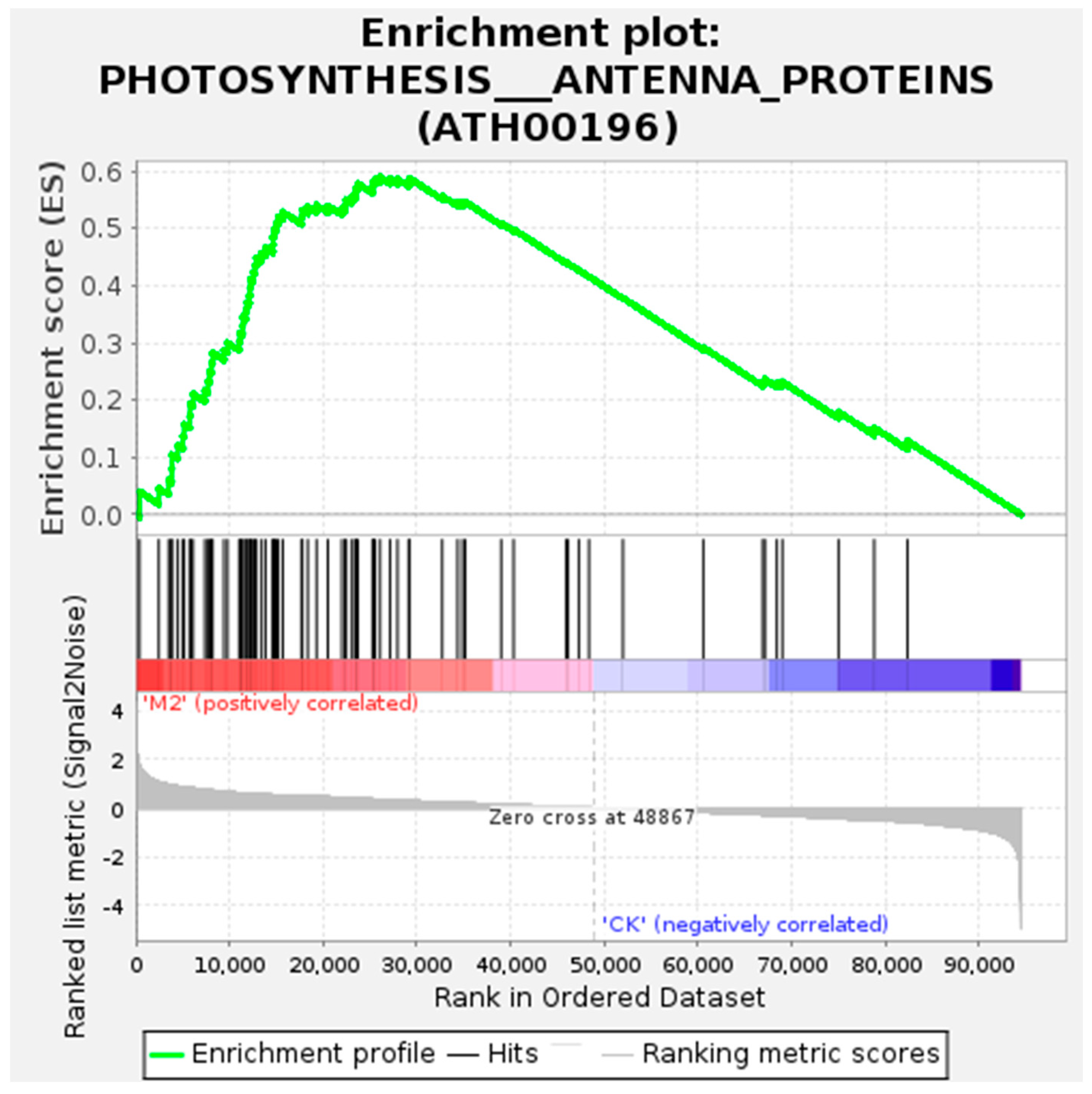
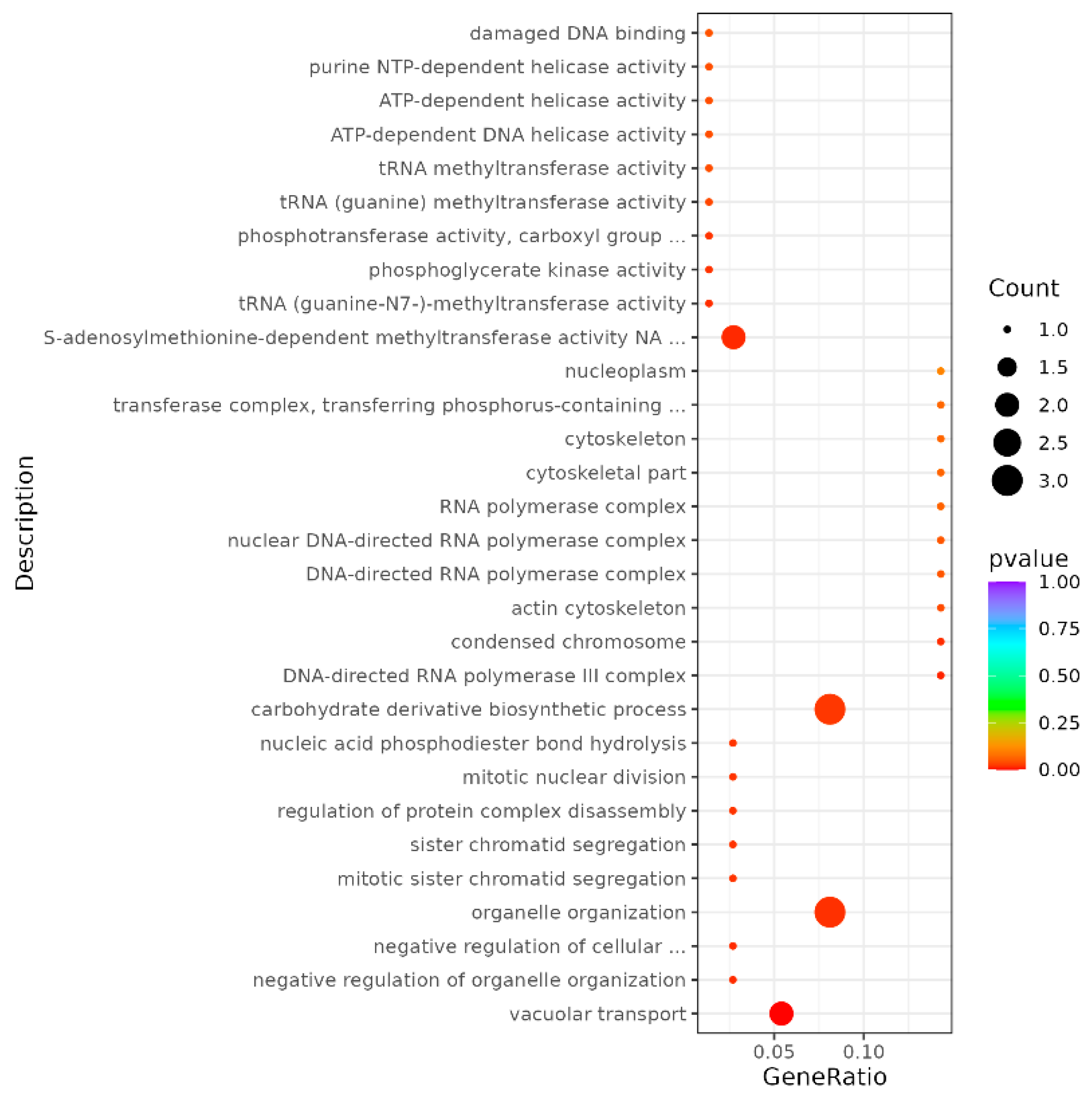
The GO and KEGG Functional Enrichment Analysis of Differentially Spliced Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEGs | Differentially expressed genes |
| FDR | False discovery rate |
| SE | Skipped exon |
| A3SS | Alternative 3′ splice site |
| A5SS | Alternative 5′ splice site |
| RI | Retained intron |
| MXE | Mutually exclusive exons |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| FPKM | Fragments per kilobase of transcript per million mapped reads |
| ES | Enrichment Score |
| GSEA | Gene Set Enrichment Analysis |
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023; FAO Publications: Rome, Italy, 2023. [Google Scholar]
- Hussain, A.; Larsson, H.; Kuktaite, R.; Olsson, M.E.; Johansson, E. Carotenoid Content in Organically Produced Wheat: Relevance for Human Nutritional Health on Consumption. Int. J. Env. Res. Public Health 2015, 12, 14068–14083. [Google Scholar] [CrossRef]
- Requena-Ramírez, M.D.; Rodríguez-Suárez, C.; Ávila, C.M.; Palomino, C.; Hornero-Méndez, D.; Atienza, S.G. Bread Wheat Biofortification for Grain Carotenoid Content by Inter-Specific Breeding. Foods 2023, 12, 1365. [Google Scholar] [CrossRef]
- Shih, C.H.; Chu, H.; Tang, L.K.; Sakamoto, W.; Maekawa, M.; Chu, I.K.; Wang, M.; Lo, C. Functional characterization of key structural genes in rice Xavonoid biosynthesis. Planta 2008, 228, 1043–1054. [Google Scholar] [CrossRef]
- Padhy, A.K.; Sharma, A.; Sharma, H.; Rajput, R.; Pandey, A.; Srivastava, P.; Kaur, S.; Kaur, H.; Singh, S.; Kashyap, L.; et al. Bread wheat with enhanced grain carotenoid content: A novel option for wheat biofortification. Mol. Breed. 2022, 42, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Xia, X.; He, Z. Carotenoids in Staple Cereals: Metabolism, Regulation, and Genetic Manipulation. Front. Plant Sci. 2016, 7, 1197. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Li, Y.; Wang, C.; Xie, Y.; Guo, T. Effect of nitrogen fertilisation and irrigation on phenolic content, phenolic acid composition, and antioxidant activity of winter wheat grain. J. Sci. Food Agric. 2015, 95, 1039–1046. [Google Scholar] [CrossRef]
- Sun, D.-X.; Ma, D.-Y.; Wang, C.-Y.; Li, Y.-G.; Liu, W.-X.; Li, Q.-X.; Feng, W.; Guo, T.-C. Effects of Irrigation and Nitrogen on Antioxidant Contents in Yumai 49-198 Grains. Acta Agron. Sin. 2014, 40, 2046–2051. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, R.; Wei, J.; Fu, X.; Zhang, S.; Zhao, Z.; Lin, H.; Xu, Y.; Tan, D.; Gao, X.; et al. Enhancing micronutrient bioavailability in wheat grain through organic fertilizer substitution. Front. Nutr. 2025, 12, 1559537. [Google Scholar] [CrossRef] [PubMed]
- Verardo, V.; Riciputi, Y.; Sorrenti, G.; Ornaghi, P.; Marangoni, B.; Caboni, M.F. Effect of nitrogen fertilisation rates on the content of fatty acids, sterols, tocopherols and phenolic compounds, and on the oxidative stability of walnuts. LWT Food Sci. Technol. 2013, 50, 732–738. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wang, D.W.; Zhang, Z.B. Progress and prospects in the research on wheat drought response and resistance mechanisms using transcriptomic and proteomic approaches. J. Triceae Crop 2016, 36, 878–887. [Google Scholar] [CrossRef]
- Wang, M.M.; Xue, S.D.; Wu, T.Q.; Luo, S.B.; Xie, D.S.; Zhong, Y.J. Effects of Illumination and Temperature Regulation on Synthesis of Carotenogenesis in Tomato (Solanum lycopersicum L.) Fruit. Mol. Plant Breed. 2020, 18, 6158. [Google Scholar] [CrossRef]
- Zheng, J.F.; Li, C.P.; Dong, S.M.; Bai, Z.Y.; Li, C.D.; Bi, C.R. The effect of phosphorus deficiency stress on chlorophyll content and corticoid content and chromosome of wheat substitution lines. Acta Agric. Boreali Sin. 2010, 25, 161–165. [Google Scholar] [CrossRef]
- Ali, Q.; Anwar, F.; Ashraf, M.; Saari, N.; Perveen, R. Ameliorating effects of exogenously applied proline on seed composition, seed oil quality and oil antioxidant activity of maize (Zea mays L.) under drought stress. Int. J. Mol. Sci. 2013, 14, 818–835. [Google Scholar] [CrossRef]
- Luo, X.Q.; Xue, J.J.; Cai, J.; An, F.F.; Zhu, W.L.; Chen, S.B. Effects of GA3 treatment on carotenoids biosynthesis in cassava. Chin. J. Trop. Crops 2022, 43, 346–352. [Google Scholar] [CrossRef]
- Xie, D.X.; Ma, X.N.; Zhao, Y.Q.; Li, J.; Fu, D.; Zhang, Z.; Ju, C.; Wang, C. Absorption, transport and regulation of manganese in plants. Sci. Sin. Vitae 2023, 53, 1199–1212. (In Chinese) [Google Scholar] [CrossRef]
- Arif, M.; Chohan, M.A.; Ali, S.; Gul, R.; Khan, D. Response of wheat to foliar application of nutrients. J. Agric. Biol. Sci. 2006, 1, 30–34. Available online: https://www.researchgate.net/publication/236620915 (accessed on 19 July 2025).
- Yang, D.Q.; Li, Y.L.; Ni, Y.L.; Yin, Y.P.; Yang, W.B.; Cui, Z.Y.; Zhang, Y.T.; Ma, R.Y.; Wang, Z.L. Effects of exogenous ABA and 6-BA on protein content and grain filling process in different types of stay-green wheat. Acta Agron. Sin. 2014, 40, 301–312. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Liang, Y.; Lu, W.; Lu, D. Spraying exogenous hormones alleviate impact of weak-light on yield by improving leaf carbon and nitrogen metabolism in fresh waxy maize. Front. Plant Sci. 2023, 14, 1220827. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Hussain, S.; Ishfaq, M.; Ali, N.; Ahmad, M.; Ihsan, F.; Sheteiwy, M.S.; Rauf, A.; Hano, C.; El-Esawi, M.A. Manganese supply improves bread wheat productivity, economic returns and grain biofortification under conventional and no tillage systems. Agriculture 2021, 11, 142. [Google Scholar] [CrossRef]
- Ma, C.; Jie, X.L.; Liu, S.L.; Liu, F.; Hua, D.L.; Cui, H.Y.; Hu, H.F.; Guo, X. Effect of manganese sulfate spurt application on the yield and quality of alfalfa. Soil Fertil. Sci. China 2011, 1, 44–48. [Google Scholar]
- Li, J.; Liu, F.J.; Zhang, G.Q.; Song, Y.; Xu, J. Effects of foliar application of manganese fertilizer on the quality of tomato fruit. Jiangsu Agric. Sci. 2011, 39, 273–274. [Google Scholar] [CrossRef]
- Henkens, C.H.; Jongman, E. The movement of manganese in the plant and the practical consequences. Neth. J. Agric. Sci. 1965, 13, 392–407. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese Deficiency in Plants: The Impact on Photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef]
- Yang, Z.B.; You, J.F.; Yang, Z.M. Manganese uptake and transportation as well as antioxidant response to excess manganese in plants. J. Plant Physiol. Mol. Biol. 2007, 33, 480–488. (In Chinese) [Google Scholar] [PubMed]
- Carvalho, E.R.; Oliveira, J.A.; Pinho, É.V.d.R.v.; Neto, J.C. Enzyme activity in soybean seeds produced under foliar application of manganese. Ciênc. Agrotec. 2014, 38, 317–327. [Google Scholar] [CrossRef]
- Huang, J.; Ebach, M.; Triantafilis, J. Cladistic analysis of Chinese Soil Taxonomy. Geoderma Reg. 2017, 10, 11–20. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.; He, Z. Establishment of ultra performance liquid chromatography (UPLC) protocol for analyzing carotenoids in common wheat. Acta Agron. Sin. 2016, 42, 706–713. [Google Scholar] [CrossRef]
- Yan, L.; Yang, M.; Guo, H.; Yang, L.; Wu, J.; Li, R.; Liu, P.; Lian, Y.; Zheng, X.; Yan, J.; et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 2013, 20, 1131–1139. [Google Scholar] [CrossRef]
- International Wheat Genome Sequencing Consortium (IWGSC). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. Available online: https://daehwankimlab.github.io/hisat2/ (accessed on 4 November 2016). [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomicfeatures. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Park, J.W.; Lu, Z.-X.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar] [CrossRef] [PubMed]
- Mao, S. Effects of different concentrations of manganese treatments on the photosynthesis characteristic of wheat seedling. J. Jiangsu Univ. Technol. 2010, 16, 14–17. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, H.; Feng, M.; Zhang, D. Influences of micronutrients on the wheat variety Linyou 2018. Shaanxi J. Agric. Sci. 2006, 5, 6–7. [Google Scholar] [CrossRef]
- Zeng, J.; Li, T.; Wen, T.; Wang, W.; Zuo, Y.; Li, J. Impact of spraying manganese fertilizer on leaf on growth, absorption and transfer of manganese in spring wheat. J. Irrigat. Drain. 2017, 36, 25–29. [Google Scholar] [CrossRef]
- Mustafa, T.; Sattar, A.; Sher, A.; Ul-Allah, S.; Ijaz, M.; Irfan, M.; Butt, M.; Cheema, M. Exogenous application of silicon improves the performance of wheat under terminal heat stress by triggering physio-biochemical mechanisms. Sci. Rep. 2021, 11, 23170. [Google Scholar] [CrossRef]
- Liu, C.; Tao, Y.; Li, M.; Zhang, X. Effect of manganese sulfate on photosynthesis and nitrogen metabolism of cultivated Indian barnyard grass seedlings under saline-alkali stress. Acta Pratacult. Sin. 2024, 33, 84–98. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, L.F.; Chai, T.Y.; Lin, D.; Zhang, H.M. Mechanisms of manganese toxicity and manganese tolerance in plants. Chin. Bull. Bot. 2010, 45, 506–520. [Google Scholar] [CrossRef]
- Nazir, M.A.; Hasan, M.; Mustafa, G.; Tariq, T.; Ahmed, M.M.; Golzari Dehno, R.; Ghorbanpour, M. Zinc oxide nano-fertilizer differentially effect on morphological and physiological identity of redox-enzymes and biochemical attributes in wheat (Triticum aestivum L.). Sci. Rep. 2024, 14, 13091. [Google Scholar] [CrossRef]
- Frąszczak, B.; Matysiak, R.; Smiglak, M.; Kukawka, R.; Spychalski, M.; Kleiber, T. Application of salicylic acid derivative in modifying the iron nutritional value of lettuce (Lactuca sativa L.). Plants 2024, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, D.; Qin, Q.; Wu, G.; Zhang, Z.; Ni, D. Advances in metabolism of chlorophylls and carotenoids in higher plants. Plant Physiol. J. 2023, 59, 793–802. [Google Scholar] [CrossRef]
- Landrau, P., Jr.; Samuels, G. The effect of fertilizers on the yield and quality of sweet potatoes. J. Agric. Univ. Puerto Rico 1969, 35, 71–87. [Google Scholar] [CrossRef]
- Han, L.J.; Yang, C.W.; Ou, Z.Y. Progress in biosynthetic pathway and its biological functions of plant carotenoid. J. Biol. 2002, 19, 1–3. [Google Scholar]
- Dong, M.; Kuerban, Z.; Lv, P.; Du, R.H.; Ye, K.; Hou, S.L.; Liu, G.Q. Transcriptome analysis and gene mining of salt tolerance in sorghum seedlings (Sorghum bicolor L. Moench). Sci. Agric. Sin. 2019, 52, 3987–4001. [Google Scholar] [CrossRef]
- Zhao, C.L.; Guo, W.M.; Chen, J.Y. Formation and Regulation of Flower Color in Higher Plants. Chin. Bull. Bot. 2005, 22, 70–81. [Google Scholar]
- Zhou, L.; Liu, L. Regulation factors of carotenoid biosythesis and their impacts on photosythesis. Tianjin Agric. Sci. 2011, 17, 5–8. [Google Scholar] [CrossRef]
- Lee, J.J.; Chen, L.; Shi, J.; Trzcinski, A.; Chen, W.N. Metabolomic profiling of Rhodosporidium toruloides grown on glycerol for carotenoid production during different growth phases. J. Agric. Food Chem. 2014, 62, 10203–10209. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Zhang, J.; Yuan, J.; Cai, A.; Ma, C. Role of alternative splicing in plant development and abiotic stress responses. J. Nucl. Agric. Sci. 2020, 34, 62–70. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant. 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, S.; Huo, Y.; Ma, G.; Sun, Z.; Li, H.; Hou, S.; Han, Y. Comparative metabolomic and transcriptomic analysis reveals a coexpression network of the carotenoid metabolism pathway in the panicle of Setariaitalica. BMC Plant Biol. 2022, 22, 105. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Ding, H.; Qin, H.; Hou, J.; Huang, X.; Xie, Y.; Guo, T. Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front. Plant Sci. 2017, 8, 00860. [Google Scholar] [CrossRef] [PubMed]
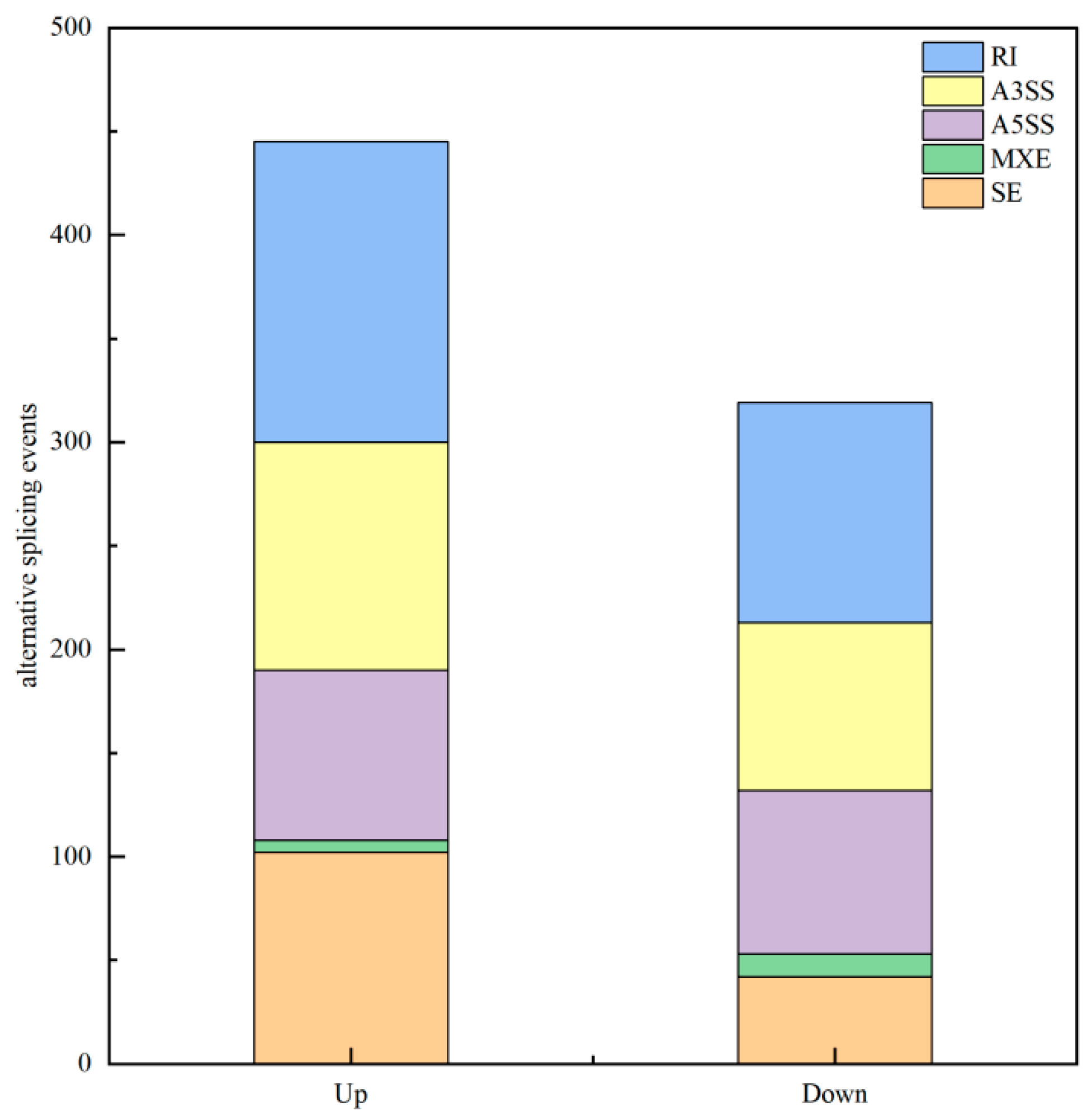

| AS Event | Gene Number | AS Number |
|---|---|---|
| SE | 7587 | 13,080 |
| MXE | 369 | 652 |
| A5SS | 3898 | 6999 |
| A3SS | 6163 | 11,459 |
| RI | 5124 | 11,204 |
| Total | 23,141 | 43,394 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Wang, K.; Zhang, J.; Jiao, X.; Yang, Z.; Wang, J.; Yang, S. Transcriptome Analysis of the Regulatory Mechanism of Exogenous Manganese Sulfate Application on Wheat Grain Yield and Carotenoids. Agronomy 2025, 15, 2190. https://doi.org/10.3390/agronomy15092190
Yang N, Wang K, Zhang J, Jiao X, Yang Z, Wang J, Yang S. Transcriptome Analysis of the Regulatory Mechanism of Exogenous Manganese Sulfate Application on Wheat Grain Yield and Carotenoids. Agronomy. 2025; 15(9):2190. https://doi.org/10.3390/agronomy15092190
Chicago/Turabian StyleYang, Na, Ke Wang, Jiancheng Zhang, Xiaoyan Jiao, Zhiguo Yang, Jian Wang, and Sha Yang. 2025. "Transcriptome Analysis of the Regulatory Mechanism of Exogenous Manganese Sulfate Application on Wheat Grain Yield and Carotenoids" Agronomy 15, no. 9: 2190. https://doi.org/10.3390/agronomy15092190
APA StyleYang, N., Wang, K., Zhang, J., Jiao, X., Yang, Z., Wang, J., & Yang, S. (2025). Transcriptome Analysis of the Regulatory Mechanism of Exogenous Manganese Sulfate Application on Wheat Grain Yield and Carotenoids. Agronomy, 15(9), 2190. https://doi.org/10.3390/agronomy15092190






