Abstract
Researching the photosynthetic activity of sunflower (Helianthus annuus L.) is essential for understanding how different genotypes respond to environmental conditions and utilise solar energy for growth and productivity. The objective of this study was to gain insight into and quantify the adaptation of ten sunflower hybrids during the flowering stage under field conditions. As part of an ongoing sunflower breeding programme, this research aimed to assess genotypic differences in photosynthetic performance and yield-related traits in response to variable environmental conditions. During the flowering stage, chlorophyll a fluorescence (ChlF) parameters revealed significant genotypic differences in energy fluxes, particularly in ABS/RC, DI0/RC, ET0/RC, and RE0/RC. Those results indicate variability in light-harvesting efficiency and electron transport capacity. Although specific photochemical efficiency indicators (e.g., TR0/RC, TR0/ABS, ET0/TR0) showed slight variation, energy dissipation and photosystem I-related parameters differed significantly among hybrids. Leaf temperature and chlorophyll content also varied and showed moderate correlations with fluorescence-based indicators. Yield components (plant height, head diameter, and seed mass per head) displayed significant differences among sunflower hybrids, with notable opposite patterns between plant height and head size. Revealed strong relationships between photosynthetic performance (PITOTAL, RE0/ABS) and yield traits, particularly plant height and number of seeds per head, were confirmed with correlation analysis. Principal Component Analysis (PCA) distinguished the hybrids into distinct groups. The analysis confirmed physiological and morphological variability among hybrids, enabling effective screening of genotypes for breeding purposes. Photosynthesis is a key physiological trait that directly influences biomass accumulation and seed yield, making it a critical parameter in evaluating the performance and adaptability of various sunflower genotypes. Thus, this study demonstrates the integrative value of combining ChlF, thermal, and agronomic traits for identifying high-performing sunflower hybrids under optimal field conditions.
1. Introduction
Sunflower is an annual plant with the Latin name Helianthus annuus L., which belongs to the Asteraceae family. Today’s domesticated sunflowers have common ancestors that originated from the same domestication centre in the middle interior latitudes of eastern North America [1]. More than 4000 years ago, Native Americans began cultivating sunflowers, marking the beginning of the domestication and breeding process of these flowers [2].
Plant breeding integrates both basic and applied sciences to develop economically valuable genotypes [3]. The concept of breeding or improving a plant species varies greatly depending on the specific plant species. It depends on the desires and needs of humanity, economic profitability, consumption of the product, and its intended use or market application [4]. The primary goals of plant breeding are to create genotypes with increased crop yield and quality, as well as resistance to environmental stresses [3]. This is achieved by generating genetic variability and creating new allele combinations through natural and artificial selection in order to create cultivars with new and beneficial properties [5]. Therefore, lines with more desirable allelic combinations are created by systematically producing plant populations through genetic separation and selection among populations. This is achieved by recurrent selection of heterozygous plants in cross-pollinated species, such as sunflowers, in contrast to pure lines that are preferred in self-pollinated species. Sunflowers and maize are among the most important crops developed through hybrid breeding techniques [6]. Sunflower is a diploid species with 2n = 34 chromosomes and a haploid genome size of approximately 3000 Mb [7]. Cross-pollination results in genotypes with diverse traits, which are then subject to material selection. For this reason, genotype testing is necessary to detect the response of the desired characteristics in different environmental conditions.
Global climate change poses a challenge for sunflower breeders, as it takes 10–15 years to develop sunflower hybrids. This means breeders must develop hybrids suited for both current and future environmental conditions. However, these selections are based on field trials conducted under current weather conditions [8]. Ahmed et al. [9] emphasise that the seed yield of sunflower represents a multifactorial trait governed by various morphological and physiological characteristics and is significantly affected by environmental variability.
Alongside soybeans, palm, and rapeseed, sunflower ranks among the world’s most important oilseed crops. Global production and harvested area have been steadily increasing, with regional fluctuations in cultivation trends reflecting the growing demand for oil production. According to FAOStat [10] in 2023, total world sunflower seed production was 58,574,867 tons, with the regional shares in sunflower seed production being as follows: Europe (72.0%), Asia (12.4%), the Americas (11.0%), Africa (4.4%), and Oceania (0.1%). The European Union accounts for approximately 15% of global sunflower production, with Croatia ranking second among EU countries in average seed yield per hectare based on long-term data from 1999 to 2018 [11]. They pointed out the need to increase sunflower crops in Croatian fields in the future.
Climate change, which has led to increasingly drier growing seasons, especially in summer with less precipitation, higher temperatures, and daily temperature maxima with a lack of precipitation [12,13], has contributed to the strategic importance of sunflower cultivation. Namely, with its morphological structure and appearance, the sunflower plant can withstand weather changes better than other crops. Still, its yield potential may be compromised by heat and water stress, which disrupts physiological and morphological processes and reduces achene yield [14]. Additive Main effects and Multiplicative Interaction (AMMI) analysis by Liović et al. [15] revealed that genotype × environment interaction (41.72%) had the greatest effect on seed yield, followed by genotype (20.75%) and environment (2.10%). Conversely, oil content was most influenced by genotype (52.82%), followed by environment (23.02%) and their interaction (17.27%).
Achieving stable and high yields under changing environmental conditions requires in-depth knowledge of crop biology, field experience, and precise implementation of agronomic practices [16]. Recently, many researchers have employed various approaches to detect the effects of changing weather conditions, which are the primary causes of plant stress. One widely used approach is chlorophyll fluorescence (ChlF) analysis, with which the authors gain insight into the structure and function of the photosynthetic apparatus using ChlF parameters. ChlF parameters have so far been used on sunflower plants when testing the effects of cadmium stress [17], tissue structure [18], phosphorus nutrition [19], the elevated temperature and light [20,21], drought and heat stress [22], herbicide effects [23], the relationship between ChlF parameters and yield components [24], exogenous application of selenium on sunflower [25], drought response [26,27], effects of NaCl Stress [28], salt-stress tolerance [29], etc.
According to Omonov et al. [30], exotic and local sunflower cultivars were compared for chlorophyll a, b, total pigment content, and carotenoids across budding, flowering, and ripening stages. The study confirmed high and significant variation among genotypes, with certain exotic lines showing higher chlorophyll content during flowering and ripening. Similarly, De la Mata et al. [31] observed that chlorophyll a and b content decreased under high irradiance (350 µmol photons m−2 s−1) during leaf ageing in sunflower, indicating accelerated senescence. On the contrary, the carotenoid content increased under high light, suggesting a protective response against photooxidative stress.
In field crops, numerous factors, such as plant density, fertilisation, water availability, and genotype, strongly influence yield components [32,33,34]. Miladinović et al. [35] confirmed that the flowering time is one of the most critical phenological growth stages in sunflower, as it directly influences yield formation, morphological development, and overall plant performance. The timing of flowering affects pollination efficiency, seed set, and the duration of the seed-filling period, all of which contribute to final yield. Additionally, environmental factors such as temperature and photoperiod interact with genetic factors to determine flowering time, making it a key trait for adaptation to diverse growing conditions.
The primary objective of this study was to gain insight into and quantify the adaptation of ten sunflower hybrids during the flowering stage. The study is part of a sunflower breeding programme that examines the effect of variable weather conditions in experimental fields to monitor the functioning of the photosynthetic apparatus and biomass traits.
2. Materials and Methods
2.1. Field Trial
The location of the experiment was the Tenja Experimental field of the Faculty of Agrobiotechnical Sciences in Osijek (Croatia). Ten different sunflower hybrids were sown in April 2020 at a depth of 4 cm. Rows were five metres long with a row spacing of 23 cm, and the spacing between rows was 70 cm. The experiment had three repetitions, and the design was a randomised complete block design (RBD). Maize (Zea mays L.) was the previous crop. A detailed soil chemical analysis was performed prior to sowing. The soil pH (in KCl) was 7.27, humus content was 2.56%, available phosphorus (P-Olsen) was 12.60 mg/kg, and available potassium (AL-K2O) was 21.68 mg/100 g of soil. Fertilisation included both basal and pre-sowing applications. Basal fertilisation was performed using 250 kg/ha of NPK 0:20:30, while pre-sowing fertilisation involved 150 kg/ha of urea (46% N). All plant protection measures were carried out in accordance with professional standards and good agricultural practice, as described in Međimurec [36]. Harvest was in September 2020.
2.2. Weather Conditions
Weather conditions during the sunflower growing season were generally favourable for plant development (Figure 1a,b). Mean monthly temperature increased steadily from April, reaching a peak of approximately 23 °C in August, which is optimal for sunflower flowering and seed filling. Rainfall distribution showed notable variation across the months. The highest precipitation was recorded in August (over 100 mm), coinciding with peak temperatures, which may have facilitated seed filling and reduced heat stress. However, precipitation was relatively low in early spring (April), which could have potentially affected early vegetative growth if irrigation had not been provided. Overall, the combination of adequate rainfall in mid to late summer and optimal temperature profiles likely contributed positively to photosynthetic activity and yield formation in sunflowers.
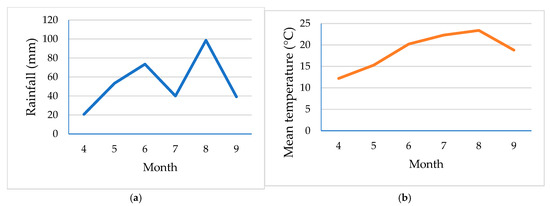
Figure 1.
The amount of (a) rainfall and (b) mean air temperature for the Osijek area [37].
2.3. Photosynthetic Activity
During the flowering stage (R5 according to Schneiter et al. [38]), physiological analyses were performed. A handy PEA device (Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK) was used to determine chlorophyll a fluorescence (ChlF) parameters. Ten randomly chosen plants of each hybrid within a single plot were tested. During the measurement, a 3200 μmol m−2 s−1 pulse saturating red light was applied to sunflower leaves. Specially designed leaf clips were attached 30 min prior to measurement to induce dark adaptation, following the protocol described by Markulj Kulundžić et al. [39]. The clamps were positioned on a sufficiently developed leaf of ten plants per hybrid, located below the sunflower heads. After measurement, the data were analysed using the JIP test [40,41], and transients were recorded by measuring chlorophyll fluorescence over a time span from 50 microseconds (μs) to 1 second (s) after illumination began. This transient curve includes distinct steps labelled O, J, I, and P, which correspond to specific physiological events in photosystem II (PSII). The structural and functional parameters selected for this research are shown in Table S1.
After ChlF measurements, at the same leaf where ChlF measurements were performed, relative chlorophyll content was determined with a chlorophyll content metre (Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK), and the leaf temperature with a Dual Focus Infrared Thermometer B+B Thermo-Technik GmbH, Donaueschingen, Germany). Those two analyses were conducted on ten leaves per hybrid. Three measurements were taken on each leaf, and the average value was calculated from these measurements. These values were then used to determine the analysis values. All measurements (ChlF, chlorophyll content and leaf temperature) were taken between 8:00 and 10:00 in the morning. During this period, the average air temperature was 23.5 °C, and the Photosynthetically Active Radiation (PAR) was around 854 µmol m−2 s−1.
During the experiment’s harvest, the following parameters were determined for ten plants per hybrid: plant height (PH), head diameter (HD), seed weight per head (SWH), number of head seeds (NHS), and 1000-seed weight (TSW).
2.4. Data Analysis
The collected data were put into the database and analysed using Statistica 12 to identify biometric differences among the sunflower hybrids. The ANOVA analysis was performed on all observed morphological parameters, and differences between means were determined using the LSD (least significant difference) test at p ≤ 0.05. Photosynthetic activity parameters and yield components were compared using Pearson’s pairwise correlation test, Principal Component analysis (PCA) and cluster analysis to evaluate the strength and significance of the relationships. Heatmap visualisation was generated using the Heatmapper [42] online tool.
3. Results and Discussion
3.1. Photosynthetic Activity Among Hybrids
Chlorophyll a fluorescence parameters observed in the leaves of ten sunflower hybrids at the flowering stage are shown in Table 1, Table 2, Table 3 and Table 4.

Table 1.
Specific energy fluxes (per reaction centre—RC) observed in the leaves of ten sunflower hybrids. The letters indicate a statistical difference at p ≤ 0.05 between hybrids.

Table 2.
Quantum efficiencies and probabilities observed in the leaves of ten sunflower hybrids. The letters indicate a statistical difference at p ≤ 0.05 between hybrids.

Table 3.
Phenomenological energy fluxes (per excited cross-section—CS) observed in the leaves of ten sunflower hybrids. The letters indicate a statistical difference at p ≤ 0.05 between hybrids.

Table 4.
Relative ratios, derived parameters and performance indices observed in the leaves of ten sunflower hybrids. The letters indicate a statistical difference at p ≤ 0.05 between hybrids.
The absorption flux per reaction centre (ABS/RC), which reflects the apparent antenna size, varied significantly among the tested sunflower hybrids (Table 1). These results indicate genotypic differences in hybrids in light-harvesting capacity. The highest ABS/RC was recorded in hybrid 3. In contrast, hybrids 1 and 2 exhibited the lowest ABS/RC values. In other crops, increased ABS/RC was associated with genotypes exposed to stress conditions [43,44,45]. The trend of hybrid behaviour in ABS/RC values was mirrored by changes in dissipated energy flux per RC (DI0/RC), suggesting that increased energy absorption was accompanied by higher energy dissipation, and vice versa. Specifically, hybrids 1 and 2, which showed the lowest ABS/RC, also had the lowest DI0/RC values, while hybrid 3 exhibited the highest values for both parameters. This parallel increase in ABS/RC and DI0/RC likely reflects adjustment of the photosynthetic apparatus in hybrid 3. A substantial portion of the absorbed energy may not have been used for photochemistry but instead dissipated as heat or fluorescence. This pattern indicates activation of photoprotective mechanisms and possibly reduced energy use efficiency [46]. High ABS/RC may point to a relatively larger apparent antenna size or increased excitation pressure on PSII reaction centres. In contrast, elevated DI0/RC suggests the engagement of non-photochemical quenching (NPQ) as a basal photoprotective process. NPQ dissipates excess excitation energy as heat, preventing photoinhibition even under moderate light levels [47]. Additionally, the xanthophyll cycle, particularly zeaxanthin-mediated energy dissipation, plays a central role in the thermal dissipation of excess photons as part of short-term photoprotection [48]. Such coordinated modulation of light absorption and energy dissipation may be characteristic of hybrid 3. It represents an energy allocation strategy that helps maintain PSII functional integrity during the early photoperiod, supporting sustained photosynthetic performance as light intensity rises later in the day. Similar correlations between these parameters have been documented in previous studies [49,50].
Interestingly, TR0/RC (trapping flux leading to QA−), which reflects the efficiency of energy trapping at the PSII reaction centre, did not differ statistically among sunflower hybrids. This could indicate a conserved mechanism of primary photochemical charge separation across hybrids [50], despite differences in ABS/RC, ET0/RC (electron transport flux beyond QA−) and RE0/RC (probability that a chlorophyll molecule functions as an RC). Therefore, TR0/RC alone is not a suitable parameter for discriminating among sunflower hybrids. Sunil et al. [51], who studied the effect of nitric oxide on PSII electron transport in pea mesophyll protoplasts treated with sodium nitroprusside (SNP) at different time points, also reported no significant differences in TR0/RC. Similarly, Antunović Dunić et al. [52], examining the effect of drought on kale, reported that the combination of stable F0 and stable TR0/RC is a sign of constant trapping on active RCs. Conversely, numerous studies have shown that TR0/RC can be extremely sensitive to stress conditions. Ghaffar et al. [53] found increased TR0/RC in wheat under drought stress in susceptible cultivars, accompanied by reduced ET0/RC. Franić et al. [54] observed increased TR0/RC in maize genotypes exposed to cadmium, while in sunflower, increased TR0/RC was found under elevated temperatures and high irradiation [20].
Notably, ET0/RC and RE0/RC exhibited significant variation among hybrids, with hybrid 7 showing the highest values and hybrid 10 the lowest. These parameters are vital indicators of the efficiency of electron flow beyond QA− and the reduction in PSI end acceptors. Lower ET0/RC in hybrid 10 and RE0/RC in hybrid 6 likely reflect impaired electron transport and reduced availability of active PSII RCs, which could limit the plant’s photosynthetic potential and productivity. In contrast, the high ET0/RC and RE0/RC in hybrids 7 and 9, respectively, suggest a more effective conversion of excitation energy into electron transport, potentially contributing to higher photosynthetic efficiency under field conditions. Those parameters have been associated with stress-induced declines in photosynthetic performance in cereals [55,56].
According to the ANOVA results, the quantum efficiency parameters ET0/ABS (quantum yield for electron transport), ET0/TR0 (probability that an absorbed photon will enter the electron transport chain, electron transport efficiency) and TR0/ABS (maximum quantum yield of PSII photochemistry) did not differ significantly between sunflower hybrids (Table 2). This indicates that there is a conserved capacity for primary photochemical efficiency and linear electron flow beyond QA− [26,50]. The TR0/ABS ratio, widely regarded as a robust measure of PSII integrity, also showed no hybrid effect. Murchie and Lawson [46] report that the invariant TR0/ABS values are consistent with its general stability under ambient and moderate stress conditions. Similarly, Cheng et al. [57] also established that the TR0/ABS fluorescence intensity remained constant for both soybean cultivars throughout the 24 h period of salt stress, with no significant differences observed between the two cultivars. Furthermore, Umar et al. [50] did not find significant differences in ET0/TR0 among sunflower hybrids under combined drought and salinity stress. On the other hand, in a study conducted by Franić et al. [54], ET0/ABS and ET0/TR0 showed statistically significant variations among the tested maize genotypes and cadmium treatments, suggesting different capacities of linear electron flow outside PSII among the hybrids.
In contrast, ΔR0 (efficiency of electron transfer to final PSI acceptors) and RE0/ABS (quantum yield of electron transport to PSI end acceptors) exhibited identical ranking patterns across hybrids. Coordination between these two parameters is often observed under stress conditions, where limitations of terminal PSI acceptors impose constraints on both [55]. A similar pattern of behaviour for these parameters was previously reported in sunflower by Markulj Kulundžić et al. [39].
The phenomenological energy fluxes per excited cross-section at t = 0 (CS0), ABS/CS0 (absorbed photon flux per excited cross-section (CS) of PSII), TR0/CS0 (trapped energy flux per cross-section), DI0/CS0 (dissipated energy flux per cross-section), ET0/CS0 (electron transport flux through PSII per excited cross-section), and RE0/CS0 (electron flux reducing end acceptors at PSI per cross-section at time 0) provides an integrated view of PSII activity across the entire sampled leaf surface, as opposed to the reaction centre parameters, specific fluxes that focus on individual active PSII units [40,58].
One-way ANOVA showed that only ET0/CS0 remained invariant among sunflower hybrids, whereas ABS/CS0, TR0/CS0, DI0/CS0 and RE0/CS0 all differed significantly (Table 3). Fisher’s LSD post hoc comparisons revealed that hybrid 5 had the lowest ABS/CS0 and TR0/CS0. In contrast, hybrid 9 exhibited the highest, suggesting substantial differences in light-harvesting capacities among genotypes. The fact that hybrid rankings for DI0/CS0 mirrored those for DI0/RC and likewise for RE0/CS0 versus RE0/RC demonstrates that energy dissipation and PSI acceptor reduction processes scale proportionally from individual reaction centres to the whole leaf cross-section [40,46].
Under various abiotic stress conditions, Faseela et al. [59] reported a consistent decrease in ET0/CS0 and RC/CS0, along with increased DI0/CS0 in rice seedlings, particularly under PEG-induced osmotic stress. In contrast, results in this study obtained under non-stress conditions revealed no significant differences in ET0/CS0 among sunflower hybrids. This suggests a conserved efficiency of electron transport across hybrids under field-grown conditions. Kalaji et al. [58] showed that under shade conditions, the barley cultivar A. Aswad is an inefficient energy user as evidenced by reduced ET0/CS0, primarily lower ABS/CS0 and TR0/CS0, along with increased DI0/CS0. In this study, although all sunflower hybrids were grown under optimal light, significant genotypic differences in ABS/CS0, TR0/CS0, and DI0/CS0 suggest that underlying light-harvesting and energy dissipation capacities are genotype-specific traits, even in non-stressful environments.
The uniform scaling of DI0/RC and DI0/CS0 across hybrids indicates consistent photoprotective regulation at both the reaction centre and cross-section levels [46]. Similarly, matching patterns of RE0/CS0 and RE0/RC highlight that limitations at the PSI acceptor side (e.g., plastocyanin or ferredoxin pool size) impose equivalent constraints whether normalised per cross-section or reaction centre [55]. Furthermore, during the wheat tillering stage of growth and under drought stress conditions, lower ABS/CS0, ET0/CS0 and DI0/CS0 values were recorded, along with higher PIABS values, which indicated greater drought tolerance of certain varieties. Conversely, cultivars with higher ABS/CS0 and ET0/CS0 under stress conditions exhibited elevated DI0/CS0, negatively affecting yield, water-use efficiency, and stress resilience [60].
PIABS (performance index of PSII on absorption basis) integrates structural and functional aspects of PSII and includes RC/ABS (quantum yield for the reduction in end electron acceptors at the PSI acceptor side), TR0/DI0 (flux ratio trapping per dissipation), and ET0/(TR0 − ET0) (electron transport further than primary acceptor QA), which reflects the efficiency of electron transport beyond the primary electron acceptor QA [61].
One-way ANOVA revealed no significant differences among sunflower hybrids in RC/ABS, ET0/(TR0 − ET0), or PIABS, indicating a uniform capacity for PSII reaction centre abundance and downstream electron transport efficiency. In contrast, TR0/DI0 significantly varied among genotypes (Table 4). Hybrids 1, 2, 4, and 5 showed the highest values of TR0/DI0, indicating more efficient energy trapping and reduced thermal dissipation. Opposite to those hybrids, hybrids 3, 7, 8, and 9 exhibited lower values, pointing to increased energy loss through non-photochemical quenching, potentially as a response to stress or reduced PSII efficiency [56]. These results imply that differences among sunflower hybrids are not primarily due to variations in PSII reaction centre density (RC/ABS), but rather to how efficiently absorbed energy is utilised. High TR0/DI0 values mark a favourable balance toward energy trapping, whereas low values indicate either structural inefficiencies or activation of protective mechanisms [40].
PITOTAL (performance index of electron flux to the final PSI electron acceptors) extends PIABS by incorporating ΔR0/(1 − ΔR0) (electron transport from plastoquinol (PQH2) to final PSI acceptors) [62]. PITOTAL values were largely consistent across hybrids, with hybrids 9 and 10 showing the highest values, reflecting intact PSII and PSI connectivity. Uniform PITOTAL across most genotypes highlights the general resilience of the full electron transport chain, with only in hybrids 3 and 6 exhibiting impaired PSI level performance [41]. In previous research [39,63] on sunflower, it has been proven that PITOTAL and ΔR0/(1 − ΔR0) are very sensitive parameters that can be used to select materials.
3.2. Chlorophyll Content and Leaf Temperature
The observed variation in chlorophyll content and leaf temperature among sunflower hybrids reflects the interplay between photosynthetic pigment status and thermoregulation capacity. Chlorophyll in all its various forms is the primary photosynthetic pigment found in higher plants. Chlorophyll content represents a fundamental physiological parameter directly linked to the photosynthetic potential of plants, as it determines the capacity for light absorption and subsequent energy conversion in the photosystems [58]. Previous studies have shown that variations in chlorophyll concentration serve as an indicator of plant health, indicate the nutrient status of the plant, and are used to assess the impact of environmental factors on plants [64,65].
At the same time, leaf temperature is also an easily measurable physiological parameter that provides an indirect means of assessing plant transpiration and is closely related to water availability [66]. The environment influences leaf mass through energy flux and interactions between the plant and its environment. The energy received by the plant can be converted into heat within the plant, affecting its temperature, or it can be utilised in photochemical and thermochemical processes associated with metabolic and physiological activities [67]. Therefore, leaf temperature is closely linked to stomatal conductance and transpiration, thus indirectly reflecting the efficiency of photosynthetic cooling mechanisms and the overall water status [68].
In the present study, chlorophyll content and leaf temperature were measured across different sunflower hybrids to evaluate their physiological status regarding ChlF parameters (Figure 2a,b). Statistically significant differences were observed among hybrids for both traits. Hybrids 10, 5, 3, and 4 exhibited the lowest chlorophyll content, whereas hybrids 9, 2, and 1 demonstrated the highest chlorophyll content (Figure 2a). Interestingly, the lowest leaf temperatures were recorded in hybrids 1 and 2, while hybrid 8 had the highest leaf temperature (Figure 2b).
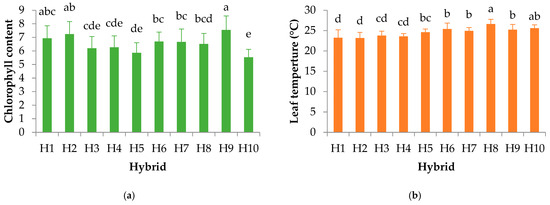
Figure 2.
Chlorophyll content (a) and leaf temperature (°C) (b) in sunflower hybrids. The letters indicate a statistical difference at p ≤ 0.05 between hybrids.
Similar coordinated changes in pigment concentration and leaf temperature have been documented in other crops. Water stress increased leaf temperature and decreased SPAD readings in both cotton and peanut, showing strong negative correlations between these traits in peanut [69]. Similarly, in sunflowers, Singh et al. [70] reported that water stress caused an increase in leaf temperature accompanied by a decline in chlorophyll content and yield attributes. Overall, Varalakshmi et al. [71] state that the positive associations between SPAD and key yield components suggest that chlorophyll content could serve as a useful indirect selection criterion in sunflower breeding for improved photosynthetic performance and productivity.
3.3. Sunflower Yield Components
The ANOVA analysis showed different influences of the sunflower yield components among hybrids (Table 5).

Table 5.
The ANOVA of sunflower yield components.
According to the results of the present study, the sunflower hybrids differed significantly (p ≤ 0.05) in all the yield components (Figure 3a–e).
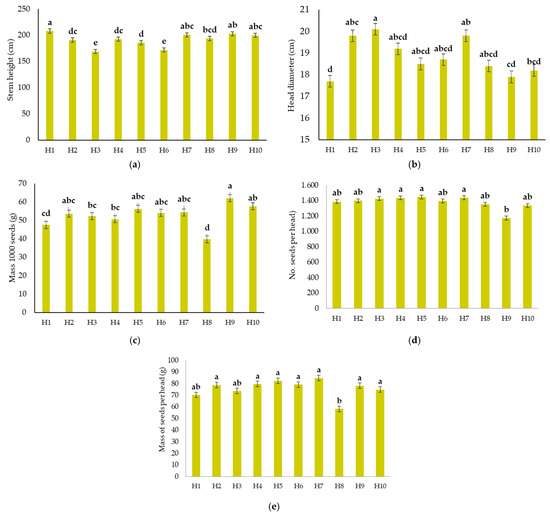
Figure 3.
The sunflower hybrids yield components as: (a) Stem height (cm); (b) Head diameter (cm); (c) 1000-seed weight (g); (d) Number of seeds per head; (e) Seed mass per plant. The letters with the same letter are not different from each other at p ≤ 0.05. Vertical bars indicate standard error.
On average, plants reached a height of 192 cm (Figure 3a), ranging from 169 cm (H3) to 208 cm (H1). The average head diameter across hybrids was 18.8 cm (Figure 3b), with values ranging from 17.7 cm (H1) to 20.1 cm (H3). The 1000-seed weight averaged 52 g (Figure 3c). The average number of seeds per head was 1380 (Figure 3d). The seed mass per head varied from 58 g (H8) to 85 g (H7), with a mean of 75 g (Figure 3e).
Sunflowers typically reach a plant height of 150–200 cm [58,72,73,74,75]. There were interesting findings for the hybrids 1 and 3 in the present study (Figure 3a). The hybrid 1 was the highest in the plant height (208 cm) and at the same time had the smallest head diameter (17.7 cm) among all hybrids. The opposite interaction was determined for the hybrid 3, which had the smallest stems (169 cm on average), but developed the largest heads with an average diameter of 20.1 cm (Figure 3b). Similarly, Kluza-Wieloch [73] states that the large plant height of sunflowers does not have a positive effect on their agronomic value. The author found that the shortest sunflower stems had the Wielkopolski genotype (124.2 cm), while the Coril genotype reached the greatest height at most growth stages (169.3 cm), followed by Frankasol (182.4 cm). In Uzbekistan, Ergasheva [76] found that the number of plants per unit area greatly affected the plant height during the growth stages. At a density of 80,000 plants ha−1, plant height ranged from 213.6 cm (Buzuluk genotype) to 234.3 cm (Rodnik genotype). In China, Li et al. [77] found that sunflower plant height increased with higher irrigation levels. In 2016 and 2017, the tallest plants reached 221.2 cm and 220.2 cm, respectively, while head diameter ranged from 16 to 20 cm. Furthermore, plant height is highly influenced by climatic and soil conditions. Drought or nutrient-poor soils can significantly reduce plant height, whereas irrigation and reduced water stress have a markedly positive effect on its growth. Handayati and Sihombing [78] stated that increasing NPK doses positively influenced the head diameter, dry head weight, total seed per head, seed weight per head, and 100-seed mass. Authors found the highest seed number and seed mass per sunflower head in the treatment with NPK 150-75-75 kg ha−1, demonstrating the beneficial effect of balanced fertilisation on sunflower yield. Hussain et al. [79] stated that nutrients as S and Zn are also essential for sunflower yield components (head diameter, number of seeds per head, and 1000 seed mass), which were strongly affected by the nutrients applied (S and Zn) and genotype of their 2-year study. According to Hladni et al. [80], the number of genes involved in the expression of a specific trait and the interrelationships among morphophysiological traits in sunflower breeding programmes are crucial. Specifically, head diameter and plant height are among the most important parameters for selecting genotypes. Authors stated that the sunflower plant height exhibited a mode of inheritance characterised by superdominance of the superior parent, while both dominance and superdominance of the better parent influenced head diameter. Ramos et al. [81] reported that due to reduced stature, dwarf sunflowers are less prone to lodging under adverse weather conditions, which can enhance yield stability. Moreover, their compact growth habit allows for optimised use of resources and may improve light interception efficiency in densely planted fields. Based on the research of Angadi and Entz [82], the factors responsible for reduced plant height in dwarf sunflower cultivars have not been fully elucidated, and their impact on root architecture and water uptake dynamics remains insufficiently understood.
Chen et al. [83] reported that drought-induced reductions in leaf-level photosynthesis, through altered gas exchange and chlorophyll fluorescence, ultimately affected seed yield components, including seed number. Based on the principal component analysis of 21 sunflower black-hulled seed hybrids, Kanwal et al. [84] reported that quality parameters, such as oil and protein content, as well as linoleic acid content, and morphometric parameters, such as plant height, head diameter, achene length, thickness and weight, contribute about 35% to the seed yield.
3.4. Correlation Analysis of Sunflower Photosynthetic Activity and Yield Components
To investigate the relationship between photosynthetic activity and sunflower yield components, a Pearson correlation analysis and a heatmap were conducted (Table S1 and Figure 4). The analysis revealed several key associations between photosynthetic parameters and agronomic traits.
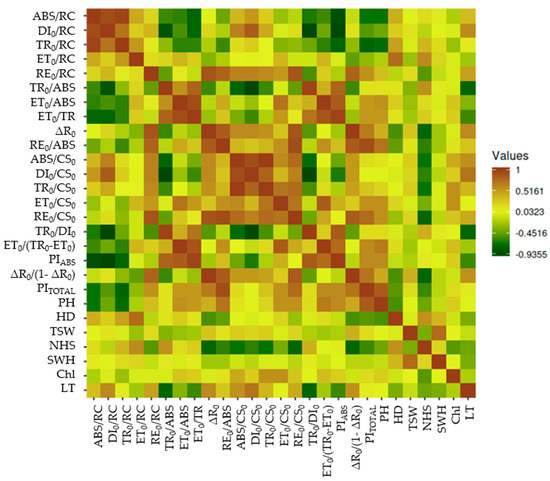
Figure 4.
Heatmap illustrating photosynthetic activity in sunflowers during the flowering stage alongside yield components. Values are expressed as Pearson pairwise correlation coefficients. Photosynthetic activity parameters are presented in Table S1; seed weight per head (SWH), plant height (PH), head diameter (HD), number of head seeds (NHS), and 1000-seed weight (TSW).
The strongest and highly significant positive correlation was found between plant height (PH) and PITOTAL (r = 0.809), indicating that higher overall photosynthetic performance is associated with taller plants. Other positive correlations were also observed with RE0/ABS (r = 0.718) and ET0/(TR0 − ET0) (r = 0.679). On the contrary, negative correlations were recorded between PH and ABS/RC (r = −0.665) and TR0/RC (r = −0.735). Furthermore, head diameter (HD) was the only significant correlation found with ET0/RC (r = 0.751), suggesting that efficient electron transport correlates with larger HD. Other correlations of sunflower head diameter with photosynthetic parameters were weak and not statistically significant.
The sunflower 1000-seed weight (TSW) showed no statistically significant correlations with any photosynthetic parameters, even though a mild (yet still non-significant) correlation was observed with ΔR0 (r = 0.288).
On the other hand, the number of seeds per sunflower head (NHS) has multiple significant negative correlations with ΔR0 (r = −0.773), ABS/CS0 (r = −0.695), TR0/CS0 (r = −0.701), RE0/CS0 (r = −0.746), and ΔR0/(1 − ΔR0) (r = −0.780). These negative relationships suggest that increased energy flux or photochemical efficiency may coincide with fewer seeds per head. In the current study, additional attention was given to the chlorophyll content.
The chlorophyll content shows generally weak correlations with most photosynthetic and agronomic traits (Table S2, Figure 4), with none reaching strong statistical significance. However, moderate positive trends were detected for ET0/ABS (r = 0.269) and ET0/TR0 (r = 0.239), suggesting that higher chlorophyll content might be associated with increased energy use efficiency in photosynthesis. A more notable positive correlation between the chlorophyll content and ABS/CS0 (r = 0.451), DI0/CS0 (r = 0.263), and RE0/CS0 (r = 0.542), which points to a possible association between chlorophyll content and light absorption as well as energy re-emission at the chlorophyll fluorescence level. Still, these correlations do not reach high significance.
Leaf temperature also shows moderate correlations with other parameters. It correlates positively with DI0/CS0 (r = 0.718). In contrast, leaf temperature is negatively correlated with TR0/ABS (r = −0.754) and TR0/DI0 (r = −0.759). Those results suggest that higher leaf temperature may be associated with reduced efficiency of energy transfer or electron transport during photosynthesis.
These findings are consistent with previous studies that emphasise the role of plant height, head diameter, and seed number as key yield-determining traits in sunflower. For instance, Göksoy and Turan [85] found strong positive correlations between seed yield and the number of seeds per head (r = 0.890), 1000-seed mass and seed yield, and sunflower head diameter. Rani et al. [86] examined 90 sunflower genotypes for correlation and path analysis of yield components. They found that 100-seed weight and head diameter had the most significant direct effects on seed yield per plant. The authors also emphasised that plant height and oil content showed positive direct influences, making them important selection criteria in sunflower breeding programmes. According to Sri et al. [87], among 22 hybrids, seed yield was significantly and positively correlated with plant height, head diameter, test weight, and volume weight. In contrast, path analysis confirmed that the plant height, head diameter, and test weight had the strongest direct contributions to the sunflower seed yield. Bonciu et al. [88] found that the key morphological traits, such as plant height, number of leaves, and head diameter of sunflower plants, are closely associated with productivity traits like seed number and weight. Authors found that plant height shows a very strong positive correlation with the number of leaves per plant and a moderate positive correlation with the head diameter and 1000-seed weight. Among six morphological traits, Hladni et al. [89] found that head diameter had the strongest negative direct effect on seed yield per plant in sunflower across two environments but still showed a significant positive correlation with yield. Plant height and total leaf area exhibited positive direct effects and were positively correlated with yield in both environments, suggesting that these traits are key for improving yield potential. Varalakshmi et al. [71] found that sunflower seed shows strong and significant positive correlations with several traits at both the genotypic and phenotypic levels (head diameter, seed weight, the number of seeds per head, plant height, the number of leaves per plant). For the SPAD index, the authors stated that SPAD values reflect chlorophyll content and indirectly photosynthetic capacity. They reported a moderate positive genotypic correlation between SPAD and plant height, suggesting that plants with higher chlorophyll content tend to grow taller, possibly due to better photosynthetic efficiency. SPAD values were also positively correlated with seed yield, although this correlation was not statistically significant. These findings support the results of this study, indicating that plant height and the number of seeds per head are the most important morphological traits.
3.5. Principal Component Analysis (PCA) of Sunflower Photosynthetic Activity and Yield Components
Principal Components Analysis (PCA) was performed to reduce the dimensionality of the dataset and to identify the main components explaining the variation in photosynthetic parameters and yield traits of sunflower hybrids (Table S3 and Figure 5). The PCA identified four principal components with eigenvalues greater than 1, explaining a total of 74.93% of the variance in the dataset (Table S3). The first two components (PC1 and PC2) accounted for 54.38% of the variance and were selected for two-dimensional interpretation and visualisation of genotypic differences.
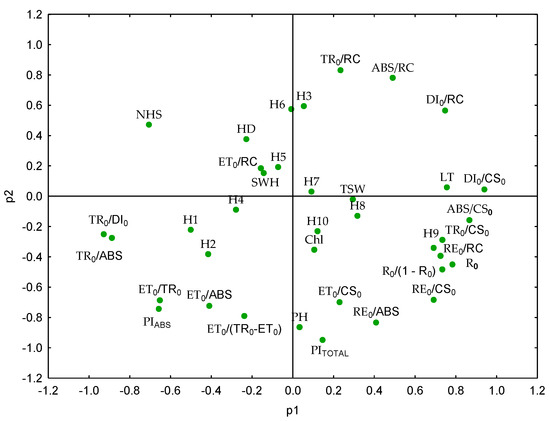
Figure 5.
Biplot of sunflower hybrids (H1–H10) and variables based on principal components (PCA and PC2). Photosynthetic activity parameters are presented in Table S1; seed weight per head (SWH), plant height (PH), head diameter (HD), number of head seeds (NHS), and 1000-seed weight (TSW), chlorophyll content (Chl), and leaf temperature (LT).
The first principal component (PC1) was strongly associated with variables indicative of photosynthetic efficiency and production potential, such as DI0/CS0, ABS/CS0, R0, RE0/RC, and TR0/CS0. These ChlF parameters reflect the entire sampled leaf surface’s ability to absorb and utilise light energy effectively in photosynthesis. In contrast, variables such as TR0/ABS, TR0/DI0, and PIABS showed strong negative loadings, suggesting lower energy conversion efficiency or higher dissipation losses in some hybrids.
The second principal component (PC2) revealed differences among hybrids in terms of energy distribution. TR0/RC, ABS/RC, and DI0/RC had positive loadings, which indicates high photosystem II activity. On the other hand, negative loadings for PITOTAL, PH, and RE0/ABS suggest alternative energy flow strategies among hybrids, which may reflect inherent physiological differences rather than stress responses.
The results of this study demonstrate that PIABS, ET0/TR0, TR0/ABS, and TR0/DI0 are the most informative and sensitive ChlF parameters for discriminating genotypic variation at the flowering stage. These parameters clustered in the same quadrant of the PCA biplot, indicating a strong intercorrelation. This shows that they accurately reflect the functional status and photochemical efficiency of PSII under the tested conditions. Their targeted application during the flowering stage phenotyping could enable a rapid, non-destructive and cost-effective assessment of photosynthetic performance, thus supporting the identification of superior genotypes before yield results are available. Incorporating these parameters into flowering stage screening protocols has the potential to increase selection accuracy, reduce the number of genotypes that will contribute to later stages of breeding, and ultimately improve the efficiency and genetic gain of sunflower breeding programmes.
The biplot (PC1 vs. PC2) further confirmed distinct clustering of hybrids based on dominant trait expression (Figure 5). Hybrids 3 and 6 were positioned in the upper right quadrant, associated with variables indicating high photosynthetic activity and efficiency, making them potential breeding material for high productivity. In contrast, hybrids 1 and 2 were located in the lower left quadrant, along with traits reflecting lower efficiency in energy transfer and absorption, suggesting a less competitive physiological profile.
Hybrids 4, 5, 7, 9, and 10 were located near the origin of the coordinate plane, showing balanced physiological profiles without extreme trait expressions, which may be desirable for broad adaptability. Hybrid 8 showed a positive contribution to PC1 but neutral values on PC2, suggesting solid photosynthetic performance with a distinct internal energy distribution pattern.
Principal component analysis has been widely used in sunflowers to characterise genetic diversity and facilitate hybrid screening. Sasikala et al. [90] reported that three principal components explained 71.45% of the total variance, identifying key traits like days to flowering and plant maturity. Zia Ullah et al. [91] found that four components accounted for 69.28% of variability, with 100-achene weight and yield per plant being major contributors. In early seedling stages, Zeinalzadeh-Tabrizi [92] showed that PC1 and PC2 together explained 80.93% of variability, enabling clear grouping of hybrids by seed vigour. These studies demonstrate the efficacy of PCA in sunflower hybrid evaluation. ChlF parameters and morphological traits in sunflower hybrids were previously studied and confirmed with PCA by Markulj Kulundžić et al. [21,39].
3.6. Cluster Analysis of Sunflower Hybrids
Cluster analysis revealed clear divisions of hybrids into groups that largely coincided with the PCA results. Using hierarchical clustering based on Euclidean distances and UPGMA connectivity, three main clusters of sunflower hybrids were identified (Figure 6). The first cluster included H1, H2, H3, H4, H5, H6 and H7. The connectivity of these hybrids indicates a high degree of similarity in their physiological and morphological traits. A second, smaller cluster was formed, containing hybrids H8 and H10, which differed moderately from the first group. In contrast, hybrid H9 positioned itself as a separate branch, indicating its unique physiological characteristics that distinguish it from all other tested hybrids.
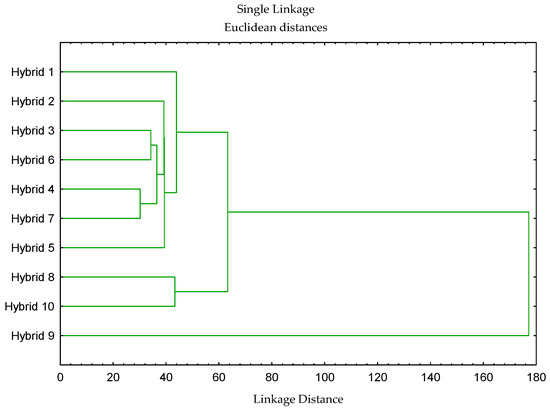
Figure 6.
Dendrogram based on Euclidean distances constructed from physiological and morphological traits of sunflower hybrids (H1–H10). Physiological parameters are listed in Table S1; morphological traits include seed weight per head (SWH), plant height (PH), head diameter (HD), number of head seeds (NHS), 1000-seed weight (TSW), chlorophyll content (Chl), and leaf temperature (LT).
This clustering pattern was corroborated by the PCA biplot (Figure 5 and Table S3), where H9 was clearly separated along PC1, driven by its divergent values for RE0/CS0, RE0/ABS, and PITOTAL. Similarly, H8 and H10, which clustered together in UPGMA, were positioned close to each other in PCA space, suggesting that they share a comparable energy allocation strategy. The combined use of PCA and cluster analysis provided us with a multivariate framework for distinguishing hybrid differences at the flowering stage of sunflower.
4. Conclusions
This study provides valuable insights into the adaptive responses of sunflower hybrids during the flowering stage, a critical stage for yield formation. The significant variation observed among hybrids in photosynthetic efficiency, energy dissipation, and leaf-level physiological traits suggests that hybrid-specific mechanisms underlie light energy utilisation and thermal regulation under field conditions.
Photosynthetic efficiency parameters, TR0/ABS and ET0/TR0, remained largely stable across hybrids, while parameters ABS/RC, DI0/RC, and RE0/RC, effectively differentiated hybrids based on their photochemical performance.
Differences in chlorophyll content and leaf temperature further supported the physiological divergence among hybrids. Those two parameters were found to be moderately associated with photosynthetic performance, suggesting their potential as indirect indicators of physiological status.
Yield component traits (plant height, head diameter, and number of seeds per head) also varied significantly. Several of them correlated strongly with chlorophyll fluorescence parameters, especially PITOTAL and RE0/ABS. These findings support the role of photosynthetic efficiency as a predictor of agronomic performance.
Principal components analysis and cluster analysis confirmed the differentiation among sunflower hybrids, which enabled the classification of hybrids with superior or balanced photosynthetic and yield characteristics. Hybrids 3 and 6 proved to be physiologically superior, while hybrids 1 and 2 showed lower photosynthetic and agronomic performance.
Overall, it can be concluded that the integration of ChlF parameters, chlorophyll content, leaf temperature, and yield components offers a robust framework for evaluating hybrid adaptation under variable environmental conditions. Within the sunflower breeding programme, this approach will improve the efficiency of selecting materials aimed at improving photosynthetic function and yield stability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15092181/s1, Table S1. JIP-test parameters; Table S2. Correlation analysis of photosynthetic parameters and sunflower yield components. Parameters that are significant at the 0.05 level are marked in red; Table S3. Principal component analysis eigenvalues, loading spreadsheet and biplot.
Author Contributions
Conceptualisation, A.M.K., D.I. and I.V.; methodology, A.M.K., D.I. and I.V.; software, A.M.K. and I.V.; formal analysis, A.M.K. and I.V.; investigation, A.M.K. and I.V.; writing—original draft preparation, A.M.K. and I.V.; writing—review and editing, A.M.K., D.I. and I.V. All authors have read and agreed to the published version of the manuscript.
Funding
The Agricultural Institute Osijek funded this research as a part of an internal project proposal: Research on sunflower genotypes titled “The effect of abiotic factors on sunflower hybrids and their parental lines”.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Blackman, B.K.; Scascitelli, M.; Kane, N.C.; Luton, H.H.; Rasmussen, D.A.; Byer, R.A.; Lentz, D.L.; Rieseberg, L.H. Sunflower domestication alleles support single domestication center in eastern North America. Proc. Natl. Acad. Sci. USA 2011, 108, 14360–14365. [Google Scholar] [CrossRef]
- Radanović, A.; Miladinović, D.; Cvejić, S.; Jocković, M.; Jocić, S. Sunflower Genetics from Ancestors to Modern Hybrids—A Review. Genes 2018, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Kozumplik, V.; Pejić, J. (Eds.) Oplemenjivanje Poljoprivrednog Bilja u Hrvatskoj; Agronomski fakultet: Zagreb, Croatia, 2012; Available online: https://urn.nsk.hr/urn:nbn:hr:204:817026 (accessed on 5 May 2025).
- Jošt, M.; Samobor, V. Oplemenjivanje bilja, proizvodnja hrane i održiva poljoprivreda. Agron. Glas. 2005, 67, 427–435. [Google Scholar]
- Salaić, M.; Galić, V.; Jambrović, A.; Zdunić, Z.; Šimić, D.; Brkić, A.; Petrović, S. Assessing Genetic Variability For NUE in Maize Lines from Agricultural Institute Osijek. Poljoprivreda 2024, 30, 13–20. [Google Scholar] [CrossRef]
- Seiler, G.J.; Qi, L.L.; Marek, L.F. Utilization of sunflower crop wild relatives for cultivated sunflower improvement. Crop Sci. 2017, 57, 1083–1101. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Pirzad, A.; Hatami-Maleki, M.; Kiani, S.P.; Sarrafi, A. Evaluation of the reaction of sunflower inbred lines and their F1 hybrids to drought conditions using various stress tolerance indices. Span. J. Agric. Res. 2010, 8, 1037–1046. [Google Scholar] [CrossRef]
- Cvejić, S.; Hrnjaković, O.; Jocković, M.; Kupusinac, A.; Doroslovački, K.; Gvozdenac, S.; Jocić, S.; Miladinović, D. Oil yield prediction for sunflower hybrid selection using different machine learning algorithms. Sci. Rep. 2023, 13, 17611. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Hassan, T.H.A.; Zahran, H.A. Heterosis for seed, oil yield and quality of some different hybrids sunflower. OCL 2021, 28, 25. [Google Scholar] [CrossRef]
- FAOstat. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 September 2025).
- Mijić, A.; Liović, I.; Sudarić, A.; Duvnjak, T.; Jug, D.; Kranjac, D.; Jovović, Z.; Markulj Kulundžić, A. Status and perspectives of sunflower production in Croatia. Agric. For. 2021, 67, 35–45. [Google Scholar]
- Jug, D.; Jug, I.; Brozović, B.; Vukadinović, V.; Stipešević, B.; Đurđević, B. The role of conservation agriculture in mitigation and adaptation to climate change. Poljoprivreda 2018, 24, 35–44. [Google Scholar] [CrossRef]
- Lisjak, M.; Ocvirk, D.; Špoljarević, M.; Teklić, T.; Liović, I.; Špoljarić Marković, S.; Volenik, M.; Mijić, A. The effect of Seed Priming with Hydrogen Sulfide on Germination and Biochemical Indicators of Drought Stress in Sunflower Seedlings. Poljoprivreda 2025, 31, 1–12. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Liović, I.; Horvat, D.; Mijić, A.; Sudarić, A.; Duvnjak, T.; Markulj Kulundžić, A. Procjena stabilnosti uroda zrna i sadržaja ulja hibrida suncokreta AMMI analizom. Poljoprivreda 2021, 27, 3–10. [Google Scholar] [CrossRef]
- Mijić, A.; Liović, I.; Sudarić, A.; Duvnjak, T.; Šimić, B.; Markulj Kulundžić, A. Makropokusi kao važan čimbenik u procjeni agronomskih svojstava hibrida suncokreta. Poljoprivreda 2022, 28, 24–31. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Shen, J.; Zhang, L.; Wei, P.; Liu, A.; Song, H. The alleviating effect on the growth, chlorophyll synthesis, and biochemical defense system in sunflowers under cadmium stress achieved through foliar application of humic acid. BMC Plant Biol. 2024, 24, 792. [Google Scholar] [CrossRef]
- Zou, Q.-Q.; Liu, D.-H.; Sang, M.; Jiang, C.-D. Sunflower Leaf Structure Affects Chlorophyll a Fluorescence Induction Kinetics In Vivo. Int. J. Mol. Sci. 2022, 23, 14996. [Google Scholar] [CrossRef]
- Plesničar, M.; Kastori, R.; Petrović, N.; Panković, D. Photosynthesis and chlorophyll fluorescence in sunflower (Helianthus annuus L.) leaves as affected by phosphorus nutrition. J. Exp. Bot. 1994, 45, 919–924. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Mijić, A.; Varga, I.; Sudarić, A.; Cesar, V.; Lepeduš, H. The combination of increased temperatures and high irradiation causes changes in photosynthetic efficiency. Plants 2021, 10, 2076. [Google Scholar] [CrossRef] [PubMed]
- Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Antunović Dunić, J.; Varga, I.; Zdunić, Z.; Sudarić, A.; Cesar, V.; Lepeduš, H. Effect of Elevated Temperature and Excess Light on Photosynthetic Efficiency, Pigments, and Proteins in the Field-Grown Sunflower during Afternoon. Horticulturae 2022, 8, 392. [Google Scholar] [CrossRef]
- Killi, D.; Raschi, A.; Bussotti, F. Lipid peroxidation and chlorophyll fluorescence of photosystem II performance during drought and heat stress is associated with the antioxidant capacities of C3 sunflower and C4 maize varieties. Int. J. Mol. Sci. 2020, 21, 4846. [Google Scholar] [CrossRef]
- Bazhenov, M.; Litvinov, D.; Kocheshkova, A.; Karlov, G.; Divashuk, M. Chlorophyll fluorescence imaging reveals the dynamics of bentazon action on sunflower (Helianthus annuus L.) plants. Agronomy 2024, 14, 1748. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Iljkić, D.; Antunović, M.; Sudarić, A.; Varga, I. The relationship between chlorophyll a fluorescence parameters and yield components in sunflower hybrids. Bot. Serb. 2023, 47, 103–111. [Google Scholar] [CrossRef]
- Ameen, M.; Zia, M.A.; Najeeb Alawadi, H.F.; Naqve, M.; Mahmood, A.; Shahzad, A.N.; Khan, B.A.; Alhammad, B.A.; Aljabri, M.; Seleiman, M.F. Exogenous application of selenium on sunflower (Helianthus annuus L.) to enhance drought stress tolerance by morpho-physiological and biochemical adaptations. Front. Plant Sci. 2024, 15, 1427420. [Google Scholar] [CrossRef] [PubMed]
- Arslan, Ö.; Balkan Nalçaiyi, A.S.; Çulha Erdal, Ş.; Pekcan, V.; Kaya, Y.; Çiçek, N.; Ekmekçi, Y. Analysis of drought response of sunflower inbred lines by chlorophyll a fluorescence induction kinetics. Photosynthetica 2020, 58, 348–357. [Google Scholar] [CrossRef]
- Çiçek, N.; Pekcan, V.; Arslan, Ö.; Çulha Erdal, Ş.; Balkan Nalçaiyi, A.S.; Çil, A.N.; Şahin, V.; Kaya, Y.; Ekmekçi, Y. Assessing drought tolerance in field-grown sunflower hybrids by chlorophyll fluorescence kinetics. Braz. J. Bot. 2019, 42, 249–260. [Google Scholar] [CrossRef]
- Heidari, A.; Toorchi, M.; Bandehagh, A.; Shakiba, M.-R. Effect of NaCl stress on growth, water relations, organic and inorganic osmolytes accumulation in sunflower (Helianthus annuus L.) lines. Univ. J. Environ. Res. Technol. 2011, 1, 351–359. [Google Scholar]
- Azevedo Neto, A.D.; Mota, K.N.A.B.; Silva, P.C.C.; Cova, A.M.W.; Ribas, R.F.; Gheyi, H.R. Selection of sunflower genotypes for salt stress and mechanisms of salt tolerance in contrasting genotypes. Ciênc. Agrotec. 2020, 44, e020120. [Google Scholar] [CrossRef]
- Omonov, O.; Amanov, B.; Muminov, K.H.; Buronov, A.; Tursunova, N. Physiological and biochemical composition of sunflower (Helianthus annuus L.). SABRAO J. Breed. Genet. 2023, 55, 2159–2167. [Google Scholar] [CrossRef]
- De la Mata, L.; Cabello, P.; De La Haba, P.; Agüera, E. Study of the senescence process in primary leaves of sunflower (Helianthus annuus L.) plants under two different light intensities. Photosynthetica 2013, 51, 85–94. [Google Scholar] [CrossRef]
- Murai, R.; Tsuchiya, H.; Tojo, S.; Chosa, T.; Kato, H. Effect of Planting Density on the Mechanical Properties of Sunflower Stem. 2012. Available online: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20133223007 (accessed on 3 May 2025).
- Desheva, G.; Valchinova, E. Ocjena stabilnosti prinosa i adaptibilnosti genotipova zobi (Avena sativa L.). Poljoprivreda 2024, 30, 3–12. [Google Scholar] [CrossRef]
- Iljkić, D.; Vuković, M.; Dvojković, K.; Horvat, D.; Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Rastija, M. Variety, Chemical Protection and Biostimulator Effect on Winter Wheat Status. Poljoprivreda 2024, 30, 28–35. [Google Scholar] [CrossRef]
- Miladinović, D.; Hladni, N.; Radanović, A.; Jocić, S.; Cvejić, S. Sunflower and Climate Change: Possibilities of Adaptation Through Breeding and Genomic Selection. In Genomic Designing of Climate-Smart Oilseed Crops; Kole, C., Ed.; Springer: Cham, Switzerland, 2019; pp. 173–238. [Google Scholar] [CrossRef]
- Međimurec, T. Results of a comparative sunflower trial at locations Koprivnica and Osijek. Sjemenarstvo 2021, 32, 47–56. [Google Scholar] [CrossRef]
- DHMZ—Croatian Meteorological and Hydrological Service. Available online: https://meteo.hr/index_en.php (accessed on 11 June 2025).
- Schneiter, A.A.; Miller, J.F.; Berglund, D.R. Stages of Sunflower Development; North Dakota State University: Fargo, ND, USA, 2019; Available online: https://www.sunflowernsa.com/uploads/10/stagesofsunflowerdevelopment.pdf (accessed on 16 May 2025).
- Markulj Kulundžić, A.; Sudarić, A.; Matoša Kočar, M.; Duvnjak, T.; Liović, I.; Mijić, A.; Varga, I.; Viljevac Vuletić, M. Detailed insight into the behaviour of chlorophyll a fluorescence transient curves and parameters during different times of dark adaptation in sunflower leaves. Agronomy 2024, 14, 954. [Google Scholar] [CrossRef]
- Strasser, R.J.; Stirbet, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterise and screen photosynthetic samples. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Heatmapper. Available online: http://heatmapper.ca/pairwise/ (accessed on 6 August 2025).
- Braga, P.C.S.; Martins, J.P.R.; Bonomo, R.; Borges, R.M.; Silva, J.V.G.; Falqueto, A.R. Differential response of photosystem II and I photochemistry in leaves of two Crambe abyssinica Hochst lineages submitted to water deficit. Photosynthetica 2020, 58, 1122–1129. [Google Scholar] [CrossRef]
- Matoša Kočar, M.; Sudarić, A.; Duvnjak, T.; Mazur, M. Soybean Genotype-Specific Cold Stress and Priming Responses: Chlorophyll a Fluorescence and Pigment-Related Spectral Reflectance Indices as Tools for Breeding. Agronomy 2025, 15, 390. [Google Scholar] [CrossRef]
- Mazur, M.; Matoša Kočar, M.; Jambrović, A.; Sudarić, A.; Volenik, M.; Duvnjak, T.; Zdunić, Z. Crop-Specific Responses to Cold Stress and Priming: Insights from Chlorophyll Fluorescence and Spectral Reflectance Analysis in Maize and Soybean. Plants 2024, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photoinhibition. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 1992, 186, 390–398. [Google Scholar] [CrossRef]
- Ji, W.; Hong, E.; Chen, X.; Li, Z.; Lin, B.; Xia, X.; Li, T.; Song, X.; Jin, S.; Zhu, X. Photosynthetic and physiological responses of different peony cultivars to high temperature. Front. Plant Sci. 2022, 13, 969718. [Google Scholar] [CrossRef]
- Umar, M.; Uddin, Z.; Siddiqui, Z.S. Responses of photosynthetic apparatus in sunflower cultivars to combined drought and salt stress. Photosynthetica 2019, 57, 627–639. [Google Scholar] [CrossRef]
- Sunil, B.; Strasser, R.J.; Raghavendra, A.S. Targets of nitric oxide during modulation of photosystems in pea mesophyll protoplasts. Photosynthetica 2020, 58, 452–459. [Google Scholar] [CrossRef]
- Antunović Dunić, J.; Štolfa Čamagajevac, I.; Teklić, T.; Parađiković, N.; Lisjak, M.; Soldo, B.; Cesar, V.; Lepeduš, H. Comparative Analysis of Primary Photosynthetic Reactions Assessed by OJIP Kinetics in Three Brassica Crops after Drought and Recovery. Appl. Sci. 2023, 13, 3078. [Google Scholar] [CrossRef]
- Ghaffar, A.; Li, J.; Munir, M.Z.; Huang, Y.; Chen, H.; Li, Y.; Ali, Q.; Aslam, H.; Sattar, A.; Xu, C.; et al. Photosynthetic Activity and Metabolic Profiling of Bread Wheat Cultivars Contrasting in Drought Tolerance. Front. Plant Sci. 2023, 14, 1123080. [Google Scholar] [CrossRef] [PubMed]
- Franić, M.; Galić, V.; Lončarić, Z.; Šimić, D. Genotypic Variability of Photosynthetic Parameters in Maize Ear-Leaves at Different Cadmium Levels in Soil. Agronomy 2020, 10, 986. [Google Scholar] [CrossRef]
- Brestič, M.; Živčák, M.; Balatová, Z.; Drevenáková, P.; Olšovská, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Goltsev, V.; Cuin, T.A.; Lazar, D.; Govindjee; Strasser, R.J. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll fluorescence measurements. Plant Physiol. Biochem. 2016, 81, 16–25. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, X.; Wei, K.; Wang, Y. Comparative Transcriptome Analysis of Salt-Tolerant and -Sensitive Soybean Cultivars under Salt Stress. Int. J. Mol. Sci. 2024, 25, 9818. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee; Bosa, K.; Allakhverdiev, S.I.; Kalaji, M.H. Chlorophyll fluorescence: Understanding of the basic concepts and a roadmap of its use in stress biology. Photosynth. Res. 2017, 133, 1–16. [Google Scholar]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a Fluorescence Parameters as Indicators of a Particular Abiotic Stress in Rice. Photosynthetica 2020, 58, 293–300. [Google Scholar] [CrossRef]
- Kovačević, J.; Mazur, M.; Drezner, G.; Lalić, A.; Sudarić, A.; Dvojković, K.; Viljevac Vuletić, M.; Josipović, M.; Josipović, A.; Markulj Kulundžić, A.; et al. Photosynthetic Efficiency Parameters as Indicators of Agronomic Traits of Winter Wheat Cultivars in Different Soil Water Conditions. Genetika 2017, 49, 891–910. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescent transient as a tool to characterise and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Živčák, M.; Brestič, M.; Olsovská, K.; Govindjee; Kalaji, H.M. Changes in chlorophyll fluorescence quenching and energy dissipation in the photosynthetic apparatus of cereals under drought stress. Photosynthetica 2014, 52, 115–124. [Google Scholar]
- Markulj Kulundžić, A.; Sudarić, A.; Matoša Kočar, M.; Mijić, A.; Liović, I.; Viljevac Vuletić, M.; Varga, I.; Cesar, V.; Lepeduš, H. Sunflower Agronomic Traits in Field Irrigation Conditions. Genetika 2022, 54, 473–489. [Google Scholar] [CrossRef]
- Torres Netto, A.; Campostrini, E.; de Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic Pigments, Nitrogen, Chlorophyll a Fluorescence and SPAD-502 Readings in Coffee Leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Jiménez-Lao, R.; Garcia-Caparros, P.; Pérez-Saiz, M.; Llanderal, A.; Lao, M.T. Monitoring Optical Tool to Determine the Chlorophyll Concentration in Ornamental Plants. Agronomy 2021, 11, 2197. [Google Scholar] [CrossRef]
- Blum, A. Breeding methods for drought resistance. In Plant Under Stress; Jones, H.G., Flowers, T.J., Jones, M.B., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 197–216. [Google Scholar]
- Gates, D.M. Transpiration and leaf temperature. Annu. Rev. Plant Physiol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Jones, H.G. Plant Water Relations and Irrigation Management; CAB International: Wallingford, UK, 1999. [Google Scholar]
- Shahenshah, H.; Isoda, A. Relationship of leaf temperature and SPAD readings in peanut under water stress. J. Agron. Crop Sci. 2010, 196, 302–311. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Singh, R.P.; Singh, A.K. Effect of water stress on chlorophyll content and physiological parameters in sunflower (Helianthus annuus L.). J. Oilseeds Res. 2014, 31, 206–210. [Google Scholar]
- Varalakshmi, K.; Neelima, S.; Sreenivasulu, K.N. Correlation and Path Coefficient Analysis for Yield and Its Component Traits in Sunflower Hybrids (Helianthus annuus L.). J. Res. ANGRAU 2019, 47, 27–35. [Google Scholar]
- Malo, D.D.; Worcester, B.K. Plant Height and Yield of Sunflowers at Different Landscape Positions. Farm Res. 1974, 31, 17–23. Available online: https://core.ac.uk/download/pdf/211311145.pdf (accessed on 7 August 2025).
- Kluza-Wieloch, M. Plant Height at Different Developmental Stages in Observed Types of Sunflower (Helianthus annuus L.) Cultivars. Rocz. Akad. Rol. Poznaniu. 2003, 6, 93–105. [Google Scholar]
- Mirzabe, A.H.; Khazaei, J.; Chegini, G.R. Measuring Some Physical Properties of Sunflower (Helianthus annuus L.) Head and Modeling Dimensions. Agric. Eng. Int. CIGR J. 2016, 18, 333–339. Available online: http://www.cigrjournal.org (accessed on 7 August 2025).
- Kotsareva, N.; Kovalenko, E. Effect of Pre-Sowing Treatment of Sunflower Seeds on Plant Height and Photosynthetic Activity of Hybrids F1 Borey and F1 Dariy under the Conditions of the Southwestern Part of the Central Black Soil Region. BIO Web Conf. 2021, 30, 04011. [Google Scholar] [CrossRef]
- Ergasheva, N. Effect of seedling thickness on stem height and number of leaves of oil sunflower cultivars. In Proceedings of the BIO Web of Conferences, Blagoveschensk, Russia, 22–25 May 2023; EDP Sciences: Les Ulis, France, 2023; Volume 65, p. 01007. [Google Scholar]
- Li, J.; Qu, Z.; Chen, J.; Yang, B.; Huang, Y. Effect of Planting Density on the Growth and Yield of Sunflower under Mulched Drip Irrigation. Water 2019, 11, 752. [Google Scholar] [CrossRef]
- Handayati, W.; Sihombing, D. Study of NPK Fertilizer Effect on Sunflower Growth and Yield. AIP Conf. Proc. 2019, 2120, 030031. [Google Scholar] [CrossRef]
- Hussain, S.; Khalili, A.; Qayyum, A.; Khan, S.U.; Mehmood, A.; Ahmad, G.; Ghazy, A.-H.; Al-Doss, A.A.; Attia, K.A.; Zeng, Y. Optimising sunflower (Helianthus annuus L.) hybrids growth, achene and oil yield through soil applied sulphur and zinc. Sci. Rep. 2025, 15, 13829. [Google Scholar] [CrossRef]
- Hladni, N.; Miklič, V.; Jocić, S.; Kraljević-Balalić, M.; Škorić, D. Mode of Inheritance and Combining Ability for Plant Height and Head Diameter in Sunflower (Helianthus annuus L.). Genetika 2014, 46, 159–168. [Google Scholar] [CrossRef]
- Ramos, M.L.; Altieri, E.; Bulos, M.; Sala, C.A. Phenotypic and Molecular Prospection of Reduced Height Sunflower Germplasm. In Proceedings of the 18th International Sunflower Association (Breeding Session), Mar del Plata, Argentina, 26 February–1 March 2012; Volume 1. [Google Scholar]
- Angadi, S.V.; Entz, M.H. Root System and Water Use Patterns of Different Height Sunflower Cultivars. Agron. J. 2002, 94, 136–145. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Teng, A.; Zhang, C.; Lei, L.; Ba, Y.; Wang, Z. Photosynthetic characteristics, yield and quality of sunflower response to deficit irrigation in a cold and arid environment. Front. Plant Sci. 2023, 14, 1280347. [Google Scholar] [CrossRef]
- Kanwal, N.; Ali, F.; Ali, Q.; Sadaqat, H.A. Phenotypic Tendency of Achene Yield and Oil Contents in Sunflower Hybrids. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 690–705. [Google Scholar] [CrossRef]
- Göksoy, A.; Turan, Z. Correlations and path analysis of yield components in synthetic varieties of sunflower (Helianthus annuus L.). Acta Agron. Hung. 2007, 55, 339–345. [Google Scholar] [CrossRef]
- Rani, R.; Sheoran, R.K.; Chander, S. Genetic Divergence Analysis among Sunflower (Helianthus annuus L.) Inbred Lines for Yield and Component Traits. Indian J. Plant Genet. Resour. 2017, 30, 66–71. [Google Scholar] [CrossRef]
- Sri, K.S.; Balakrishna, D.; Mahalakshmi, V. Correlation and path coefficient analysis for yield and its components in sunflower (Helianthus annuus L.). J. Exp. Agric. Int. 2025, 47, 14–21. [Google Scholar] [CrossRef]
- Bonciu, E.; Iancu, P.; Soare, M. The Yield Relationships in Sunflower (Helianthus annuus). 2010, pp. 123–128. Available online: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20103319052 (accessed on 3 May 2025).
- Hladni, N.; Škoric, D.; Kraljević-Balalić, M.; Ivanović, M.; Sakac, Z.; Jovanović, D. Correlation of Yield Components and Seed Yield per Plant in Sunflower (Helianthus annuus). In Proceedings of the 16th International Sunflower Conference, Fargo, ND, USA, 29 August–2 September 2004; pp. 491–496. [Google Scholar]
- Sasikala, R.; Ramesh, S.; Pushpa, R. Principal component analysis for yield attributing traits of sunflower (Helianthus annuus L.) genotypes. J. Oilseeds Res. 2020, 37, 106–110. Available online: https://epubs.icar.org.in/index.php/JOR/article/view/139687 (accessed on 18 July 2025). [CrossRef]
- Zia, Z.U.; Sadaqat, H.A.; Ahmad, S.; Nazeer, W.; Ali, I.; Jabbar, A.; Bibi, A.; Hussain, N. Grouping and Selection of 32 Single Cross Sunflower Hybrids Using Principal Component Analysis. Eurasian J. Agric. Res. 2018, 2, 4–12. [Google Scholar]
- Zeinalzadeh Tabrizi, H.; Şahin, E.; Haliloğlu, K. Principal Components Analysis of Some F1 Sunflower Hybrids at Germination and Early Seedling Growth Stage. J. Agric. Fac. Atatürk Univ. 2011, 42, 103–109. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).