Variation of Protein and Protein Fraction Content in Wheat in Relation to NPK Mineral Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Location and Experimental Conditions
2.2. Biological Material and Experimental Variants
2.3. Analysis of Quality Indices
2.3.1. Determination of Protein and Gluten Content
2.3.2. Determination of Glutenin and Gliadin Content
2.4. Statistical Analysis of Experimental Data
3. Results
3.1. Quality Indices in Relation to Mineral Fertilization
3.2. Comparative Analysis of the Results
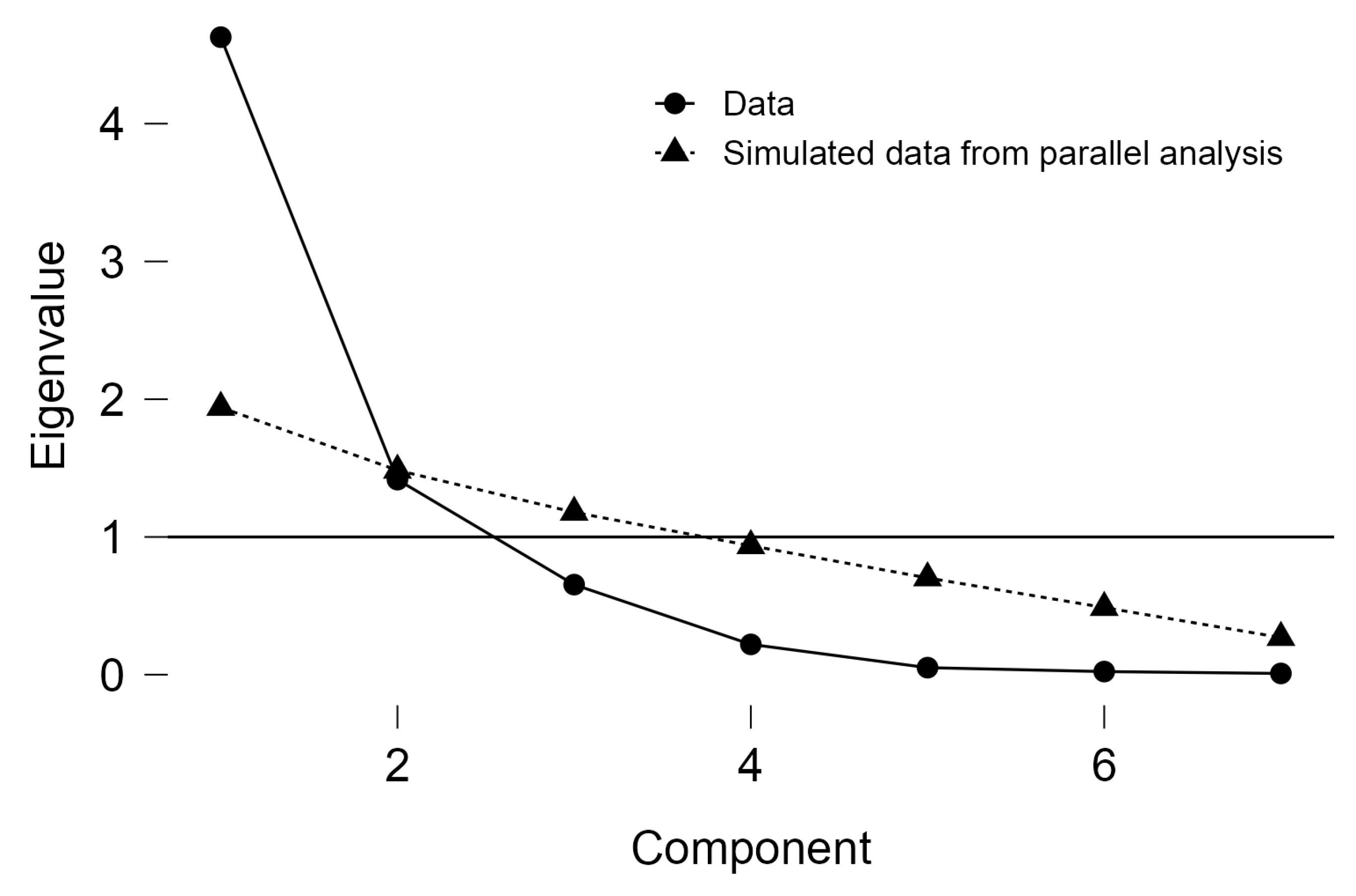
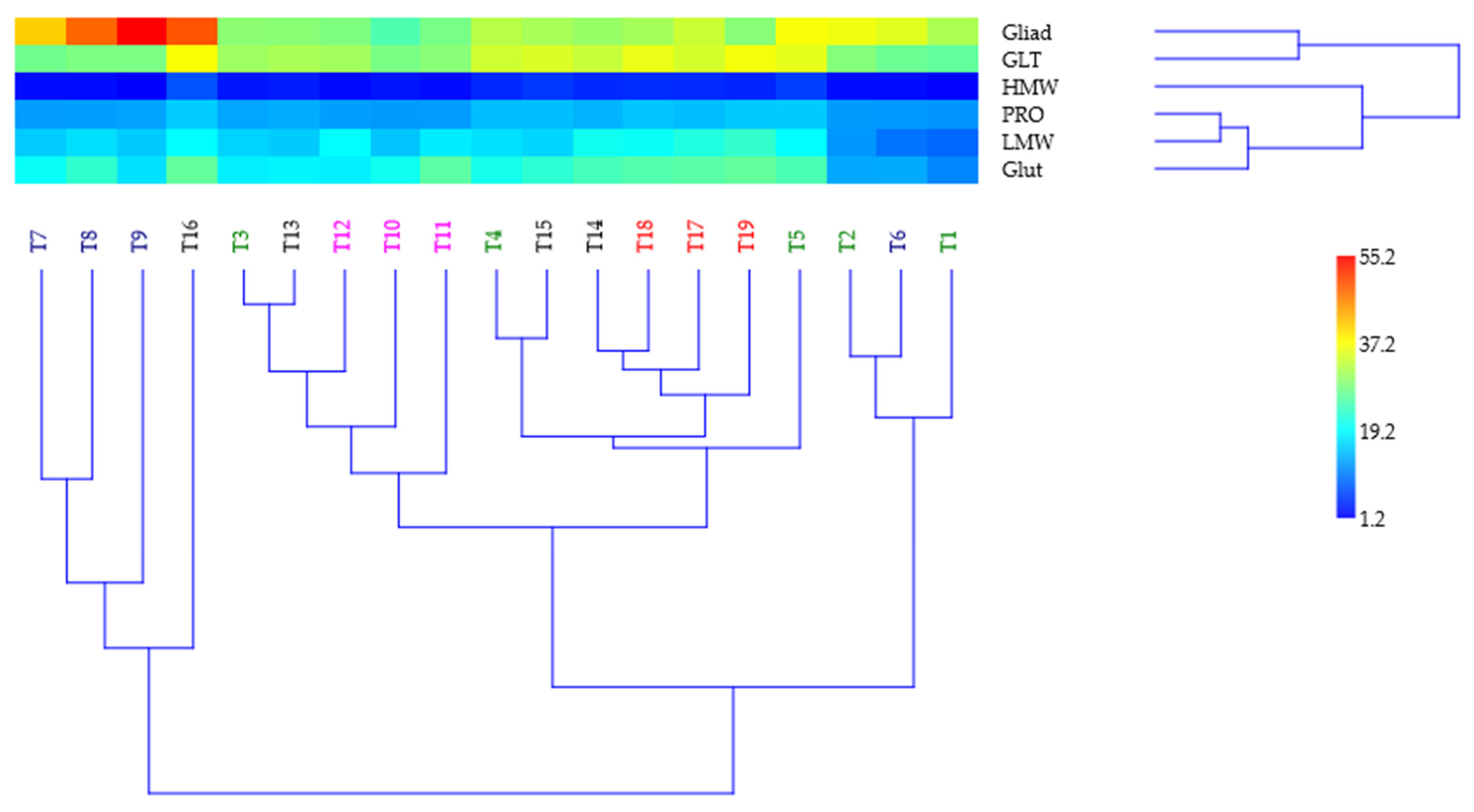
3.3. Multivariate Analysis for Explaining Quality Indices Position
3.4. Correlation Analysis Describing the Interaction of Quality Indices
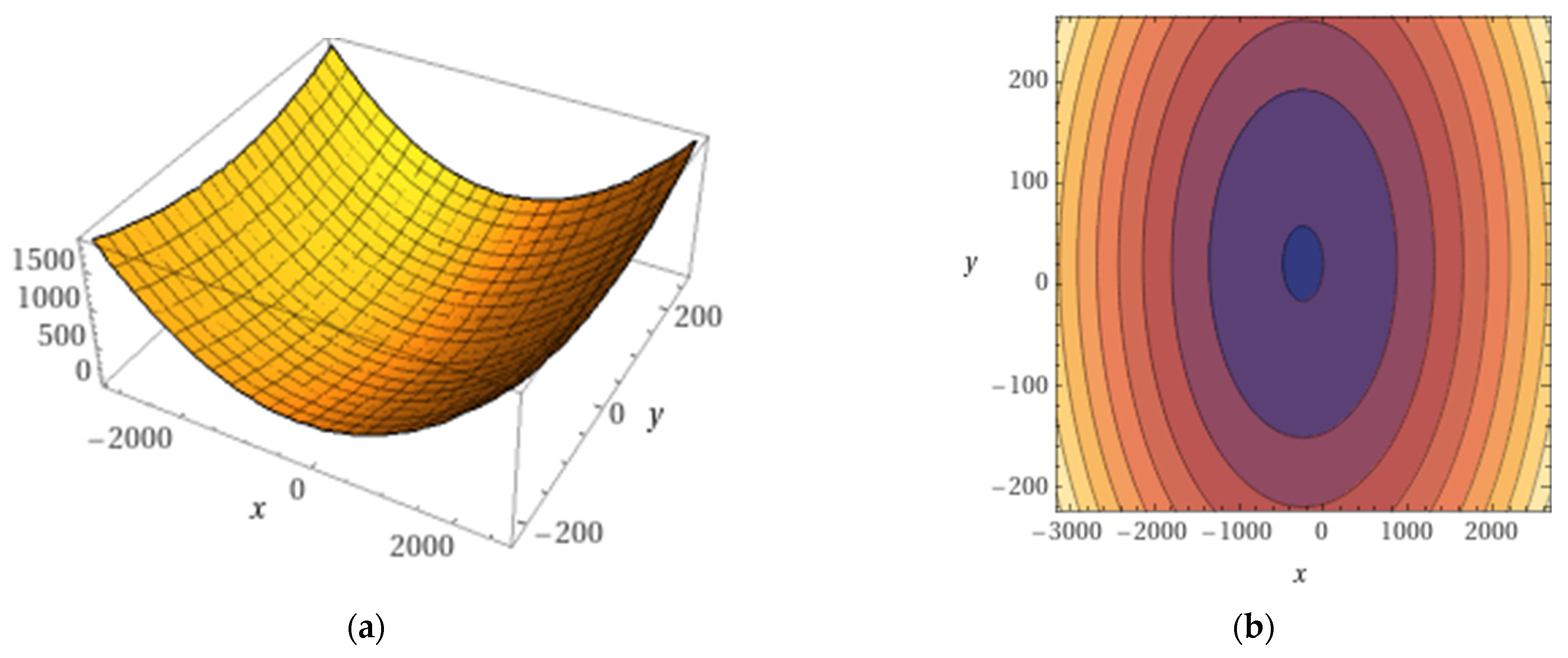
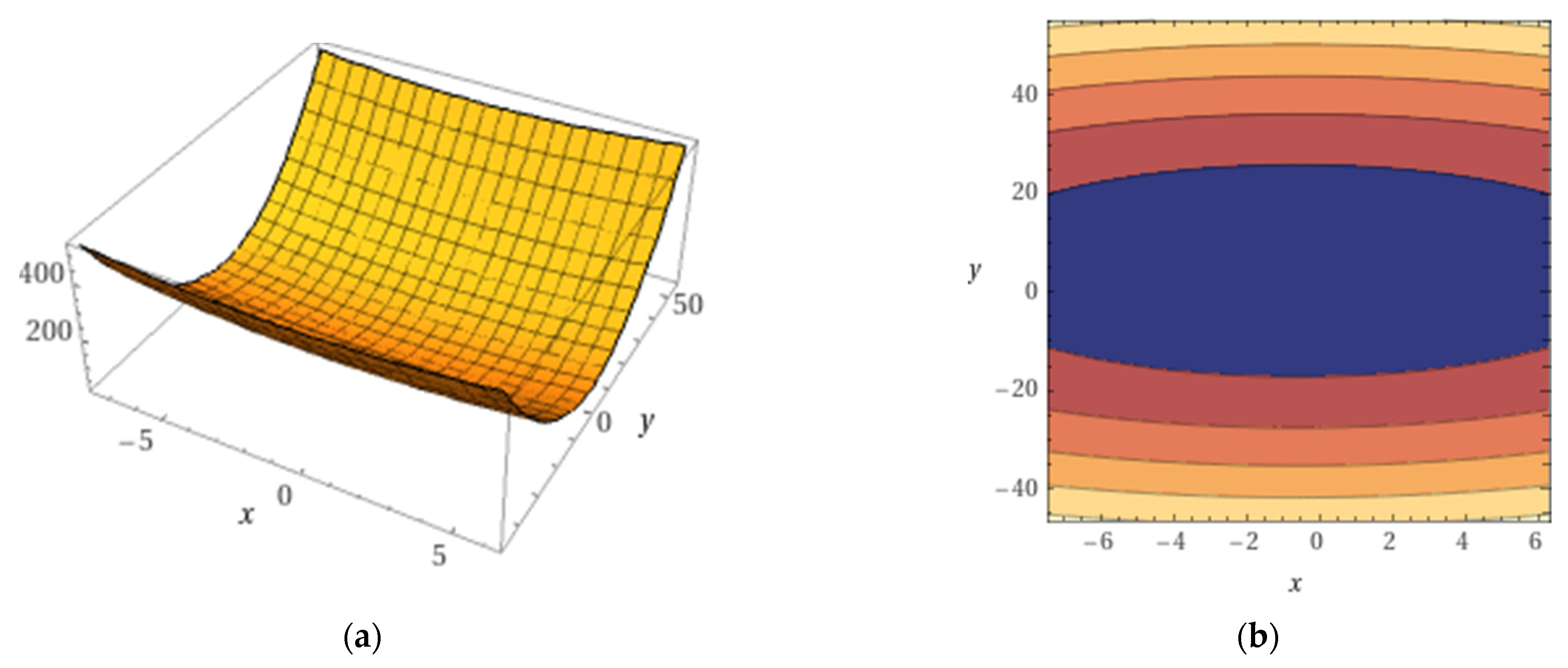
3.5. Variation Models of Quality Indices in Relation to NPK in Fertilizers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRO | Protein content |
| GLT | Gluten content |
| Gliad | Gliadins content |
| Glut | Glutenins content |
| HMW | High-molecular-weight subunits |
| LMW | Low-molecular-weight subunits |
| a.s. | Active substance |
| Sig. | Significant |
| ns | Not significant |
| me | Milliequivalent |
| Mmhos | Millimhos |
References
- Cho, K.; Beom, H.-R.; Jang, Y.-R.; Altenbach, S.B.; Vensel, W.H.; Simon-Buss, A.; Lim, S.-H.; Kim, M.G.; Lee, J.-Y. Proteomic profiling and epitope analysis of the complex α-, γ-, and ω-gliadin families in a commercial bread wheat. Front. Plant Sci. 2018, 9, 818. [Google Scholar] [CrossRef]
- Lakhneko, O.; Danchenko, M.; Morgun, B.; Kováč, A.; Majerová, P.; Škultéty, Ľ. Comprehensive comparison of clinically relevant grain proteins in modern and traditional bread wheat cultivars. Int. J. Mol. Sci. 2020, 21, 3445. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E.; Hashemi, S.M.B. Gliadin and glutenin genomes and their effects on the technological aspect of wheat-based products. Curr. Res. Food Sci. 2023, 7, 100622. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Quantitative composition. Cereal Chemi. 2023, 100, 36–55. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Plessis, A.; Ravel, C.; Bordes, J.; Balfourier, F.; Martre, P. Association study of wheat grain protein composition reveals that gliadin and glutenin composition are trans-regulated by different chromosome regions. J. Exp. Bot. 2013, 64, 3627–3644. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, J.; Shi, J.; Gao, Y.; Ma, H.; Wang, Y.; Ma, H. Molecular characterization and marker development of the HMW-GS gene from Thinopyrum elongatum for improving wheat quality. Int. J. Mol. Sci. 2023, 24, 11072. [Google Scholar] [CrossRef]
- Shewry, P.R. What is gluten–Why is it special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Chang, H.-C.; Rowe, M.H.; Yu, X.B.; Simon-Buss, A.; Seabourn, B.W.; Green, P.H.; Alaedini, A. Reducing the immunogenic potential of wheat flour: Silencing of alpha gliadin genes in a U.S. wheat cultivar. Front. Plant Sci. 2020, 11, 20. [Google Scholar] [CrossRef]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat gluten functionality as a quality determinant in cereal-based food products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef]
- Payne, P.I. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Ann. Rev. Plant Physiol. 1987, 38, 141–153. [Google Scholar] [CrossRef]
- Ma, S.; Han, W.; Li, L.; Zheng, X.; Wang, X. The thermal stability, structural changeability, and aggregability of glutenin and gliadin proteins induced by wheat bran dietary fiber. Food Funct. 2019, 10, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates. Biophys. Rev. 2018, 10, 435–443. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Liu, Q.; Sun, L.; Chen, X.; Lv, L.; Liu, Y.; Jia, X.; Li, H. Deletion of high-molecular-weight glutenin subunits in wheat significantly reduced dough strength and bread-baking quality. BMC Plant Biol. 2018, 18, 319. [Google Scholar] [CrossRef] [PubMed]
- Nucia, A.; Okoń, S.; Tomczyńska-Mleko, M. Characterization of HMW glutenin subunits in European spring common wheat (Triticum aestivum L.). Genet. Resour. Crop Evol. 2019, 66, 579–588. [Google Scholar] [CrossRef]
- Franaszek, S.; Salmanowicz, B. Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough. Open Life Sci. 2021, 16, 641–652. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Shen, Q.; Yang, D. High-molecular-weight glutenin subunits: Genetics, structures, and relation to end use qualities. Int. J. Mol. Sci. 2021, 22, 184. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, C.; Zhang, Z.; Wang, Z.; Chen, Y.; Rossi, V.; Chen, W.; Xin, M.; Su, Z.; Du, J.; et al. Unprocessed wheat γ-gliadin reduces gluten accumulation associated with the endoplasmic reticulum stress and elevated cell death. New Phytol. 2022, 236, 146–164. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Khatkar, B.S. Relationship of gliadin and glutenin proteins with dough rheology, flour pasting and bread making performance of wheat varieties. LWT-Food Sci. Technol. 2013, 51, 211–217. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Khatkar, B.S. Influence of gliadin and glutenin fractions on rheological, pasting, and textural properties of dough. Int. J. Food Prop. 2014, 17, 1428–1438. [Google Scholar] [CrossRef]
- Dhaka, V.; Khatkar, B.S. Effects of gliadin/glutenin and HMW-GS/LMW-GS ratio on dough rheological properties and bread-making potential of wheat varieties. J. Food Qual. 2015, 38, 71–82. [Google Scholar] [CrossRef]
- Schopf, M.; Wehrli, M.C.; Becker, T.; Jekle, M.; Scherf, K.A. Fundamental characterization of wheat gluten. Eur. Food Res. Technol. 2021, 247, 985–997. [Google Scholar] [CrossRef]
- Chaudhary, N.; Virdi, A.S.; Dangi, P.; Khatkar, B.S.; Mohanty, A.K.; Singh, N. Protein, thermal and functional properties of α-, γ- and ω-gliadins of wheat and their effect on bread making characteristics. Food Hydrocoll. 2022, 124 Pt A, 107212. [Google Scholar] [CrossRef]
- Cinco-Moroyoqui, F.J.; MacRitchie, F. Quantitation of LMW-GS to HMW-GS ratio in wheat flours. Cereal Chem. 2008, 85, 824–829. [Google Scholar] [CrossRef]
- Verbruggen, I.M.; Veraverbeke, W.S.; Delcour, J.A. Significance of LMW-GS and HMW-GS for dough extensibility: «Addition» versus «Incorporation» protocols. J. Cereal Sci. 2001, 33, 253–260. [Google Scholar] [CrossRef]
- Li, M.; Yue, Q.; Liu, C.; Zheng, X.; Hong, J.; Li, L.; Bian, K. Effect of gliadin/glutenin ratio on pasting, thermal, and structural properties of wheat starch. J. Cereal Sci. 2020, 93, 102973. [Google Scholar] [CrossRef]
- Zhao, L.; Song, L.; Li, L.; Li, X. Effects of HMW-GS Dx2 absence on the protein aggregation characteristics and thermal stability of wheat flour during maturation. Food Qual. Saf. 2023, 7, fyad019. [Google Scholar] [CrossRef]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative study on gluten protein composition of ancient (einkorn, emmer and spelt) and modern wheat species (durum and common wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef]
- García-Molina, M.D.; Barro, F. Characterization of changes in gluten proteins in low-gliadin transgenic wheat lines in response to application of different nitrogen regimes. Front. Plant Sci. 2017, 8, 257. [Google Scholar] [CrossRef]
- Rathan, N.D.; Mahendru-Singh, A.; Govindan, V.; Ibba, M.I. Impact of high and low-molecular-weight glutenins on the processing quality of a set of biofortified common wheat (Triticum aestivum L.) lines. Front. Sustain. Food Syst. 2020, 4, 583367. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Desktop, version Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2025.
- Șandru, I.; Pușcă, I. Stațiunea de Cercetări Agricole Lovrin; Editura Brumar: Timisoara, Romania, 2002; pp. 10–11. [Google Scholar]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants e BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001; 158p. [Google Scholar]
- Goetz, H.; Kuschel, M.; Wulff, T.; Sauber, C.; Miller, C.; Fisher, S.; Woodward, C. Comparison of selected analytical techniques for protein sizing, quantitation and molecular weight determination. J. Biochem. Biophys. Methods 2004, 60, 281–293. [Google Scholar] [CrossRef]
- Hey, J.S. Advances in microscale protein fractionation and analysis. Am. Biotechnol. Lab. 2007, 25, 12–16. [Google Scholar]
- Živančev, D.R.; Nikolovski, B.G.; Torbica, A.M.; Mastilović, J.S.; Dukić, N.H. Lab-on-a-chip method uncertainties in determination of high-molecular- weight glutenin subunits. Chem. Ind. Chem. Eng. Q. 2013, 19, 553–561. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- JASP, Version 0.16.2; JASP Team: Washington, DC, USA, 2022.
- Mathematica, Version 14.2; Wolfram Research, Inc.: Champaign, IL, USA, 2024.
- Farrer, D.C.; Weisz, R.; Heiniger, R.; Murphy, J.P.; White, J.G. Minimizing protein variability in soft red winter wheat: Impact of nitrogen application timing and rate. Agron. J. 2006, 98, 1137–1145. [Google Scholar] [CrossRef]
- Maryami, Z.; Huertas-García, A.B.; Azimi, M.R.; Hernández-Espinosa, N.; Payne, T.; Cervantes, F.; Govindan, V.; Ibba, M.I.; Guzman, C. Variability for glutenins, gluten quality, iron, zinc and phytic acid in a set of one hundred and fifty-eight common wheat landraces from Iran. Agronomy 2020, 10, 1797. [Google Scholar] [CrossRef]
- Zhen, S.; Deng, X.; Xu, X.; Liu, N.; Zhu, D.; Wang, Z.; Yan, Y. Effect of high-nitrogen fertilizer on gliadin and glutenin subproteomes during kernel development in wheat (Triticum aestivum L.). Crop J. 2020, 8, 38–52. [Google Scholar] [CrossRef]
- Jiang, H.M.; Yu, S.L.; Yu, Z.W.; Zhao, Q.N.; Qiu, H.J.; Ding, X.Y. Response of wheat glutenin polymerization to increased nitrogen fertilization. J. Triticum Crops 2003, 23, 43–46, (In Chinese with English abstract). [Google Scholar]
- Zhang, D.Y.; Zhang, Y.Q.; Yang, W.D.; Yan, C.P.; Hu, T.J. Effect of nitrogen application on high molecular weight glutenin subunit expression and grain quality of winter wheat. Plant Nutr. Fertil. Sci. 2008, 14, 235–241. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.L.; Yu, Z.W. Effects of nitrogen fertilizer application on protein components contents and processing quality of different wheat genotypes. Plant Nutr. Fertil. Sci. 2010, 16, 33–40, (In Chinese with English abstract). [Google Scholar]
- Trevisan, S.; Khorshidi, A.S.; Scanlon, M.G. Relationship between nitrogen functionality and wheat flour dough rheology: Extensional and shear approaches. Food Res. Int. 2022, 162 Pt B, 112049. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, Q.; Wang, Q.; Wang, X.; Cai, J.; Huang, M.; Jiang, D. Effects of nitrogen fertilizer on quality characteristics of wheat with the absence of different individual high-molecular-weight glutenin subunits (HMW-GSs). Int. J. Mol. Sci. 2022, 23, 2178. [Google Scholar] [CrossRef]
- An, H.-Y.; Han, J.-J.; He, Q.-N.; Zhu, Y.-L.; Wu, P.; Wang, Y.-C.; Gao, Z.-Q.; Du, T.-Q.; Xue, J.-F. Influence of nitrogen application rate on wheat grain protein content and composition in china: A meta-analysis. Agronomy 2024, 14, 1164. [Google Scholar] [CrossRef]
- Hu, X.; Tian, P.; Fu, W.; Tian, Z.; Du, M.; Chang, Z.; Ye, Y.; Meng, X.; Wang, Y. Effects of nitrogen fertilizer application on the lodging resistance traits, yield, and quality of two gluten types of wheat. Agriculture 2025, 15, 637. [Google Scholar] [CrossRef]
- Rossmann, A.; Buchner, P.; Savill, G.P.; Hawkesford, M.J.; Scherf, K.A.; Mühling, K.H. Foliar N application at anthesis alters grain protein composition and enhances baking quality in winter wheat only under a low N fertiliser regimen. Eur. J. Agron. 2019, 109, 125909. [Google Scholar] [CrossRef]
- Ahmadi, H.; Mirseyed Hosseini, H.; Moshiri, F.; Alikhani, H.A.; Ftesami, H. Impact of varied tillage practices and phosphorus fertilization regimes on wheat yield and grain quality parameters in a five-year corn-wheat rotation system. Sci. Rep. 2024, 14, 14717. [Google Scholar] [CrossRef]
- Tóth, B.; van Biljon, A.; Labuschagne, M. Influence of low soil nitrogen and phosphorus on gluten polymeric and monomeric protein distribution in two high quality spring wheat cultivars. J. Cereal Sci. 2020, 91, 102867. [Google Scholar] [CrossRef]
- Bouacha, O.D.; Nouaigui, S.; Rezgui, S. Effects of N and K fertilizers on durum wheat quality in different environments. J. Cereal Sci. 2014, 59, 9–14. [Google Scholar] [CrossRef]
- Zang, F.; Zhang, M.; Zhou, Q.; Wang, X.; Zhong, Y.; Huang, M.; Dai, T.; Jiang, D.; Cai, J. Effect of sulfur and potassium foliar applications on wheat grain protein quality. Field Crops Res. 2024, 319, 109639. [Google Scholar] [CrossRef]
- Ahmad, S.; Pasha, I.; Saeed, M.; Shahid, M. Principal component analysis and correlation studies of spring wheats in relation to cookie making quality. Int. J. Food Prop. 2017, 20, 2299–2313. [Google Scholar] [CrossRef][Green Version]
- Spanic, V.; Cosic, J.; Zdunic, Z.; Drezner, G. Characterization of agronomical and quality traits of winter wheat (Triticum aestivum L.) for fusarium head blight pressure in different environments. Agronomy 2021, 11, 213. [Google Scholar] [CrossRef]
- Sánchez-León, S.; Giménez, M.J.; Barro, F. The α-gliadins in bread wheat: Effect of nitrogen treatment on the expression of the major celiac disease immunogenic complex in two RNAi low-gliadin lines. Front. Plant Sci. 2021, 12, 663653. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Gullì, M.; Visioli, G.; Marti, A. Gluten aggregation properties as a tool for durum wheat quality assessment: A chemometric approach. LWT 2021, 142, 111048. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Z.; Xu, J.; Huang, C.; Dang, H.; Mu, W.; Zhang, L.; Hou, S.; Huang, N.; Li, C.; et al. Nutrient requirements determined by grain yield and protein content to optimize N, P, and K fertilizer management in China. Sci. Total Environ. 2024, 946, 174187. [Google Scholar] [CrossRef] [PubMed]
- Hailegiorgis, D.; Mesfin, M.; Genet, T. Genetic divergence analysis on some bread wheat genotypes grown in Ethiopia. J. Cent. Eur. Agric. 2011, 12, 344–352. [Google Scholar] [CrossRef]
- Hasan, M.M.; Mia, M.A.B.; Ahmed, J.U.; Karim, M.A.; Islam, A.K.M.A.; Moho-Ud-Din, M. Heat stress tolerance in wheat seedling: Clustering genotypes and identifying key traits using multivariate analysis. Heliyon 2024, 10, e38623. [Google Scholar] [CrossRef]
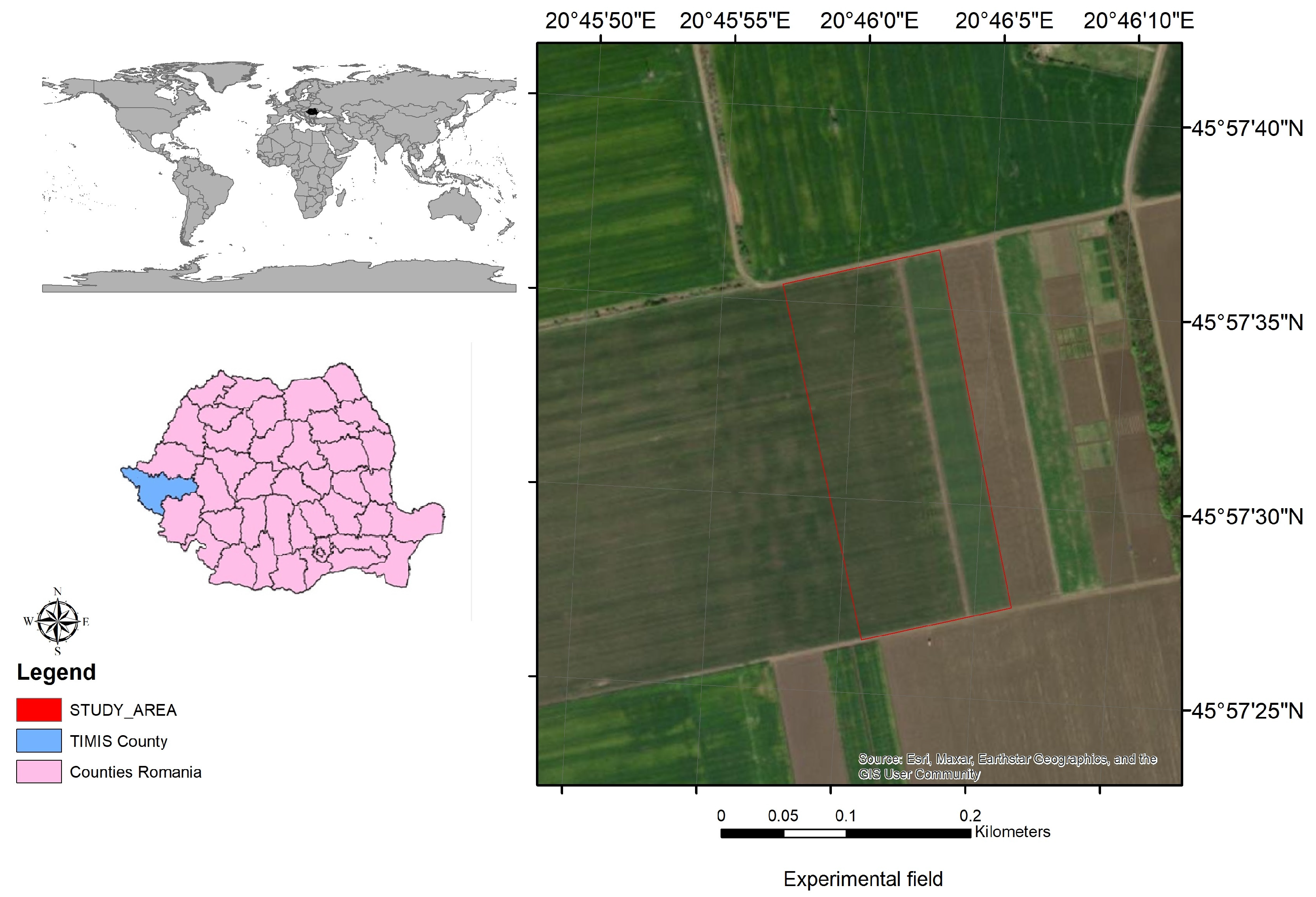
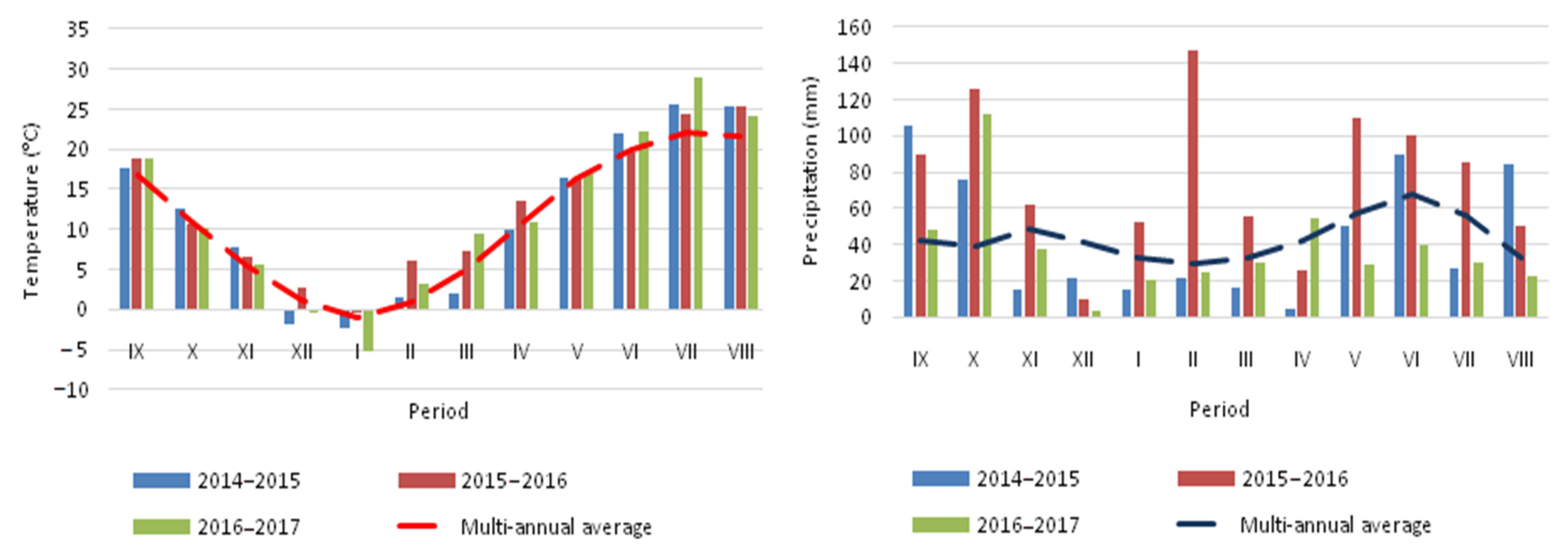



| Parameters | Soil Profile Horizons | ||||
|---|---|---|---|---|---|
| Ap | Am | A/C | Cca | C/Ca gr-ac | |
| Depths (cm) | 0–26 | 26–47 | 47–49 | 79–123 | 123–200 |
| Rough sand (2.0–0.2 mm, %) | 1.0 | 0.9 | 0.5 | 0.6 | 0.3 |
| Fine sand (0.2–0.02 mm, %) | 34.3 | 36.4 | 35.2 | 38.5 | 24.7 |
| Dust (0.02–0.002 mm, %) | 27.7 | 26.5 | 28.9 | 31.3 | 42.6 |
| Clay (<0.002, %) | 37.0 | 36.2 | 35.4 | 29.6 | 32.4 |
| Physical clay (<0.01 mm, %) | 51.8 | 51.7 | 51.0 | 46.8 | 57.0 |
| Specific density (D, g/cm3) | 2.43 | 2.55 | 2.56 | 2.63 | – |
| Apparent density (AD, g/cm3) | 1.35 | 1.40 | 1.33 | 1.27 | – |
| Total porosity (TP, %) | 45.00 | 45 | 48 | 50 | – |
| Aeration porosity (AP, %) | 10.69 | 9.0 | 13.0 | 16.0 | – |
| Coefficient of hygroscopicity (CH, %) | 8.79 | 8.0 | 7.8 | 7.0 | – |
| Coefficient of withering (CW, %) | 13.18 | 12.0 | 11.7 | 10.5 | – |
| Fields capacity (FC, %) | 25.90 | 26.0 | 26.0 | 25.4 | – |
| Useful water capacity (UC, %) | 12.75 | 14.0 | 14.3 | 14.8 | – |
| Total water capacity (TC, %) | 33.83 | 32.2 | 36.1 | 37.9 | – |
| pH in H2O | 6.90 | 7.20 | 8.45 | 9.40 | 9.45 |
| Carbonates (CaCO3, %) | – | 0.4 | 9.8 | 19.3 | 16.0 |
| Hydraulic conductivity (K, mm/h) | 3.47 | 11.9 | 10.3 | 12.8 | – |
| Humus (H, %) | 3.47 | 3.28 | 2.73 | – | – |
| Bacteria no. (mil/ 100 g dry soil) | 772 | – | – | – | – |
| C:N | 13.7 | 13.9 | 13.7 | – | – |
| Total nitrogen (%) | 0.171 | 0.159 | 0.120 | – | – |
| Mobile phosphorus (ppm) | 75.7 | 31.7 | 8.7 | – | – |
| Mobile potassium (ppm) | 205 | 202 | 163 | – | – |
| Exchange bases (EB me for 100 g soil) | 27.6 | 27.6 | 20.3 | – | – |
| Changeable hydrogen (CH me for 100 g soil) | 4.35 | – | – | – | – |
| Cationic exchange capacity (CEC, me for 100 g soil) | 32.0 | 27.6 | 21.9 | – | – |
| Bases saturation degree (V, %) | 86.4 | 100 | 100 | 100 | 100 |
| EC (Mmhos/cm) | 0.78 | 0.57 | 1.68 | – | – |
| Na+ (me for 100 g soil) | 0.04 | 0.10 | 0.66 | – | – |
| Exchange Na+ (% of CEC) | 0.6 | 0.5 | 3.5 | 13.1 | 13.0 |
| Trial | N | p | K | PRO | GLT | Gliad | Glut | HMW | LMW | Gliad/Glut |
|---|---|---|---|---|---|---|---|---|---|---|
| (kg a.s. ha−1) | (%) | (g 100 g−1) | (Ratio) | |||||||
| T1 | 0 | 0 | 0 | 11.70 | 26.10 | 31.40 | 10.80 | 1.30 | 8.53 | 2.91 |
| T2 | 30 | 0 | 0 | 12.10 | 28.60 | 36.20 | 13.10 | 2.10 | 11.80 | 2.76 |
| T3 | 60 | 0 | 0 | 12.90 | 30.40 | 29.10 | 18.10 | 2.70 | 16.10 | 1.61 |
| T4 | 90 | 0 | 0 | 14.60 | 33.70 | 32.20 | 20.60 | 4.00 | 17.10 | 1.56 |
| T5 | 120 | 0 | 0 | 15.50 | 35.40 | 36.70 | 24.50 | 5.70 | 19.20 | 1.50 |
| T6 | 0 | 40 | 0 | 12.00 | 26.80 | 35.00 | 13.20 | 2.00 | 9.30 | 2.65 |
| T7 | 0 | 80 | 0 | 12.30 | 27.10 | 40.70 | 19.70 | 2.00 | 15.60 | 2.07 |
| T8 | 0 | 120 | 0 | 12.40 | 28.00 | 48.00 | 22.60 | 2.10 | 16.90 | 2.12 |
| T9 | 0 | 160 | 0 | 12.70 | 27.90 | 55.20 | 17.10 | 1.20 | 15.50 | 3.23 |
| T10 | 0 | 0 | 40 | 12.10 | 27.80 | 24.80 | 20.30 | 2.70 | 15.10 | 1.22 |
| T11 | 0 | 0 | 80 | 12.30 | 28.80 | 27.70 | 25.70 | 2.00 | 17.90 | 1.08 |
| T12 | 0 | 0 | 120 | 12.40 | 30.70 | 28.20 | 18.10 | 1.80 | 19.30 | 1.56 |
| T13 | 30 | 80 | 0 | 13.40 | 31.20 | 28.90 | 18.50 | 3.20 | 15.50 | 1.70 |
| T14 | 60 | 80 | 0 | 13.90 | 33.20 | 30.00 | 24.10 | 4.10 | 20.30 | 1.30 |
| T15 | 90 | 80 | 0 | 14.60 | 34.50 | 31.00 | 22.30 | 5.10 | 16.30 | 1.50 |
| T16 | 120 | 80 | 0 | 15.60 | 36.80 | 49.20 | 26.10 | 7.10 | 19.30 | 2.00 |
| T17 | 60 | 80 | 80 | 14.50 | 34.20 | 33.50 | 25.40 | 4.20 | 21.50 | 1.40 |
| T18 | 120 | 80 | 40 | 15.10 | 35.70 | 30.90 | 25.00 | 4.30 | 19.80 | 1.30 |
| T19 | 120 | 80 | 80 | 15.40 | 36.40 | 28.80 | 26.10 | 3.80 | 22.80 | 1.50 |
| SE | ±0.31 | ±0.83 | ±1.88 | ±1.08 | ±0.37 | ±0.87 | ±0.14 | |||
| Trial | Protein | Gluten | Gliadins | Glutenins | HMW | LMW | Gliadin/ Glutenin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference | Sig | Difference | Sig | Difference | Sig | Difference | Sig | Difference | Sig. | Difference | Sig. | Difference | Sig | |
| T1 | −1.75 | ooo | −5.14 | ooo | −3.21 | ns | −9.79 | ooo | −1.93 | ooo | −8.2 | ooo | 1.07 | *** |
| T2 | −1.35 | ooo | −2.64 | oo | 1.59 | ns | −7.49 | ooo | −1.13 | ooo | −4.93 | ooo | 0.92 | *** |
| T3 | −0.55 | ns | −0.84 | ns | −5.51 | oo | −2.49 | ooo | −0.53 | ns | −0.63 | ns | −0.23 | ns |
| T4 | 1.15 | ** | 2.46 | ** | −2.41 | ns | 0.01 | ns | 0.77 | ns | 0.37 | ns | −0.28 | ns |
| T5 | 2.05 | *** | 4.16 | *** | 2.09 | ns | 3.91 | ** | 2.47 | *** | 2.47 | * | −0.34 | o |
| T6 | −1.45 | ooo | −4.44 | ooo | 0.39 | ns | −7.39 | ooo | −1.23 | oo | −7.43 | ooo | 0.81 | *** |
| T7 | −1.15 | oo | −4.14 | ooo | 6.09 | ** | −0.89 | ns | −1.23 | oo | −1.13 | ns | 0.23 | ns |
| T8 | −1.05 | oo | −3.24 | oo | 13.39 | *** | 2.01 | ns | −1.13 | oo | 0.17 | ns | 0.28 | ns |
| T9 | −0.75 | o | −3.34 | ooo | 20.59 | *** | −3.49 | oo | −2.03 | ooo | −1.23 | ns | 1.39 | *** |
| T10 | −1.35 | ooo | −3.44 | ooo | −9.81 | ooo | −0.29 | ns | −0.53 | ns | −1.63 | ns | −0.62 | ooo |
| T11 | −1.15 | oo | −2.44 | oo | −6.91 | oo | 5.11 | *** | −1.23 | oo | 1.17 | ns | −0.76 | ooo |
| T12 | −1.05 | oo | −0.54 | ns | −6.41 | oo | −2.49 | o | −1.43 | oo | 2.57 | ** | −0.28 | ns |
| T13 | −0.05 | ns | −0.04 | ns | −5.71 | oo | −2.09 | ns | −0.03 | ns | −1.23 | ns | −0.14 | ns |
| T14 | 0.45 | ns | 1.96 | * | −4.61 | o | 3.51 | ** | 0.87 | * | 3.57 | *** | −0.54 | oo |
| T15 | 1.15 | ** | 3.26 | *** | −3.61 | ns | 1.71 | ns | 1.87 | *** | −0.43 | ns | −0.34 | o |
| T16 | 2.15 | *** | 5.56 | *** | 14.59 | *** | 5.51 | *** | 3.87 | *** | 2.57 | ** | 0.16 | ns |
| T17 | 1.05 | ** | 2.96 | ** | −1.11 | ns | 4.81 | *** | 0.97 | * | 4.77 | *** | −0.44 | oo |
| T18 | 1.65 | *** | 4.46 | *** | −3.71 | ns | 4.41 | *** | 1.07 | ** | 3.07 | ** | −0.54 | oo |
| T19 | 1.95 | *** | 5.16 | *** | −5.81 | oo | 5.51 | *** | 0.57 | ns | 6.07 | *** | −0.34 | o |
| Title 1 | PC1 | PC2 | Uniqueness |
|---|---|---|---|
| PRO | 0.955 | 0.088 | |
| GLT | 0.947 | 0.090 | |
| HMW | 0.896 | 0.196 | |
| Glutenin | 0.869 | 0.185 | |
| LMW | 0.836 | 0.225 | |
| Gliadin | 0.936 | 0.113 | |
| Gliad/Glut | 0.798 | 0.059 |
| Components | Unrotated Solution | Rotated Solution | ||||
|---|---|---|---|---|---|---|
| Eigenvalue | Proportion var. | Cumulative | SumSq. Loadings | Proportion var. | Cumulative | |
| Component 1 | 4.628 | 0.661 | 0.661 | 4.381 | 0.626 | 0.626 |
| Component 2 | 1.416 | 0.202 | 0.863 | 1.663 | 0.238 | 0.863 |
| Quality Index | Fitting Equation | Statistical Safety Parameters | ||
|---|---|---|---|---|
| R2 | F | p | ||
| PRO | 0.911 | 171.05 | p < 0.001 | |
| GLT | 0.986 | 1251.8 | p < 0.001 | |
| Gliad | 0.770 | 57.73 | p < 0.001 | |
| Glut | 0.836 | 86.077 | p < 0.001 | |
| HMW | 0.968 | 525.61 | p < 0.001 | |
| LMW | 0.820 | 77.586 | p < 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agapie, A.L.; Horablaga, M.N.; Gorinoiu, G.; Horablaga, A.; Herbei, M.V.; Sala, F. Variation of Protein and Protein Fraction Content in Wheat in Relation to NPK Mineral Fertilization. Agronomy 2025, 15, 2076. https://doi.org/10.3390/agronomy15092076
Agapie AL, Horablaga MN, Gorinoiu G, Horablaga A, Herbei MV, Sala F. Variation of Protein and Protein Fraction Content in Wheat in Relation to NPK Mineral Fertilization. Agronomy. 2025; 15(9):2076. https://doi.org/10.3390/agronomy15092076
Chicago/Turabian StyleAgapie, Alina Laura, Marinel Nicolae Horablaga, Gabriela Gorinoiu, Adina Horablaga, Mihai Valentin Herbei, and Florin Sala. 2025. "Variation of Protein and Protein Fraction Content in Wheat in Relation to NPK Mineral Fertilization" Agronomy 15, no. 9: 2076. https://doi.org/10.3390/agronomy15092076
APA StyleAgapie, A. L., Horablaga, M. N., Gorinoiu, G., Horablaga, A., Herbei, M. V., & Sala, F. (2025). Variation of Protein and Protein Fraction Content in Wheat in Relation to NPK Mineral Fertilization. Agronomy, 15(9), 2076. https://doi.org/10.3390/agronomy15092076







