Spatial Distribution Characteristics of Soil Nutrients and Stoichiometric Ratios in Eragrostis minor Distribution Areas of Gansu Province, Northwestern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Research Setup
2.3. Soil Sampling and Sample Preparation
2.4. Analysis of Soil pH, OM, Nutrients and Stoichiometric Ratios
2.5. Statistical Analysis
3. Results
3.1. Variability in Soil Nutrients and Stoichiometry Ratios Across E. minor Distribution Sites
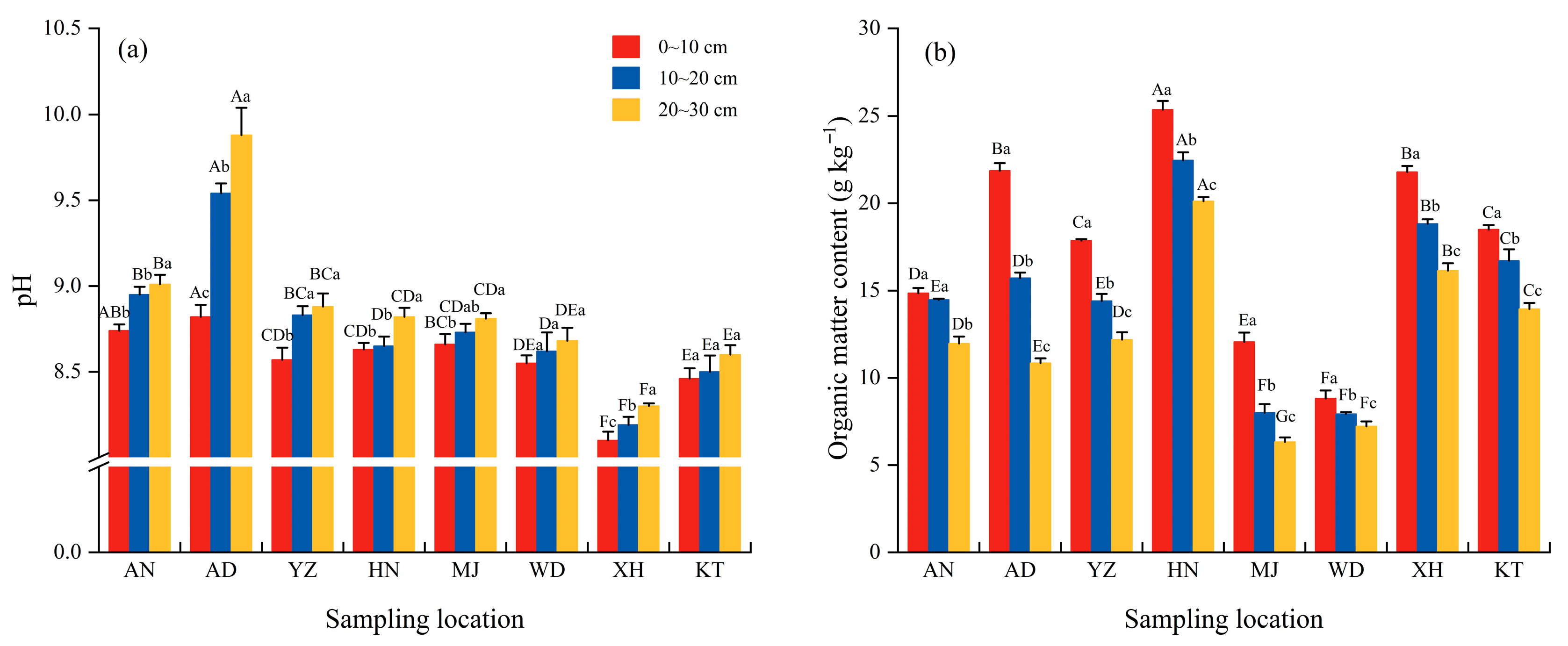
3.2. Vertical Distribution of Soil pH and OM Under E. minor Sites
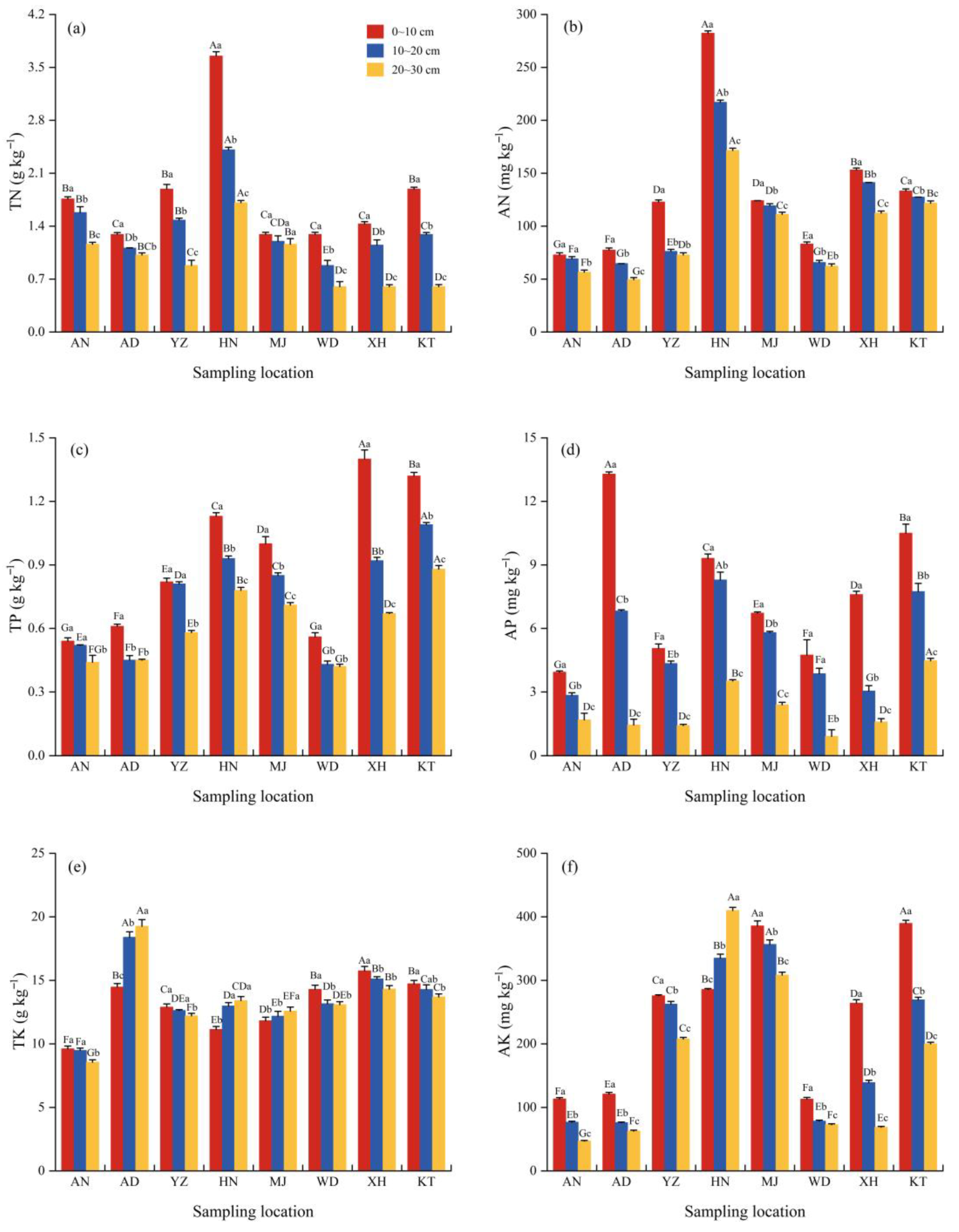
3.3. Vertical Distribution of Soil Nutrients Under E. minor Sites
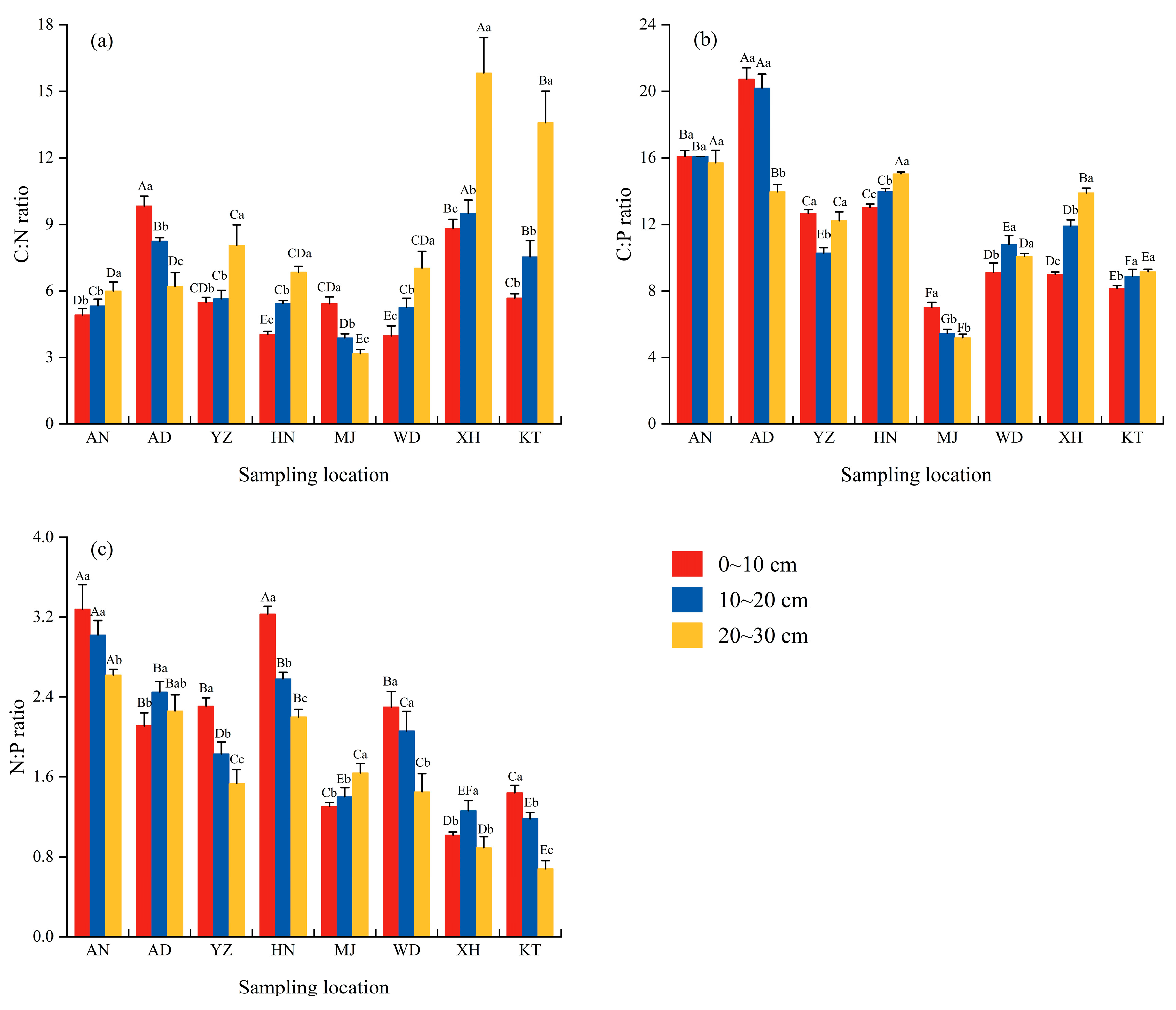
3.4. Vertical Distribution of Soil Stoichiometric Ratios Under E. minor Sites
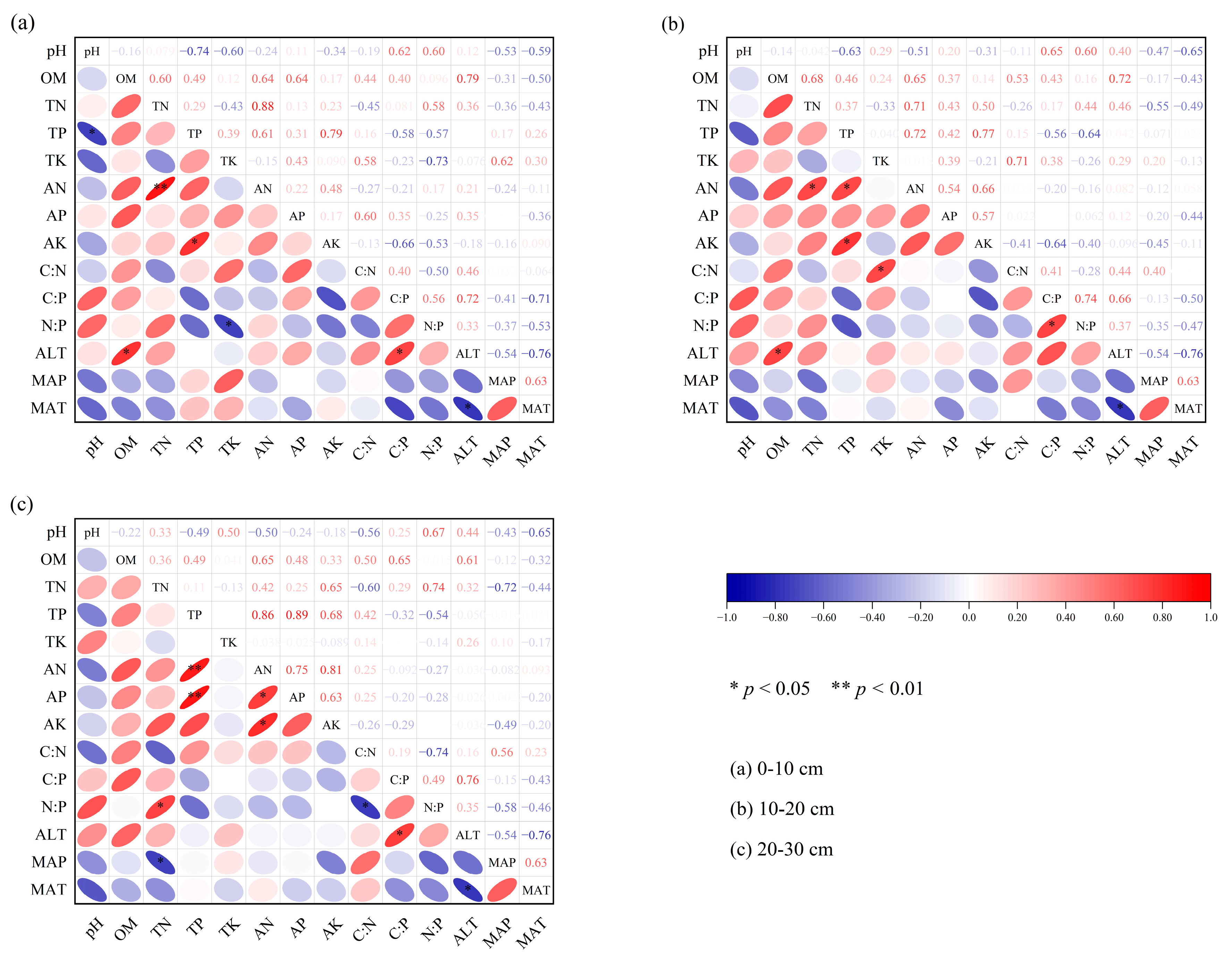
3.5. Correlation Analysis
4. Discussion
4.1. Effects of Spatial Distribution and Soil Depth on Soil pH and Nutrient Content
4.2. Effects of Spatial Distribution and Soil Depth on Soil Stoichiometric Ratios
4.3. Factors Influencing Soil Nutrients and Stoichiometric Ratios
4.4. Ecological Adaptation Strategies of E. minor Driven by Soil Nutrient Heterogeneity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, H.B.; Wu, J.; Liu, W.F.; Yuan, Y.H.; Hu, L.; Cai, Q.K. Linkages of Plant and Soil C:N:P Stoichiometry and Their Relationships to Forest Growth in Subtropical Plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Wasaya, A.; Tahir, M.; Ali, H.; Hussain, M.; Yasir, T.A.; Sher, A.; Ijaz, M.; Sattar, A. Influence of Varying Tillage Systems and Nitrogen Application on Crop Allometry, Chlorophyll Contents, Biomass Production and Net Returns of Maize (Zea mays L.). Soil Tillage Res. 2017, 170, 18–26. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, H.S.; Zhang, W.; Wang, K.L. Soil Nutrients and Stoichiometric Ratios as Affected by Land Use and Lithology at County Scale in a Karst Area, Southwest China. Sci. Total Environ. 2018, 619–620, 1299–1307. [Google Scholar] [CrossRef]
- Joshi, R.K.; Garkoti, S.C. Influence of Vegetation Types on Soil Physical and Chemical Properties, Microbial Biomass and Stoichiometry in the Central Himalaya. Catena 2023, 222, 106835. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Sun, J.; Ren, C.J.; Kang, D.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Land Use Change Influences Soil C, N and P Stoichiometry under ‘Grain-to-Green Program’ in China. Sci. Rep. 2015, 5, 10195. [Google Scholar] [CrossRef]
- Yang, Z.P.; Baoyin, T.; Li, F.Y. Long-Term Effects of Restoration Measures on Soil C and C: Nutrient Ratios in a Semiarid Steppe. Ecol. Eng. 2020, 153, 105913. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, X.B.; Zhang, S.H.; Lu, H.Y.; Shao, H.B. Soil Nutrient Stoichiometry on Linear Sand Dunes from a Temperate Desert in Central Asia. Catena 2020, 195, 104847. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Xie, J.S.; Lyu, M.K.; Xiong, D.C.; Wang, J.; Chen, Y.M.; Li, Y.; Wang, M.K.; Yang, Y.S. Short-Term Effects of Soil Warming and Nitrogen Addition on the N:P Stoichiometry of Cunninghamia lanceolata in Subtropical Regions. Plant Soil 2017, 411, 395–407. [Google Scholar] [CrossRef]
- Chen, Z.X.; Xu, X.; Wen, Y.L.; Cheng, M.; Wang, X. The Critical Role of Soil Ecological Stoichiometric Ratios: How Does Reforestation Improve Soil Nitrogen and Phosphorus Availability? Plants 2024, 13, 2320. [Google Scholar] [CrossRef]
- Kousar, R.; Lone, A.H.; Shah, Z.A.; Dar, E.A.; Mir, M.S.; Alkeridis, L.A.; Al-Shuraym, L.A.; Ganie, M.A.; Bhat, J.A.; Alshehri, M.A.; et al. Farm-Scale Soil Spatial Variability at a Mountain Research Centre in Northwestern Himalayas. Sci. Rep. 2025, 15, 19705. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M.L. Linkages of C: N: P Stoichiometry between Soil and Leaf and Their Response to Climatic Factors along Altitudinal Gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, Y.W.; Jiang, H.L.; Li, X.; Liu, Y.H. Research Progress of the Effects of Wind Speed Change on Grassland Ecosystem. Acta Ecol. Sin. 2017, 37, 4289–4298. [Google Scholar] [CrossRef]
- Huang, Y.P.; Wang, Q.Q.; Zhang, W.J.; Zhu, P.; Xiao, Q.; Wang, C.J.; Wu, L.; Tian, Y.F.; Xu, M.G.; Gunina, A. Stoichiometric Imbalance of Soil Carbon and Nutrients Drives Microbial Community Structure under Long-Term Fertilization. Appl. Soil Ecol. 2021, 168, 104119. [Google Scholar] [CrossRef]
- Committee of Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1990. [Google Scholar]
- Wróbel, A.; Nobis, M.; Nowak, A. Patterns of the Lemma Micromorphology: A Useful Tool in Taxonomy of the Middle Asian Eragrostis Species (Poaceae). Bot. Lett. 2017, 164, 253–262. [Google Scholar] [CrossRef]
- Li, X.R.; Zhou, H.Y.; Wang, X.P.; Liu, L.C.; Zhang, J.; Chen, G.X.; Zhang, Z.S.; Liu, Y.B.; Tan, H.J.; Guo, Y.H. Ecological Restoration and Recovery in Arid Desert Regions of China: A Review for 60-Year Research Progresses of Shapotou Desert Research and Experiment Station, Chinese Academy of Sciences. J. Desert. Res. 2016, 36, 247–264. [Google Scholar]
- Hutang, G.R.; Tong, Y.; Zhu, X.; Gao, L.Z. Genome Size Variation and Polyploidy Prevalence in the Genus Eragrostis Are Associated with the Global Dispersal in Arid Area. Front. Plant Sci. 2023, 14, 1066925. [Google Scholar] [CrossRef]
- Zhu, F. Chemical Composition and Food Uses of Teff (Eragrostis tef). Food Chem. 2018, 239, 402–415. [Google Scholar] [CrossRef]
- Carloto, B.W.; Buriol, G.A.; Dornelles, S.H.B.; Trivisiol, V.S.; Peripolli, M.; Escobar, O.S. Morphological and Phenological Responses of Eragrostis plana Nees and Eragrostis pilosa (L.) P. Beauv. Plants Subjected to Different Soil Moisture Conditions. Planta Daninha 2019, 37, e019217246. [Google Scholar] [CrossRef]
- Barretto, R.; Buenavista, R.M.; Rivera, J.L.; Wang, S.; Prasad, P.V.; Siliveru, K. Teff (Eragrostis tef) Processing, Utilization and Future Opportunities: A Review. Int. J. Food Sci. Technol. 2021, 56, 3125–3137. [Google Scholar] [CrossRef]
- Habtegebrial, K.; Singh, B.R.; Haile, M. Impact of Tillage and Nitrogen Fertilization on Yield, Nitrogen Use Efficiency of Tef (Eragrostis tef (Zucc.) Trotter) and Soil Properties. Soil Tillage Res. 2007, 94, 55–63. [Google Scholar] [CrossRef]
- Fiorenza, M.; Dotto, D.B.; Boligon, A.A.; Boligon, A.A.; Athayde, M.L.; Vestena, S. Phytochemical analysis and allelopathic activity of Eragrostis plana nees (capim-annoni). Iheringia Ser. Bot. 2016, 71, 193–200. [Google Scholar]
- Favaretto, A.; Cantrell, C.L.; Fronczek, F.R.; Duke, S.O.; Wedge, D.E.; Ali, A.; Scheffer-Basso, S.M. New Phytotoxic Cassane-like Diterpenoids from Eragrostis plana. J. Agric. Food Chem. 2019, 67, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Klein Hendges, A.P.P.; dos Santos, E.F.; Teixeira, S.D.; Santana, F.S.; Trezzi, M.M.; Batista, A.N.L.; Batista, J.M., Jr.; de Lima, V.A.; Marques, F.D.A.; Sales Maia, B.H.L.N. Phytotoxic Neocassane Diterpenes from Eragrostis plana. J. Nat. Prod. 2020, 83, 3511–3518. [Google Scholar] [CrossRef]
- Haddadi, B.S.; Fang, R.; Girija, A.; Kattupalli, D.; Widdowson, E.; Beckmann, M.; Yadav, R.; Mur, L.A.J. Metabolomics Targets Tissue-Specific Responses in Alleviating the Negative Effects of Salinity in Tef (Eragrostis tef) during Germination. Planta 2023, 258, 67. [Google Scholar] [CrossRef]
- Xie, B.; Wang, Q.; Huang, B.; Chen, Y.; Yang, J.; Qi, P. Coordinated State Analysis and Differential Regulation of Territorial Spatial Functions in Underdeveloped Regions: A Case Study of Gansu Province, China. Sustainability 2022, 14, 950. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Publishing House: Beijing, China, 2000. [Google Scholar]
- LY/T 1237-1999; Determination of Organic Matter in Forest Soil and Calculation of Carbon-Nitrogen Ratio. State Forestry Administration: Beijing, China, 1999.
- LY/T 1228-2015; Nitrogen Determination Methods of Forest Soils. State Forestry Administration: Beijing, China, 2015.
- NY/T 88-1988; Method for Determination of Soil Total Phosphorus. Ministry of Agriculture: Beijing, China, 1988.
- LY/T 1232-2015; Phosphorus Determination Methods of Forest Soils. State Forestry Administration: Beijing, China, 2015.
- NY/T 87-1988; Method for Determination of Total Potassium in Soils. Ministry of Agriculture: Beijing, China, 1988.
- LY/T 1234-2015; Potassium Determination Methods of Forest Soils. State Forestry Administration: Beijing, China, 2015.
- Song, S.M.; Zhou, H.Y.; Li, P.X.; Zhang, L.; Su, D.W.; Zheng, D.; Zhang, Z.X.; Luo, Z.Z.; Yu, S.K.; Liu, B.; et al. Carbon, Nitrogen, and Phosphorus Ecological Stoichiometry in Cenchrus fungigraminus and Soil under Waterlogging Stress. Plant Soil 2025. [Google Scholar] [CrossRef]
- Wu, W.; Liu, H.B. Estimation of Soil pH with Geochemical Indices in Forest Soils. PLoS ONE 2019, 14, e0223764. [Google Scholar] [CrossRef]
- Hartemink, A.E.; Barrow, N.J. Soil pH—Nutrient Relationships: The Diagram. Plant Soil 2023, 486, 209–215. [Google Scholar] [CrossRef]
- Liu, R.S.; Wang, D.M. Soil C, N, P and K Stoichiometry Affected by Vegetation Restoration Patterns in the Al-pine Region of the Loess Plateau, Northwest China. PLoS ONE 2020, 15, e0241859. [Google Scholar] [CrossRef]
- Zewd, I.; Siban, M. The Effects of Alkalinity on Physical and Chemical Properties of Soil. J. Plant Biol. Agric. Sci. 2021, 3, 1–5. [Google Scholar]
- Tian, H.Q.; Chen, G.S.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and Variation of C:N:P Ratios in China’s Soils: A Synthesis of Observational Data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xing, X.; Zhang, H.; An, S.S. Soil Ecological Stoichiometry under Different Vegetation Area on Loess Hilly-Gully Region. Acta Ecol. Sin. 2013, 33, 4674–4682. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.H.; Guo, L.P.; Bai, L.; Zhang, J.R.; Chen, Z.H.; Ahmad, S. Variation in Soil Nutrients in Grasslands along the Kunes River in Xinjiang, China. Chem. Ecol. 2015, 31, 111–122. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Ma, Z.W.; Li, L.H. Soil Nutrient Contents and Stoichiometry as Affected by Land-Use in an Agro-Pastoral Region of Northwest China. Catena 2017, 150, 146–153. [Google Scholar] [CrossRef]
- McLauchlan, K. The Nature and Longevity of Agricultural Impacts on Soil Carbon and Nutrients: A Review. Ecosystems 2006, 9, 1364–1382. [Google Scholar] [CrossRef]
- Wang, H.F.; Zhu, X.A.; Zakari, S.; Chen, C.F.; Liu, W.J.; Jiang, X.J. Assessing the Effects of Plant Roots on Soil Water Infiltration Using Dyes and Hydrus-1D. Forests 2022, 13, 1095. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Bore-al Forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Fadeyibi, M.; Sawyerr, H.O.; Opasola, O.A. Enhancing Soil-Derived Bioactive Metabolites for Controlling Staphylococcal Nosocomial Infections through Environmental Interactions. Discov. Appl. Sci. 2025, 7, 615. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Chen, L.J.; Chen, X.H.; Tan, M.L.; Duan, Z.H.; Wu, Z.J.; Li, X.J.; Fan, X.H. Response of Soil Enzyme Activity to Long-Term Restoration of Desertified Land. Catena 2015, 133, 64–70. [Google Scholar] [CrossRef]
- Wang, C.Q.; Kuzyakov, Y. Soil Organic Matter Priming: The PH Effects. Glob. Change Biol. 2024, 30, e17349. [Google Scholar] [CrossRef]
- Yu, Y.H.; Chi, Y.K. Ecological Stoichiometric Characteristics of Soilat Different Depths in a Karst Plateau Mountain Area of China. Pol. J. Environ. Stud. 2019, 29, 969–978. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sui, Y.Y.; Zhang, X.D.; Meng, K.; Herbert, S.J. Spatial Variability of Nutrient Properties in Black Soil of Northeast China. Pedosphere 2007, 17, 19–29. [Google Scholar] [CrossRef]
- Tian, L.M.; Zhao, L.; Wu, X.D.; Fang, H.B.; Zhao, Y.H.; Hu, G.J.; Yue, G.; Sheng, Y.; Wu, J.C.; Chen, J.; et al. Soil Moisture and Texture Primarily Control the Soil Nutrient Stoichiometry across the Tibetan Grassland. Sci. Total Environ. 2018, 622–623, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chang, S.L.; Zhang, Y.T.; Wang, H.J.; Song, C.C.; He, P.; Sun, X.J. Plant and Soil Ecological Stoichiometry with Vertical Zonality on the Northern Slope of the Middle Tianshan Mountains. Acta Ecol. Sin. 2016, 36, 4363–4372. [Google Scholar]
- Exbrayat, J.-F.; Pitman, A.J.; Zhang, Q.; Abramowitz, G.; Wang, Y.-P. Examining Soil Carbon Uncertainty in a Global Model: Response of Microbial Decomposition to Temperature, Moisture and Nutrient Limitation. Biogeosciences 2013, 10, 7095–7108. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Yang, X.; Yao, H.J.; Deng, X.Q.; Wang, Y.Q.; An, S.S.; Kuzyakov, Y.; Chang, S.X. Plant and Soil Elemental C:N:P Ratios Are Linked to Soil Microbial Diversity during Grassland Restoration on the Loess Plateau, China. Sci. Total Environ. 2022, 806, 150557. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Kang, D.; Han, X.H.; Yang, G.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Soil Stoichiometry and Carbon Storage in Long-Term Afforestation Soil Affected by Understory Vegetation Diversity. Ecol. Eng. 2015, 74, 415–422. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P Stoichiometry in Soil: Is There a “Redfield Ratio” for the Microbial Biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, P.; Zhu, T.B.; Li, Q.; Cao, J.H. The Characteristics of Soil C, N, and P Stoichiometric Ratios as Affected by Geological Background in a Karst Graben Area, Southwest China. Forests 2019, 10, 601. [Google Scholar] [CrossRef]
- Long, Y.Y.; Zhang, D.D.; Wu, H.M.; Li, J.S.; Xiong, P.F.; Zhao, G.H.; Liu, H.; Wu, B.R.; Zhang, Z. Patterns and Driving Mechanisms of Soil Organic Carbon, Nitrogen, and Phosphorus, and Their Stoichiometry in Lime-stone Mines of Anhui Province, China. Forests 2024, 15, 1969. [Google Scholar] [CrossRef]
- Shen, A.H.; Zhao, N.; Shi, Y.; Mi, W.; She, J.; Zhang, F.H.; Guo, R.; Wu, T.; Li, Z.G.; Li, J.; et al. Soil Ecological Stoichiometry in Varied Micro-Topographies of an Alluvial Fan at Eastern Helan Mountains, Northwest China. J. Arid Land 2024, 16, 1648–1663. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R. Effects of Planting Caragana Shrubs on Soil Nutrients and Stoichiometries in Desert Steppe of Northwest China. Catena 2019, 183, 104213. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhang, G.; Zhang, B.J. Growth Characteristics of Typical Plant Communities on Gully Slopes in the Loess Hilly-Gully Region. Res. Soil. Water Conserv. 2019, 26, 62–67. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xue, X.; Zhang, Y.; Qin, S.G.; You, Q.G.; Duan, Y.L.; Wang, L.L.; Chen, J.; Liu, J.; Yao, B.; et al. Divergent Mechanisms Driving Nutrient Stoichiometry in Surface and Deep Soils of Desert Ecosystems on the Qinghai–Tibetan Plateau. Catena 2024, 246, 108417. [Google Scholar] [CrossRef]
- Liao, K.H.; Lai, X.; Zhou, Z.W.; Zhu, Q. Applying Fractal Analysis to Detect Spatio-Temporal Variability of Soil Moisture Content on Two Contrasting Land Use Hillslopes. Catena 2017, 157, 163–172. [Google Scholar] [CrossRef]
- Zhu, Q.; Liao, K.H.; Lai, X.; Lv, L.G. Scale-dependent Effects of Environmental Factors on Soil Organic Carbon, Soil Nutrients and Stoichiometry under Two Contrasting Land-use Types. Soil Use Manag. 2021, 37, 243–256. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Kongsurakan, P.; Hatano, R. Assessing Soil Organic Carbon, Soil Nutrients and Soil Erodibility under Terraced Paddy Fields and Upland Rice in Northern Thailand. Agronomy 2022, 12, 537. [Google Scholar] [CrossRef]
- Zhu, F.F.; Lu, X.K.; Liu, L.; Mo, J.M. Phosphate Addition Enhanced Soil Inorganic Nutrients to a Large Extent in Three Tropical Forests. Sci. Rep. 2015, 5, 7923. [Google Scholar] [CrossRef]
- Chen, Y.P.; Li, S.K.; Zeng, L.; An, B.; Xiao, T.Q.; Mao, R.; Zhang, Y. Effects of Mycorrhizal and Extraradical Hyphae of Subtropical Native Tree Species on Soil Enzyme Activities and Their Stoichiometric Ratios. Forests 2023, 14, 2112. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zhao, J.; Xiao, D.; Chen, M.F.; He, X.Y. Higher Colonization but Lower Diversity of Root-Associated Arbuscular Mycorrhizal Fungi in the Topsoil than in Deep Soil. Appl. Soil Ecol. 2024, 194, 105195. [Google Scholar] [CrossRef]
- Maherali, H.; Klironomos, J.N. Influence of Phylogeny on Fungal Community Assembly and Ecosystem Functioning. Science 2007, 316, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Eissenstat, D.M.; Kucharski, J.M.; Zadworny, M.; Adams, T.S.; Koide, R.T. Linking Root Traits to Nutrient Foraging in Arbuscular Mycorrhizal Trees in a Temperate Forest. New Phytol. 2015, 208, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.J.; Qian, Q.; Liang, Y.M.; Wang, K.L.; Zhang, W. Spatial Variations in Fine Root Turnover, Biomass, and Necromass of Two Vegetation Types in a Karst Ecosystem, Southwestern China. Forests 2022, 13, 611. [Google Scholar] [CrossRef]
- Serrano, K.; Bezrutczyk, M.; Goudeau, D.; Dao, T.; O’Malley, R.; Malmstrom, R.R.; Visel, A.; Scheller, H.V.; Cole, B. Spatial Co-Transcriptomics Reveals Discrete Stages of the Arbuscular Mycorrhizal Symbiosis. Nat. Plants 2024, 10, 673–688. [Google Scholar] [CrossRef]
- Wu, X.; Liang, Y.M.; Zhao, W.; Pan, F.J. Root and Mycorrhizal Nutrient Acquisition Strategies in the Succession of Subtropical Forests under N and P Limitation. BMC Plant Biol. 2025, 25, 8. [Google Scholar] [CrossRef] [PubMed]

| Sampling Location | Code | Longitude | Latitude | ALT (m) | MAP (mm) | MAT (°C) | Habitat |
|---|---|---|---|---|---|---|---|
| Lanzhou Anning | AN | 103°41′ E | 36°08′ N | 1535.5 | 294.1 | 19.92 | Edge of a farmland |
| Dingxi Anding | AD | 104°38′ E | 35°56′ N | 1750.1 | 288.3 | 16.50 | Side of the highway |
| Lanzhou Yuzhong | YZ | 103°58′ E | 36°00′ N | 1733.3 | 119.8 | 16.17 | Under the Shrubs |
| Baiyin Huining | HN | 104°46′ E | 36°04′ N | 1623.1 | 207.0 | 18.25 | The apple orchard |
| Tianshui Maiji | MJ | 105°51′ E | 34°34′ N | 1084.5 | 212.1 | 25.08 | Roadside |
| Longnan Wudu | WD | 104°54′ E | 33°24′ N | 1008.2 | 669.9 | 26.25 | Edge of a farmland |
| Longnan Xihe | XH | 105°19′ E | 34°02′ N | 1528.9 | 531.0 | 26.92 | The cherry orchard |
| Pingliang Kongtong | KT | 106°43′ E | 35°33′ N | 1339.8 | 561.6 | 20.17 | Roadside |
| Parameters | Minimum | Maximum | Mean | Standard Deviation | Coefficient of Variation (%) |
|---|---|---|---|---|---|
| pH | 8.10 | 9.88 | 8.73 | 0.38 | 4.32 |
| OM (g kg−1) | 6.32 | 25.37 | 14.93 | 5.28 | 35.33 |
| TN (g kg−1) | 0.60 | 3.65 | 1.39 | 0.65 | 46.90 |
| AN (mg kg−1) | 49.47 | 282.25 | 111.97 | 54.88 | 49.01 |
| TP (g kg−1) | 0.42 | 1.40 | 0.76 | 0.28 | 37.10 |
| AP (mg kg−1) | 0.90 | 13.29 | 5.05 | 3.20 | 63.33 |
| TK (g kg−1) | 8.56 | 19.26 | 13.34 | 2.45 | 18.40 |
| AK (mg kg−1) | 47.00 | 410.00 | 204.94 | 121.69 | 59.38 |
| C:N ratio | 3.17 | 15.82 | 6.90 | 3.00 | 43.40 |
| C:P ratio | 5.18 | 20.74 | 12.02 | 4.09 | 34.02 |
| N:P ratio | 0.68 | 3.28 | 1.92 | 0.73 | 38.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Bai, X.; Wang, H.; Ran, F.; Ruan, Q.; Sadiq, M.; Ding, S. Spatial Distribution Characteristics of Soil Nutrients and Stoichiometric Ratios in Eragrostis minor Distribution Areas of Gansu Province, Northwestern China. Agronomy 2025, 15, 1996. https://doi.org/10.3390/agronomy15081996
Hu S, Bai X, Wang H, Ran F, Ruan Q, Sadiq M, Ding S. Spatial Distribution Characteristics of Soil Nutrients and Stoichiometric Ratios in Eragrostis minor Distribution Areas of Gansu Province, Northwestern China. Agronomy. 2025; 15(8):1996. https://doi.org/10.3390/agronomy15081996
Chicago/Turabian StyleHu, Shuiqin, Xiaoming Bai, Hanrui Wang, Fu Ran, Qian Ruan, Mahran Sadiq, and Siyuan Ding. 2025. "Spatial Distribution Characteristics of Soil Nutrients and Stoichiometric Ratios in Eragrostis minor Distribution Areas of Gansu Province, Northwestern China" Agronomy 15, no. 8: 1996. https://doi.org/10.3390/agronomy15081996
APA StyleHu, S., Bai, X., Wang, H., Ran, F., Ruan, Q., Sadiq, M., & Ding, S. (2025). Spatial Distribution Characteristics of Soil Nutrients and Stoichiometric Ratios in Eragrostis minor Distribution Areas of Gansu Province, Northwestern China. Agronomy, 15(8), 1996. https://doi.org/10.3390/agronomy15081996





