Stage-Specific Light Intensity Optimization for Yield and Energy Efficiency in Plant Factory Potato Pre-Basic Seed Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Growth Parameters and Energy Efficiency
2.3. Photosynthetic Parameters
2.4. Measurement of Photosynthates
2.5. Carbon Metabolism Enzymes and Relative Expression Level of StSP6A
2.6. Statistical Analyses
3. Results
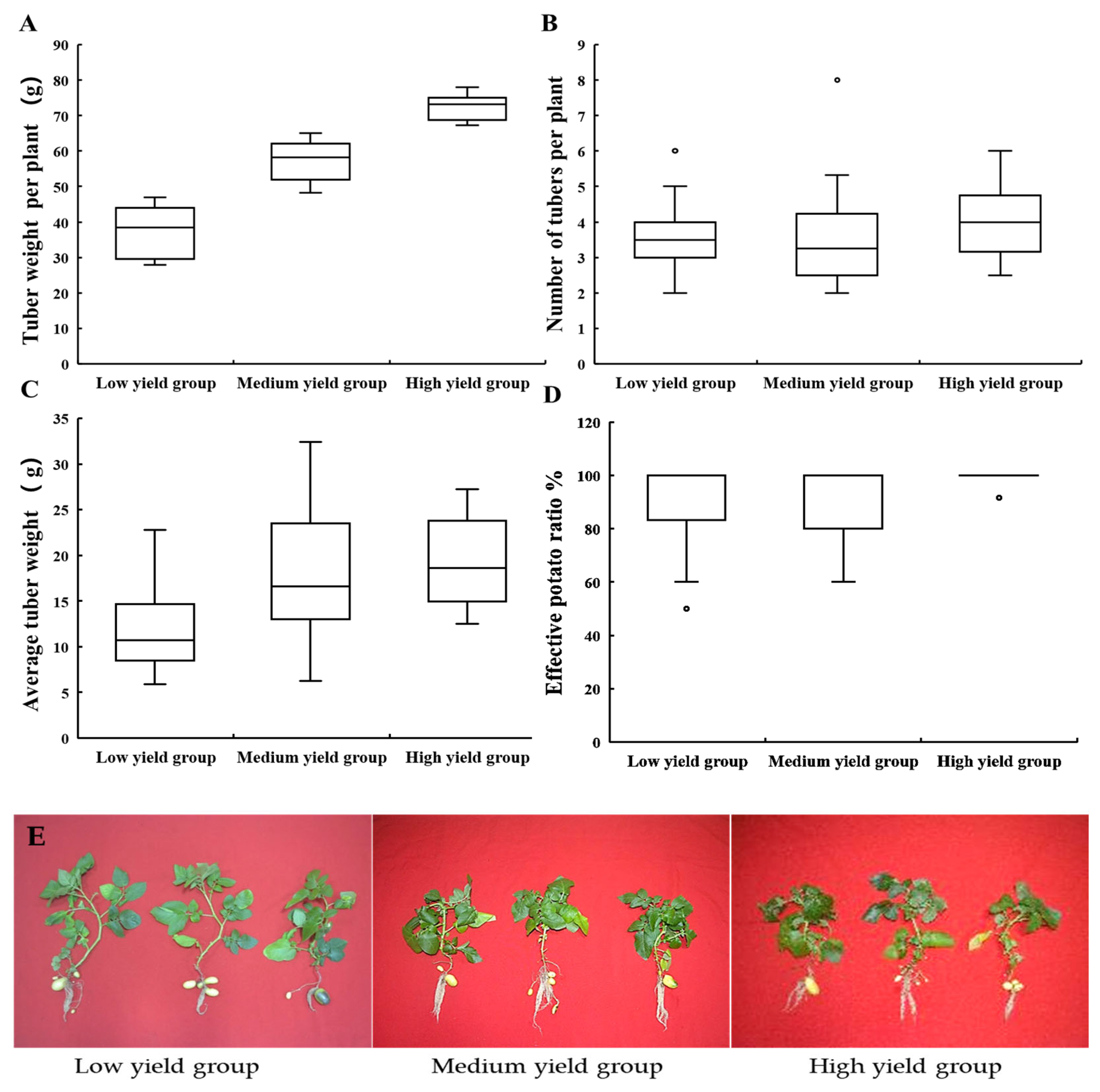
3.1. Potato Among Different Yield Groups
3.1.1. Yield Traits
3.1.2. Pearson Correlation Analysis Between Yield Traits and Light Intensity
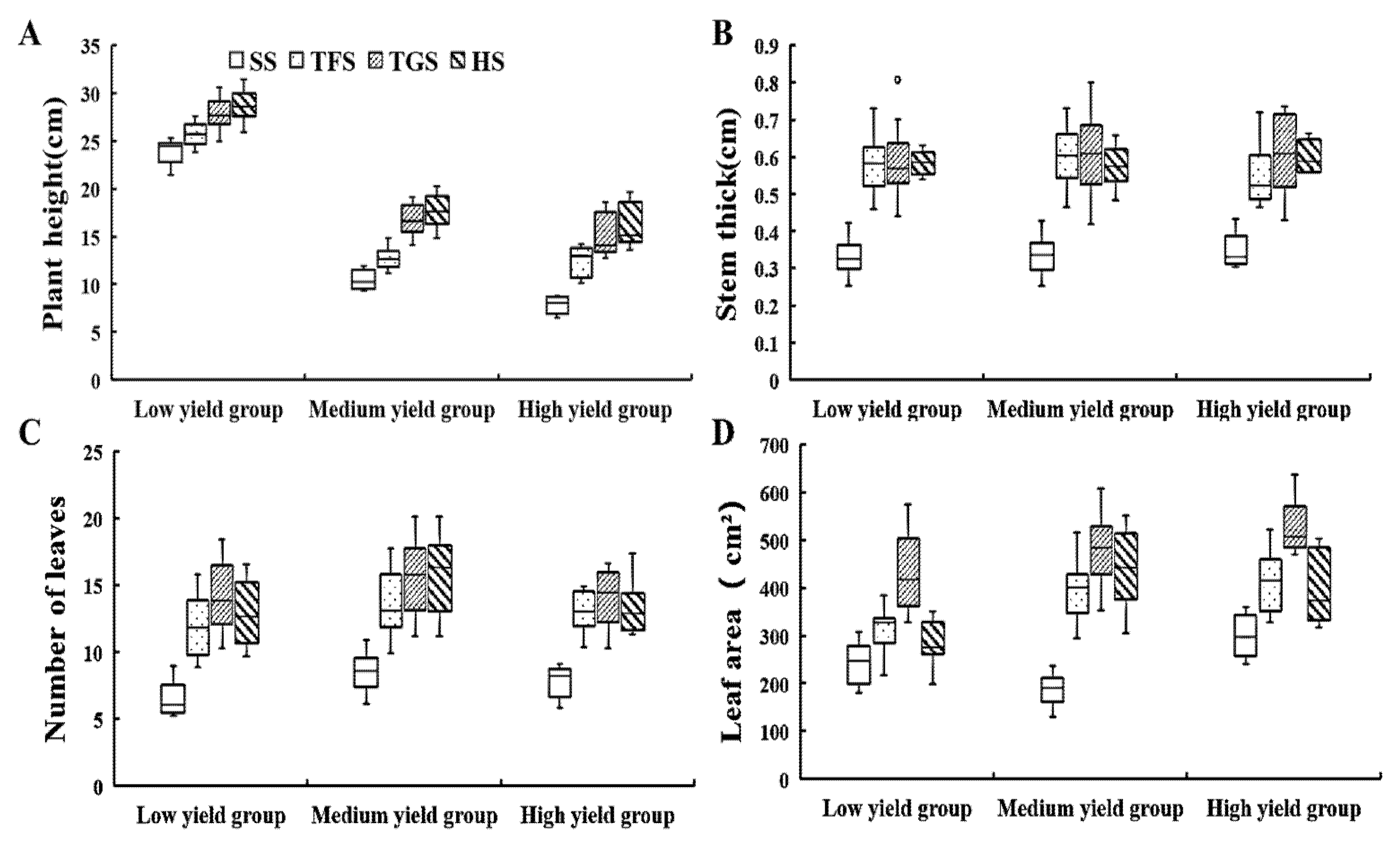
3.1.3. Growth Parameters
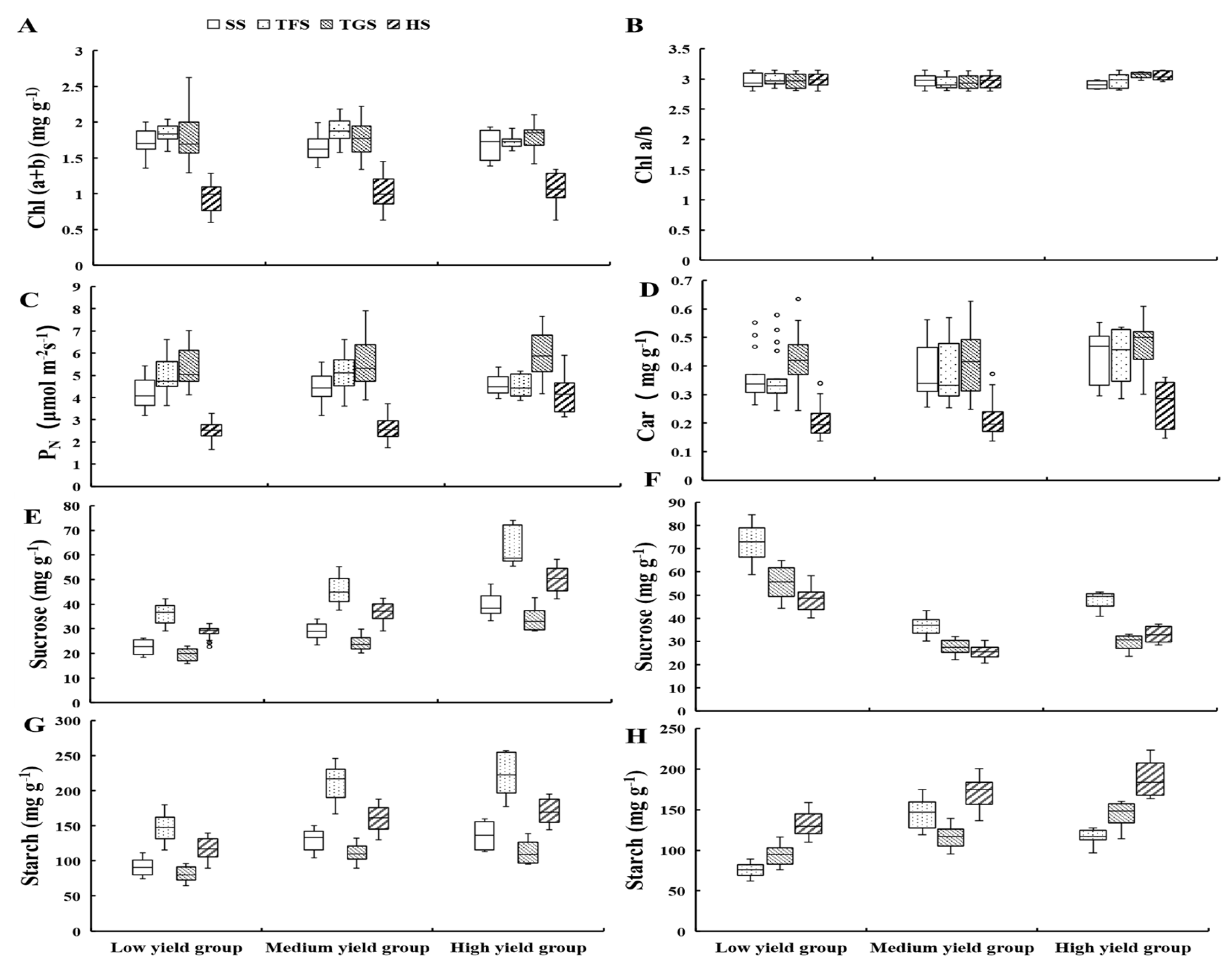
3.1.4. Photosynthetic Characteristics
3.2. Typical Treatment
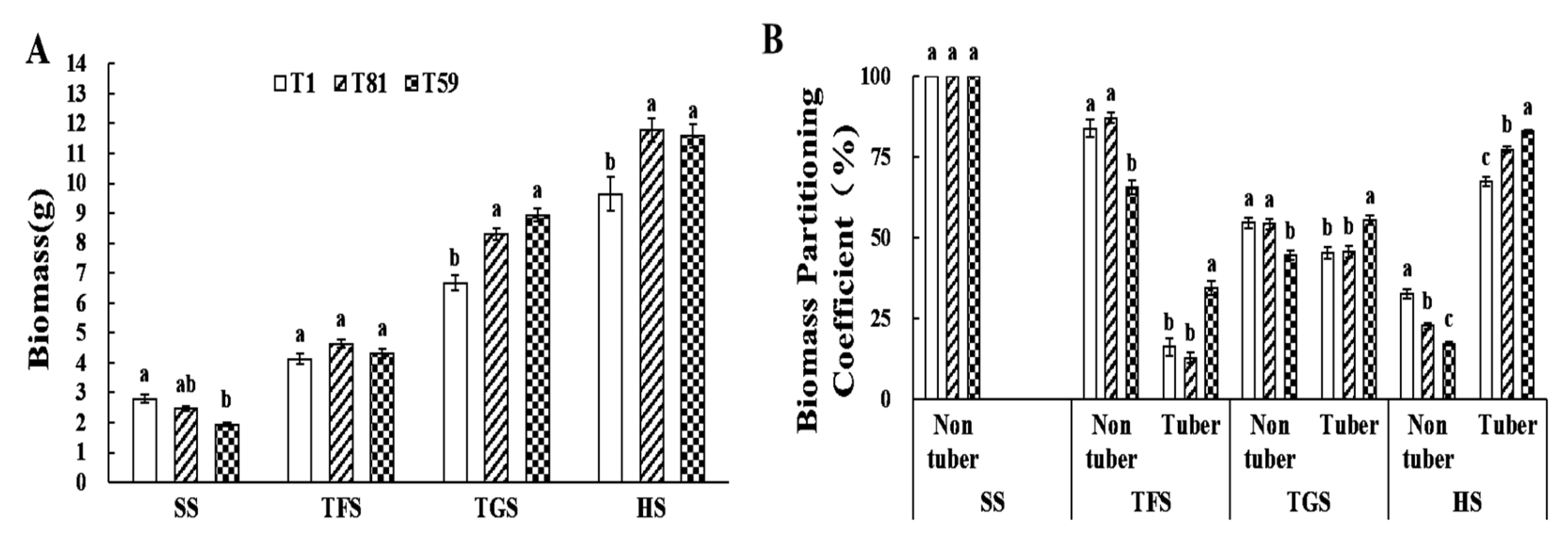
3.2.1. Biomass and Yield Traits
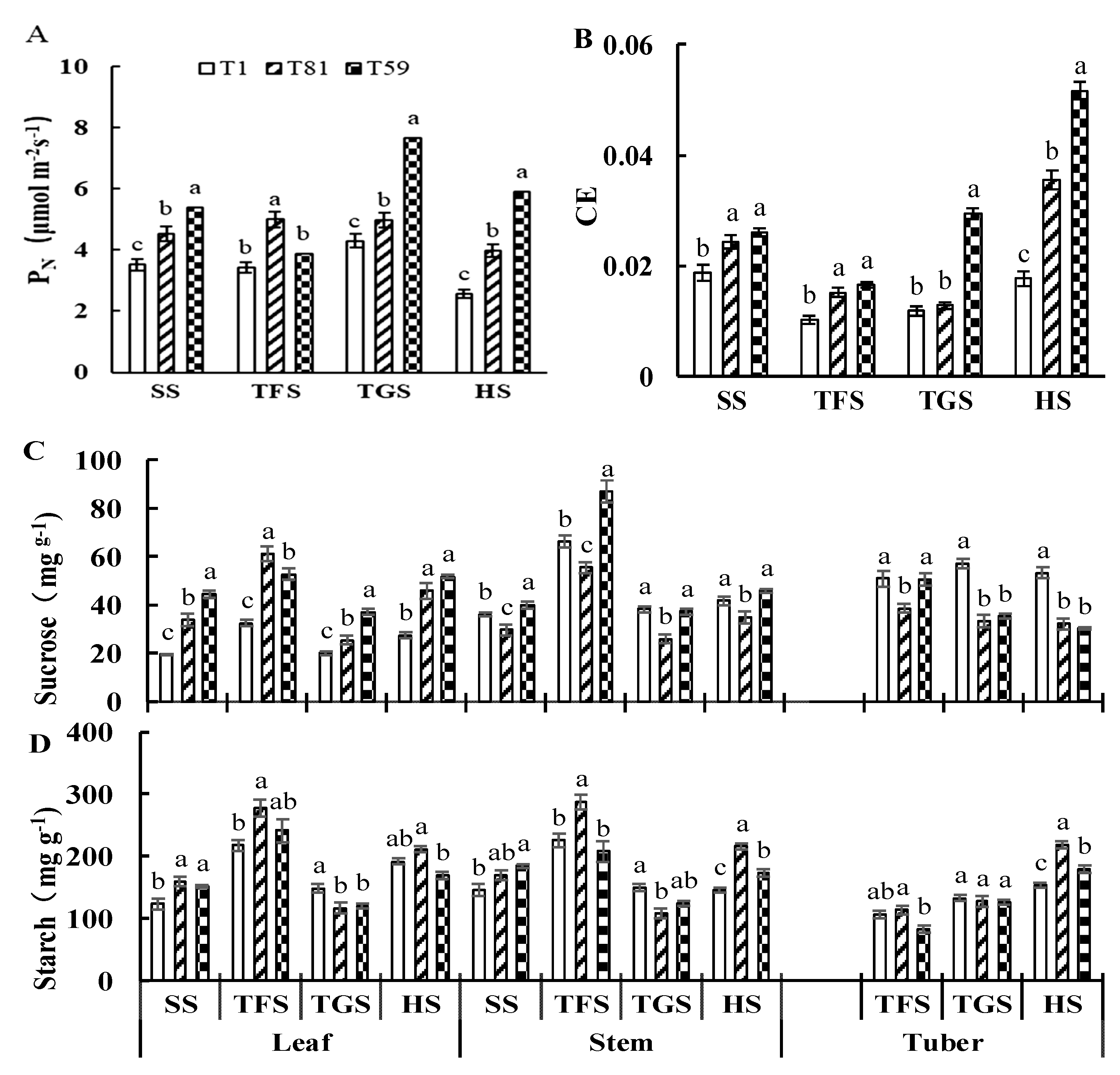
3.2.2. Typical Treatment Photosynthetic Characteristics
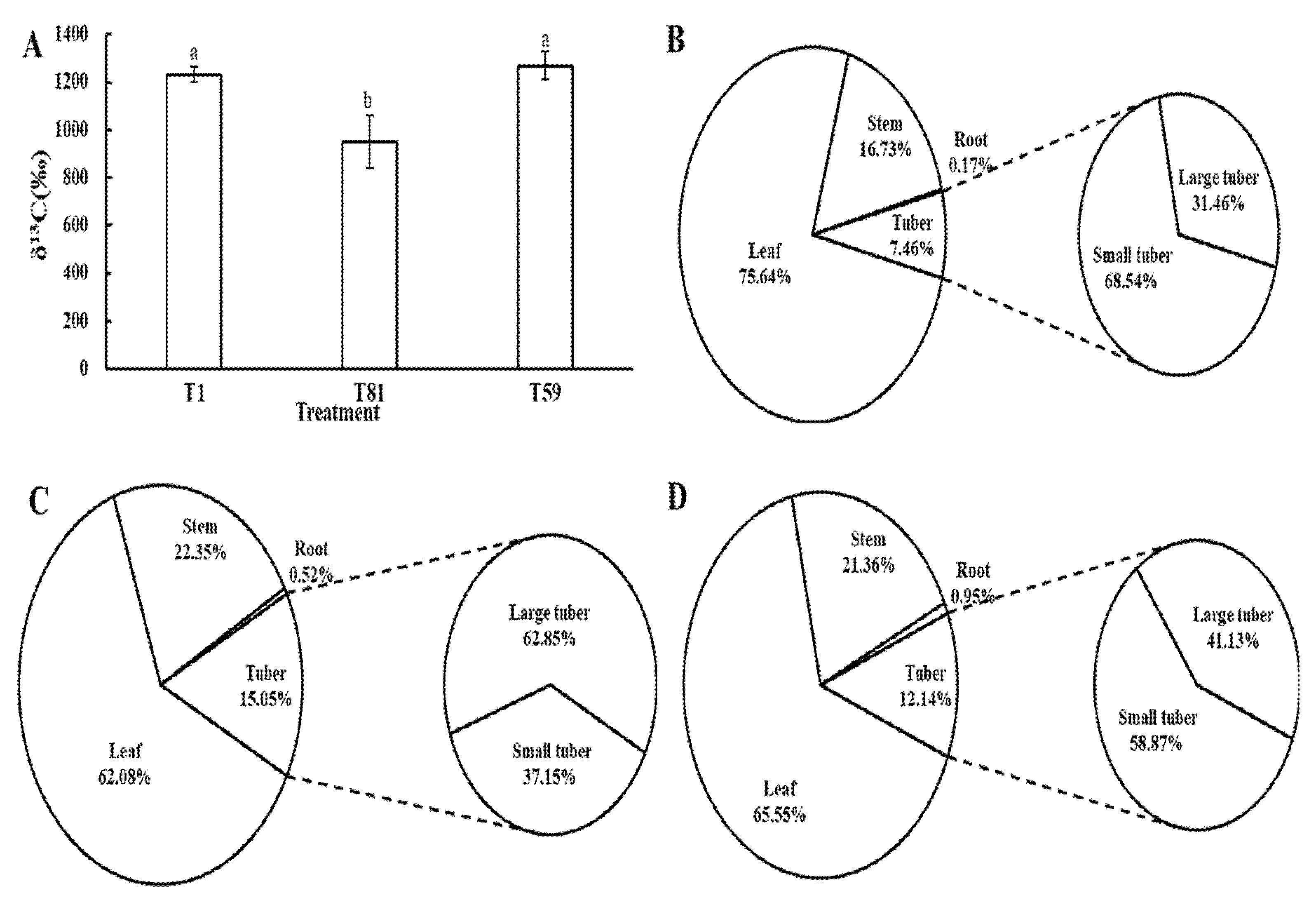
3.2.3. 13C Metabolism of Potato Plants at the Tuber Formation Stage
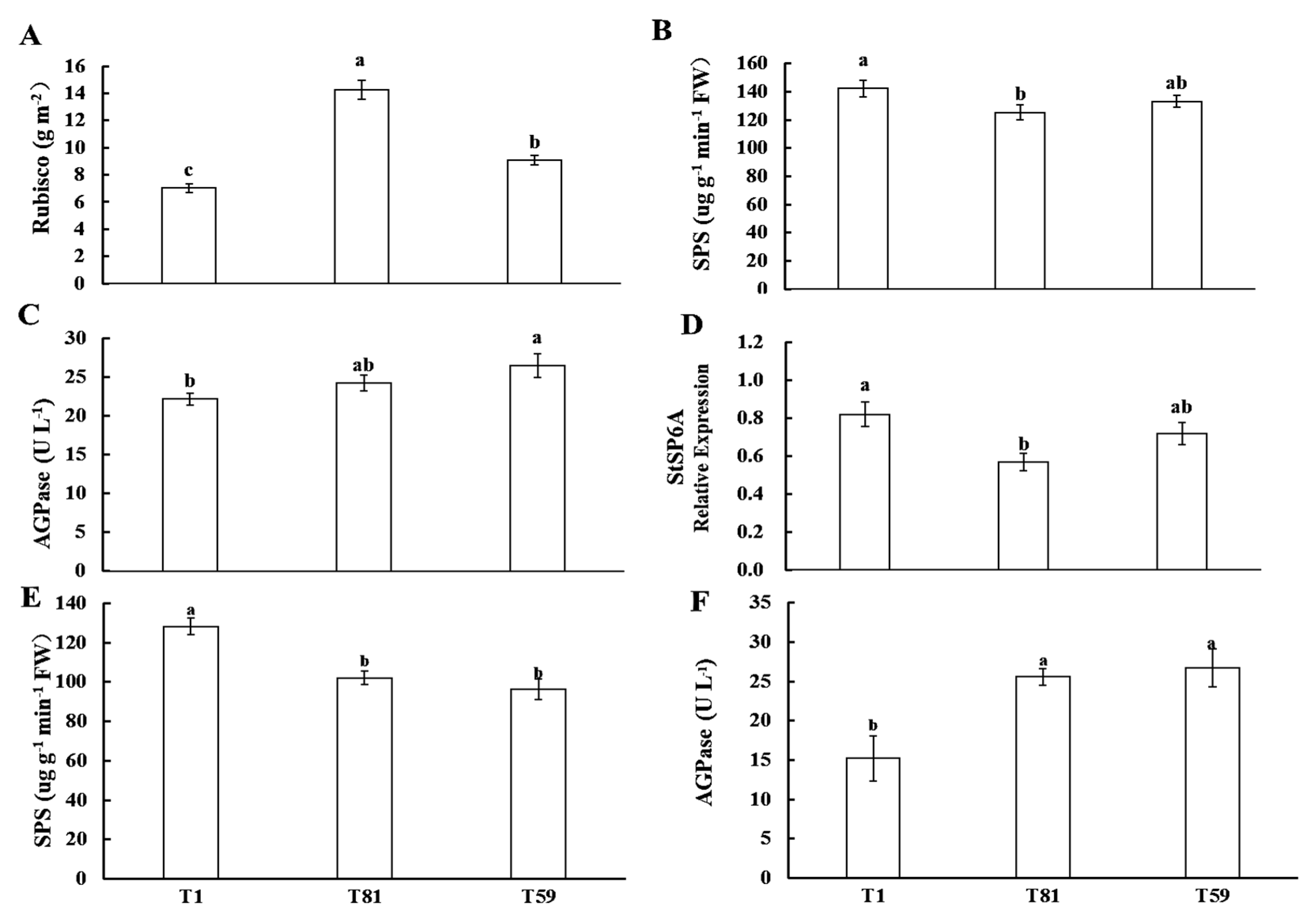
3.2.4. Carbon Metabolism Enzymes and Related Genes at the Tuber Formation Stage
3.2.5. Fluorescence Parameters at the Tuber Formation Stage and Tuber Growth Stage
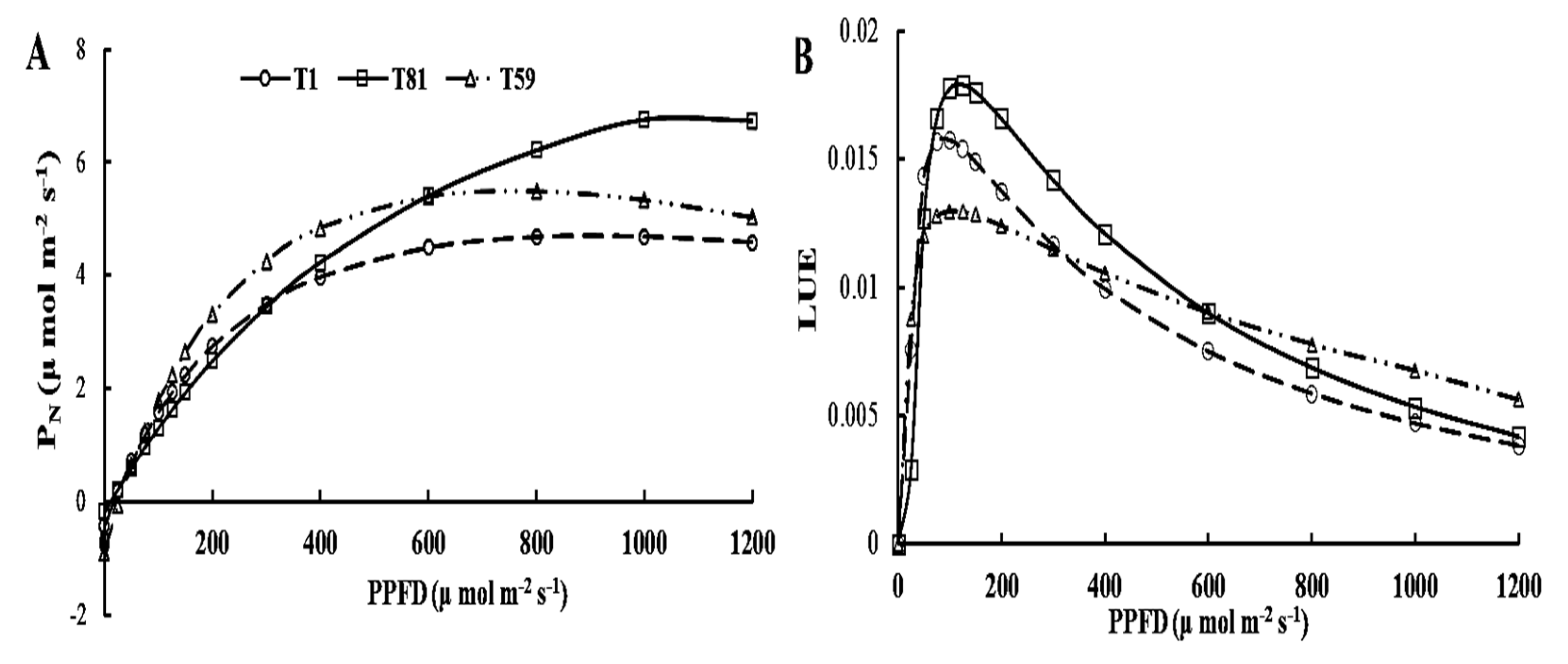
3.2.6. Light Response Curve at Tuber Formation Stage
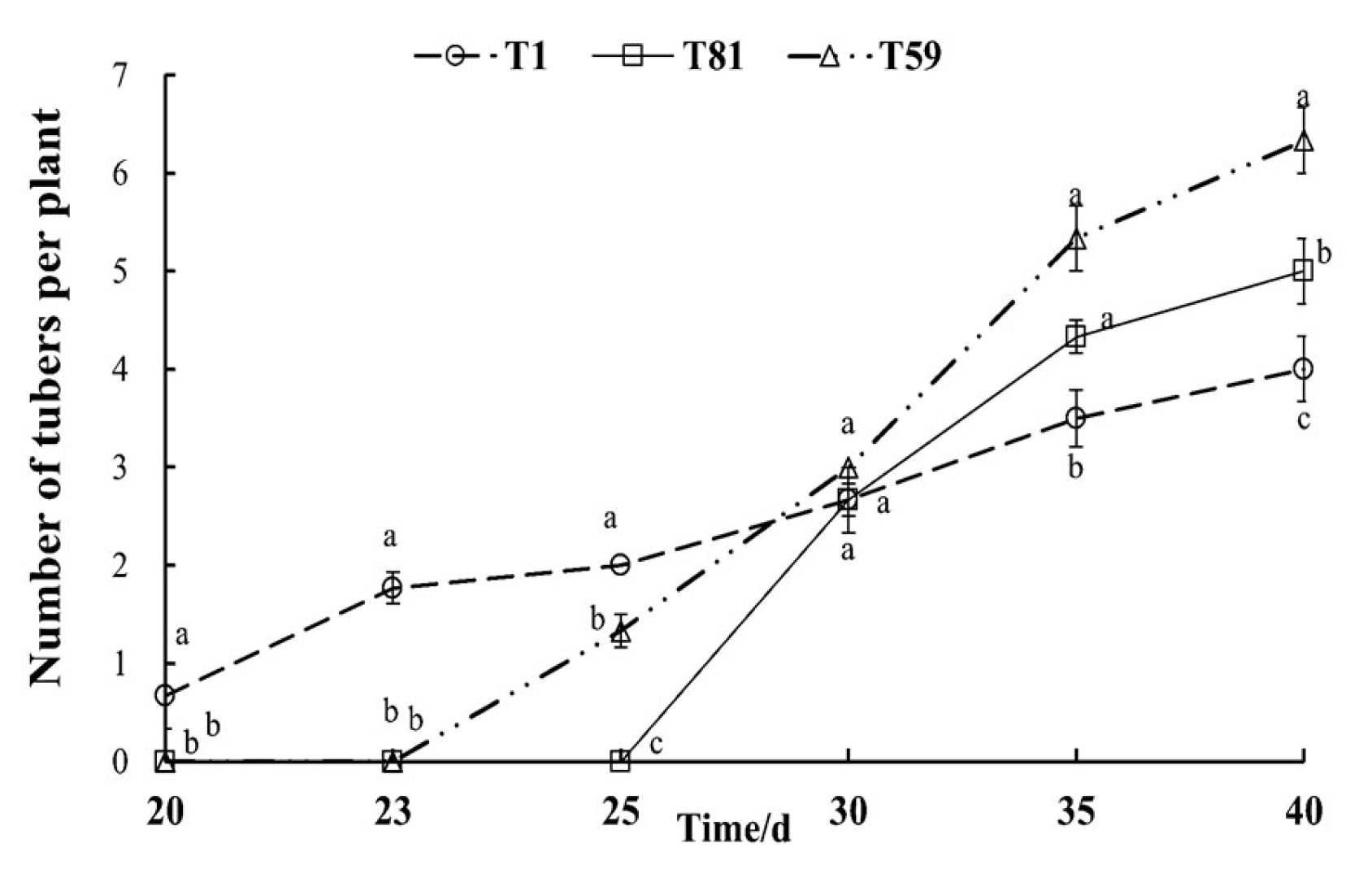
3.2.7. Tuber Development at Tuber Formation Stage
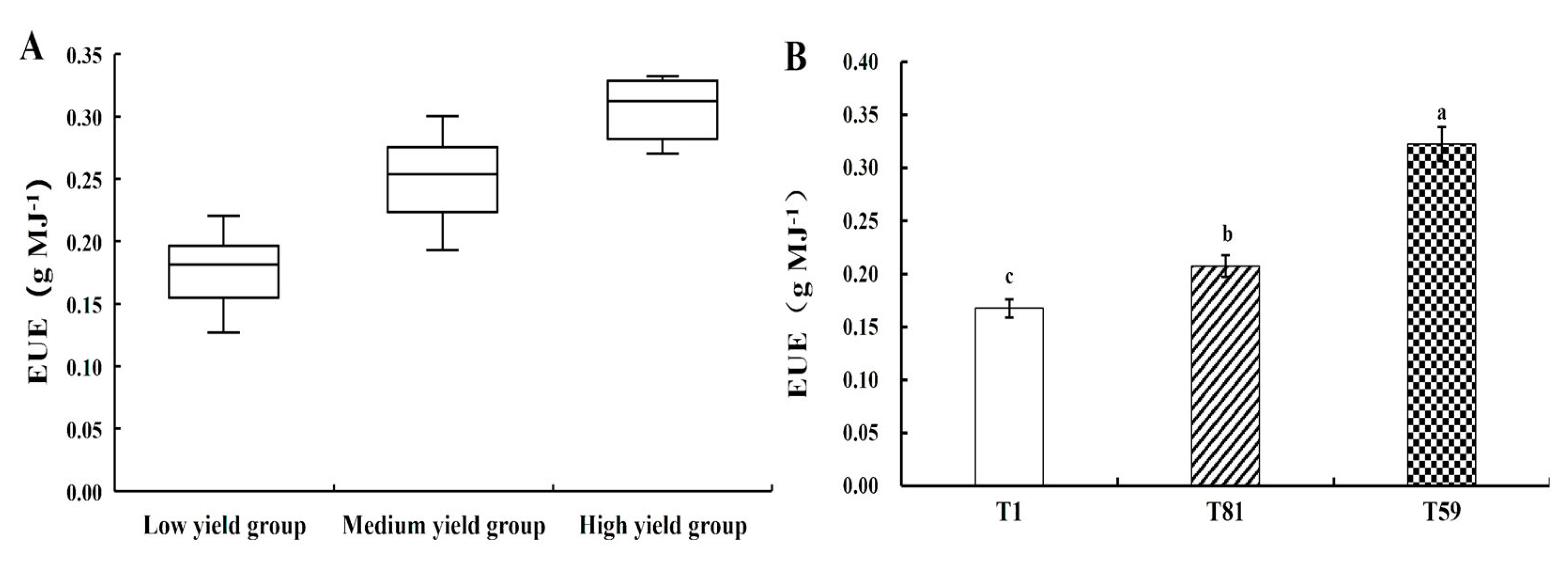
3.3. EUE
4. Discussion
4.1. Stage-Specific Light Intensity Modulation Enhances Potato Yield by Coordinating Photosynthetic Efficiency and Source-Sink Dynamics
4.2. Precision Regulation of Light Intensity on the Mechanism of Potato Tuber Formation
4.3. Energy Efficiency Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
- Enzyme Extraction.
- Sucrose Phosphate Synthase (SPS) Activity Assay.
- ADP-glucose pyrophosphorylase (AGPase) Activity Assay.
- Rubisco Activity Assay.
- StSP6A relative expression Assay.
References
- Chen, S.; Wang, D.; Lin, J.; Xu, Z. The Comprehensive Regulation of Light Intensity and Photoperiod on Growth and Yield of Virus-Free Potato Under the Same Daily Light Integral. Agronomy 2025, 15, 898. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Cruz-Oró, E.; Franco-Zorrilla, J.M.; Prat, S. Potato StCONSTANS-like1 suppresses storage organ formation by directly activating the FT-like StSP5G repressor. Curr. Biol. 2016, 26, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Pu, M.; Li, J.; Xu, Z.G.; Gan, L. Potato Tuber Growth and Yield Under Red and Blue LEDs in Plant Factories. J. Plant Growth Regul. 2021, 41, 40–51. [Google Scholar] [CrossRef]
- Xie, X.; Cheng, H.; Hou, C.; Ren, M. Integration of Light and Auxin Signaling in Shade Plants: From Mechanisms to Opportunities in Urban Agriculture. Int. J. Mol. Sci. 2022, 23, 3422. [Google Scholar] [CrossRef]
- Ma, L.; Li, G. Auxin-Dependent Cell Elongation During the Shade Avoidance Response. Front. Plant Sci. 2019, 10, 914. [Google Scholar] [CrossRef]
- Guo, X.; Wang, D.; Shakeel, M. Transcriptome analysis reveals light-induced anthocyanin synthesis candidate genes in rabbiteye blueberry (Vaccinium ashei: Reade). Biotechnol. Biotechnol. Equip. 2021, 35, 746–757. [Google Scholar] [CrossRef]
- Miki, S.; Wada, K.C.; Takeno, K. A possible role of an anthocyanin filter in low-intensity light stress-induced flowering in Perilla frutescens var. crispa. J. Plant Physiol. 2015, 175, 157–162. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Heuvelink, E.; Prinzenberg, A.E.; Marcelis, L.F. Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Sci. Rep. 2016, 6, 31252. [Google Scholar] [CrossRef]
- Yang, F.; Feng, L.; Liu, Q.; Wu, X.; Fan, Y.; Raza, M.A.; Cheng, Y.; Chen, J.; Wang, X.; Yong, T.; et al. Effect of Interactions between Light Intensity and Red-to- Far-Red Ratio on the Photosynthesis of Soybean Leaves under Shade Condition. Environ. Exp. Bot. 2018, 150, 79–87. [Google Scholar] [CrossRef]
- Sekhar, S.; Panda, D.; Kumar, J.; Mohanty, N.; Biswal, M.; Baig, M.J.; Kumar, A.; Umakanta, N.; Samantaray, S.; Pradhan, S.K.; et al. Comparative Transcriptome Profiling of Low Light Tolerant and Sensitive Rice Varieties Induced by Low Light Stress at Active Tillering Stage. Sci. Rep. 2019, 9, 5753. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C. Spectral effects of artiffcial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- d’aquino, L.; Cozzolino, R.; Malorni, L.; Bodhuin, T.; Gambale, E.; Sighicelli, M.; Della Mura, B.; Matarazzo, C.; Piacente, S.; Montoro, P.; et al. Light Flux Density and Photoperiod Affect Growth and Secondary Metabolism in Fully Expanded Basil Plants. Foods 2024, 13, 2273. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Tan, T.; Zhang, X.; Guo, X.; Zhu, Y.; Song, Z.; Wang, D. Effects of Light Intensity on Endogenous Hormones and Key Enzyme Activities of Anthocyanin Synthesis in Blueberry Leaves. Horticulturae 2023, 9, 618. [Google Scholar] [CrossRef]
- Yamori, W.; Sakata, N.; Suzuki, Y.; Shikanai, T.; Makino, A. Cyclic Electron Flow around Photosystem I via Chloroplast NAD(P)H Dehydrogenase (NDH) Complex Performs a Significant Physiological Role during Photosynthesis and Plant Growth at Low Temperature in Rice. Plant J. 2011, 68, 966–976. [Google Scholar] [CrossRef]
- Yamori, W.; Nagai, T.; Makino, A. The Rate-Limiting Step for CO2 Assimilation at Different Temperatures Is Influenced by the Leaf Nitrogen Content in Several C3 Crop Species. Plant Cell Environ. 2011, 34, 764–777. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T.L. A Meta-Analysis of Plant Responses to Light Intensity for 70 Traits Ranging from Molecules to Whole Plant Performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F.M. Light Regulates Ascorbate in Plants: An Integrated View on Physiology and Biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional Quality, Mineral and Antioxidant Content in Lettuce Affected by Interaction of Light Intensity and Nutrient Solution Concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Liu, W. LED Red and Blue Light Intensity Affected Productivity and Nitrogen Metabolisms of Ryegrass. J. Plant Nutr. 2023, 47, 705–717. [Google Scholar] [CrossRef]
- Lee, R.J.; Bhandari, S.R.; Lee, G.; Lee, J.G. Optimization of Temperature and Light, and Cultivar Selection for the Production of High-Quality Head Lettuce in a Closed-Type Plant Factory. Hortic. Environ. Biotechnol. 2019, 60, 207–216. [Google Scholar] [CrossRef]
- Rahman, M.; Vasiliev, M.; Alameh, K. LED Illumination Spectrum Manipulation for Increasing the Yield of Sweet Basil (Ocimum basilicum L.). Plants 2021, 10, 344. [Google Scholar] [CrossRef]
- Fan, X.; Zang, J.; Xu, Z.; Guo, S.; Jiao, X.; Liu, X.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Ye, Z.; Suggett, D.J.; Robakowski, P.; Kang, H. A mechanistic model for the photosynthesis–light response based on the photosynthetic electron transport of photosystem II in C3 and C4 species. New Phytol. 2013, 199, 110–120. [Google Scholar] [CrossRef]
- He, W.; Miao, C.; You, J.; Gan, L.; Xu, Z.-G. Effects of Red and Blue Light with Supplemental White Light on Growth, Carbohydrate Metabolism, and Yield of Virus-Free Potato in Plant Factories. Am. J. Potato Res. 2020, 97, 554–564. [Google Scholar] [CrossRef]
- Cai, X.A.; Sun, G.C.; Zhao, P. High nitrogen and low light intensity limit photosynthetic energy utilization in successional tree species of south subtropical forests. Sci. China Ser. C Life Sci. 2008, 51, 464–474. [Google Scholar] [CrossRef]
- Alan, M.J. Using light to improve commercial value. Hortic. Res. 2018, 5, 47. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, X.; Guo, K.; Zheng, M.; Cao, T.; Yang, Y.; Song, J.; Cen, J.; Zhang, J.; Jiang, Y.; et al. A rapid aureochrome opto-switch enables diatom acclimation to dynamic light. Nat. Commun. 2024, 15, 5578. [Google Scholar] [CrossRef] [PubMed]
- Nestby, R.; Trandem, N. LED light in primocane raspberries grown in polytunnel at high latitudes. Acta Hortic. 2016, 1117, 149–156. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, L.; He, Z. Understanding the regulation of cereal grain filling: The way forward. J. Integr. Plant Biol. 2023, 65, 526–547. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, K.; Na, T.; Ye, G.; Han, S.; Wang, J. Transcriptomic profiles reveal hormonal regulation of sugar-induced stolon initiation in potato. Sci. Rep. 2025, 15, 19122. [Google Scholar] [CrossRef] [PubMed]
- Chincinska, I.A.; Liesche, J.; Krügel, U.; Michalska, J.; Geigenberger, P.; Grimm, B.; Kühn, C. Sucrose transporter StSUT4from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol. 2008, 146, 515–528. [Google Scholar] [CrossRef]
- Ou, Y.; Song, B.; Liu, X.; Xie, C.; Li, M.; Lin, Y. Promoter regions of potato vacuolar invertase gene in response to sugars and hormones. Plant Physiol. Biochem. 2013, 69, 9–16. [Google Scholar] [CrossRef]
- Kim, Y.U.; Seo, B.S.; Choi, D.H.; Ban, H.Y.; Lee, B.W. Impact of high temperatures on the marketable tuber yield and related traits of potato. Eur. J. Agron. 2017, 89, 46–52. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Gan, X.; Qian, Y.; Wu, X.; Yang, Q. Assessing nutritional status of Festuca arundinacea by monitoring photosynthetic pigments from hyperspectral data. Comput. Electron. Agric. 2009, 70, 52–59. [Google Scholar] [CrossRef]
- Rong, L.; An, J.; Chen, X.; Wang, C.; Wu, J.; Wang, P.; Zheng, Y.; Wang, X.; Chai, X.; Li, W.; et al. LTD Coordinates Chlorophyll Biosynthesis and Light-Harvesting Chlorophyll A/B-Binding Protein Transport. Plant Cell 2025, 37, 068. [Google Scholar] [CrossRef]
- Xiao, X.; Duan, B.; Huang, F.; Zhi, X.; Jiang, Z.; Ma, N. Analysis of canopy light utilization efficiency in high-yielding rapeseed varieties. Sci. Rep. 2024, 14, 31243. [Google Scholar] [CrossRef]
- Wang, W.; Cui, W.; Xu, K.; Gao, H.; Wei, H.; Zhang, H. Effects of Early- and Late-Sowing on Starch Accumulation and Associated Enzyme Activities During Grain Filling Stage in Rice. Rice Sci. 2021, 28, 191–199. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I.A. Supplemental Light-Emitting Diode Inter-Lighting Increases Tomato Fruit Growth Through Enhanced Photosynthetic Light Use Efficiency and Modulated Root Activity. Front. Plant Sci. 2019, 10, 1656. [Google Scholar] [CrossRef]
- Stockem, J.E.; Bus, M.D.; de Vries, M.E.; Struik, P.C. Opening Eyes on Seedling Tuber Quality in Potato: Size Matters. Potato Res. 2024, 67, 1691–1715. [Google Scholar] [CrossRef]
- Guo, R.; Jin, Y.; Liu, J.; Yang, H.; Cheng, L.; Yu, B. Harnessing Light Quality for Potato Production: Red and Blue Light as Key Regulators of Growth and Yield. Plants 2025, 14, 1039. [Google Scholar] [CrossRef] [PubMed]
- Agbodjato, A.N.; Babalola, O.O. Promoting sustainable agriculture by exploiting plant growth-promoting rhizobacteria (PGPR) to improve maize and cowpea crops. PeerJ 2024, 12, e16836. [Google Scholar] [CrossRef]
- Edlinger, A.; Garland, G.; Hartman, K.; Banerjee, S.; Degrune, F.; García-Palacios, P.; Hallin, S.; Valzano-Held, A.; Herzog, C.; Jansa, J.; et al. Agricultural management and pesticide use reduce the functioning of beneficial plant symbionts. Nat. Ecol. Evol. 2022, 6, 1145. [Google Scholar] [CrossRef]
- van Dijk, L.C.; de Vries, M.E.; Lommen, W.J.; Struik, P.C. Transplanting Hybrid Potato Seedlings at Increased Densities Enhances Tuber Yield and Shifts Tuber-Size Distributions. Potato Res. 2021, 65, 307–331. [Google Scholar] [CrossRef]
- Aliche, E.B.; Oortwijn, M.; Theeuwen, T.P.J.M.; Bachem, C.W.B.; van Eck, H.J.; Visser, R.G.F.; van der Linden, C.G. Genetic mapping of tuber size distribution and marketable tuber yield under drought stress in potatoes. Euphytica 2019, 215, 186. [Google Scholar] [CrossRef]
- Lincoin, T.; Eduardo, Z. Plant Physiology; Science Press: Beijing, China, 2015; 163p. [Google Scholar]
- Abelenda, J.A.; Bergonzi, S.; Oortwijn, M.; Sonnewald, S.; Du, M.; Visser, R.G.; Sonnewald, U.; Bachem, C.W. Source-sink regulation is mediated by interaction of an FT homolog with a SWEET protein in potato. Curr. Biol. 2019, 29, 1178–1186. [Google Scholar] [CrossRef]
- Bao, X.; Zhu, Y.; Li, G.; Liu, L. Regulation of storage organ formation by long-distance tuberigen signals in potato. Hortic. Res. 2025, 12, uhae360. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wu, S.Q. Influence of sugar metabolism on the dialogue between arbuscular mycorrhizal fungi and plants. Hortic. Adv. 2023, 1, 2. [Google Scholar] [CrossRef]
- Daiva, J.; Grazina, K. Influence of Different Intensities of Tillage on Physiological Characteristics and Productivity of Crop-Rotation Plants. Plants 2022, 11, 3107. [Google Scholar] [CrossRef]
- Taki, M.; Rohani, A.; Rahmati-Joneidabad, M. Solar thermal simulation and applications in greenhouse. Inf. Process. Agric. 2018, 5, 83–113. [Google Scholar] [CrossRef]
- Pannico, A.; Arouna, N.; Fusco, G.M.; Santoro, P.; Caporale, A.G.; Nicastro, R.; Pagliaro, L.; De Pascale, S.; Paradiso, R. Enhancing tuber yield and nutraceutical quality of potato by supplementing sunlight with LED red-blue light. Front. Plant Sci. 2025, 16, 1517074. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Role of the plant factory with artiffcial lighting (PFAL) in Urban Areas. In Plant Factory; Academic Press: Cambridge, MA, USA, 2016; Chapter 2; pp. 7–34. [Google Scholar]
| Light Intensity (μmol m−2 s−1) | Cluster | Light Intensity (μmol m−2 s−1) | Cluster | Light Intensity (μmol m−2 s−1) | Cluster | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | SS | TFS | TGS | HS | Treatment | SS | TFS | TGS | HS | Treatment | SS | TFS | TGS | HS | |||
| T1 | 200 | 200 | 200 | 200 | 1 | T28 | 300 | 200 | 200 | 200 | 2 | T55 | 400 | 200 | 200 | 200 | 1 |
| T2 | 200 | 200 | 200 | 300 | 1 | T29 | 300 | 200 | 200 | 300 | 2 | T56 | 400 | 200 | 200 | 300 | 1 |
| T3 | 200 | 200 | 200 | 400 | 1 | T30 | 300 | 200 | 200 | 400 | 2 | T57 | 400 | 200 | 200 | 400 | 1 |
| T4 | 200 | 200 | 300 | 200 | 1 | T31 | 300 | 200 | 300 | 200 | 2 | T58 | 400 | 200 | 300 | 200 | 1 |
| T5 | 200 | 200 | 300 | 300 | 1 | T32 | 300 | 200 | 300 | 300 | 2 | T59 | 400 | 200 | 300 | 300 | 3 |
| T6 | 200 | 200 | 300 | 400 | 1 | T33 | 300 | 200 | 300 | 400 | 3 | T60 | 400 | 200 | 300 | 400 | 2 |
| T7 | 200 | 200 | 400 | 200 | 1 | T34 | 300 | 200 | 400 | 200 | 2 | T61 | 400 | 200 | 400 | 200 | 2 |
| T8 | 200 | 200 | 400 | 300 | 2 | T35 | 300 | 200 | 400 | 300 | 2 | T62 | 400 | 200 | 400 | 300 | 2 |
| T9 | 200 | 200 | 400 | 400 | 1 | T36 | 300 | 200 | 400 | 400 | 2 | T63 | 400 | 200 | 400 | 400 | 2 |
| T10 | 200 | 300 | 200 | 200 | 2 | T37 | 300 | 300 | 200 | 200 | 1 | T64 | 400 | 300 | 200 | 200 | 2 |
| T11 | 200 | 300 | 200 | 300 | 2 | T38 | 300 | 300 | 200 | 300 | 1 | T65 | 400 | 300 | 200 | 300 | 1 |
| T12 | 200 | 300 | 200 | 400 | 1 | T39 | 300 | 300 | 200 | 400 | 1 | T66 | 400 | 300 | 200 | 400 | 1 |
| T13 | 200 | 300 | 300 | 200 | 2 | T40 | 300 | 300 | 300 | 200 | 1 | T67 | 400 | 300 | 300 | 200 | 2 |
| T14 | 200 | 300 | 300 | 300 | 2 | T41 | 300 | 300 | 300 | 300 | 3 | T68 | 400 | 300 | 300 | 300 | 2 |
| T15 | 200 | 300 | 300 | 400 | 2 | T42 | 300 | 300 | 300 | 400 | 2 | T69 | 400 | 300 | 300 | 400 | 2 |
| T16 | 200 | 300 | 400 | 200 | 1 | T43 | 300 | 300 | 400 | 200 | 2 | T70 | 400 | 300 | 400 | 200 | 2 |
| T17 | 200 | 300 | 400 | 300 | 2 | T44 | 300 | 300 | 400 | 300 | 1 | T71 | 400 | 300 | 400 | 300 | 2 |
| T18 | 200 | 300 | 400 | 400 | 2 | T45 | 300 | 300 | 400 | 400 | 2 | T72 | 400 | 300 | 400 | 400 | 2 |
| T19 | 200 | 400 | 200 | 200 | 2 | T46 | 300 | 400 | 200 | 200 | 2 | T73 | 400 | 400 | 200 | 200 | 2 |
| T20 | 200 | 400 | 200 | 300 | 2 | T47 | 300 | 400 | 200 | 300 | 1 | T74 | 400 | 400 | 200 | 300 | 1 |
| T21 | 200 | 400 | 200 | 400 | 2 | T48 | 300 | 400 | 200 | 400 | 3 | T75 | 400 | 400 | 200 | 400 | 2 |
| T22 | 200 | 400 | 300 | 200 | 2 | T49 | 300 | 400 | 300 | 200 | 2 | T76 | 400 | 400 | 300 | 200 | 2 |
| T23 | 200 | 400 | 300 | 300 | 1 | T50 | 300 | 400 | 300 | 300 | 3 | T77 | 400 | 400 | 300 | 300 | 1 |
| T24 | 200 | 400 | 300 | 400 | 2 | T51 | 300 | 400 | 300 | 400 | 3 | T78 | 400 | 400 | 300 | 400 | 2 |
| T25 | 200 | 400 | 400 | 200 | 1 | T52 | 300 | 400 | 400 | 200 | 3 | T79 | 400 | 400 | 400 | 200 | 2 |
| T26 | 200 | 400 | 400 | 300 | 1 | T53 | 300 | 400 | 400 | 300 | 2 | T80 | 400 | 400 | 400 | 300 | 2 |
| T27 | 200 | 400 | 400 | 400 | 3 | T54 | 300 | 400 | 400 | 400 | 2 | T81 | 400 | 400 | 400 | 400 | 2 |
| Tuber Weight of per Plant | Number of Tubers per Plant | Average Tuber Weight | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low Yield Group | Medium Yield Group | High Yield Group | Low Yield Group | Medium Yield Group | High Yield Group | Low Yield Group | Medium Yield Group | High Yield Group | ||
| Number of tubers per plant | 0.16 | 0.30 | 0.27 | |||||||
| Average tuber weight | 0.38 * | 0.27 | −0.12 | −0.80 * | −0.88 * | −0.95 * | ||||
| Light intensity | SS | 0.33 | 0.12 | 0.29 | −0.23 | 0.20 | 0.49 | 0.42 | −0.11 | −0.28 |
| TFS | 0.53 * | −0.04 | −0.23 | 0.27 | −0.03 | −0.79 * | 0.04 | 0.14 | 0.68 | |
| TGS | 0.34 | 0.39 * | −0.54 | 0.49 * | 0.20 | 0.20 | −0.27 | −0.19 | −0.42 | |
| HS | −0.20 | 0.10 | 0.02 | 0.25 | 0.15 | −0.27 | −0.30 | −0.13 | 0.38 | |
| Number of Tubers per Plant | Average Tuber Weight (g) | Tuber Weight per Plant (g) | Effective Tuber Ratio (%) | Proportion of Large Tubers (%) | Proportion of Small Tubers (%) | |
|---|---|---|---|---|---|---|
| T1 | 2.83 b | 9.74 b | 27.31 c | 95.68 a | 35.34 ab | 64.66 ab |
| T81 | 4.00 b | 14.70 a | 58.15 b | 81.71 b | 50.00 a | 50.00 b |
| T59 | 6.50 a | 11.31 ab | 72.91 a | 98.335 a | 23.08 b | 76.92 a |
| T1 | T81 | T59 | ||
|---|---|---|---|---|
| TFS | Fv/Fm | 0.86 a | 0.75 b | 0.85 a |
| PhiPSII | 0.76 a | 0.77 a | 0.77 a | |
| ETR | 64.07 b | 129.73 a | 64.52 b | |
| Jc | 31.62 c | 54.01 a | 34.65 b | |
| Jo | 32.44 b | 75.72 a | 29.87 c | |
| TGS | Fv/Fm | 0.79 a | 0.78 a | 0.78 a |
| PhiPSII | 0.77 a | 0.78 a | 0.78 a | |
| ETR | 64.9 c | 130.62 a | 97.99 b | |
| Jc | 33.87 b | 56.06 a | 54.52 a | |
| Jo | 31.03 c | 74.56 a | 43.47 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Lin, J.; Xu, Z. Stage-Specific Light Intensity Optimization for Yield and Energy Efficiency in Plant Factory Potato Pre-Basic Seed Production. Agronomy 2025, 15, 1976. https://doi.org/10.3390/agronomy15081976
Chen S, Lin J, Xu Z. Stage-Specific Light Intensity Optimization for Yield and Energy Efficiency in Plant Factory Potato Pre-Basic Seed Production. Agronomy. 2025; 15(8):1976. https://doi.org/10.3390/agronomy15081976
Chicago/Turabian StyleChen, Song, Jiating Lin, and Zhigang Xu. 2025. "Stage-Specific Light Intensity Optimization for Yield and Energy Efficiency in Plant Factory Potato Pre-Basic Seed Production" Agronomy 15, no. 8: 1976. https://doi.org/10.3390/agronomy15081976
APA StyleChen, S., Lin, J., & Xu, Z. (2025). Stage-Specific Light Intensity Optimization for Yield and Energy Efficiency in Plant Factory Potato Pre-Basic Seed Production. Agronomy, 15(8), 1976. https://doi.org/10.3390/agronomy15081976





