Responses of Rhizospheric Microbial Communities to Brevibacillus laterosporus-Enhanced Reductive Soil Disinfestation in Continuous Cropping Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Samples for Pot Experiment
2.2. Experimental Treatment
2.3. Plant Materials and Microbial Strains
2.4. Determination of Soil Properties
2.5. Genomic DNA Extraction and High-Throughput Sequencing
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
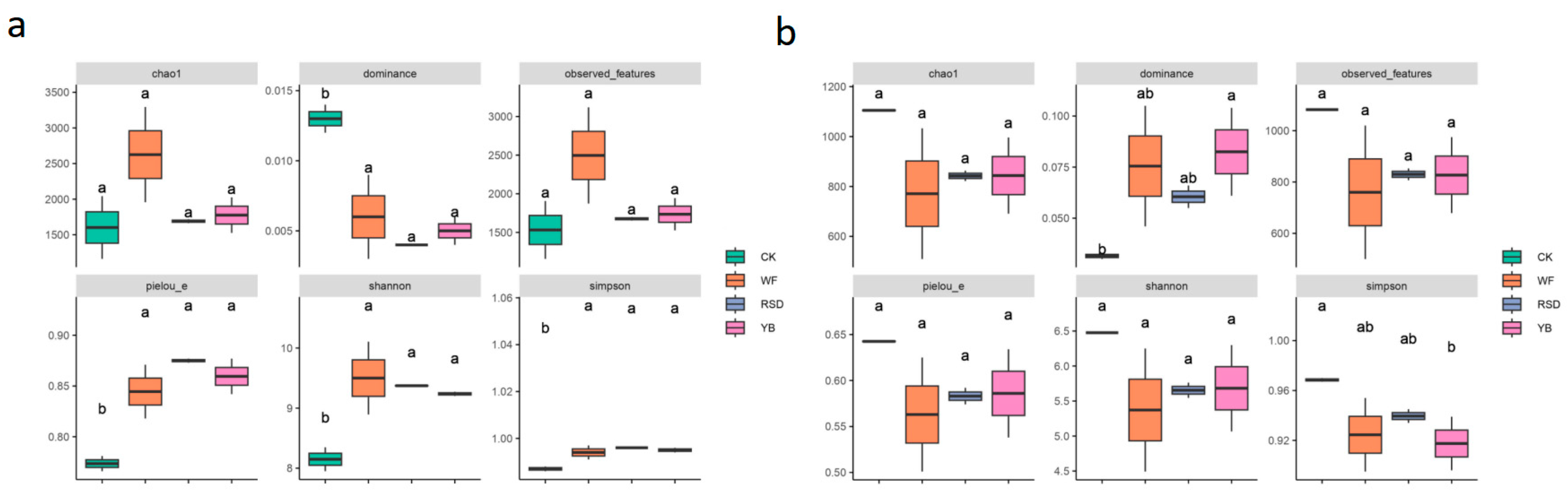
3.2. Microbial Alpha Diversity
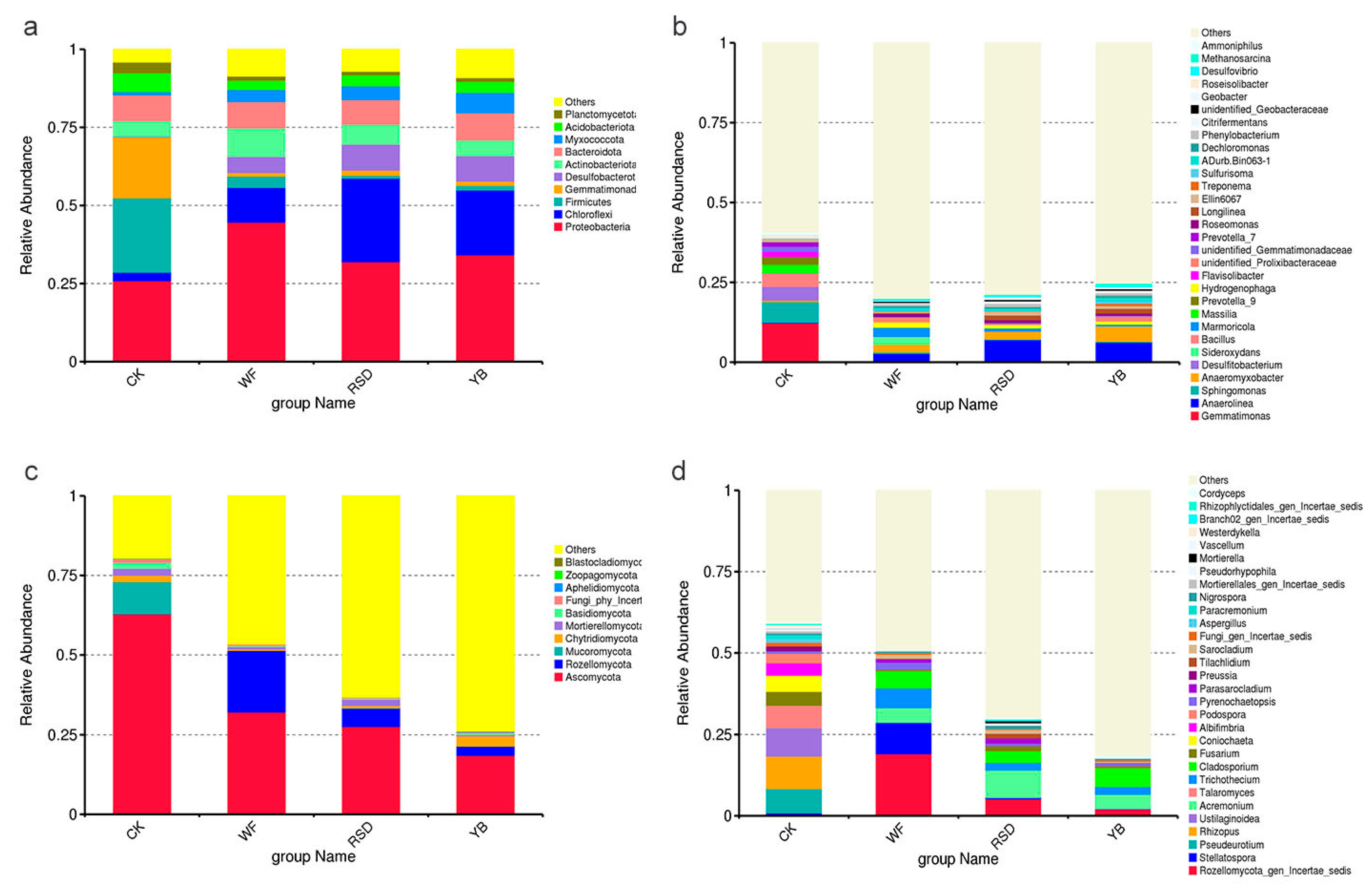
3.3. Microbial Community Composition
3.4. LEfSe Analysis of Microbial Communities
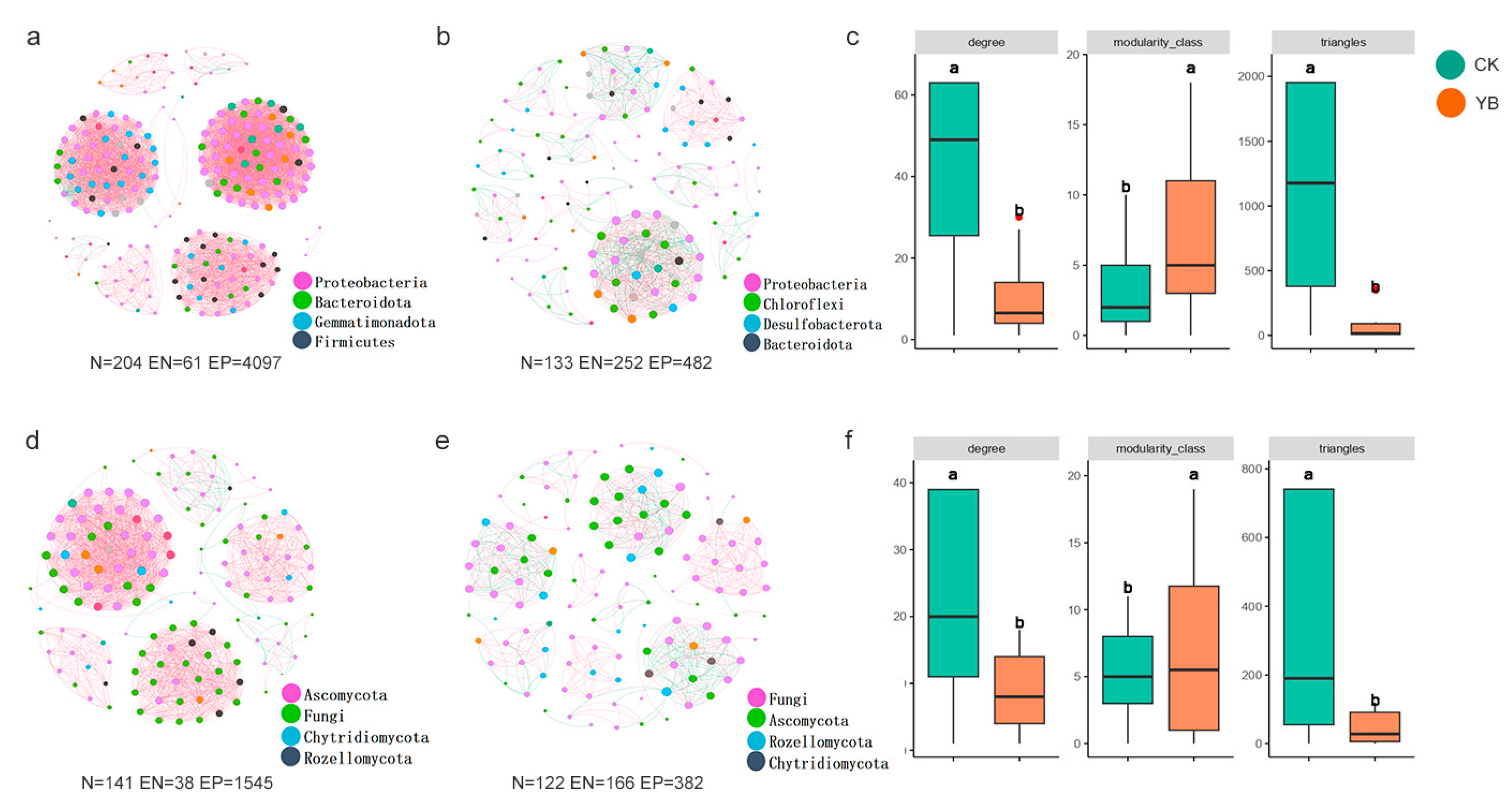
3.5. Microbial Network Properties
3.6. Relationships Between Soil Properties and Bacterial Communities
4. Discussion
4.1. Brevibacillus Laterosporus Inoculation Improved the Soil Environment
4.2. Influence of Brevibacillus laterosporus Inoculation and RSD on Bacterial and Fungal Community Diversity and Compositions in Rhizosphere Soil
4.3. Effect of B. laterosporus-Enhanced RSD on Microbial Network Keystone Taxa, Complexity, and Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teng, Y.; Ren, W.J.; Li, Z.G.; Wang, X.B.; Liu, W.X.; Luo, Y.M. Advance in mechanism of peanut continuous cropping obstacle. Soils 2015, 47, 259–265. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Liu, J.; Li, X.; Wang, X.; Dai, C.; Li, X. Crop rotation and native microbiome inoculation restore soil capacity to suppress a root disease. Nat. Commun. 2023, 14, 8126. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Linquist, B.A.; Lazicki, P.A. Effect of fertilization on soil microorganisms in paddy rice systems—A Meta Analysis. Soil Biol. Biochem. 2017, 115, 452–460. [Google Scholar] [CrossRef]
- Li, X.G.; Ding, C.F.; Hua, K.; Zhang, T.L. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2024, 78, 149–159. [Google Scholar] [CrossRef]
- Lopes, E.A.; Canedo, E.J.; Gomes, V.A.; Vieira, B.S.; Parreira, D.F.; Neves, W.S. Anaerobic soil disinfestation for the management of soilborne pathogens: A review. Appl. Soil Ecol. 2022, 174, 104408. [Google Scholar] [CrossRef]
- Ueki, A.; Kaku, N.; Ueki, K. Role of anaerobic bacteria in biological soil disinfestation for elimination of soil-borne plant pathogens in agriculture. Appl. Microbiol. Biotechnol. 2018, 102, 6309–6318. [Google Scholar] [CrossRef]
- Ali, A.; Elrys, A.S.; Liu, L. Deciphering the synergies of reductive soil disinfestation combined with biochar and antagonistic microbial inoculation in cucumber Fusarium Wilt suppression through rhizosphere microbiota structure. Microb. Ecol. 2023, 85, 980–997. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Z.H.; Zhang, J.Q.; Zhang, J.B.; Cai, Z.C.; Zhao, J. Effects of reductive soil disinfestation and Bacillus subtilis inoculant on soil phenolic acids of Lily. Soils 2023, 55, 1016–1024. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, Y.; Xu, R.; Song, J.; Wang, Y. Short-term responses of soil organic carbon and chemical composition of particle-associated organic carbon to anaerobic soil disinfestation in degraded greenhouse soils. Land Degr. Dev. 2023, 34, 4428–4440. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Li, Y.; Song, J.; Liang, Y.; Chen, F.; Chen, Y. Linking bacterial life strategies with the distribution pattern of antibiotic resistance genes in soil aggregates after straw addition. J. Hazard. Mater. 2024, 471, 134355. [Google Scholar] [CrossRef]
- Luo, X.; Sun, K.; Li, H.R.; Zhang, X.Y.; Pan, Y.T.; Luo, D.L.; Zhang, W. Depletion of protective microbiota promotes the incidence of fruit disease. ISME J. 2024, 8, 071. [Google Scholar] [CrossRef]
- Xu, R.; Li, K.; Chen, A.; Chen, Y.; Sheng, R.; Chen, C.; Zhu, B. Responses of soil microbial communities to abandoned paddy fields with different fertilization histories. Land Degr. Dev. 2025, 15, 625–633. [Google Scholar] [CrossRef]
- Fan, Y.P.; Song, B.Q.; Wang, C.X. Progress of research on alleviating obstacles of continuous cropping by soil sterilization and arbuscular mycorrhizal fungi. J. Agr. Sci. Tech. 2024, 26, 158–167. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.; Wang, J.; Lv, D.; Ma, Y.; Zhou, B.; Wang, B. Biocontrol effects of Brevibacillus laterosporus AMCC100017 on potato common scab and its impact on rhizosphere bacterial communities. Biol. Control. 2017, 106, 89–98. [Google Scholar] [CrossRef]
- Ruiu, L. Brevibacillus laterosporus, a pathogen of invertebrates and a broad-spectrum antimicrobial species. Insects 2013, 4, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Fan, H.; Li, Y.; Yao, H. Influence of reductive soil disinfestation or biochar amendment on bacterial communities and their utilization of plant-derived carbon in the rhizosphere of tomato. Appl. Microbiol. Biotech. 2021, 105, 815–825. [Google Scholar] [CrossRef]
- Shen, B.; Wang, X.; Zhang, Y.; Zhang, M.; Wang, K.; Ji, H. The optimum pH and Eh for simultaneously minimizing bioavailable cadmium and arsenic contents in soils under the organic fertilizer application. Sci. Total Environ. 2022, 711, 135229. [Google Scholar] [CrossRef]
- Fan, R.; Ma, W.; Zhang, H. Microbial community responses to soil parameters and their effects on petroleum degradation during bio-electrokinetic remediation. Sci. Total Environ. 2020, 748, 142463. [Google Scholar] [CrossRef]
- Kang, E.; Li, Y.; Zhang, X. Soil pH and nutrients shape the vertical distribution of microbial communities in an Alpine Wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Zhao, H.; Brearley, F.Q.; Huang, L.; Tang, J.; Xu, Q.; Li, X.; Li, N. Abundant and rare taxa of planktonic fungal community exhibit distinct assembly patterns along coastal eutrophication gradient. Microb. Ecol. 2023, 85, 495–507. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Fang, J.; Chu, H.; Adams, J.M. Soil microbial network complexity varies with pH as a continuum, not a threshold, across the North China Plain. Front. Microb. 2022, 13, 895687. [Google Scholar] [CrossRef]
- Ding, J.N.; Shaopeng, Y. Impacts of land use on soil nitrogen-cycling microbial communities: Insights from community structure, functional gene abundance, and network complexity. Life 2025, 15, 466. [Google Scholar] [CrossRef]
- Xiao, X.; Pei, M.; Liu, X. Planktonic algal bloom significantly alters sediment bacterial community structure. J. Soils Sediments 2017, 17, 2547–2556. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Huttenhower, C. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. IMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]


| EC | pH | Eh | SOC | NO3− | NH4+ | AP | |
|---|---|---|---|---|---|---|---|
| CK | 500.3 ± 11.6 a 1 | 8.5 ± 0.1 a | 510.7 ± 7.5 a | 5.9 ± 0.3 a | 212 ± 4.7 a | 1.7 ± 0.1 a | 51 ± 3.5 a |
| WF | 448.7 ± 10.4 b | 8.3 ± 0.1 a | −55.3 ± 3.2 b | 5.7 ± 0.4 a | 135 ± 5.1 b | 2.5 ± 0.2 a | 53 ± 3.5 ab |
| RSD | 168 ± 9.2 c | 8.0 ± 0.1 b | −85.7 ± 4.1 c | 9.4 ± 0.1 b | 127.3 ± 4.6 b | 2.9 ± 0.1 b | 67 ± 6.4 bc |
| YB | 144 ± 6.8 c | 7.7 ± 0.1 c | −107.3 ± 3.3 d | 10.1 ± 0.2 b | 89.3 ± 4.7 c | 3.0 ± 0.1 c | 71.67 ± 4.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Liu, H.; Chen, Y.; Guo, Z.; Liu, J.; Li, Y.; Mei, J.; Ma, T.; Chen, Y. Responses of Rhizospheric Microbial Communities to Brevibacillus laterosporus-Enhanced Reductive Soil Disinfestation in Continuous Cropping Systems. Agronomy 2025, 15, 1775. https://doi.org/10.3390/agronomy15081775
Xu R, Liu H, Chen Y, Guo Z, Liu J, Li Y, Mei J, Ma T, Chen Y. Responses of Rhizospheric Microbial Communities to Brevibacillus laterosporus-Enhanced Reductive Soil Disinfestation in Continuous Cropping Systems. Agronomy. 2025; 15(8):1775. https://doi.org/10.3390/agronomy15081775
Chicago/Turabian StyleXu, Risheng, Haijiao Liu, Yafei Chen, Zhen Guo, Juan Liu, Yue Li, Jingyi Mei, Tengfei Ma, and Yanlong Chen. 2025. "Responses of Rhizospheric Microbial Communities to Brevibacillus laterosporus-Enhanced Reductive Soil Disinfestation in Continuous Cropping Systems" Agronomy 15, no. 8: 1775. https://doi.org/10.3390/agronomy15081775
APA StyleXu, R., Liu, H., Chen, Y., Guo, Z., Liu, J., Li, Y., Mei, J., Ma, T., & Chen, Y. (2025). Responses of Rhizospheric Microbial Communities to Brevibacillus laterosporus-Enhanced Reductive Soil Disinfestation in Continuous Cropping Systems. Agronomy, 15(8), 1775. https://doi.org/10.3390/agronomy15081775






