Organic Materials Promote Soil Phosphorus Cycling: Metagenomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site
2.2. Experimental Design and Sample Collection
2.3. Soil Properties and Enzyme Activity Characterization
2.4. Determination of Soil Bioavailable Phosphorus
2.5. Metagenomic Sequencing
2.6. Statistical Analysis
3. Result
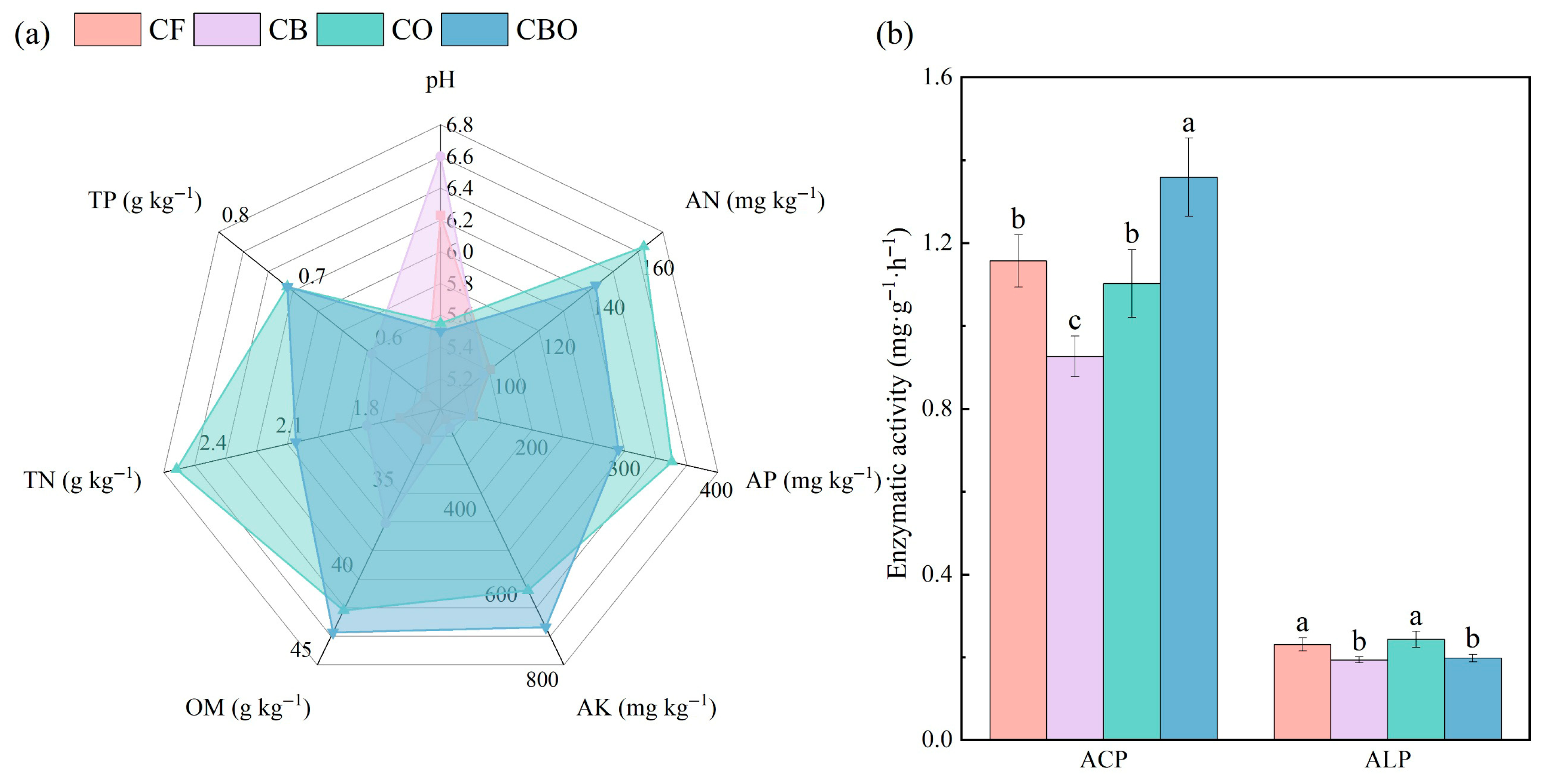
3.1. Effects on Edaphic Factors and Enzyme Activities
3.2. Effects on Bioavailable Phosphorus Content
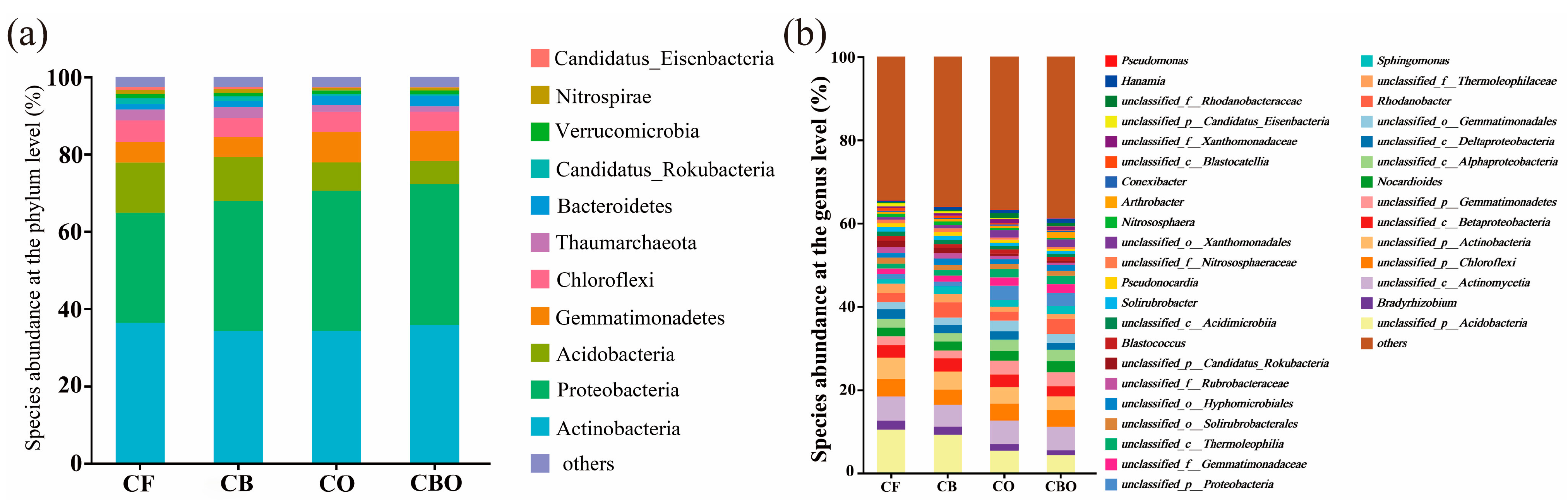
3.3. Effects on Microbial Communities
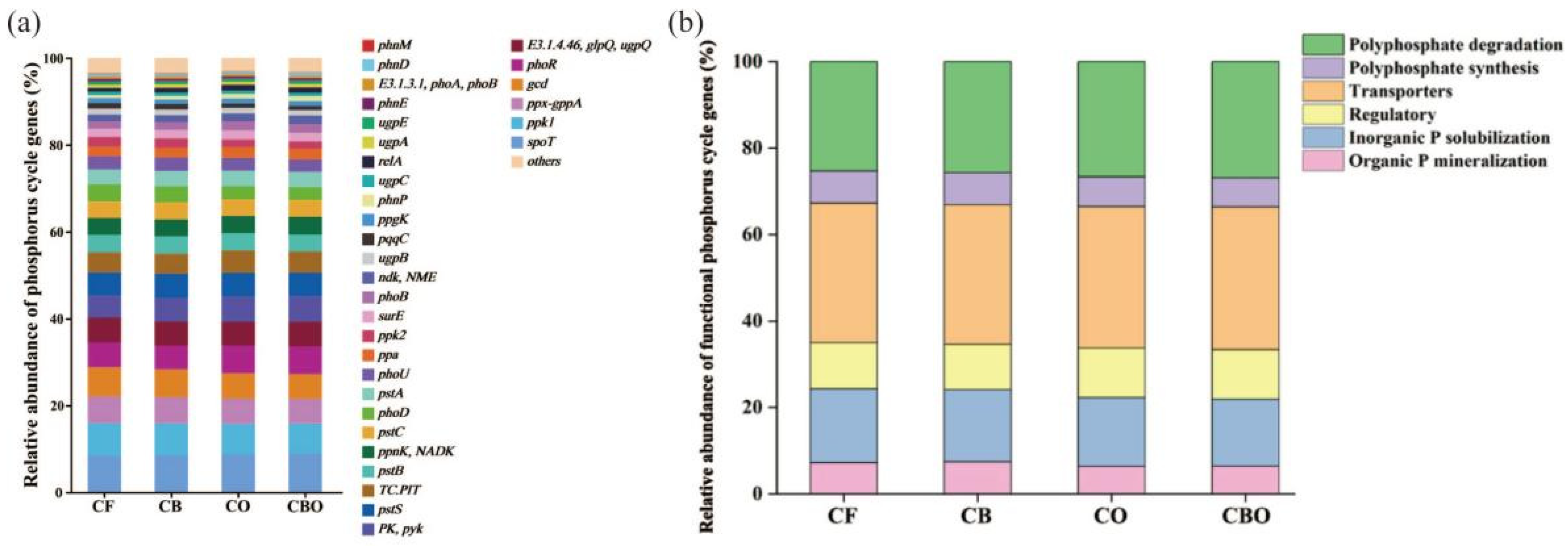
3.4. Effects on Phosphorus Cycling Genes
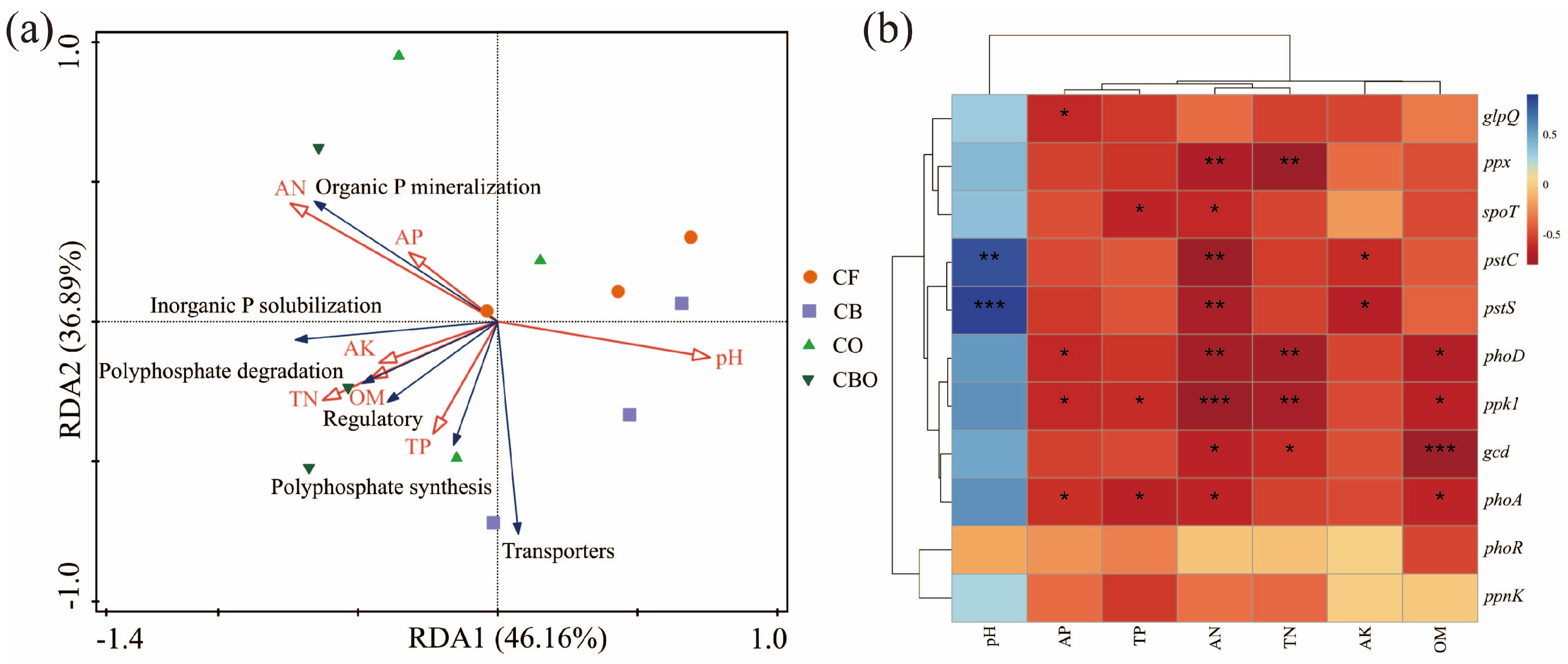
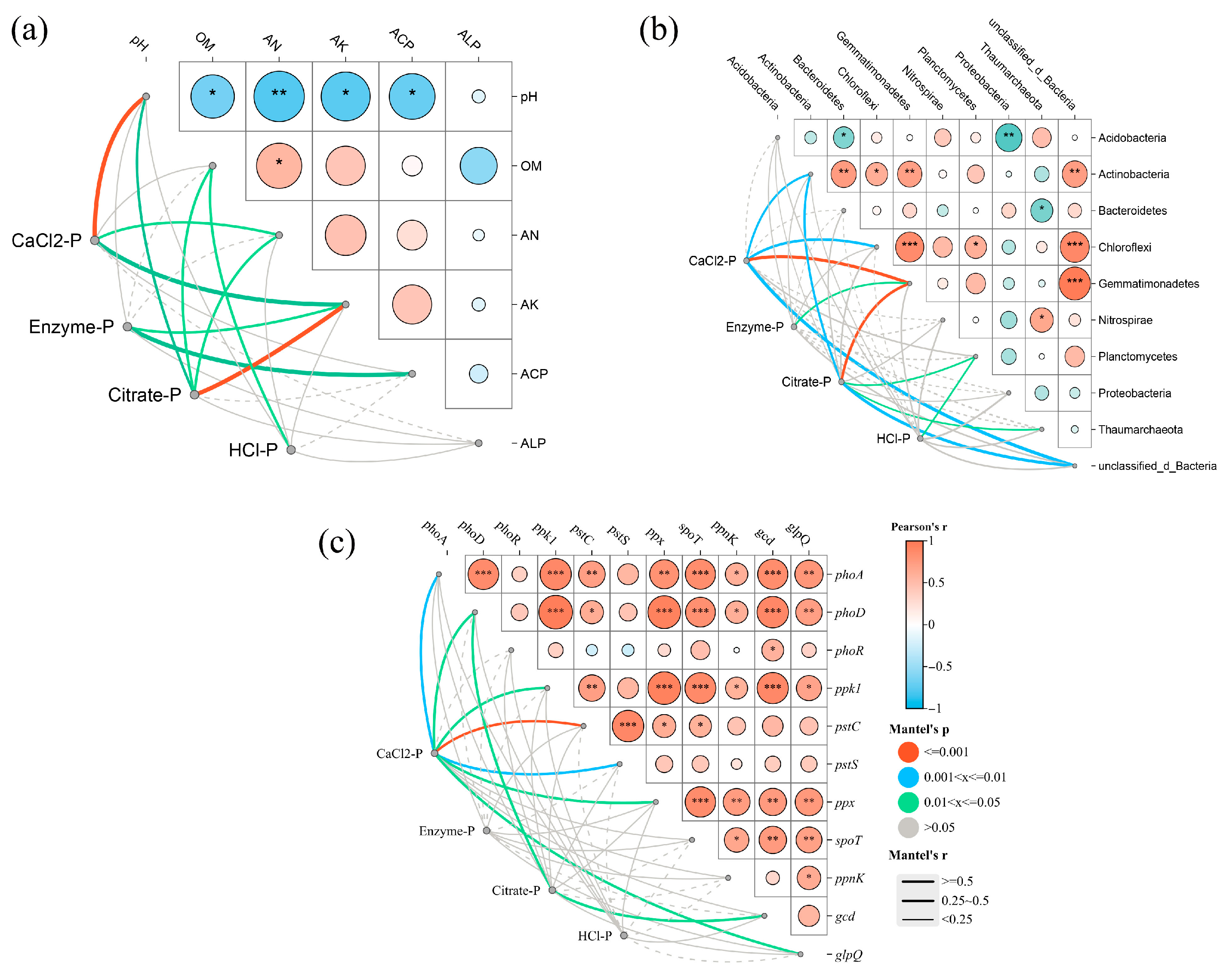
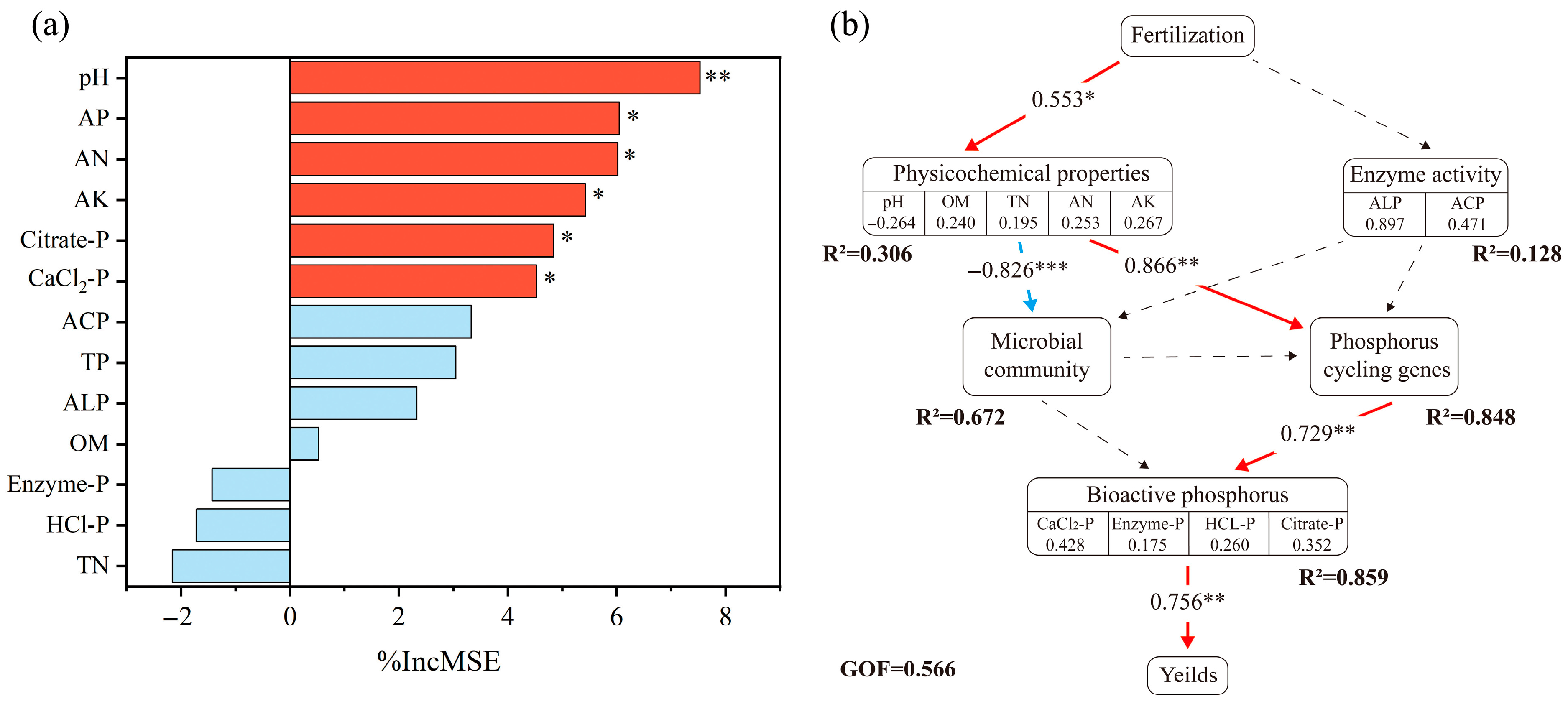
3.5. Relationship Among Edaphic Factors, Enzyme Activities, Bioavailable Phosphorus, and Maize Yield
4. Discussion
4.1. Effect of Combined Applications on Maize Yield and Bioavailable Phosphorus Content
4.2. Effect of Combined Applications on Phosphorus Cycling Microorganisms
4.3. Effect of Combined Applications on Phosphorus Cycling Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, J.; Yang, F.; Yang, W.; Zhang, M.; He, S.; Li, Z. Fertilizers and Manures Enhance the Bioavailability of Soil Phosphorus Fractions in Karst Grassland. Agronomy 2024, 14, 1429. [Google Scholar] [CrossRef]
- Shen, F.; Fei, L.; Tuo, Y.; Peng, Y.; Yang, Q.; Zheng, R.; Wang, Q.; Liu, N.; Fan, Q. Effects of water and fertilizer regulation on soil physicochemical properties, bacterial diversity and community structure of Panax notoginseng. Sci. Hortic. 2024, 326, 112777. [Google Scholar] [CrossRef]
- Lee, C.H.; Das, S.; Park, M.H.; Kim, S.Y.; Kim, P.J. Long-term application of silicate fertilizer alters microbe-mediated phosphorus cycling in paddy soils. Agric. Ecosyst. Environ. 2024, 374, 109175. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Ali, M.; Abdullah, R.; Chowdhury, A.J.K.; Salleh, N.T.; Musah, A.A. Co-application of triple super phosphate and chicken litter biochar improves phosphorus availability of mineral tropical acid soils to reduce water pollution. Desalin. Water Treat. 2022, 264, 40–53. [Google Scholar] [CrossRef]
- Tkaczyk, P.; Mocek-Płóciniak, A.; Skowrońska, M.; Bednarek, W.; Kuśmierz, S.; Zawierucha, E. The mineral fertilizer-dependent chemical parameters of soil acidification under field conditions. Sustainability 2020, 12, 7165. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, X.; Zhou, B.; Lei, L.; Xiao, W. Variations in rhizosphere soil total phosphorus and bioavailable phosphorus with respect to the stand age in Pinus massoniana Lamb. Front. Plant Sci. 2022, 13, 939683. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Glanville, H.C.; Harris, M.; Emmett, B.A.; Pingree, M.R.A.; de Sosa, L.L.; Cerdá-Moreno, C.; Jones, D.L. A novel biologically-based approach to evaluating soil phosphorus availability across complex landscapes. Soil Biol. Biochem. 2015, 88, 110–119. [Google Scholar] [CrossRef]
- Chen, X.; Condron, L.M.; Dunfield, K.E.; Wakelin, S.A.; Chen, L. Impact of grassland afforestation with contrasting tree species on soil phosphorus fractions and alkaline phosphatase gene communities. Soil Biol. Biochem. 2021, 159, 108274. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, Y.; Sun, Q.; Liu, K.; Chen, X.; Ge, T.; Zhu, B.; Zhu, Z.; Zhang, Z.; Su, Y. Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci. Total Environ. 2018, 628–629, 53–63. [Google Scholar] [CrossRef]
- Gichangi, E.M.; Mnkeni, P.N.S.; Brookes, P.C. Goat manure application improves phosphate fertilizer effectiveness through enhanced biological cycling of phosphorus. Soil Sci. Plant Nutr. 2010, 56, 853–860. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Xiangmin, R.; Fei, J.; Peng, J.; Luo, G. Coupling amendment of biochar and organic fertilizers increases maize yield and phosphorus uptake by regulating soil phosphatase activity and phosphorus-acquiring microbiota. Agric. Ecosyst. Environ. 2023, 355, 108582. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, D.; Hao, H.; Zhao, X.; Hao, W.; Liu, Q. Photosynthetically active radiation determining yields for an intercrop of maize with cabbage. Eur. J. Agron. 2015, 69, 32–40. [Google Scholar] [CrossRef]
- Sui, L.; Tang, C.; Cheng, K.; Yang, F. Biochar addition regulates soil phosphorus fractions and improves release of available phosphorus under freezing-thawing cycles. Sci. Total Environ. 2022, 848, 157748. [Google Scholar] [CrossRef]
- Gross, A.; Lin, Y.; Weber, P.K.; Pett-Ridge, J.; Silver, W.L. The role of soil redox conditions in microbial phosphorus cycling in humid tropical forests. Ecology 2020, 101, e02928. [Google Scholar] [CrossRef] [PubMed]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J.; Chen, K.; Lan, W.; Ye, Y.; Ma, X.; Lin, K. Responses of soil phosphorus cycling-related microbial genes to thinning intensity in Cunninghamia lanceolata plantations. Forests 2024, 15, 440. [Google Scholar] [CrossRef]
- Yang, P.X.; Ma, L.; Chen, M.H.; Xi, J.Q.; He, F.; Duan, C.Q.; Mo, M.H.; Fang, D.H.; Duan, Y.Q.; Yang, F.X. Phosphate solubilizing ability and phylogenetic diversity of bacteria from P-rich soils around Dianchi Lake drainage area of China. Pedosphere 2012, 22, 707–716. [Google Scholar] [CrossRef]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ. Microbiol. 2016, 18, 2767–2779. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Li, C.; Ma, X.; Wang, Y.; Sun, Q.; Chen, M.; Zhang, C.; Ding, S.; Dai, Z. Root-mediated acidification, phosphatase activity and the phosphorus-cycling microbial community enhance phosphorus mobilization in the rhizosphere of wetland plants. Water Res. 2024, 255, 121548. [Google Scholar] [CrossRef] [PubMed]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.-X.; Dong, D.-F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Cao, N.; Zhi, M.L.; Zhao, W.Q.; Pang, J.Y.; Hu, W.; Zhou, Z.G.; Meng, Y.L. Straw retention combined with phosphorus fertilizer promotes soil phosphorus availability by enhancing soil P-related enzymes and the abundance of phoC and phoD genes. Soil Tillage Res. 2022, 220, 105390. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Ruan, Y.; Sun, W.; Wang, S.; Wang, H.; Zhang, Y.; Guo, J.; Wang, Y.; Guo, H.; et al. Metagenomes reveal the effect of crop rotation systems on phosphorus cycling functional genes and soil phosphorus availability. Agric. Ecosyst. Environ. 2024, 364, 108886. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, J.; Yang, X.; Han, X.; Lan, Y.; Cao, D.; Sun, Q.; Cui, X.; Meng, J.; Chen, W. Responses of soil respiration and C sequestration efficiency to biochar amendment in maize field of Northeast China. Soil Tillage Res. 2022, 223, 105442. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Q.; Yuan, J.; Fu, S.; Lan, Y.; Jiang, X.; Meng, J.; Han, X.; Chen, W. Successive corn stover and biochar applications mitigate N2O emissions by altering soil physicochemical properties and N-cycling-related enzyme activities: A five-year field study in Northeast China. Agric. Ecosyst. Environ. 2022, 340, 108183. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Luo, G.; Sun, B.; Li, L.; Li, M.; Liu, M.; Zhu, Y.; Guo, S.; Ling, N.; Shen, Q. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD- and phoC-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Lan, W.; Liu, H.; Weng, R.; Zeng, Y.; Lou, J.; Xu, H.; Yu, Y.; Jiang, Y. Microbial community of municipal drinking water in Hangzhou using metagenomic sequencing. Environ. Pollut. 2024, 342, 123066. [Google Scholar] [CrossRef]
- Su, J.; Xia, Y.; Yao, H.; Li, Y.; An, X.; Singh, B.K.; Zhang, T.; Zhu, Y. Metagenomic assembly unravel microbial response to redox fluctuation in acid sulfate soil. Soil Biol. Biochem. 2017, 105, 244–252. [Google Scholar] [CrossRef]
- Yasir, M. Analysis of microbial communities and pathogen detection in domestic sewage using metagenomic sequencing. Diversity 2021, 13, 6. [Google Scholar] [CrossRef]
- Adiningrat, A.; Maulana, I.; Fadhlurrahman, A.G.; Yumoto, H. Probiotic bacteria from asymptomatic necrotic tooth can regulate the microbiome homeostasis. Microb. Pathog. 2025, 206, 107791. [Google Scholar] [CrossRef] [PubMed]
- Carver, S.M.; Nikulin, N.; Kao-Kniffin, J. Uncovering Plant Growth-Mediating Allelochemicals Produced by Soil Microorganisms. Weed Sci. 2016, 64, 119–128. [Google Scholar] [CrossRef]
- Pereira, M.B.; Wallroth, M.; Jonsson, V.; Kristiansson, E. Comparison of normalization methods for the analysis of metagenomic gene abundance data. BMC Genom. 2018, 19, 274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, S.; Zhao, Y.; Jing, M.; Xu, Y.; Chen, J. A field experiment on enhancement of crop yield by rice straw and corn stalk-derived biochar in northern China. Sustainability 2015, 7, 13713–13728. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Rong, X.; Han, Y.; Yang, Z.; Hou, K.; Zhao, H.; Hu, W. Responses of maize yield, nitrogen and phosphorus runoff losses and soil properties to biochar and organic fertilizer application in a light-loamy fluvo-aquic soil. Agric. Ecosyst. Environ. 2021, 314, 107433. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Zuo, W.; Xu, L.; Qiu, M.; Yi, S.; Wang, Y.; Shen, C.; Zhao, Y.; Li, Y.; Gu, C.; Shan, Y.; et al. Effects of different exogenous organic materials on improving soil fertility in coastal saline-alkali soil. Agronomy 2023, 13, 61. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Li, Y.; Liu, S. Metagenomics of the effect of long-term straw return on the phosphorus cycle in meadow black soil. Agronomy 2023, 13, 3003. [Google Scholar] [CrossRef]
- Shi, R.; Liu, Z.; Li, Y.; Jiang, T.; Xu, M.; Li, J.; Xu, R. Mechanisms for increasing soil resistance to acidification by long-term manure application. Soil Tillage Res. 2019, 185, 77–84. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Straw and straw biochar differently affect phosphorus availability, enzyme activity and microbial functional genes in an Ultisol. Sci. Total Environ. 2022, 805, 150325. [Google Scholar] [CrossRef] [PubMed]
- Pingree, M.R.A.; Makoto, K.; DeLuca, T.H. Interactive effects of charcoal and earthworm activity increase bioavailable phosphorus in sub-boreal forest soils. Biol. Fertil. Soils 2017, 53, 873–884. [Google Scholar] [CrossRef]
- Gao, S.; Hoffman-Krull, K.; Bidwell, A.L.; DeLuca, T.H. Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric. Ecosyst. Environ. 2016, 233, 43–54. [Google Scholar] [CrossRef]
- Thomas, E.; Borchard, N.; Sarmiento, C.; Atkinson, R.; Ladd, B. Key factors determining biochar sorption capacity for metal contaminants: A literature synthesis. Biochar 2020, 2, 151–163. [Google Scholar] [CrossRef]
- Wu, J.; Sha, C.; Wang, M.; Ye, C.; Li, P.; Huang, S. Effect of Organic Fertilizer on Soil Bacteria in Maize Fields. Land 2021, 10, 328. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Tang, X.; Yan, L.; El-Desouki, Z.; Li, Y.; Wang, X.; Cuncang, J. Insight into mechanisms of biochar-fertilizer induced of microbial community and microbiology of nitrogen cycle in acidic soil. J. Environ. Manag. 2023, 336, 117602. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.W.; Sun, C.J. Different genotypes of tartary buckwheat can regulate the transformation of nitrogen through the secretion of organic acids under low nitrogen stress. Eur. J. Soil Biol. 2023, 118, 103543. [Google Scholar] [CrossRef]
- Touhami, D.; Condron, L.M.; McDowell, R.W.; Moss, R. Effects of long-term phosphorus fertilizer inputs and seasonal conditions on organic soil phosphorus cycling under grazed pasture. Soil Use Manag. 2023, 39, 385–401. [Google Scholar] [CrossRef]
- Tian, J.; Kuang, X.; Tang, M.; Chen, X.; Huang, F.; Cai, Y.; Cai, K. Biochar application under low phosphorus input promotes soil organic phosphorus mineralization by shifting bacterial phoD gene community composition. Sci. Total Environ. 2021, 779, 146556. [Google Scholar] [CrossRef]
- Jin, Y.; Liang, X.; He, M.; Liu, Y.; Tian, G.; Shi, J. Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: A microcosm incubation study. Chemosphere 2016, 142, 128–135. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Qiang, R.; Lu, E.; Li, C.; Zhang, J.; Gao, Q. Response of Soil Microbial Community Structure to Phosphate Fertilizer Reduction and Combinations of Microbial Fertilizer. Front. Environ. Sci. 2022, 10, 899727. [Google Scholar] [CrossRef]
- Ji, L.; Si, H.; He, J.; Fan, L.; Li, L. The shifts of maize soil microbial community and networks are related to soil properties under different organic fertilizers. Rhizosphere 2021, 19, 100388. [Google Scholar] [CrossRef]
- Li, M.; Chen, C.; Zhang, H.; Wang, Z.; Song, N.; Li, J.; Liang, X.; Yi, K.; Gu, Y.; Guo, X. Effects of biochar amendment and organic fertilizer on microbial communities in the rhizosphere soil of wheat in Yellow River Delta saline-alkaline soil. Front. Microbiol. 2023, 14, 1250453. [Google Scholar] [CrossRef]
- Li, R.; Khafipour, E.; Krause, D.O.; Entz, M.H.; de Kievit, T.R.; Fernando, W.G.D. Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PLoS ONE 2012, 7, e51897. [Google Scholar] [CrossRef] [PubMed]
- Widdig, M.; Schleuss, P.M.; Weig, A.R.; Guhr, A.; Biederman, L.A.; Borer, E.T.; Crawley, M.J.; Kirkman, K.P.; Seabloom, E.W.; Wragg, P.D.; et al. Nitrogen and phosphorus additions alter the abundance of phosphorus-solubilizing bacteria and phosphatase activity in grassland soils. Front. Environ. Sci. 2019, 7, 185. [Google Scholar] [CrossRef]
- Tian, W.; Wang, L.; Li, Y.; Zhuang, K.; Li, G.; Zhang, J.; Xiao, X.; Xi, Y. Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agric. Ecosyst. Environ. 2015, 213, 219–227. [Google Scholar] [CrossRef]
- Wu, X.; Peng, J.; Liu, P.; Bei, Q.; Rensing, C.; Li, Y.; Yuan, H.; Liesack, W.; Zhang, F.; Cui, Z. Metagenomic insights into nitrogen and phosphorus cycling at the soil aggregate scale driven by organic material amendments. Sci. Total Environ. 2021, 785, 147329. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Chen, M.; Fei, C.; Zhang, W.; Li, Y.; Ding, X. Fe-modified biochar combined with mineral fertilization promotes soil organic phosphorus mineralization by shifting the diversity of phoD-harboring bacteria within soil aggregates in saline-alkaline paddy soil. J. Soils Sediments 2023, 23, 619–633. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Tang, C.; Xu, J. Organic carbon inputs shift the profiles of phosphorus cycling-related genes in maize rhizosphere. Plant Soil 2024, 503, 595–609. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Ma, L.; Luan, H.; Tang, J.; Li, R.; Li, M.; Huang, S.; Wang, L. Long-term partial substitution of chemical fertilizer by organic amendments influences soil microbial functional diversity of phosphorus cycling and improves phosphorus availability in greenhouse vegetable production. Agric. Ecosyst. Environ. 2023, 341, 108193. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, N.; Chen, Z.; Tian, J.; Sun, N.; Xu, M.; Chen, L. Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials. Appl. Soil Ecol. 2017, 119, 197–204. [Google Scholar] [CrossRef]
- Luo, G.; Ling, N.; Nannipieri, P.; Chen, H.; Raza, W.; Wang, M.; Guo, S.; Shen, Q. Long-term fertilization regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fertil. Soils 2017, 53, 375–388. [Google Scholar] [CrossRef]
- Ludueña, L.M.; Anzuay, M.S.; Magallanes-Noguera, C.; Tonelli, M.L.; Ibañez, F.J.; Angelini, J.G.; Fabra, A.; McIntosh, M.; Taurian, T. Effects of P limitation and molecules from peanut root exudates on pqqE gene expression and pqq promoter activity in the phosphate-solubilizing strain Serratia sp. S119. Res. Microbiol. 2017, 168, 710–721. [Google Scholar] [CrossRef]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Nakanishi, T.M.; Thibaud, M.C. Phosphate import in plants: Focus on the PHT1 transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.X.; Zhang, D.P.; Wang, Y.; Hao, X.L.; Wadaan, M.A.M.; Hozzein, W.N.; Peñuelas, J.; Zhu, Y.G.; Yang, X.R. Responses to soil pH gradients of inorganic phosphate solubilizing bacteria community. Sci. Rep. 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, J.; Yi, Q.; Li, J.; Li, Z.; Wu, S.; Zhang, W.; Wang, K. Metagenomic insights into the effects of organic and inorganic agricultural managements on soil phosphorus cycling. Agric. Ecosyst. Environ. 2023, 343, 108281. [Google Scholar] [CrossRef]
- Zhang, Y.; He, G.; Yang, L.; Wen, S.; Yan, J.; Min, B.; Peng, T.; Ji, L. Phosphorus fertilizer application shifts the rhizosphere bacterial community and their carbon, nitrogen and phosphorus-cycle genes in a Phoebe bournei young plantation. Appl. Soil Ecol. 2024, 198, 105391. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Jiang, Y.; Zhang, J.; Wang, W.; Liu, X.; Jin, Y.; Li, S.; Qu, J.; Zhu, Y. Organic Materials Promote Soil Phosphorus Cycling: Metagenomic Analysis. Agronomy 2025, 15, 1693. https://doi.org/10.3390/agronomy15071693
Yang W, Jiang Y, Zhang J, Wang W, Liu X, Jin Y, Li S, Qu J, Zhu Y. Organic Materials Promote Soil Phosphorus Cycling: Metagenomic Analysis. Agronomy. 2025; 15(7):1693. https://doi.org/10.3390/agronomy15071693
Chicago/Turabian StyleYang, Wei, Yue Jiang, Jiaqi Zhang, Wei Wang, Xuesheng Liu, Yu Jin, Sha Li, Juanjuan Qu, and Yuanchen Zhu. 2025. "Organic Materials Promote Soil Phosphorus Cycling: Metagenomic Analysis" Agronomy 15, no. 7: 1693. https://doi.org/10.3390/agronomy15071693
APA StyleYang, W., Jiang, Y., Zhang, J., Wang, W., Liu, X., Jin, Y., Li, S., Qu, J., & Zhu, Y. (2025). Organic Materials Promote Soil Phosphorus Cycling: Metagenomic Analysis. Agronomy, 15(7), 1693. https://doi.org/10.3390/agronomy15071693




