Potassium Fulvate Alleviates Salinity and Boosts Oat Productivity by Modifying Soil Properties and Rhizosphere Microbial Communities in the Saline–Alkali Soils of the Qaidam Basin
Abstract
1. Introduction
2. Materials and Methods
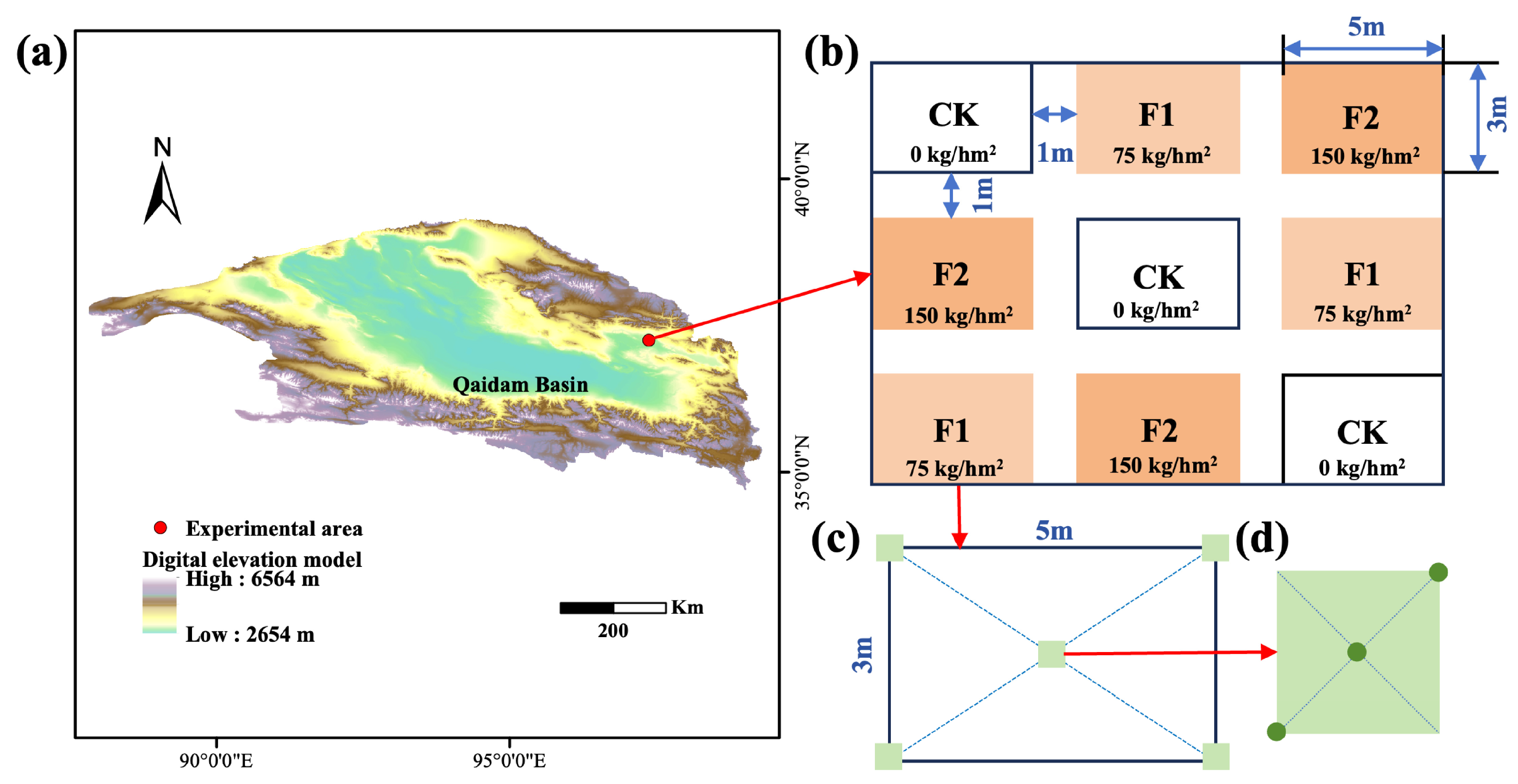
2.1. Experimental Site
2.2. Experimental Materials and Design
2.3. Soil Sampling for Chemical and Microbiological Studies
2.4. Plant and Soil Measurements
2.5. DNA Extraction and Sequencing
2.6. Statistical Analysis
3. Results
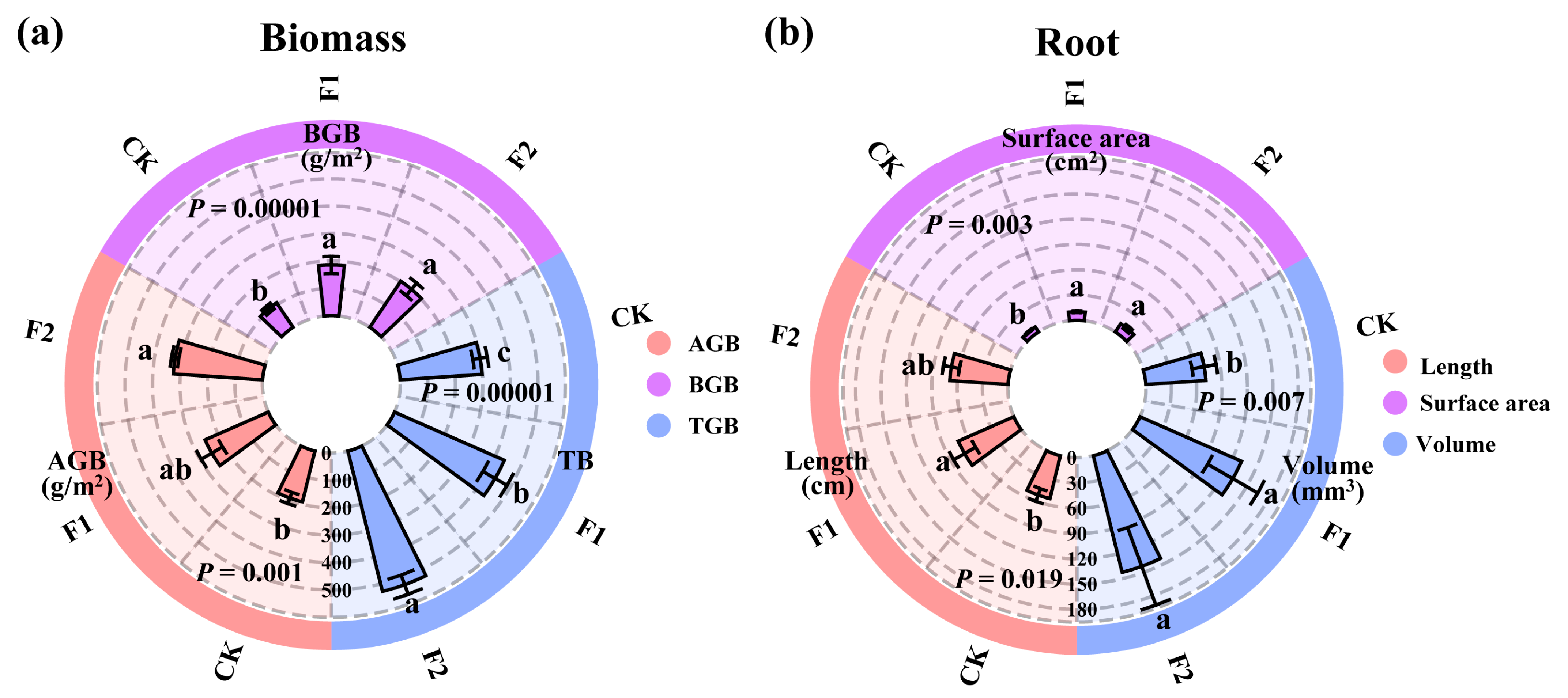
3.1. Effects of Potassium Fulvate Application on Oat Growth
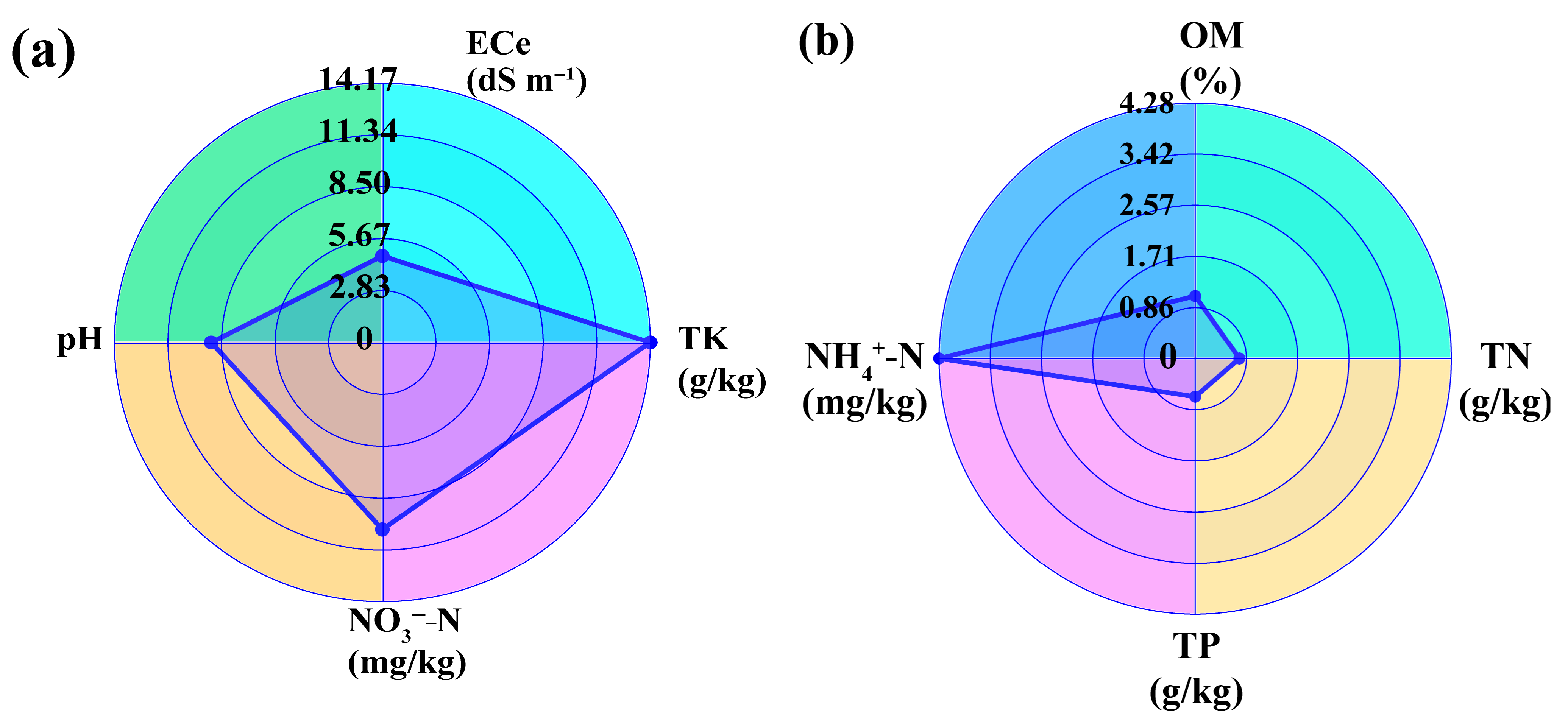
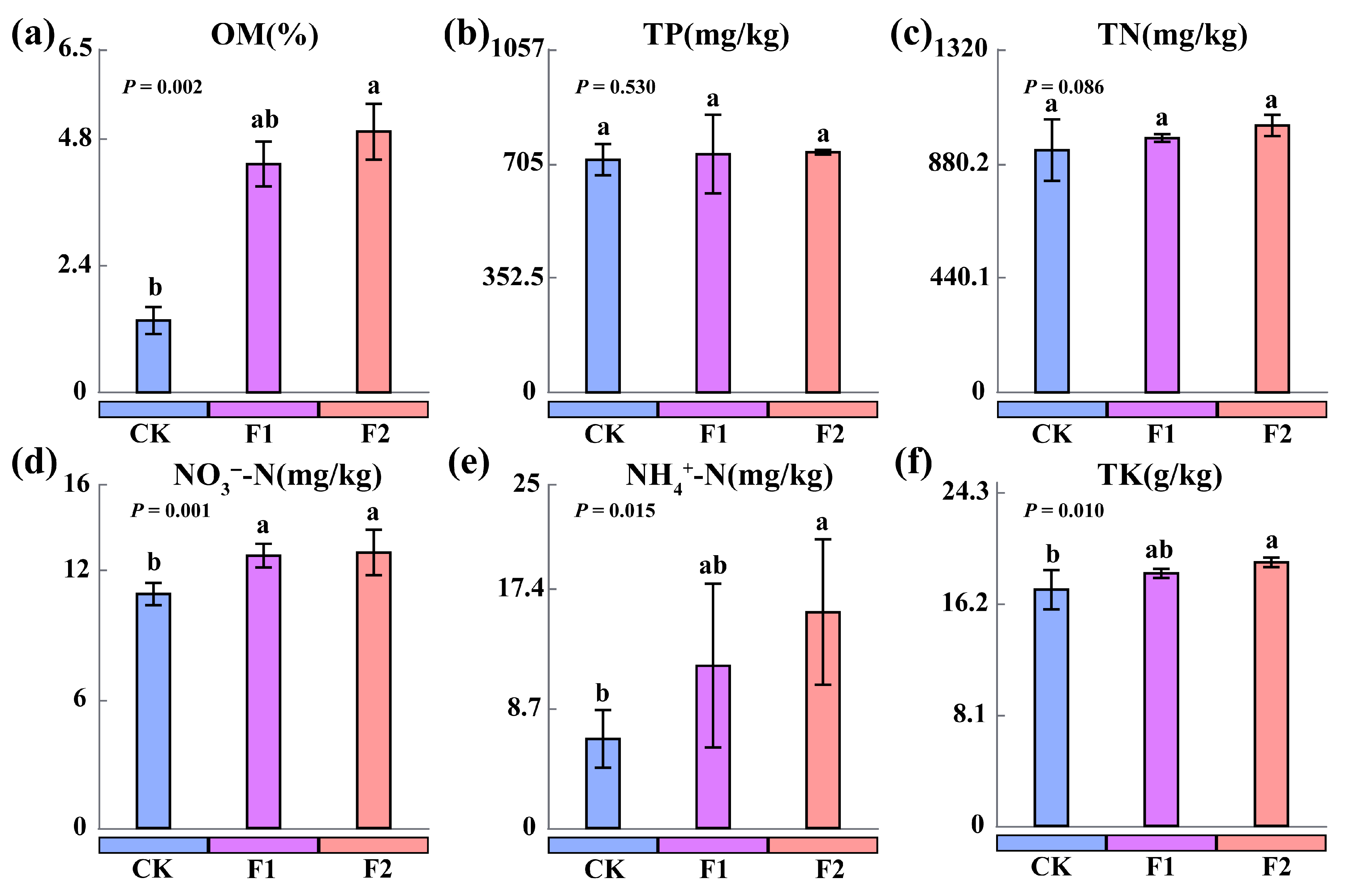
3.2. Effects of Potassium Fulvate Application on Rhizosphere Soil
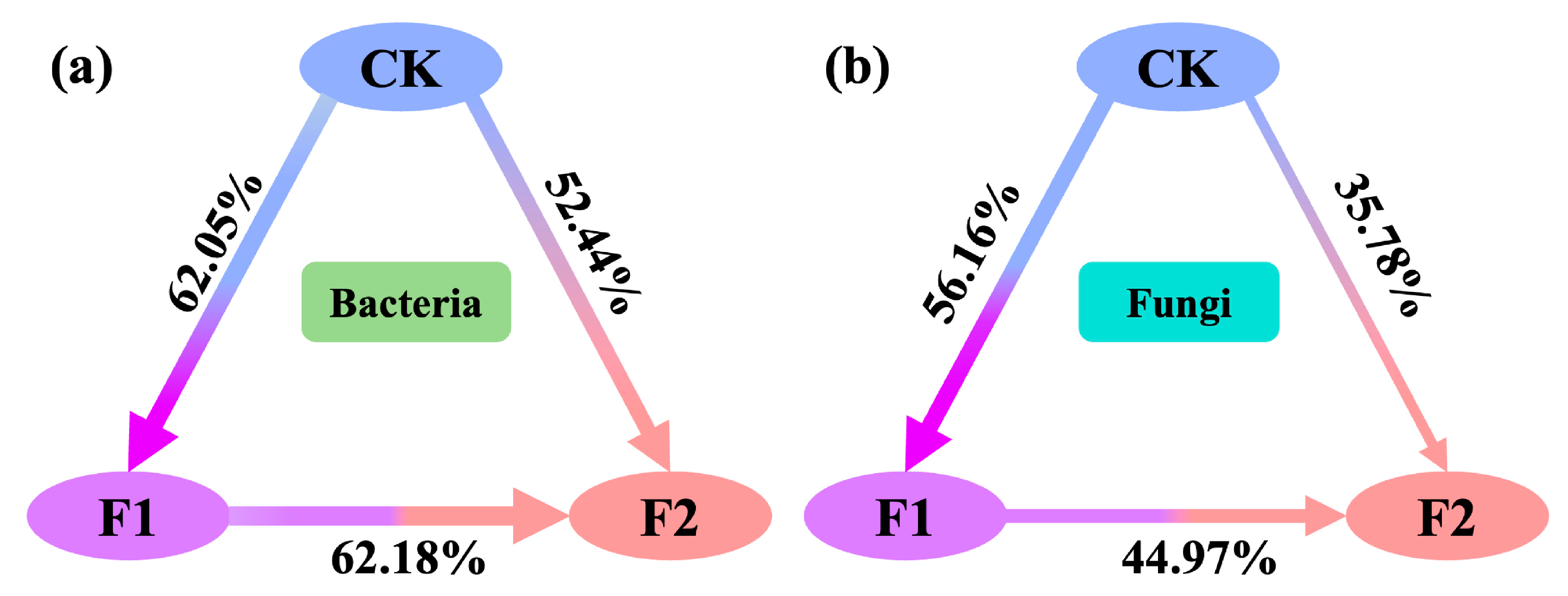
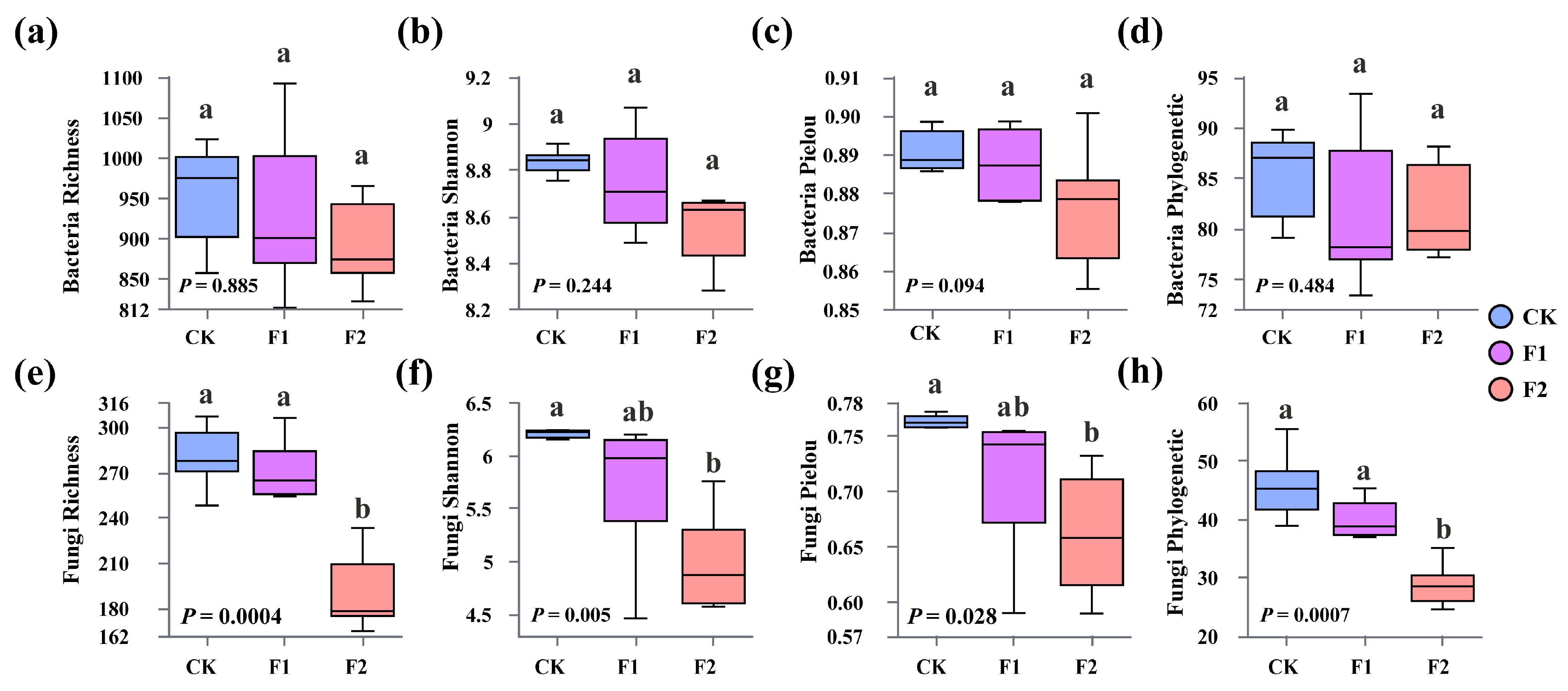
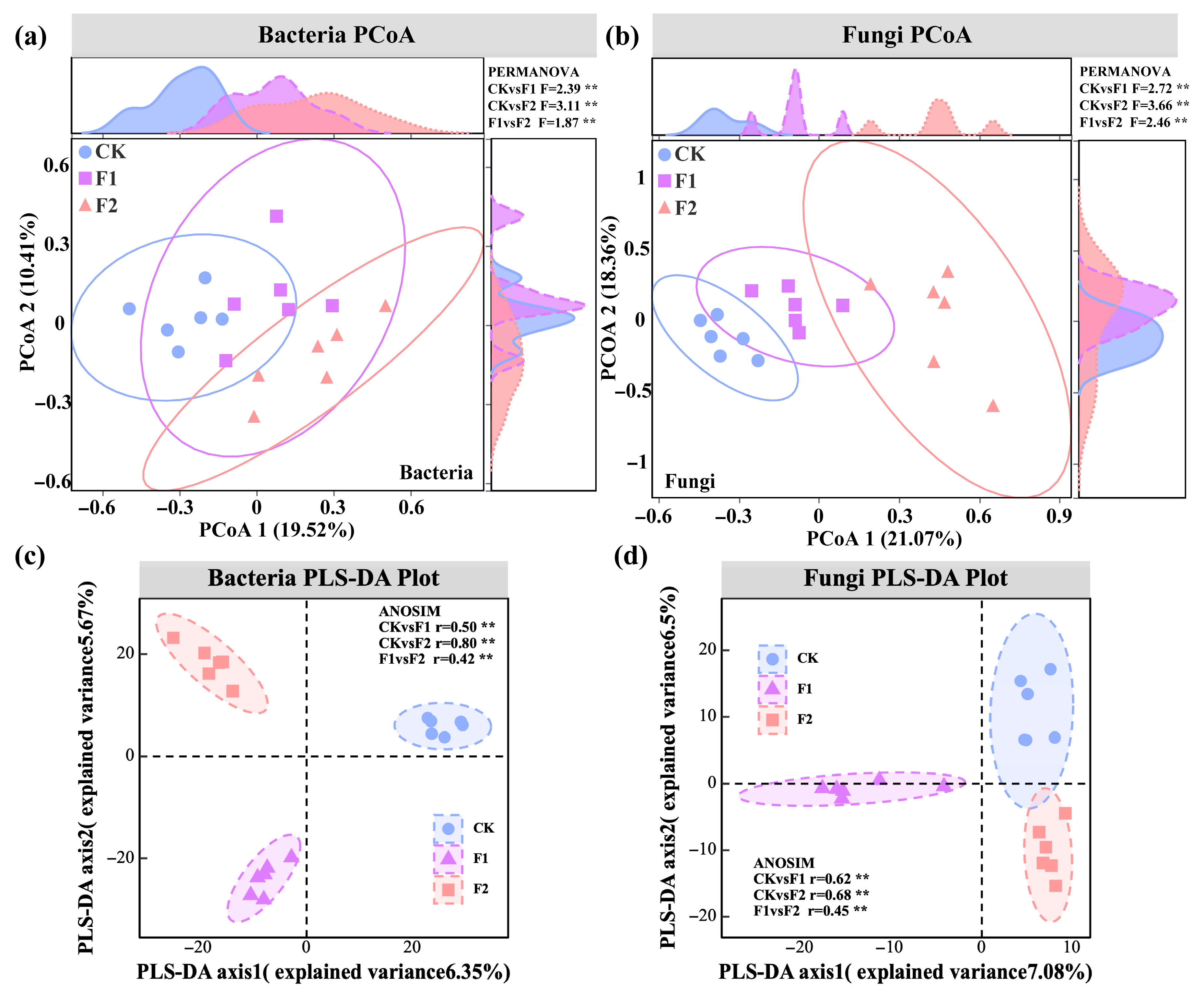
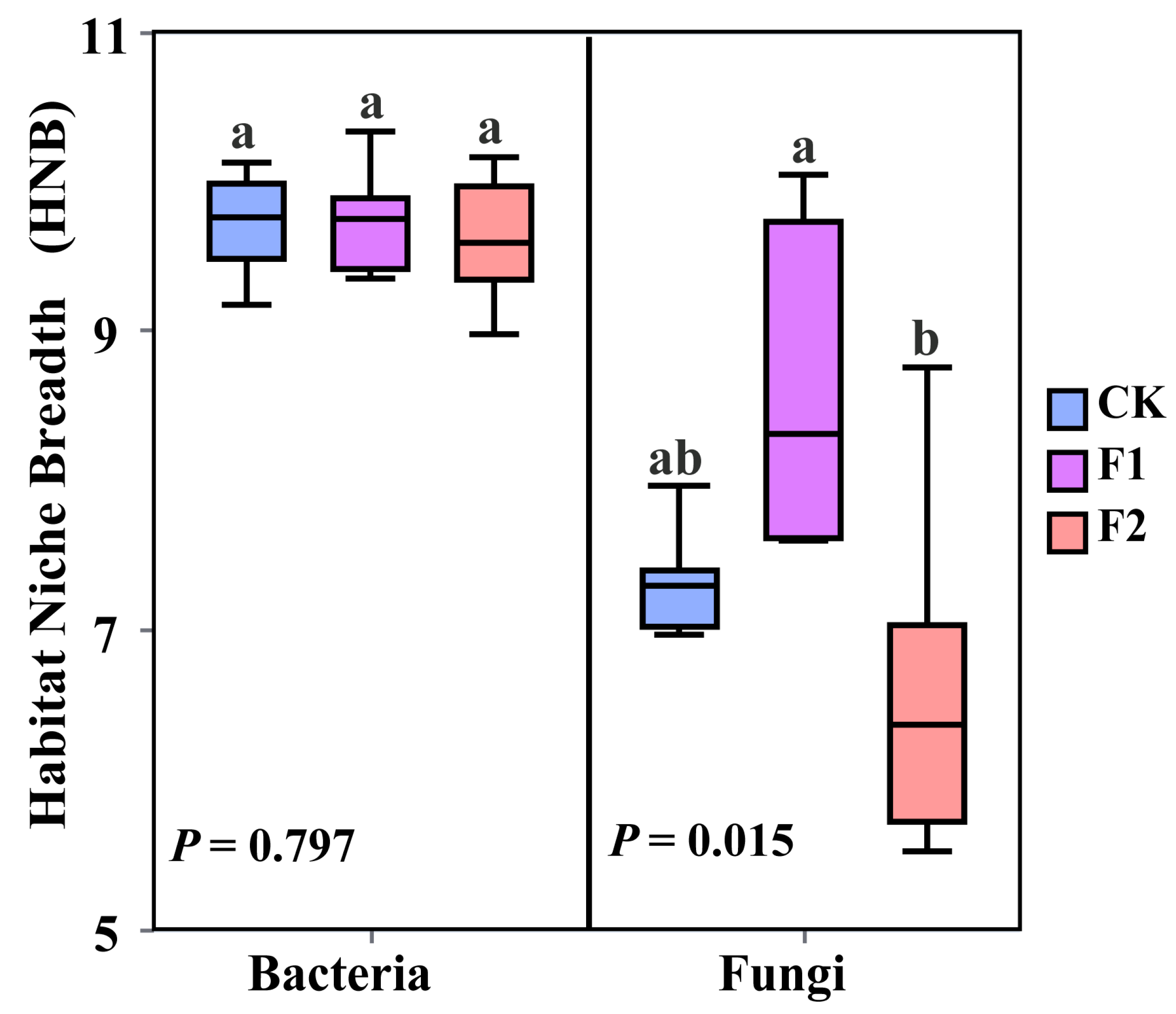
3.3. Effects of Potassium Fulvate Application on Rhizosphere Microorganisms
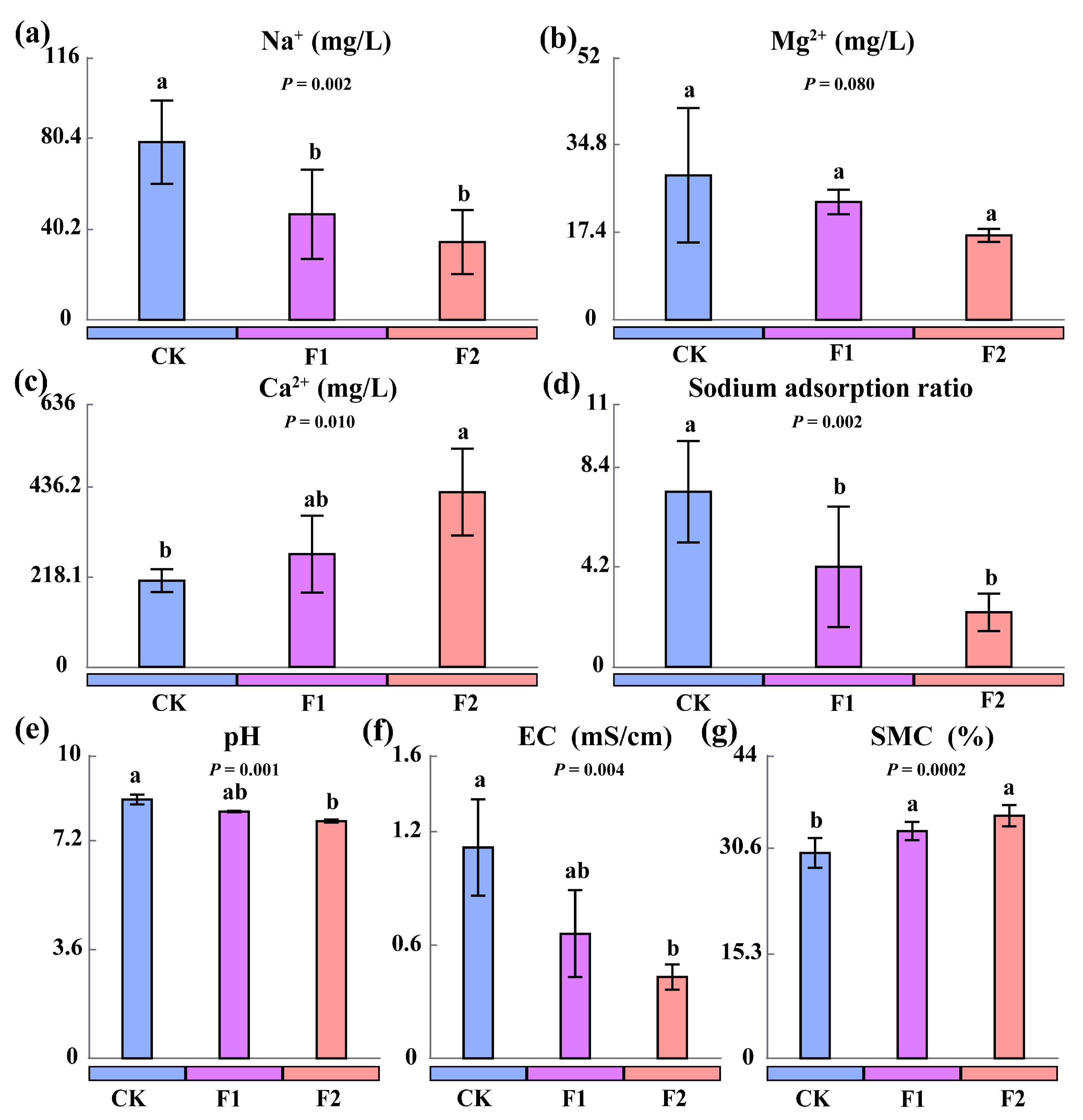
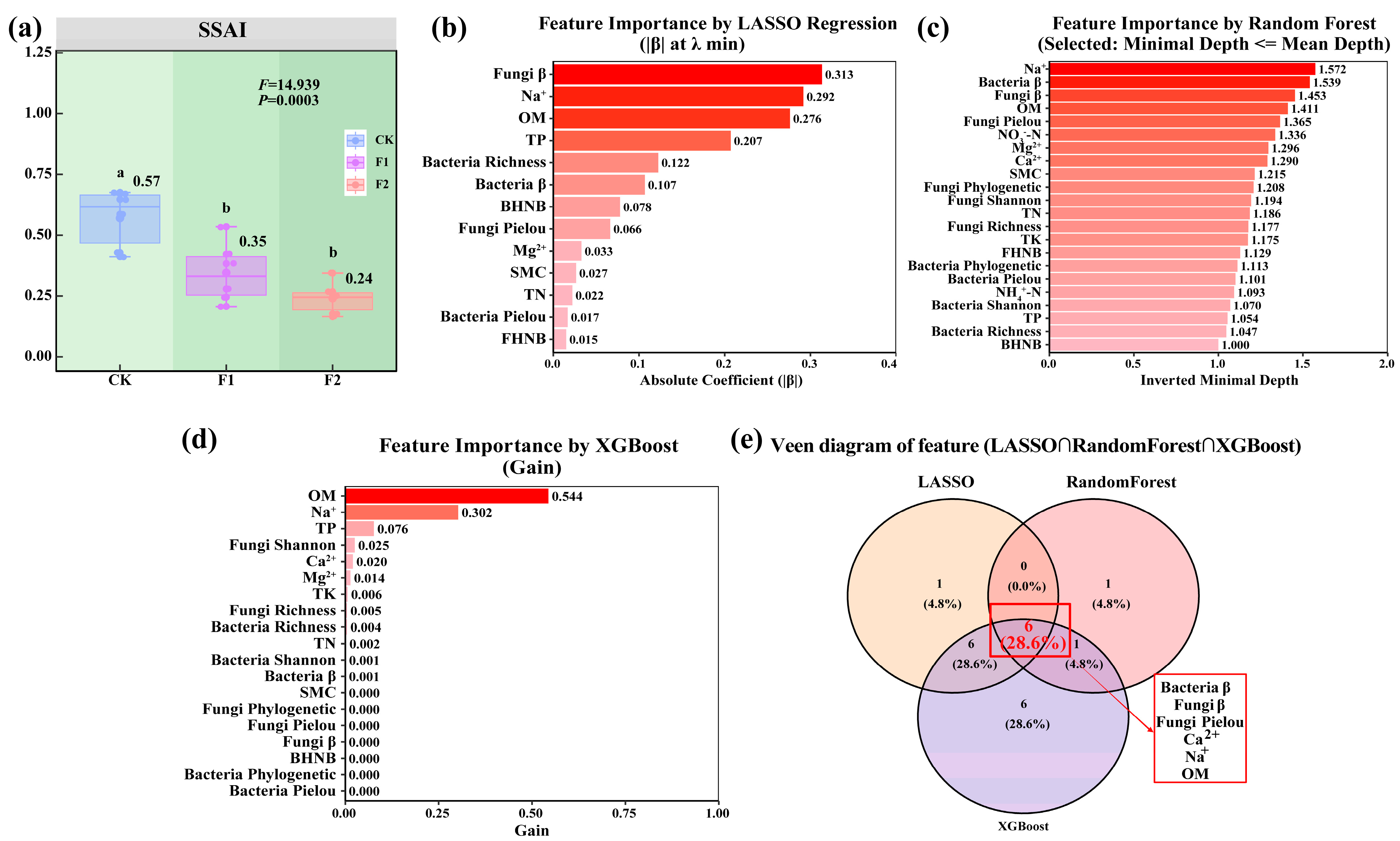
3.4. Effects of Potassium Fulvate Application on Rhizosphere Soil Salinity and Alkalinity
4. Discussion
4.1. Potassium Fulvate Promotes Plant Growth and Optimizes Rhizospheric Soil
4.2. Potassium Fulvate Alters Community Turnover and Reshapes Microbial Community Structure
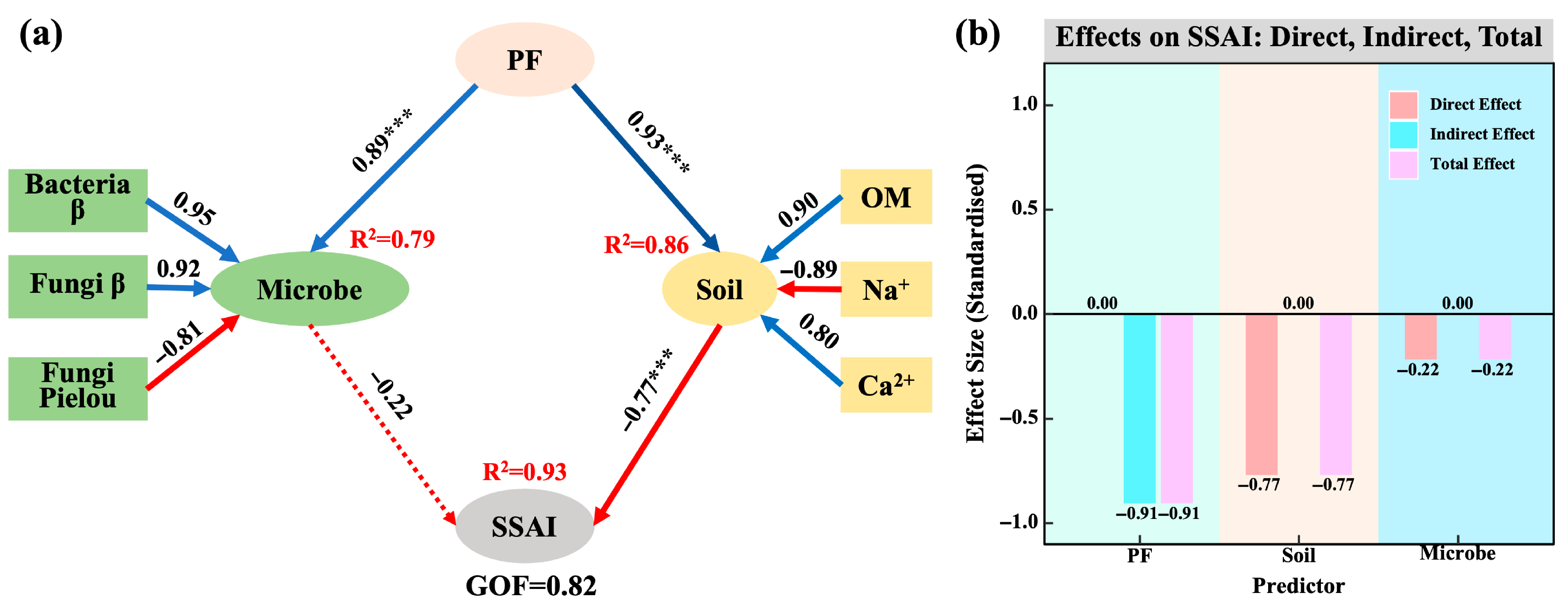
4.3. Construction of the Salinization–Alkalization Stress Index (SSAI) and Its Ecological Regulation Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shang, X.; Geng, L.; Yang, J.; Zhang, Y.; Xu, W. Transcriptome Analysis Reveals the Mechanism of Alkalinity Exposure on Spleen Oxidative Stress, Inflammation and Immune Function of Luciobarbus capito. Ecotoxicol. Environ. Saf. 2021, 225, 112748. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Kawaoka, A.; Nishikubo, N.; Osakabe, K. Responses to Environmental Stresses in Woody Plants: Key to Survive and Longevity. J. Plant Res. 2012, 125, 1–10. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; He, X.; Lin, E.; Yang, G. Winter Irrigation Effects on Soil Moisture, Temperature and Salinity, and on Cotton Growth in Salinized Fields in Northern Xinjiang, China. Sustainability 2020, 12, 7573. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, K.; Qian, H.; Gao, Y.; Fang, Y.; Xiao, S.; Tang, S.; Zhang, Q.; Qu, W.; Ren, W. Characterization of Soil Salinization and Its Driving Factors in a Typical Irrigation Area of Northwest China. Sci. Total Environ. 2022, 837, 155808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.; Sun, H.; Yang, F. In the Qaidam Basin, Soil Nutrients Directly or Indirectly Affect Desert Ecosystem Stability under Drought Stress through Plant Nutrients. Plants 2024, 13, 1849. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, G.; Mu, X.; Lan, Z.; Jiao, J.; An, S.; Wu, Y.; Miping, P. Integrated Assessments of Land Degradation on the Qinghai-Tibet Plateau. Ecol. Indic. 2023, 147, 109945. [Google Scholar] [CrossRef]
- Cuevas, J.; Daliakopoulos, I.N.; del Moral, F.; Hueso, J.J.; Tsanis, I.K. A Review of Soil-Improving Cropping Systems for Soil Salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef]
- Howe, C.W.; Goemans, C.; Howe, C.W.; Goemans, C. Water Transfers and Their Impacts: Lessons from Three Colorado Water Markets. J. Am. Water Resour. Assoc. 2003, 39, 1055–1065. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, N. Study on the Harm of Saline Alkali Land and Its Improvement Technology in China. IOP Conf. Ser. Earth Environ. Sci. 2021, 692, 42053. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.; Zhao, X.; Li, Z.; Li, M.; Zhang, J.; Wei, K. Field Irrigation Using Magnetized Brackish Water Affects the Growth and Water Consumption of Haloxylon ammodendron Seedlings in an Arid Area. Front. Plant Sci. 2022, 13, 929021. [Google Scholar] [CrossRef]
- Haddeland, I.; Heinke, J.; Biemans, H.; Eisner, S.; Flörke, M.; Hanasaki, N.; Konzmann, M.; Ludwig, F.; Masaki, Y.; Schewe, J.; et al. Global Water Resources Affected by Human Interventions and Climate Change. Proc. Natl. Acad. Sci. USA 2014, 111, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, W.; Zhang, H.; Sun, J.; Zhao, Q. Experimental Study on the Effect of Deep Pine Technology on Water and Salt Transport in Soda Saline Land. Arab. J. Geosci. 2021, 14, 571. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, C.; Jia, X.; Ma, Q. Characteristics of Evapotranspiration and Water Consumption of Different Underlying Surfaces in Qaidam Basin. Water 2022, 14, 3469. [Google Scholar] [CrossRef]
- Hui, R.; Tan, H. Divergence of Nutrients, Salt Accumulation, Bacterial Community Structure and Diversity in Soil after 8 Years of Flood Irrigation with Surface Water and Groundwater. BMC Microbiol. 2024, 24, 477. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective Strategies for Reclamation of Saline-Alkali Soil and Response Mechanisms of the Soil-Plant System. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, Y.; Wang, Z.; Mu, Y. Influence of Different Phytoremediation on Soil Microbial Diversity and Community Composition in Saline-Alkaline Land. Int. J. Phytorem. 2022, 24, 507–517. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, J.; Wu, H.; Zhu, Y.; Nimir, N.E.A.; Zhou, G. The Optimum Mixed Cropping Ratio of Oat and Alfalfa Enhanced Plant Growth, Forage Yield, and Forage Quality in Saline Soil. Plants 2024, 13, 3103. [Google Scholar] [CrossRef]
- Guo, C.; Xu, C.; Pu, X.; Zhao, Y.; Wang, J.; Fu, Y.; Wang, W. Oat and Forage Pea Mixed Sowing Improves Soil Chemical Fertility and Fresh and Dry Mass Yield in Light Saline–Alkali Land: Preliminary Results. Agronomy 2025, 15, 297. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, A.; Ma, F.; Liu, J.; Xiao, G.; Xu, X. Amendment of Saline–Alkaline Soil with Flue-Gas Desulfurization Gypsum in the Yinchuan Plain, Northwest China. Sustainability 2023, 15, 8658. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, B.; Wang, H.; Duan, M.; Feng, L. Effects of Different Soil Amendments on Physicochemical Property of Soda Saline-Alkali Soil and Crop Yield in Northeast China. Int. J. Agric. Biol. Eng. 2022, 15, 192–198. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Meng, Q.; Zeng, X.; Zhang, J.; Li, D.; Wang, J.; Du, W.; Ma, X. Additional Application of Aluminum Sulfate with Different Fertilizers Ameliorates Saline-Sodic Soil of Songnen Plain in Northeast China. J. Soils Sediments 2019, 19, 3521–3533. [Google Scholar] [CrossRef]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.S.; Alzuaibr, F.M.; Gharib, H.S.; et al. Applications of Humic and Fulvic Acid under Saline Soil Conditions to Improve Growth and Yield in Barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, Q.; Guo, X.; Li, H.; Chen, X.; Men, K.; Liu, X.; Shang, X.; Gao, Y.; Zhang, L.; et al. Effect of Potassium Fulvate on Continuous Tobacco Cropping Soils and Crop Growth. Front. Plant Sci. 2024, 15, 1457793. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Yao, R.; Chen, X.; Wang, X. Biochar and Fulvic Acid Amendments Mitigate Negative Effects of Coastal Saline Soil and Improve Crop Yields in a Three Year Field Trial. Sci. Rep. 2020, 10, 8946. [Google Scholar] [CrossRef]
- El-Shaboury, H.; Baddour, A.G.A. Effects of Various Organic Fertilizer Sources and External Applications of Potassium Fulvate and Potassium Citrate on the Yield and Quality of Two Barley Varieties Grown under Salt Affected Soil. J. Soil Sci. Agric. Eng. 2024, 15, 117–123. [Google Scholar] [CrossRef]
- Lumactud, R.A.; Gorim, L.Y.; Thilakarathna, M.S. Impacts of Humic-Based Products on the Microbial Community Structure and Functions toward Sustainable Agriculture. Front. Sustain. Food Syst. 2022, 6, 977121. [Google Scholar] [CrossRef]
- Whitton, M.M.; Ren, X.; Yu, S.J.; Irving, A.D.; Trotter, T.; Bajagai, Y.S.; Stanley, D. Humate Application Alters Microbiota–Mineral Interactions and Assists in Pasture Dieback Recovery. Heliyon 2023, 9, e13327. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, X.; Zhan, T.; Zhang, Z.; Chen, L.; Chen, L.; Ji, M. Rapid Warming and Increasing Moisture Levels in the Qaidam Basin. Theor. Appl. Climatol. 2024, 155, 7121–7132. [Google Scholar] [CrossRef]
- Lin, X.; Pan, S.; Zhang, W.; Jia, X.; Chen, X.; An, K.; Wu, L.; Huang, J.; Chen, X.; Chen, H. Establishing the Modern-like Differential Climate of the Qaidam Basin, Northeast Tibetan Plateau, since ca. 9 Ma: Implications of the Effect of Interactions between Tectonics and Atmospheric Circles on Paleoclimate. GSA Bull. 2024, 137, 2258–2270. [Google Scholar] [CrossRef]
- Shi, X.Z.; Yu, D.S.; Warner, E.D.; Pan, X.Z.; Petersen, G.W.; Weindorf, D.C. Soil Database of 1:1,000,000 Digital Soil Survey and Reference System of the Chinese Genetic Soil Classification System. Soil Surv. Horiz. 2004, 45, 129–136. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; ISBN 979-8-9862451-1-9. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Paz, A.M.; Amezketa, E.; Canfora, L.; Castanheira, N.; Falsone, G.; Goncalves, M.C.; Gould, I.; Hristov, B.; Mastrorilli, M.; Ramos, T.; et al. Salt-Affected Soils: Field-Scale Strategies for Prevention, Mitigation, and Adaptation to Salt Accumulation. Ital. J. Agron. 2023, 18, 2166. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, N.; Zhou, G.; Dang, P.; Yang, X.; Qiu, L.; Huang, M.; Gong, Y.; Zhao, S.; Chen, J. Response of Soil Microbial Community to Plant Composition Changes in Broad-Leaved Forests of the Karst Area in Mid-Subtropical China. PeerJ 2022, 10, e12739. [Google Scholar] [CrossRef]
- Zhu, G.; Schmidt, O.; Luan, L.; Xue, J.; Fan, J.; Geisen, S.; Sun, B.; Jiang, Y. Bacterial Keystone Taxa Regulate Carbon Metabolism in the Earthworm Gut. Microbiol. Spectr. 2022, 10, e0108122. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Sun, S.; Liu, W.; Zhu, L.; Yan, X. Different Forms and Proportions of Exogenous Nitrogen Promote the Growth of Alfalfa by Increasing Soil Enzyme Activity. Plants 2022, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Qin, Y.; Lu, X.; Zhang, J.; Chen, G.; Li, X. Fractal Dimension of Particle-Size Distribution and Their Relationships with Alkalinity Properties of Soils in the Western Songnen Plain, China. Sci. Rep. 2020, 10, 20603. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Lv, Y.; Liu, F.; Peng, J.; Shen, T.; Zhao, Y.; Tang, Y.; Luo, S. Quantitative Analysis of Nutrient Elements in Soil Using Single and Double-Pulse Laser-Induced Breakdown Spectroscopy. Sensors 2018, 18, 1526. [Google Scholar] [CrossRef]
- Bi, B.; Wang, K.; Zhang, H.; Wang, Y.; Fei, H.; Pan, R.; Han, F. Plants Use Rhizosphere Metabolites to Regulate Soil Microbial Diversity. Land Degrad. Dev. 2021, 32, 5267–5280. [Google Scholar] [CrossRef]
- Ciccazzo, S.; Esposito, A.; Rolli, E.; Zerbe, S.; Daffonchio, D.; Brusetti, L. Safe-Site Effects on Rhizosphere Bacterial Communities in a High-Altitude Alpine Environment. Biomed. Res. Int. 2014, 2014, 480170. [Google Scholar] [CrossRef]
- Schmidt Jennifer, E.; Vannette Rachel, L.; Igwe, A.; Blundell, R.; Casteel Clare, L.; Gaudin Amélie, C.M. Effects of Agricultural Management on Rhizosphere Microbial Structure and Function in Processing Tomato Plants. Appl. Environ. Microbiol. 2019, 85, e01064-19. [Google Scholar] [CrossRef]
- Shenhav, L.; Thompson, M.; Joseph, T.A.; Briscoe, L.; Furman, O.; Bogumil, D.; Mizrahi, I.; Pe’er, I.; Halperin, E. FEAST: Fast Expectation-Maximization for Microbial Source Tracking. Nat. Methods 2019, 16, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Wu, X.; Yan, Y.; Wei, C.; Luo, M.; Xiao, Y.; Zhang, Y. Evaluation of Groundwater Quality for Drinking and Irrigation Purposes Using GIS-Based IWQI, EWQI and HHR Model. Water 2023, 15, 2233. [Google Scholar] [CrossRef]
- Khan, M.H.; Xiao, Y.; Yang, H.; Zhang, Y.; Wang, L.; Wang, J.; Hu, W.; Chen, F.; Shrestha, R. Geochemical Characteristics, Mechanisms, and Suitability of Groundwater Resource for Sustainable Water Supply in Quetta Valley. Water Supply 2024, 24, 1802–1824. [Google Scholar] [CrossRef]
- Kreyling, J.; Jentsch, A.; Beier, C. Beyond Realism in Climate Change Experiments: Gradient Approaches Identify Thresholds and Tipping Points. Ecol. Lett. 2014, 17, 125-e1. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Huang, K.; Hao, G.; Li, J. Research on Optimal Control of Non-Point Source Pollution: A Case Study from the Danjiang River Basin in China. Environ. Sci. Pollut. Res. 2022, 29, 15582–15602. [Google Scholar] [CrossRef]
- Bhatt, A.G.; Kumar, A.; Trivedi, P.R. Integration of Multivariate Statistics and Water Quality Indices to Evaluate Groundwater Quality and Its Suitability in Middle Gangetic Floodplain, Bihar. SN Appl. Sci. 2021, 3, 426. [Google Scholar] [CrossRef]
- Basu, S.; Kumbier, K.; Brown, J.B.; Yu, B. Iterative Random Forests to Discover Predictive and Stable High-Order Interactions. Proc. Natl. Acad. Sci. USA 2018, 115, 1943–1948. [Google Scholar] [CrossRef]
- Shi, Y.; Fang, J.; Li, J.; Yu, K.; Zhu, J.; Lu, Y. Fracture Risk Prediction in Diabetes Patients Based on Lasso Feature Selection and Machine Learning. Comput. Methods Biomech. Biomed. Eng. 2024. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wei, C.; Deng, P.; Peng, T.; Hu, X. Deep Exploration of Random Forest Model Boosts the Interpretability of Machine Learning Studies of Complicated Immune Responses and Lung Burden of Nanoparticles. Sci. Adv. 2021, 7, eabf4130. [Google Scholar] [CrossRef]
- Lin, X.; Gao, F.; Lin, H.; Yao, W.; Wang, Y. XGBoost-Based Nomogram for Predicting Lymph Node Metastasis in Endometrial Carcinoma. Am. J. Cancer Res. 2024, 14, 5769–5783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Chen, B.; Yu, Y.; Wang, T.; Xu, N.; Fan, X.; Penuelas, J.; Fu, Z.; Deng, Y.; et al. Global Biogeography of Microbes Driving Ocean Ecological Status under Climate Change. Nat. Commun. 2024, 15, 4657. [Google Scholar] [CrossRef]
- Lu, M.; He, G.; Fan, L.; Liu, G.; Wu, J.; Liu, W.; Ma, L. Temperature Sensitivity of Aerobic and Anaerobic Organic Carbon Mineralization Varies with Climate and Soil Depth in Riparian Zones. Soil Biol. Biochem. 2024, 195, 109455. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z. Research on Influencing Factors of Soybean Yield in China’s Northeast Black Soil Region Based on PLS-SEM. Front. Sustain. Food Syst. 2024, 8, 1436224. [Google Scholar] [CrossRef]
- Mosaad, I.S.M.; Selim, E.-M.M.; Gaafar, D.E.M.; Al-Anoos, M.A.T. Effects of Humic and Fulvic Acids on Forage Production and Grain Quality of Triticale under Various Soil Salinity Levels. Cereal Res. Commun. 2024. [Google Scholar] [CrossRef]
- Abbas, G.; Rehman, S.; Siddiqui, M.H.; Ali, H.M.; Farooq, M.A.; Chen, Y. Potassium and Humic Acid Synergistically Increase Salt Tolerance and Nutrient Uptake in Contrasting Wheat Genotypes through Ionic Homeostasis and Activation of Antioxidant Enzymes. Plants 2022, 11, 263. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Wang, X.; Feng, J.; Zhu, S. Potassium Fulvic Acid Alleviates Salt Stress of Citrus by Regulating Rhizosphere Microbial Community, Osmotic Substances and Enzyme Activities. Front. Plant Sci. 2023, 14, 1161469. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Mao, G.; Zheng, H.; Wang, W.; Tang, Q. Growth Changes of Tomato Seedlings Responding to Sodium Salt of α-Naphthalene Acetic Acid and Potassium Salt of Fulvic Acid. Sci. Rep. 2023, 13, 4024. [Google Scholar] [CrossRef]
- Fan, J.; Lv, Q.; Zhou, T.; Wang, T.; Gao, H.; Zhou, W.; Ren, X.; Hu, S. Calcium Lactate as a Soil Amendment: Mechanistic Insights into Its Effect on Salinity, Alkalinity, and Aggregation in Saline-Alkaline Soils. Soil Tillage Res. 2025, 248, 106459. [Google Scholar] [CrossRef]
- Zhu, Y.; Ali, A.; Dang, A.; Wandel, A.P.; Bennett, J.M. Re-Examining the Flocculating Power of Sodium, Potassium, Magnesium and Calcium for a Broad Range of Soils. Geoderma 2019, 352, 422–428. [Google Scholar] [CrossRef]
- El-Kamar, F. Effect of Humic Acid and Yeast Waste Application on Fababean (Vicia faba) Yield, Yield Components and Some Soil Properties of Salt Affected Soil. J. Soil Sci. Agric. Eng. 2020, 11, 483–488. [Google Scholar] [CrossRef]
- Song, H.; Nam, K. Development of a Potassium-Based Soil Washing Solution Using Response Surface Methodology for Efficient Removal of Cesium Contamination in Soil. Chemosphere 2023, 332, 138854. [Google Scholar] [CrossRef]
- Canini, F.; Zucconi, L.; Pacelli, C.; Selbmann, L.; Onofri, S.; Geml, J. Vegetation, pH and Water Content as Main Factors for Shaping Fungal Richness, Community Composition and Functional Guilds Distribution in Soils of Western Greenland. Front. Microbiol. 2019, 10, 2348. [Google Scholar] [CrossRef]
- Yao, R.; Yang, J.; Zhu, W.; Li, H.; Yin, C.; Jing, Y.; Wang, X.; Xie, W.; Zhang, X. Impact of Crop Cultivation, Nitrogen and Fulvic Acid on Soil Fungal Community Structure in Salt-Affected Alluvial Fluvo-Aquic Soil. Plant Soil 2021, 464, 539–558. [Google Scholar] [CrossRef]
- Glassman, S.I.; Wang, I.J.; Bruns, T.D. Environmental Filtering by pH and Soil Nutrients Drives Community Assembly in Fungi at Fine Spatial Scales. Mol. Ecol. 2017, 26, 6960–6973. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Winston, G.C.; Goulden, M.L.; Treseder, K.K. Environmental Filtering Affects Soil Fungal Community Composition More than Dispersal Limitation at Regional Scales. Fungal Ecol. 2014, 12, 14–25. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Siri, M.; Liu, C.; Feng, C.; Shao, X.; Liu, K. Calcium-Modified Biochar Rather than Original Biochar Decreases Salinization Indexes of Saline-Alkaline Soil. Environ. Sci. Pollut. Res. 2023, 30, 74966–74976. [Google Scholar] [CrossRef] [PubMed]
- Sarani, F.; Ahangar, A.G.; Shabani, A. Predicting ESP and SAR by Artificial Neural Network and Regression Models Using Soil pH and EC Data (Miankangi Region, Sistan and Baluchestan Province, Iran). Arch. Agron. Soil Sci. 2016, 62, 127–138. [Google Scholar] [CrossRef]
- Zhang, Z.; Chi, Y.; Fu, Z.; Li, T.; Hu, R.; Zhang, Y.; Lu, Z.; Sun, J. Indirect Prediction Based on Machine Learning and Remote Sensing of Ecological Stoichiometric Ratio Superior to Direct Prediction. Land Degrad. Dev. 2025. [Google Scholar] [CrossRef]
- Yang, X.; Dai, Z.; Yuan, R.; Guo, Z.; Xi, H.; He, Z.; Wei, M. Effects of Salinity on Assembly Characteristics and Function of Microbial Communities in the Phyllosphere and Rhizosphere of Salt-Tolerant Avicennia Marina Mangrove Species. Microbiol. Spectr. 2023, 11, e03000–e03022. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xiao, Y.; Dong, J.; Xing, J.; Tang, F.; Shi, F. Variety-Driven Rhizosphere Microbiome Bestows Differential Salt Tolerance to Alfalfa for Coping with Salinity Stress. Front. Plant Sci. 2023, 14, 1324333. [Google Scholar] [CrossRef]
- Li, R.; Jiao, H.; Sun, B.; Song, M.; Yan, G.; Bai, Z.; Wang, J.; Zhuang, X.; Hu, Q. Understanding Salinity-Driven Modulation of Microbial Interactions: Rhizosphere versus Edaphic Microbiome Dynamics. Microorganisms 2024, 12, 683. [Google Scholar] [CrossRef]
- Wang, S.; Gao, P.; Zhang, Q.; Shi, Y.; Guo, X.; Lv, Q.; Wu, W.; Zhang, X.; Li, M.; Meng, Q. Application of Biochar and Organic Fertilizer to Saline-Alkali Soil in the Yellow River Delta: Effects on Soil Water, Salinity, Nutrients, and Maize Yield. Soil Use Manag. 2022, 38, 1679–1692. [Google Scholar] [CrossRef]
- Xie, Y.; Ning, H.; Zhang, X.; Zhou, W.; Xu, P.; Song, Y.; Li, N.; Wang, X.; Liu, H. Reducing the Sodium Adsorption Ratio Improves the Soil Aggregates and Organic Matter in Brackish-Water-Irrigated Cotton Fields. Agronomy 2024, 14, 2169. [Google Scholar] [CrossRef]
- Liu, J.; Xie, W.; Yang, J.; Yao, R.; Wang, X.; Li, W. Effect of Different Fertilization Measures on Soil Salinity and Nutrients in Salt-Affected Soils. Water 2023, 15, 3274. [Google Scholar] [CrossRef]
- Li, D.; Qiu, H.; Tian, G.; Zhao, Y.; Zhou, X.; He, S. Soil Salinity Is the Main Factor Influencing the Soil Bacterial Community Assembly Process under Long-Term Drip Irrigation in Xinjiang, China. Front. Microbiol. 2023, 14, 1291962. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jin, X.; Liu, X.; Fu, Y.; Bao, K.; Quan, Z.; Xu, C.; Wang, W.; Lu, G.; Zhang, H. Potassium Fulvate Alleviates Salinity and Boosts Oat Productivity by Modifying Soil Properties and Rhizosphere Microbial Communities in the Saline–Alkali Soils of the Qaidam Basin. Agronomy 2025, 15, 1673. https://doi.org/10.3390/agronomy15071673
Wang J, Jin X, Liu X, Fu Y, Bao K, Quan Z, Xu C, Wang W, Lu G, Zhang H. Potassium Fulvate Alleviates Salinity and Boosts Oat Productivity by Modifying Soil Properties and Rhizosphere Microbial Communities in the Saline–Alkali Soils of the Qaidam Basin. Agronomy. 2025; 15(7):1673. https://doi.org/10.3390/agronomy15071673
Chicago/Turabian StyleWang, Jie, Xin Jin, Xinyue Liu, Yunjie Fu, Kui Bao, Zhixiu Quan, Chengti Xu, Wei Wang, Guangxin Lu, and Haijuan Zhang. 2025. "Potassium Fulvate Alleviates Salinity and Boosts Oat Productivity by Modifying Soil Properties and Rhizosphere Microbial Communities in the Saline–Alkali Soils of the Qaidam Basin" Agronomy 15, no. 7: 1673. https://doi.org/10.3390/agronomy15071673
APA StyleWang, J., Jin, X., Liu, X., Fu, Y., Bao, K., Quan, Z., Xu, C., Wang, W., Lu, G., & Zhang, H. (2025). Potassium Fulvate Alleviates Salinity and Boosts Oat Productivity by Modifying Soil Properties and Rhizosphere Microbial Communities in the Saline–Alkali Soils of the Qaidam Basin. Agronomy, 15(7), 1673. https://doi.org/10.3390/agronomy15071673




