Flowering and Morphology Responses of Greenhouse Ornamentals to End-of-Day Blue-Dominant Lighting with Different Phytochrome Photostationary States
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Experimental Design and Spectrum Treatments
2.3. Flowering, Morphology, and Biomass Measurements
2.4. Statistical Analysis
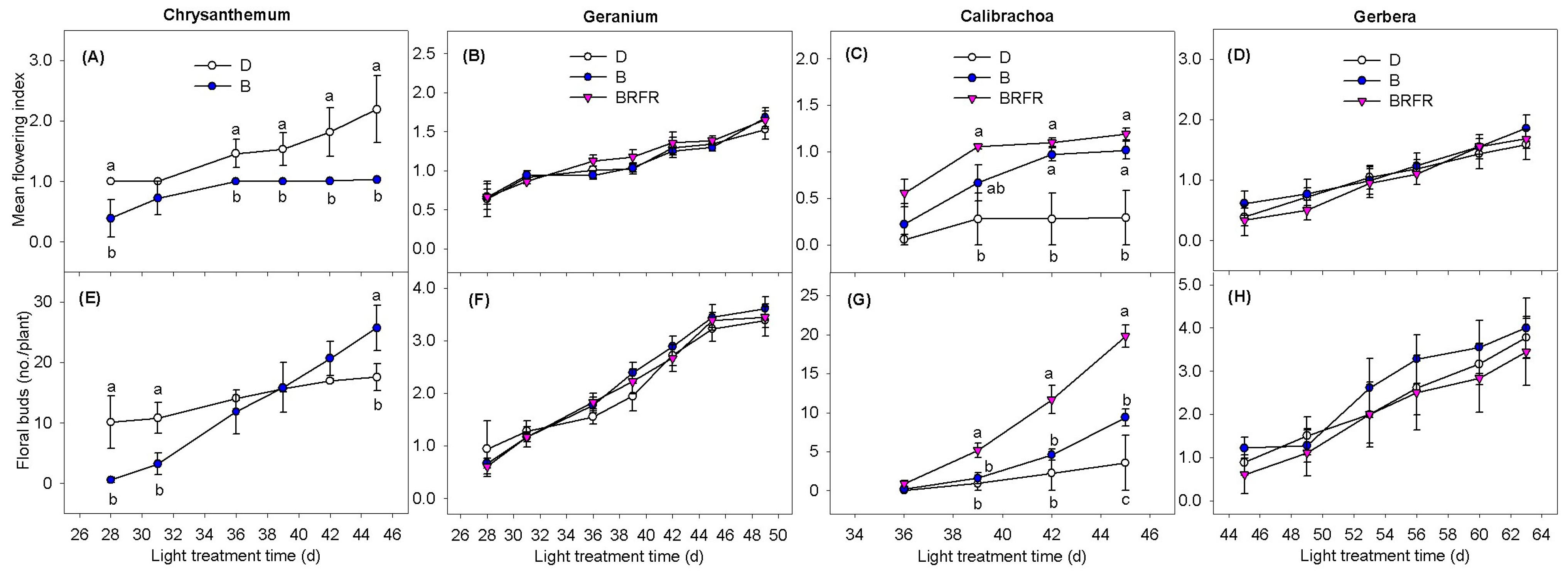
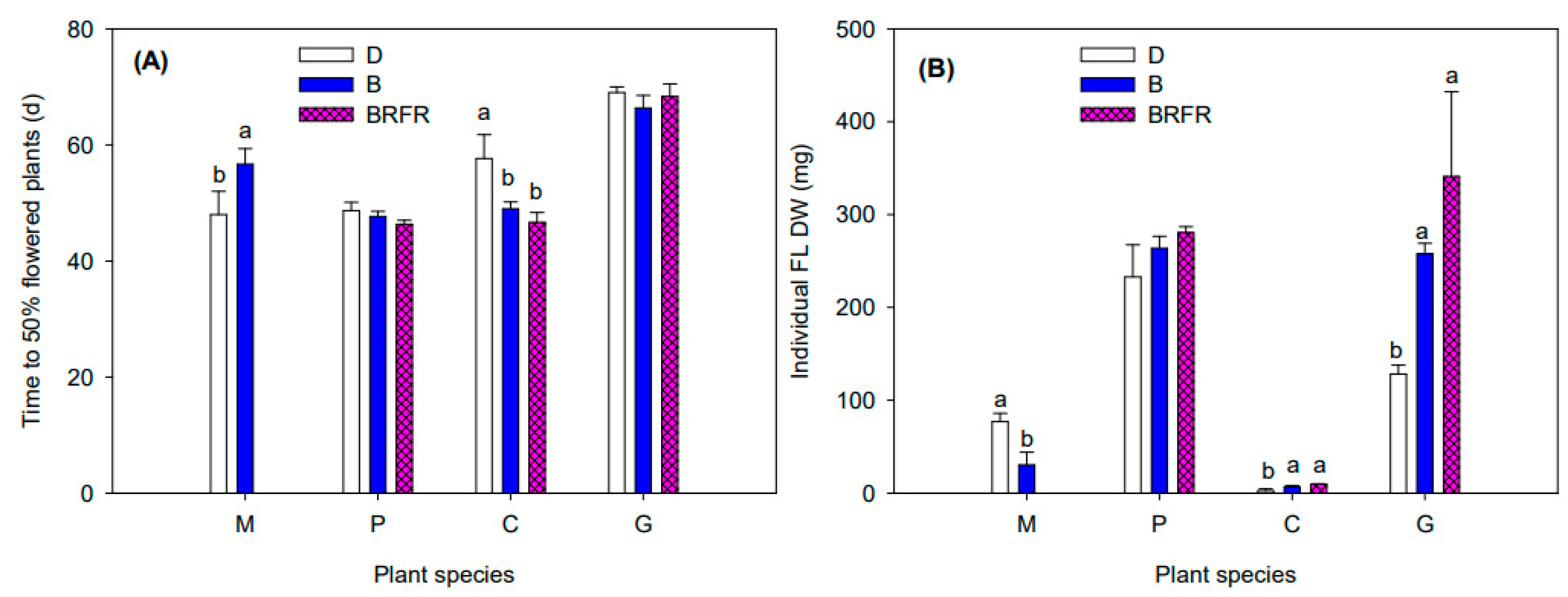
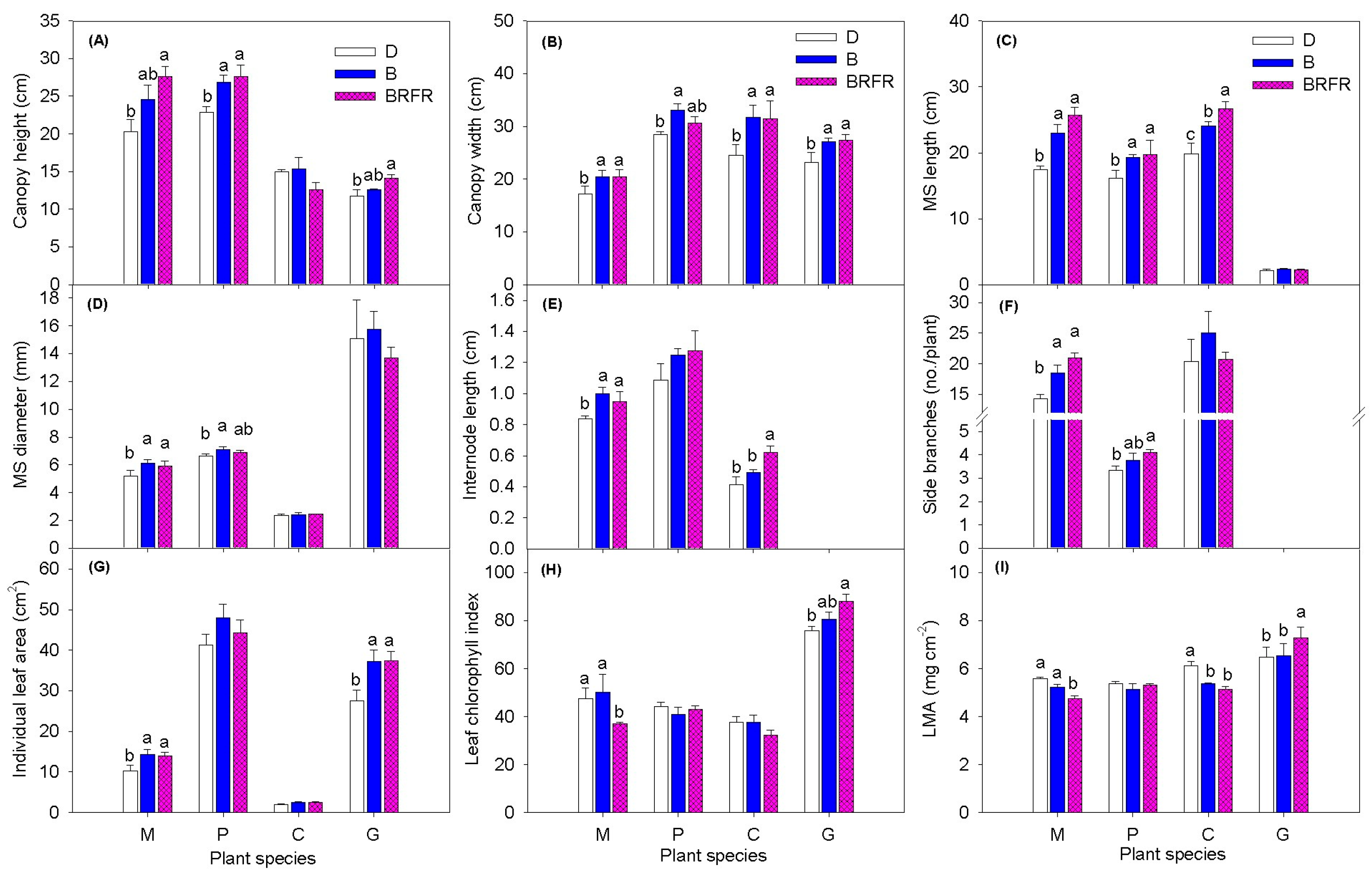
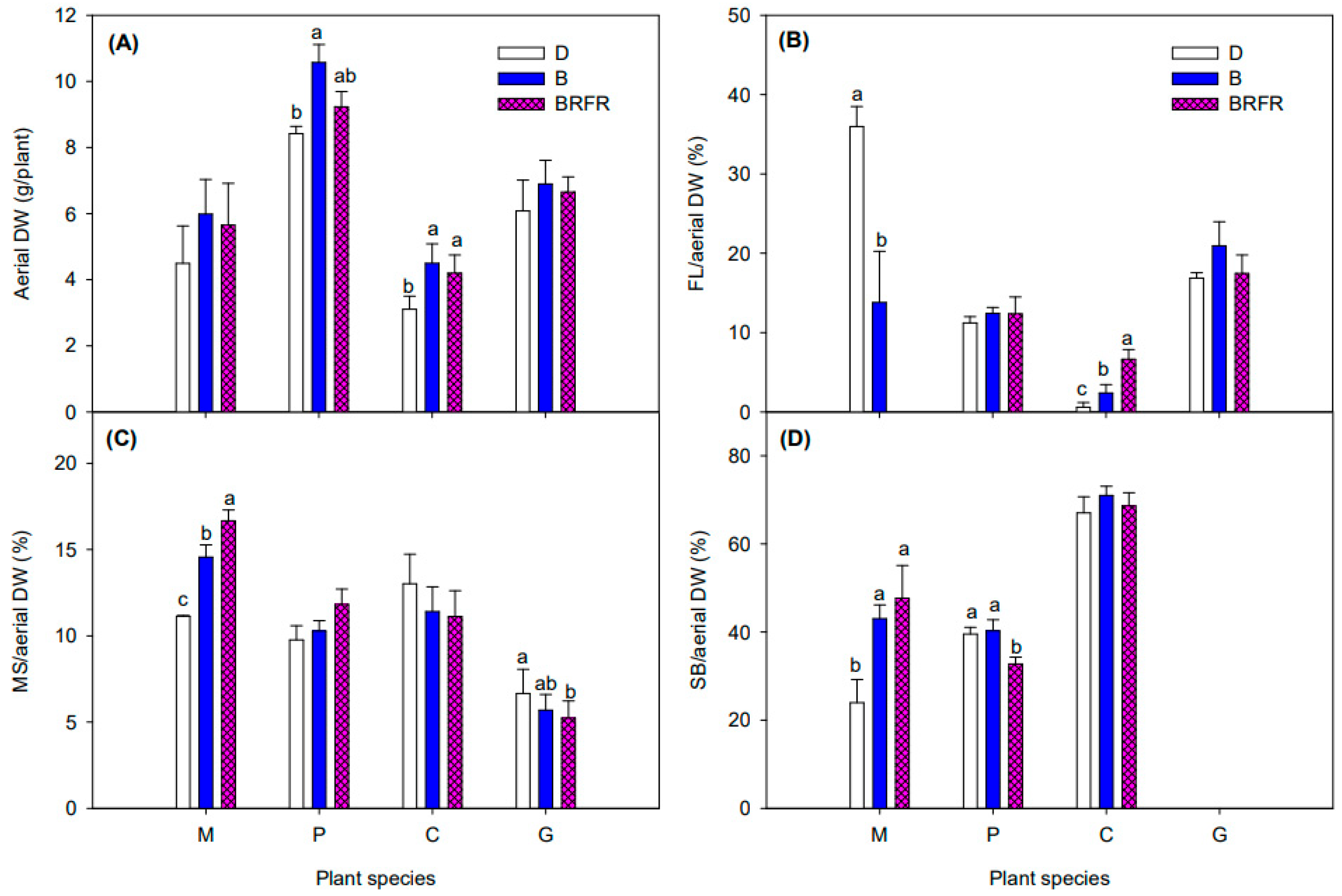
3. Results
3.1. Flowering Responses
3.2. Plant Morphology
3.3. Plant Biomass and Its Allocation
4. Discussion
4.1. Flowering of Obligate SDP Chrysanthemum Mainly Depends on the PPS of the Blue-Dominant Spectrum from EOD Lighting
4.2. EOD Lighting Using Impure B Spectrum with Higher PPS Had Greater Flowering Promotion Effects in LDP Calibrachoa than Pure B Spectrum with Lower PPS
4.3. EOD Lighting with Blue-Dominant Spectra Did Not Affect Flowering of DNP Geranium and Facultative SDP Gerbera but Modified Plant Morphology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Runkle, E.S.; Heins, R.D. Manipulating the light environment to control flowering and morphogenesis of herbaceous plants. Acta Hortic. 2006, 711, 51–60. [Google Scholar] [CrossRef]
- Kohyama, F.; Whitman, C.; Runkle, E.S. Comparing flowering responses of long-day plants under incandescent and two commercial light-emitting diode lamps. HortTechnology 2014, 24, 490–495. [Google Scholar] [CrossRef]
- Erwin, J.; Mattson, N.; Warner, R. Light effects on bedding plants. Light Management in Controlled Environments; Meister Media Worldwide: Willoughby, OH, USA, 2017; pp. 119–134. [Google Scholar]
- Papadopoulos, A.P.; Demers, D.A.; Theriault, J. The Canadian greenhouse vegetable industry with special emphasis on artificial lighting. Acta Hortic. 2002, 580, 29–33. [Google Scholar] [CrossRef]
- Moe, R.; Grimstad, S.O.; Gislerod, H.R. The use of artificial light in year round production of greenhouse crops in Norway. Acta Hortic. 2006, 711, 35–42. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Control of flowering using night-interruption and day-extension LED lighting. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E.S., Eds.; Springer Science + Business Media: Singapore, 2016; pp. 191–201. [Google Scholar] [CrossRef]
- Simpson, G.G.; Dean, C. Arabidopsis, the Rosetta stone of flowering time? Science 2002, 296, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Putterill, J.; Laurie, R.; Macknight, R. It’s time to flower: The genetic control of flowering time. Bioessays 2004, 26, 363–373. [Google Scholar] [CrossRef]
- Thomas, B. Light signals and flowering. J. Exp. Bot. 2006, 57, 3387–3393. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Jeong, S.W.; Hogewoning, S.W.; van Ieperen, W. Responses of supplemental blue light on flowering and stem extension growth of cut chrysanthemum. Sci. Hortic. 2014, 165, 69–74. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. How supplementary or night-interrupting low-intensity blue light affects the flower induction in chrysanthemum, a qualitative short-day plant. Plants 2020, 9, 1694. [Google Scholar] [CrossRef]
- Neo, D.C.J.; Ong, M.M.X.; Lee, Y.Y.; Teo, E.J.; Ong, Q.; Tanoto, H.; Xu, J.; Ong, K.S.; Suresh, V. Shaping and tuning lighting conditions in controlled environment agriculture: A review. ACS Agric. Sci. Technol. 2022, 2, 3–16. [Google Scholar] [CrossRef]
- Heuvelink, E.; Hemming, S.; Marcelis, L.F. Some recent developments in controlled-environment agriculture: On plant physiology, sustainability, and autonomous control. J. Hortic. Sci. Biotechnol. 2024, 1–11. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Van Ieperen, W. Plant morphological and developmental responses to light quality in a horticultural context. Acta Hortic. 2012, 956, 131–139. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Blue light associated with low phytochrome activity can promote flowering: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 433–440. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue-light-promoted elongation and flowering are not artifacts from 24-h lighting: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 659–666. [Google Scholar] [CrossRef]
- Singh, M.C.; van Ieperen, W.; Heuvelink, E.P. Effect of LEDs on flower bud induction in Chrysanthemum morifolium cv. Zembla. HortFlora Res. Spectr. 2013, 2, 185–188. [Google Scholar]
- Park, Y.G.; Jeong, B.R. Night interruption light quality changes morphogenesis, flowering, and gene expression in Dendranthema grandiflorum. Hortic. Environ. Biotechnol. 2019, 60, 167–173. [Google Scholar] [CrossRef]
- Higuchi, Y.; Sumitomo, K.; Oda, A.; Shimizu, H.; Hisamatsu, T. Day light quality affects the night-break response in the short-day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. J. Plant Physiol. 2012, 169, 1789–1796. [Google Scholar] [CrossRef]
- Kohler, A.E.; Birtell, E.M.; Runkle, E.S.; Meng, Q. Day-extension blue light inhibits flowering of chrysanthemum when the short main photoperiod includes far-red light. J. Am. Soc. Hortic. Sci. 2023, 148, 89–98. [Google Scholar] [CrossRef]
- SharathKumar, M.; Luo, J.; Xi, Y.; van Ieperen, W.; Marcelis, L.F.; Heuvelink, E. Several short-day species can flower under blue-extended long days, but this response is not universal. Sci. Hortic. 2024, 325, 112657. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Moderate-intensity blue radiation can regulate flowering, but not extension growth, of several photoperiodic ornamental crops. Environ. Exp. Bot. 2017, 134, 12–20. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Low-intensity blue light in night-interruption lighting does not influence flowering of herbaceous ornamentals. Sci. Hortic. 2015, 186, 230–238. [Google Scholar] [CrossRef]
- Lopez, R.G.; Meng, Q.; Runkle, E.S. Blue radiation signals and saturates photoperiodic flowering of several long-day plants at crop-specific photon flux densities. Sci. Hortic. 2020, 271, 109470. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Young, R.E.; McMahon, M.J.; Oi, R. Plant height control by photoselective filters: Current status and future prospects. HortTechnology 1999, 9, 618–624. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Photocontrol of flowering and extension growth in the long-day plant pansy. J. Am. Soc. Hortic. Sci. 2003, 128, 479–485. [Google Scholar] [CrossRef]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. Am. Soc. Agric. Eng. 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Phototropin is partly involved in blue-light-mediated stem elongation, flower initiation, and leaf expansion: A comparison of phenotypic responses between wild Arabidopsis and its phototropin mutants. Environ. Exp. Bot. 2020, 171, 103967. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Low-activity cryptochrome 1 plays a role in promoting stem elongation and flower initiation of mature Arabidopsis under blue light associated with low phytochrome activity. Can. J. Plant Sci. 2022, 102, 755–759. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.; Van Ieperen, W. Floral induction in the short-day plant chrysanthemum under blue and red extended long-days. Front. Plant Sci. 2021, 11, 610041. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.; Kong, Y.; Zheng, Y. Applying blue light alone, or in combination with far-red light, during nighttime increases elongation without compromising yield and quality of indoor-grown microgreens. HortScience 2020, 55, 876–881. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Overnight supplemental blue, rather than far-red, light improves microgreen yield and appearance quality without compromising nutritional quality during winter greenhouse production. HortScience 2020, 55, 1468–1474. [Google Scholar] [CrossRef]
- Llewellyn, D.; Schiestel, K.; Zheng, Y. Increasing levels of supplemental LED light enhances the rate flower development of greenhouse-grown cut gerbera but does not affect flower size and quality. Agronomy 2020, 10, 1332. [Google Scholar] [CrossRef]
- Mah, J.J.; Llewellyn, D.; Zheng, Y. Blackout reduces height of easter lily but end-of-day red light treatment using light-emitting diodes does not. HortTechnology 2020, 30, 156–162. [Google Scholar] [CrossRef]
- Meier, U. BBCH-Monograph: Growth Stages of Mono- and Dicotyledonous Plants; Julius Kühn-Institut (JKI): Quedlinburg, Germany, 2018. [Google Scholar]
- Kong, Y.; Zheng, Y. Diverse flowering response to blue light manipulation: Application of electric lighting in controlled-environment plant production. Horticulturae 2024, 10, 578. [Google Scholar] [CrossRef]
- Nissim-Levi, A.; Kitron, M.; Nishri, Y.; Ovadia, R.; Forer, I.; Oren-Shamir, M. Effects of blue and red LED lights on growth and flowering of Chrysanthemum morifolium. Sci. Hortic. 2019, 254, 77–83. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Complex signaling networks underlying blue-light-mediated floral transition in plants. Plants 2025, 14, 1533. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. The flowering of SDP chrysanthemum in response to intensity of supplemental or night-interruptional blue light is modulated by both photosynthetic carbon assimilation and photoreceptor-mediated regulation. Front. Plant Sci. 2022, 13, 981143. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Blue light supplemented at intervals in long-day conditions intervenes in photoperiodic flowering, photosynthesis, and antioxidant properties in chrysanthemums. Antioxidants 2022, 11, 2310. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Low-intensity blue light supplemented during photoperiod in controlled environment induces flowering and antioxidant production in kalanchoe. Antioxidants 2022, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, J.; Park, Y.G.; Jeong, B.R. Both the positioned supplemental or night-interruptional blue light and the age of leaves (or tissues) are important for flowering and vegetative growth in chrysanthemum. Plants 2024, 13, 2874. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zheng, Y. Multiple signals can be integrated into pathways of blue-light-mediated floral transition: Possible explanations on diverse flowering responses to blue light manipulation. Agronomy 2025, 15, 1534. [Google Scholar] [CrossRef]
- Stutte, G.W. Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Craig, D.S.; Runkle, E.S. A moderate to high red to far-red light ratio from light-emitting diodes controls flowering of short-day plants. J. Am. Soc. Hortic. Sci. 2013, 138, 167–172. [Google Scholar] [CrossRef]
- Higuchi, Y.; Narumi, T.; Oda, A.; Nakano, Y.; Sumitomo, K.; Fukai, S.; Hisamatsu, T. The gated induction system of a systemic floral inhibitor, antiflorigen, determines obligate short-day flowering in chrysanthemums. Proc. Natl. Acad. Sci. USA 2013, 110, 17137–17142. [Google Scholar] [CrossRef] [PubMed]
- Eskins, K. Light quality effects on Arabidopsis development: Red, blue and far-red regulation of flowering and morphology. Physiol. Plant. 1992, 86, 439–444. [Google Scholar] [CrossRef]
- Fukuda, N.; Ajima, C.; Yukawa, T.; Olsen, J.E. Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 2016, 121, 102–111. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Mockler, T.C.; Lin, C. Regulation of flowering time by Arabidopsis photoreceptors. Science 1998, 279, 1360–1363. [Google Scholar] [CrossRef]
- Lin, C.; Yang, H.; Guo, H.; Mockler, T.; Chen, J.; Cashmore, A.R. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 1998, 95, 2686–2690. [Google Scholar] [CrossRef]
- Lin, C. Photoreceptors and regulation of flowering time. Plant Physiol. 2000, 123, 39–50. [Google Scholar] [CrossRef]
- Zeevaart, J.A.D. Physiology of flower formation. Annu. Rev. Plant Physiol. 1976, 27, 321–348. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific functions of red, far red, and blue light in flowering and stem extension of long-day plants. J. Am. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar] [CrossRef]
- Thomas, B.; Vince-Prue, D. Do long-day plants and short-day plants perceive daylength in the same way? Flower. Newsl. 1995, 50–58. Available online: https://www.jstor.org/stable/43008156 (accessed on 29 May 2025).
- Lane, H.; Cathey, H.; Evans, L. The dependence of flowering in several long-day plants on the spectral composition of light extending the photoperiod. Am. J. Bot. 1965, 52, 1006–1014. [Google Scholar]
- Kohler, A.E.; Lopez, R.G. Duration of light-emitting diode (LED) supplemental lighting providing far-red radiation during seedling production influences subsequent time to flower of long-day annuals. Sci. Hortic. 2021, 281, 109956. [Google Scholar] [CrossRef]
- Lagercrantz, U. At the end of the day: A common molecular mechanism for photoperiod responses in plants? J. Exp. Bot. 2009, 60, 2501–2515. [Google Scholar] [CrossRef]
- Hughes, J.; Morgan, D.; Lambton, P.; Black, C.; Smith, H. Photoperiodic time signals during twilight. Plant Cell Environ. 1984, 7, 269–277. [Google Scholar] [CrossRef]
- Holmes, M.; Smith, H. The function of phytochrome in the natural environment—I. Characterization of daylight for studies in photomorphogenesis and photoperiodism. Photochem. Photobiol. 1977, 25, 533–538. [Google Scholar] [CrossRef]
- Smith, H.; Whitelam, G.C. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Soundararajan, P.; Manivnnan, A.; Jeong, B.R. Light quality during night interruption affects morphogenesis and flowering in geranium. Hortic. Environ. Biotechnol. 2017, 58, 212–217. [Google Scholar] [CrossRef]
- Singh, A.K. Flower Crops: Cultivation and Management; New India Publishing: New Delhi, India, 2006. [Google Scholar]

| Parameter | Compartment | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Day temperature (°C) | 21.5 ± 0.43 | 21.6 ± 0.48 | 21.9 ± 1.22 |
| Day RH (%) | 53.5 ± 4.83 | 54.9 ± 3.34 | 52.6 ± 4.08 |
| Night temperature (°C) | 18.4 ± 0.35 | 18.5 ± 0.37 | 18.4 ± 0.45 |
| Night RH (%) | 58.7 ± 3.36 | 57.6 ± 2.31 | 55.9 ± 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Li, Q.; Llewellyn, D.; Zheng, Y. Flowering and Morphology Responses of Greenhouse Ornamentals to End-of-Day Blue-Dominant Lighting with Different Phytochrome Photostationary States. Agronomy 2025, 15, 1649. https://doi.org/10.3390/agronomy15071649
Kong Y, Li Q, Llewellyn D, Zheng Y. Flowering and Morphology Responses of Greenhouse Ornamentals to End-of-Day Blue-Dominant Lighting with Different Phytochrome Photostationary States. Agronomy. 2025; 15(7):1649. https://doi.org/10.3390/agronomy15071649
Chicago/Turabian StyleKong, Yun, Qingming Li, David Llewellyn, and Youbin Zheng. 2025. "Flowering and Morphology Responses of Greenhouse Ornamentals to End-of-Day Blue-Dominant Lighting with Different Phytochrome Photostationary States" Agronomy 15, no. 7: 1649. https://doi.org/10.3390/agronomy15071649
APA StyleKong, Y., Li, Q., Llewellyn, D., & Zheng, Y. (2025). Flowering and Morphology Responses of Greenhouse Ornamentals to End-of-Day Blue-Dominant Lighting with Different Phytochrome Photostationary States. Agronomy, 15(7), 1649. https://doi.org/10.3390/agronomy15071649









