Optimizing Effects of Organic Farming and Moderately Low Nitrogen Levels on Soil Carbon and Nitrogen Pools
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Materials
2.2. Tillage Practices
2.3. Soil Sample Collection
2.4. Measurements
2.4.1. Determination of Soil Nutrient Content
2.4.2. Determination of Soil Enzyme Activity
2.4.3. Determination of Soil Active Carbon and Nitrogen Indices
2.4.4. Determination of Soil Aggregates
2.4.5. Determination of Soil Humus Content
2.5. Data Analysis Methods
3. Results
3.1. Soil Organic Carbon
3.2. Soil Carbon Pool
3.3. Soil Nitrogen Pool
3.4. Soil Humus
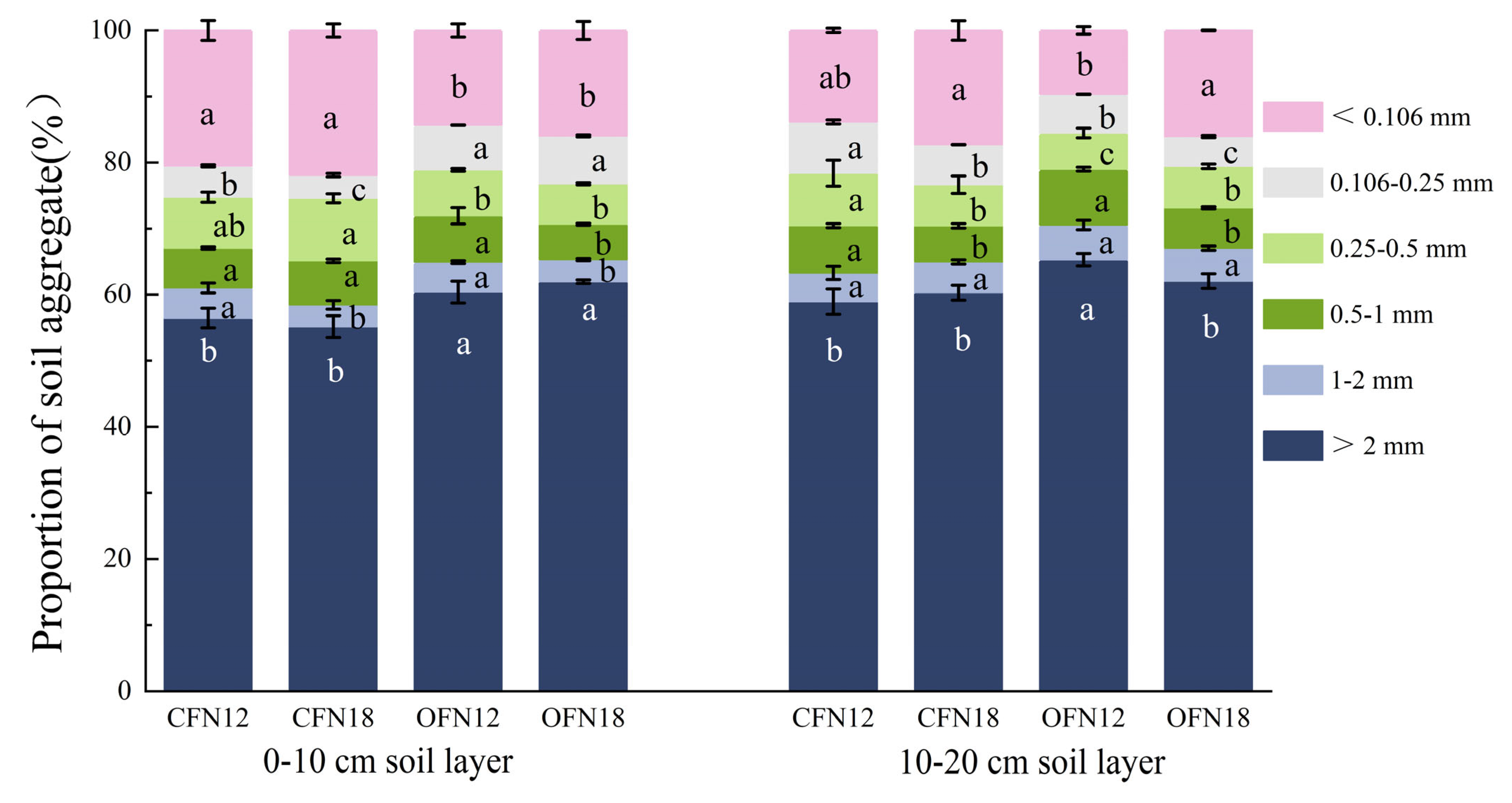
3.5. Soil Aggregates
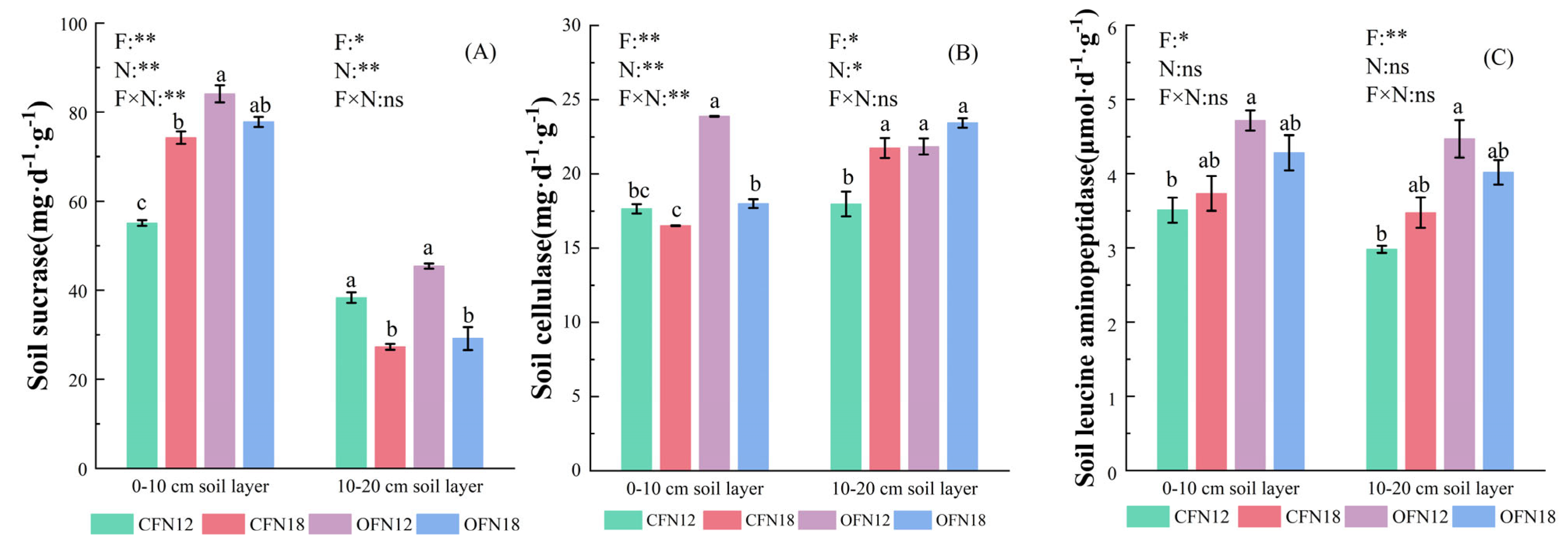
3.6. Soil Enzyme Activity
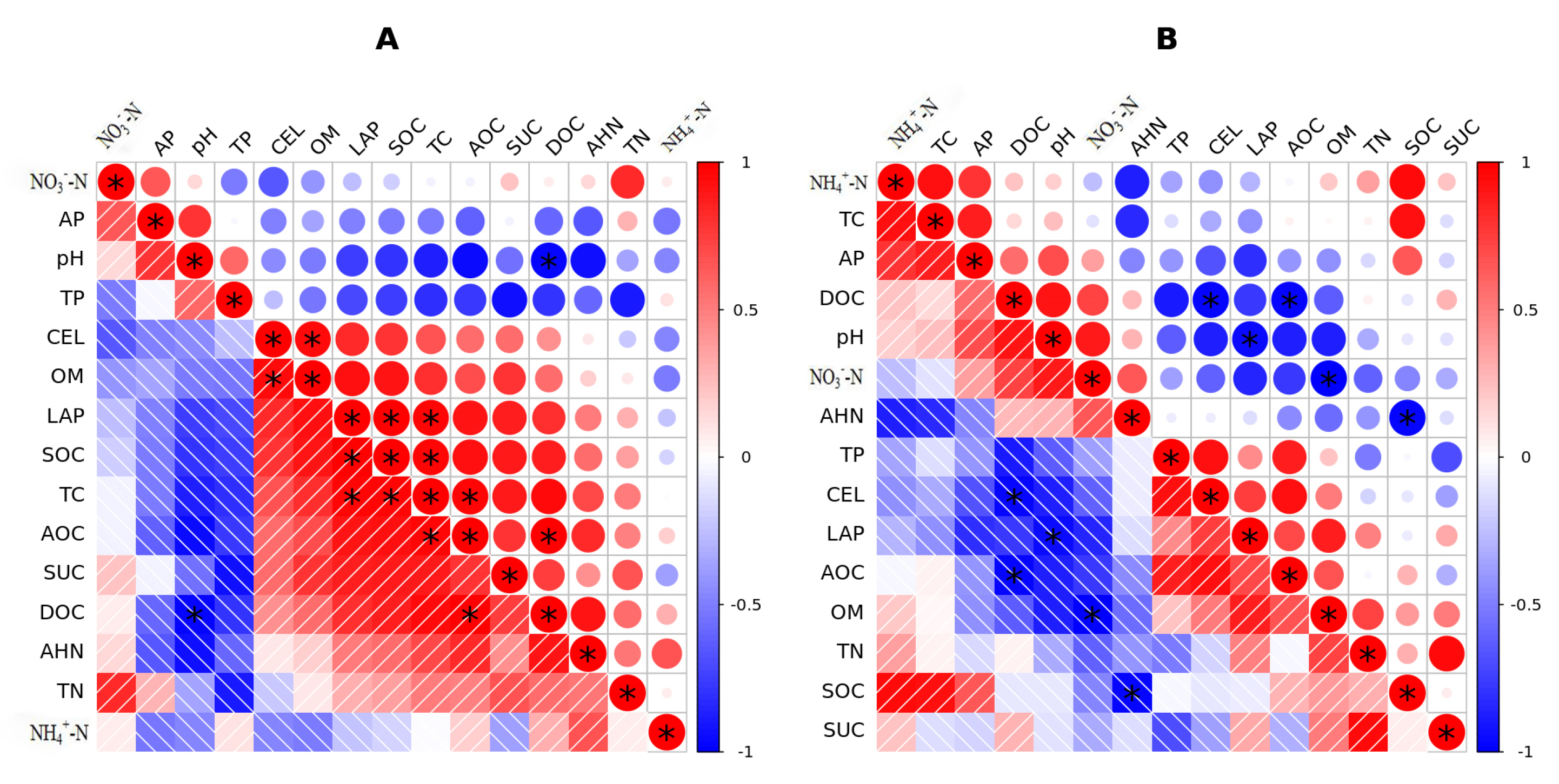
3.7. Correlation Analysis of Soil Indicators
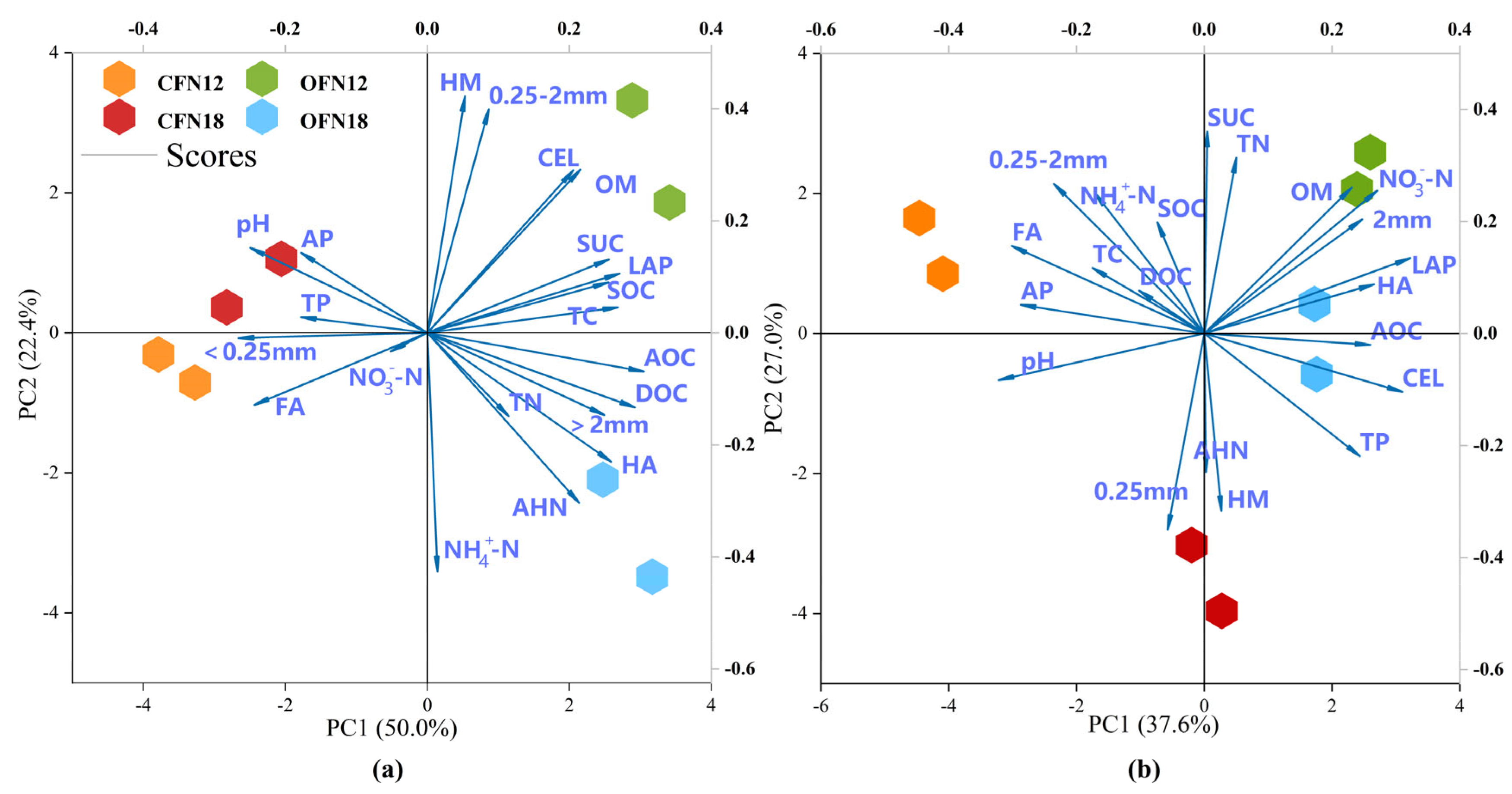
3.8. Principal Component Analysis of Soil Indicators
4. Discussion
4.1. Influence of Organic Cultivation and Reduced Nitrogen Fertilization on Soil Carbon and Nitrogen Pools
4.2. Influence of Organic Cultivation and Reduced Nitrogen Fertilization on Soil Aggregates and Humus
4.3. Impact of Organic Cultivation and Reduced Nitrogen Fertilization on Soil Enzyme Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, Z.; Zhang, K.; Khan, M.N.; Zhu, K.; Hu, L. Effects of Biochar Persistence on Soil Physiochemical Properties, Enzymatic Activities, Nutrient Utilization, and Crop Yield in a Three-Year Rice-Rapeseed Crop Rotation. Eur. J. Agron. 2024, 154, 127096. [Google Scholar] [CrossRef]
- Paudel, D.; Wang, L.; Poudel, R.; Acharya, J.P.; Victores, S.; De Souza, C.H.L.; Rios, E.; Wang, J. Elucidating the Effects of Organic vs. Conventional Cropping Practice and Rhizobia Inoculation on Rhizosphere Microbial Diversity and Yield of Peanut. Environ. Microbiome 2023, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, F.; Ni, X.; Yue, K.; Wei, X.; Zhang, X.; Zhang, X. Responses of Soil Nitrogen Pools to Litter Input Under Nitrogen Addition: A Meta-Analysis. JGR Biogeosci. 2024, 129, e2023JG007699. [Google Scholar] [CrossRef]
- Wang, L. Leaching of Soil Legacy Nitrogen in Intact Soil Columns and Significance of Soil Macropore Structure. Sci. Total Environ. 2024, 906, 167546. [Google Scholar] [CrossRef]
- Duan, P.; Song, Y.; Li, S.; Xiong, Z. Responses of N2O Production Pathways and Related Functional Microbes to Temperature across Greenhouse Vegetable Field Soils. Geoderma 2019, 355, 113904. [Google Scholar] [CrossRef]
- Ma, Y.; Woolf, D.; Fan, M.; Qiao, L.; Li, R.; Lehmann, J. Global Crop Production Increase by Soil Organic Carbon. Nat. Geosci. 2023, 16, 1159–1165. [Google Scholar] [CrossRef]
- Smith, P. An Overview of the Permanence of Soil Organic Carbon Stocks: Influence of Direct Human-induced, Indirect and Natural Effects. Eur. J. Soil Sci. 2005, 56, 673–680. [Google Scholar] [CrossRef]
- Zhao, C.; He, X.; Dan, X.; He, M.; Zhao, J.; Meng, H.; Cai, Z.; Zhang, J. Soil Dissolved Organic Matters Mediate Bacterial Taxa to Enhance Nitrification Rates under Wheat Cultivation. Sci. Total Environ. 2022, 828, 154418. [Google Scholar] [CrossRef]
- Liang, S.; Sun, N.; Meersmans, J.; Longdoz, B.; Colinet, G.; Xu, M.; Wu, L. Impacts of Climate Change on Crop Production and Soil Carbon Stock in a Continuous Wheat Cropping System in Southeast England. Agric. Ecosyst. Environ. 2024, 365, 108909. [Google Scholar] [CrossRef]
- Thomaz, E.L.; Araujo-Junior, C.F.; Vendrame, P.R.S.; De Melo, T.R. Mechanisms of Aggregate Breakdown in (Sub) Tropical Soils: Effects of the Hierarchical Resistance. CATENA 2022, 216, 106377. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Liang, B.; Liu, J.; Zong, H.; Guo, X.; Wang, X.; Song, N. Variations in Soil Aggregate Distribution and Associated Organic Carbon and Nitrogen Fractions in Long-Term Continuous Vegetable Rotation Soil by Nitrogen Fertilization and Plastic Film Mulching. Sci. Total Environ. 2022, 835, 155420. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhu, L.; Lv, Y.; Zhu, K.; Liu, X.; Zhao, R. Response of Organic Carbon Fractions and Microbial Community Composition of Soil Aggregates to Long-Term Fertilizations in an Intensive Greenhouse System. J. Soils Sediments 2020, 20, 641–652. [Google Scholar] [CrossRef]
- Hu, N.; Liu, C.; Chen, Q.; Fan, J.; Wang, Y.; Sun, H. Substitution of Chemical Fertilizer with Organic Fertilizer Can Affect Soil Labile Organic Carbon Fractions and Garlic Yield by Mediating Soil Aggregate-Associated Organic Carbon. Agronomy 2023, 13, 3062. [Google Scholar] [CrossRef]
- Leri, A.C.; Ravel, B. Abiotic Bromination of Soil Organic Matter. Environ. Sci. Technol. 2015, 49, 13350–13359. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, B.; Chu, W.; Mao, J.; Olk, D.C.; Xin, X.; Zhang, J. Altered Humin Compositions under Organic and Inorganic Fertilization on an Intensively Cultivated Sandy Loam Soil. Sci. Total Environ. 2017, 601–602, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.N.D.; Bonetti, J.D.A.; Souza, E.D.D.; Paulino, H.B.; Carneiro, M.A.C. Management Systems and Soil Use on Fractions and Stocks of Organic Carbon and Nitrogen Total in Cerrado Latosol. Biosci. J. 2016, 32, 1482–1492. [Google Scholar] [CrossRef]
- Wang, R.; Li, D.; Deng, F.; Cao, Z.; Zheng, G. Production of Artificial Humic Acid from Rice Straw for Fertilizer Production and Soil Improvement. Sci. Total Environ. 2024, 906, 167548. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, Y.; Xu, L.; Chen, B.; Liu, C.; Ma, X.; Meng, Q.; Liu, B.; Huang, Z.; Jiao, Y.; et al. Responses of Soil Humus Composition and Humic Acid Structural Characteristics to the Addition of Different Types of Biochar in Phaeozems. J. Soil Sci. Plant Nutr. 2023, 23, 1611–1618. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, L.; Wei, X.; Li, T.; Tang, Y.; Wen, J.; Peng, Z.; Zhang, Y.; Wang, Y.; Zeng, X.; et al. Effects of Organic Material Addition on Carbon Cycling and Soil Fertility in Paddy Soil. J. Environ. Manag. 2025, 379, 124898. [Google Scholar] [CrossRef]
- Egli, M.; Sartori, G.; Mirabella, A.; Favilli, F.; Giaccai, D.; Delbos, E. Effect of North and South Exposure on Organic Matter in High Alpine Soils. Geoderma 2009, 149, 124–136. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, W.; Yang, Z.; Huang, X.; Zhou, H. Effects of Manure and Chemical Fertilizer on Bacterial Community Structure and Soil Enzyme Activities in North China. Agronomy 2021, 11, 1017. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Wu, J.; Zhang, Y.; Li, Q.; Liu, S.; Gao, Y. The Effects of Localized Plant–Soil–Microbe Interactions on Soil Nitrogen Cycle in Maize Rhizosphere Soil under Long-Term Fertilizers. Agronomy 2023, 13, 2114. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, M.; Li, Y.; Zhang, X.; Wang, G.; Jin, J.; Ding, G.; Zeng, X.; Liu, X. Crop Residue Return Rather Than Organic Manure Increases Soil Aggregate Stability under Corn–Soybean Rotation in Surface Mollisols. Agriculture 2022, 12, 265. [Google Scholar] [CrossRef]
- GB/T 19630-2019; Organic Products—Requirements for Production, Processing, Labeling and Management System. Standards Press of China: Beijng, China, 2019. (In Chinese)
- Bao, S.D. Methods for Soil Agricultural and Chemical Analysis; China Agricultural Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- McKee, L.S. Measuring Enzyme Kinetics of Glycoside Hydrolases Using the 3,5-Dinitrosalicylic Acid Assay. In Protein-Carbohydrate Interactions; Abbott, D.W., Lammerts Van Bueren, A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1588, pp. 27–36. ISBN 978-1-4939-6898-5. [Google Scholar]
- Leng, F.; Sun, S.; Jing, Y.; Wang, F.; Wei, Q.; Wang, X.; Zhu, X. A Rapid and Sensitive Method for Determination of Trace Amounts of Glucose by Anthrone-Sulfuric Acid Method. Bulg. Chem. Commun. 2016, 48, 109–113. [Google Scholar]
- Mathieu, C.; Hagmann, D.; Krumins, J.; Goodey, N. Leucine-aminopeptidase Activities in Heavy-metal Contaminated Soils from Brownfields of Liberty State Park (583.6). FASEB J. 2014, 28, 583.6. [Google Scholar] [CrossRef]
- Sato, J.H.; Figueiredo, C.C.D.; Marchão, R.L.; Madari, B.E.; Benedito, L.E.C.; Busato, J.G.; Souza, D.M.D. Methods of Soil Organic Carbon Determination in Brazilian Savannah Soils. Sci. Agric. 2014, 71, 302–308. [Google Scholar] [CrossRef]
- Pu, C.; Kan, Z.; Liu, P.; Ma, S.; Qi, J.; Zhao, X.; Zhang, H. Residue Management Induced Changes in Soil Organic Carbon and Total Nitrogen under Different Tillage Practices in the North China Plain. J. Integr. Agric. 2019, 18, 1337–1347. [Google Scholar] [CrossRef]
- Rovira, P.; Ramón Vallejo, V. Labile, Recalcitrant, and Inert Organic Matter in Mediterranean Forest Soils. Soil Biol. Biochem. 2007, 39, 202–215. [Google Scholar] [CrossRef]
- Su, Y. Revisiting Carbon, Nitrogen, and Phosphorus Metabolisms in Microalgae for Wastewater Treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Palkar, K.P.; Meshram, N.A.; Kasture, M.C.; Pinjari, S.S.; Dodake, S.B. Effect of Crop Residue and Fertilizers on Humus Fractions and Rice (Oryza sativa) Yield. Indian J. Agric. Sci. 2022, 92, 249–253. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; De Goede, R. Sensitivity of Labile Carbon Fractions to Tillage and Organic Matter Management and Their Potential as Comprehensive Soil Quality Indicators across Pedoclimatic Conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Wang, Y.; Liang, C.; An, S.; Soromotin, A.; Kuzyakov, Y. Increasing Contribution of Microbial Residues to Soil Organic Carbon in Grassland Restoration Chronosequence. Soil Biol. Biochem. 2022, 170, 108688. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Fu, Y.; Lin, D.; Hou, M.; Li, X.; Hu, D.; Wang, Z. Transformation of Soil Organic Matter Subjected to Environmental Disturbance and Preservation of Organic Matter Bound to Soil Minerals: A Review. J. Soils Sediments 2023, 23, 1485–1500. [Google Scholar] [CrossRef]
- Xu, J.M.; Cheng, H.H.; Koskinen, W.C.; Molina, J.A.E. Characterization of Potentially Bioreactive Soil Organic Carbon and Nitrogen by Acid Hydrolysis. Nutr. Cycl. Agroecosyst. 1997, 49, 267–271. [Google Scholar] [CrossRef]
- Sao, S.; Ann, V.; Nishiyama, M.; Praise, S.; Watanabe, T. Tracing the Pathways by Which Flood Duration Impacts Soil Bacteria through Soil Properties and Water-Extractable Dissolved Organic Matter: A Soil Column Experiment. Sci. Total Environ. 2023, 902, 166524. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Ye, R.; Qiao, Y.; Zhu-Barker, X.; Doane, T.A.; Horwath, W.R. The Solubility of Carbon Inputs Affects the Priming of Soil Organic Matter. Plant Soil 2017, 410, 129–138. [Google Scholar] [CrossRef]
- Chen, H.; Kong, W.; Shi, Q.; Wang, F.; He, C.; Wu, J.; Lin, Q.; Zhang, X.; Zhu, Y.; Liang, C.; et al. Patterns and Drivers of the Degradability of Dissolved Organic Matter in Dryland Soils on the Tibetan Plateau. J. Appl. Ecol. 2022, 59, 884–894. [Google Scholar] [CrossRef]
- Al-Hazmi, N.E.; Naguib, D.M. Agricultural Wastes Polysaccharides Promising Soil Fertilizer Improves Plant Growth and Resistance against Soil-Borne Pathogens. Plant Soil 2024, 495, 675–697. [Google Scholar] [CrossRef]
- Fu, B.; Xu, Z.; Lei, Y.; Dong, R.; Wang, Y.; Guo, X.; Zhu, H.; Cao, Y.; Yan, Z. A Novel Secreted Protein, NISP1, Is Phosphorylated by Soybean Nodulation Receptor Kinase to Promote Nodule Symbiosis. J. Integr. Plant Biol. 2023, 65, 1297–1311. [Google Scholar] [CrossRef]
- Moffett, B.F.; Nicholson, F.A.; Uwakwe, N.C.; Chambers, B.J.; Harris, J.A.; Hill, T.C.J. Zinc Contamination Decreases the Bacterial Diversity of Agricultural Soil. FEMS Microbiol. Ecol. 2003, 43, 13–19. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.; Liu, Q.; Jia, Z.; Wright, A.L.; Cao, Z. Soil N Mineralization, Nitrification and Dynamic Changes in Abundance of Ammonia-Oxidizing Bacteria and Archaea along a 2000 Year Chronosequence of Rice Cultivation. Plant Soil 2013, 365, 59–68. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Xia, Y.; Zheng, S.; Ma, C.; Rui, Y.; He, H.; Huang, D.; Zhang, Z.; Ge, T.; et al. Contrasting Pathways of Carbon Sequestration in Paddy and Upland Soils. Glob. Chang. Biol. 2021, 27, 2478–2490. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Bartkowski, B.; Dönmez, C.; Don, A.; Mayer, S.; Steffens, M.; Weigl, S.; Wiesmeier, M.; Wolf, A.; Helming, K. Carbon Farming: Are Soil Carbon Certificates a Suitable Tool for Climate Change Mitigation? J. Environ. Manag. 2023, 330, 117142. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.-R.; Virk, A.L.; Wu, G.; Qi, J.-Y.; Ma, S.-T.; Wang, X.; Zhao, X.; Lal, R.; Zhang, H.-L. Priming Effect Intensity of Soil Organic Carbon Mineralization under No-till and Residue Retention. Appl. Soil Ecol. 2020, 147, 103445. [Google Scholar] [CrossRef]
- Yang, R.; Su, Y.; Wang, T.; Yang, Q. Effect of Chemical and Organic Fertilization on Soil Carbon and Nitrogen Accumulation in a Newly Cultivated Farmland. J. Integr. Agric. 2016, 15, 658–666. [Google Scholar] [CrossRef]
- Xing, H.; Zhou, W.; Wang, C.; Li, L.; Li, X.; Cui, N.; Hao, W.; Liu, F.; Wang, Y. Excessive Nitrogen Application under Moderate Soil Water Deficit Decreases Photosynthesis, Respiration, Carbon Gain and Water Use Efficiency of Maize. Plant Physiol. Biochem. 2021, 166, 1065–1075. [Google Scholar] [CrossRef]
- Schnabel, R.R.; Dell, C.J.; Shaffer, J.A. Filter, Inoculum and Time Effects on Measurements of Biodegradable Water Soluble Organic Carbon in Soil. Soil Biol. Biochem. 2002, 34, 737–739. [Google Scholar] [CrossRef]
- Ma, F.; Liu, L.; Han, X.; Wang, Y.; Wan, H.; Xia, J.; Lu, X. Using a Novel Vector Length Stability Index (VLSI) to Evaluate Soil Aggregate Stability: A Preliminary Study. CATENA 2024, 240, 107995. [Google Scholar] [CrossRef]
- Gioacchini, P.; Baldi, E.; Montecchio, D.; Mazzon, M.; Quartieri, M.; Toselli, M.; Marzadori, C. Effect of Long-Term Compost Fertilization on the Distribution of Organic Carbon and Nitrogen in Soil Aggregates. CATENA 2024, 240, 107968. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Bradford, M.A.; Wood, S.A. Global Meta-Analysis of the Relationship between Soil Organic Matter and Crop Yields. Soil 2019, 5, 15–32. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, M.; Zhong, S.; Tong, H.; Li, F. Soil Humic Acid Stimulates Potentially Active Dissimilatory Arsenate-Reducing Bacteria in Flooded Paddy Soil as Revealed by Metagenomic Stable Isotope Probing. Environ. Sci. Technol. 2024, 58, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cai, C.; Zhu, J. Changes of Soil Water–Stable Aggregates after Rice–Crawfish Rotation in Low-Lying Paddy Fields: A Case Study in Jianghan Plain of China. Commun. Soil Sci. Plant Anal. 2021, 52, 2358–2372. [Google Scholar] [CrossRef]
- Kou, B.; Hui, K.; Miao, F.; He, Y.; Qu, C.; Yuan, Y.; Tan, W. Differential Responses of the Properties of Soil Humic Acid and Fulvic Acid to Nitrogen Addition in the North China Plain. Environ. Res. 2022, 214, 113980. [Google Scholar] [CrossRef]
- Zhang, Y.; Hui, K.; Li, Y.; Yuan, Y.; Tan, W. Electron Transfer Capacity of Humic Acid in Soil Micro and Macro Aggregates in Response to Mulching Years. Sci. Total Environ. 2022, 831, 154927. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Rhizosphere Humic Acid Interacts with Root Cell Walls to Reduce Hydraulic Conductivity and Plant Development. Plant Soil 2010, 336, 313–322. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Gong, Z.; Wang, H.; Huang, H.; Shi, Y.; Zhao, X.; Gao, S. Humic Acid and Fulvic Acid Hinder Long-Term Weathering of Microplastics in Lake Water. Environ. Sci. Technol. 2021, 55, 15810–15820. [Google Scholar] [CrossRef]
- Yang, S.; Wang, K.; Yu, X.; Xu, Y.; Ye, H.; Bai, M.; Zhao, L.; Sun, Y.; Li, X.; Li, Y. Fulvic Acid More Facilitated the Soil Electron Transfer than Humic Acid. J. Hazard. Mater. 2024, 469, 134080. [Google Scholar] [CrossRef]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.S.; Alzuaibr, F.M.; Gharib, H.S.; et al. Applications of Humic and Fulvic Acid under Saline Soil Conditions to Improve Growth and Yield in Barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.; Bauddh, K.; Korstad, J. Humic Substances: Structure, Function and Benefits for Agroecosystems—A Review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Z.; Weng, Z.; Sun, K.; Zhang, Y.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.; Luo, Y.; et al. Formation of Soil Organic Carbon Pool Is Regulated by the Structure of Dissolved Organic Matter and Microbial Carbon Pump Efficacy: A Decadal Study Comparing Different Carbon Management Strategies. Glob. Chang. Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, H.; Yang, Z.; Zhu, Q.; Yan, B.; Fei, J.; Rong, X.; Peng, J.; Luo, G. Intercropping-Driven Nitrogen Trade-off Enhances Maize Productivity in a Long-Term Experiment. Field Crops Res. 2022, 287, 108671. [Google Scholar] [CrossRef]
- Cardinali, F.; Botta, C.; Harasym, J.; Reale, A.; Ferrocino, I.; Boscaino, F.; Orkusz, A.; Milanović, V.; Garofalo, C.; Rampanti, G.; et al. Tasting of Traditional Polish Fermented Cucumbers: Microbiology, Morpho-Textural Features, and Volatilome. Food Res. Int. 2024, 177, 113851. [Google Scholar] [CrossRef]
- Parellada, J.; Suárez, G.; Guinea, M. Inhibition of Zinc Metallopeptidases by Flavonoids and Related Phenolic Compounds: Structure-Activity Relationships. J. Enzym. Inhib. 1998, 13, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S. Organic Cultivation Induced Regulation in Yield Formation, Grain Quality Attributes, and Volatile Organic Compounds of Fragrant Rice. Food Chem. 2023, 405, 134845. [Google Scholar] [CrossRef] [PubMed]
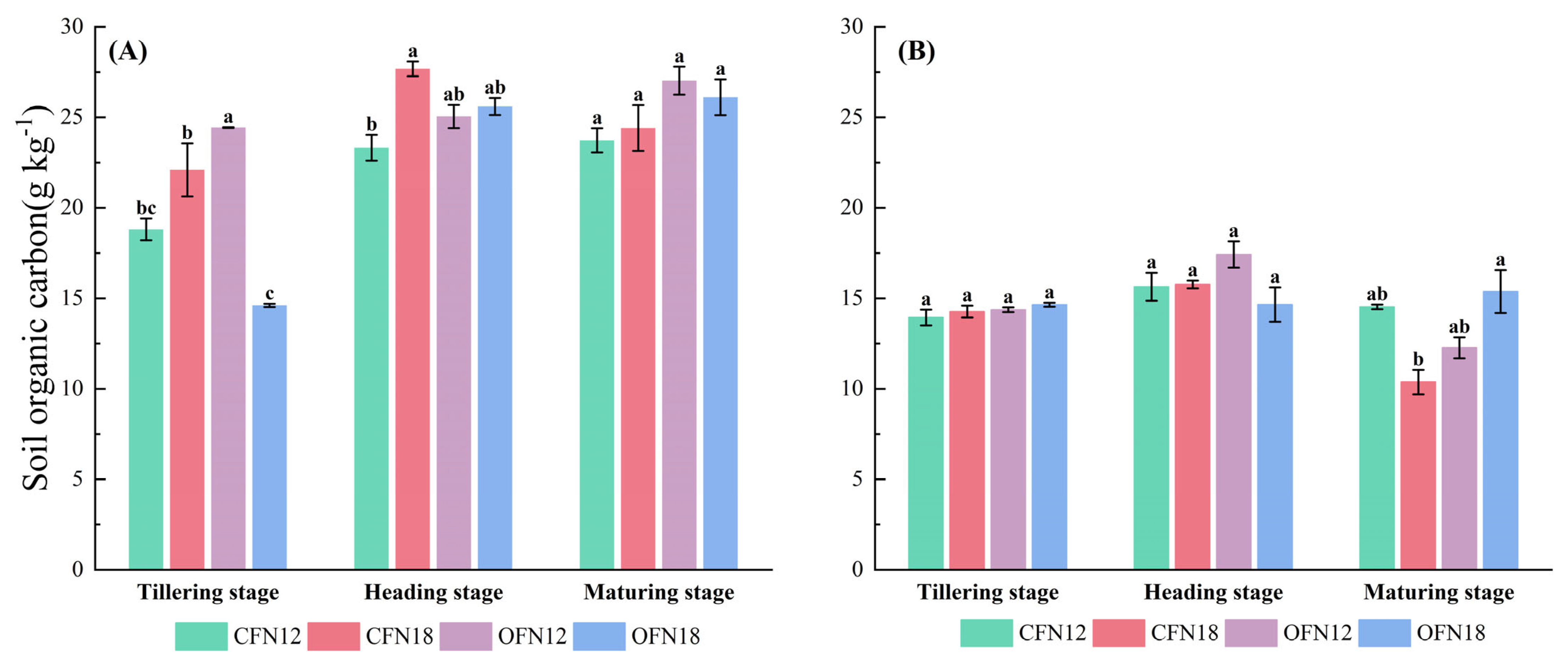
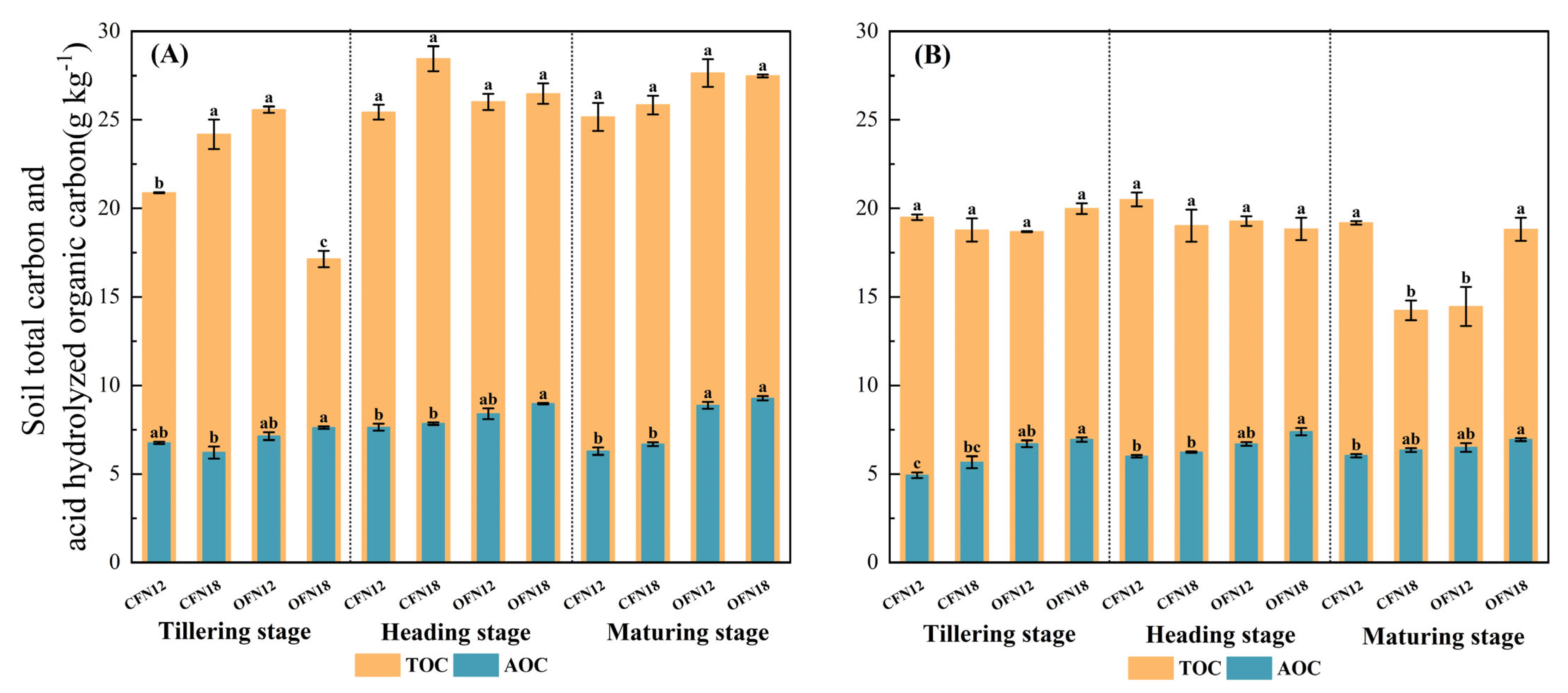
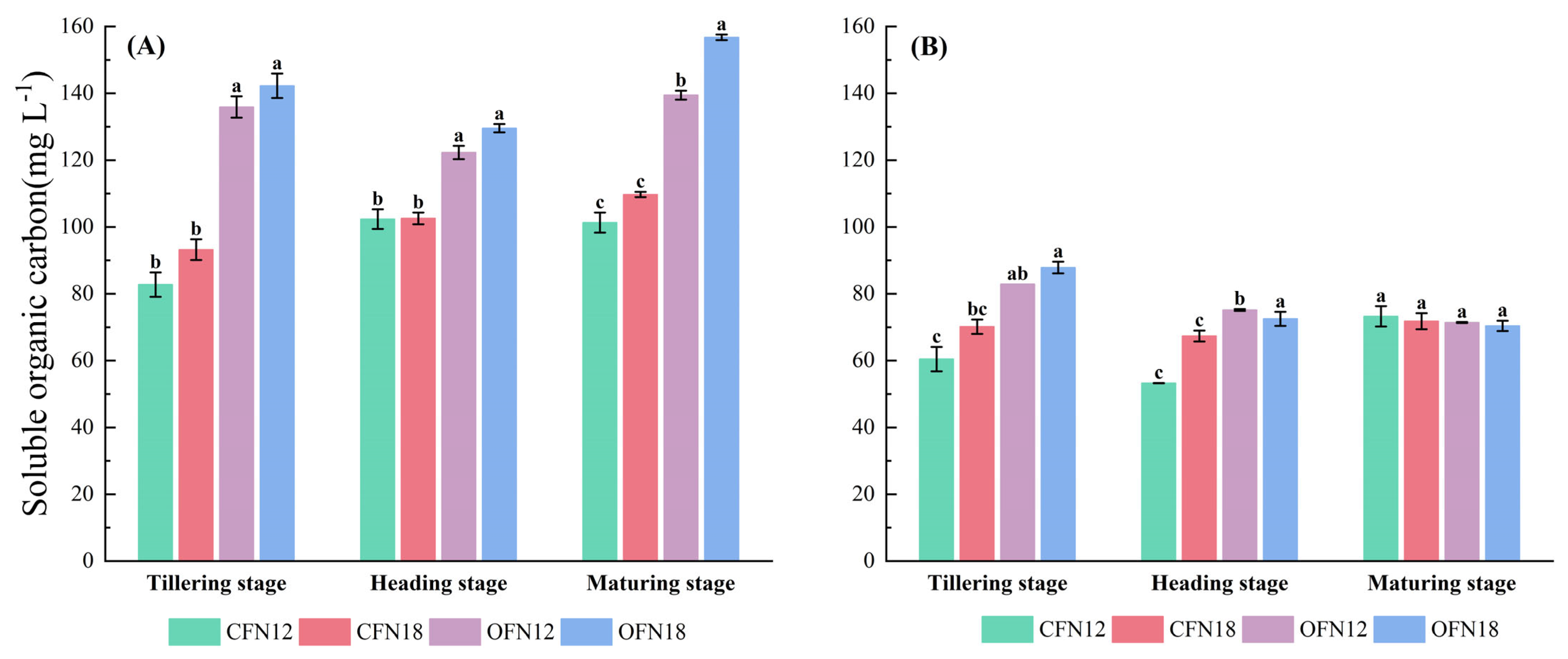
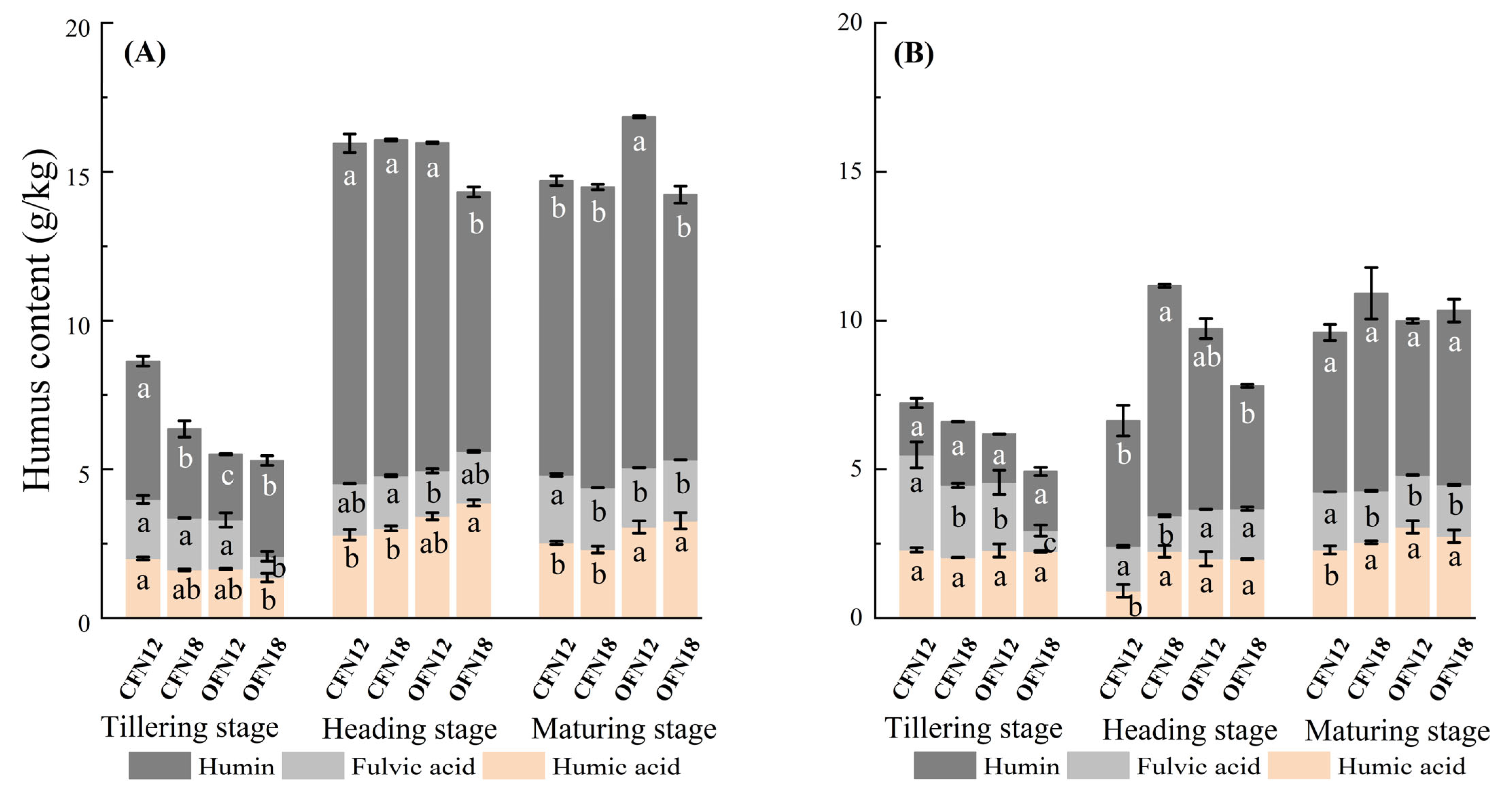
| Cultivate Way | Nitrogen Application Level | Total | Basal Fertilizer | Topdressing Fertilizer | ||||
|---|---|---|---|---|---|---|---|---|
| Compound Fertilizer (15%N) | Milk Vetch (0.33%N) | Rapeseed Cake (4.6%N) | Bio-Organic Fertilizer (4%N) | Urea (46%N) | Bio-Organic Fertilizer (4%N) | |||
| CF | N12 | 180 | 500 | / | / | / | 229 | / |
| N18 | 270 | 750 | / | / | / | 343 | / | |
| OF | N12 | 180 | / | 12,000 | 1200 | 1200 | / | 930 |
| N18 | 270 | / | 12,000 | 2400 | 1200 | / | 1800 | |
| Soil Layer (cm) | Treatment | Soil Total Nitrogen (STN) (g/kg) | Soil Alkali-Hydrolyzable Nitrogen (SAN) (mg/kg) | ||||
|---|---|---|---|---|---|---|---|
| TS | HS | MS | TS | HS | MS | ||
| 0–10 cm | CFN12 | 2.44 ± 0.03 a | 2.19 ± 0.01 b | 2.20 ± 0.02 a | 173.27 ± 3.26 ab | 164.78 ± 3.10 a | 178.91 ± 1.90 b |
| CFN18 | 2.05 ± 0.01 b | 2.11 ± 0.02 b | 2.41 ± 0.01 a | 155.14 ± 1.14 b | 172.34 ± 7.61 a | 178.12 ± 1.99 b | |
| OFN12 | 2.20 ± 0.07a b | 2.25 ± 0.07 b | 2.31 ± 0.10 a | 198.48 ± 10.48 a | 172.83 ± 3.07 a | 182.05 ± 1.30 ab | |
| OFN18 | 1.92 ± 0.07 b | 2.57 ± 0.01 a | 2.43 ± 0.03 a | 147.15 ± 7.59 b | 163.06 ± 3.54 a | 191.19 ± 2.13 a | |
| 10–20 cm | CFN12 | 1.28 ± 0.01 a | 1.29 ± 0.01 b | 1.54 ± 0.03 a | 110.68 ± 0.38 a | 131.26 ± 1.21 ab | 111.99 ± 0.96 c |
| CFN18 | 1.31 ± 0.00 a | 1.23 ± 0.04 b | 1.42 ± 0.04 a | 113.33 ± 1.53 a | 115.42 ± 4.38 c | 127.78 ± 0.90 a | |
| OFN12 | 1.18 ± 0.04 a | 1.58 ± 0.01 a | 1.62 ± 0.10 a | 116.77 ± 0.89 a | 141.15 ± 1.38 a | 116.75 ± 0.37 b | |
| OFN18 | 1.31 ± 0.01 a | 1.28 ± 0.06 b | 1.50 ± 0.03 a | 117.09 ± 2.70 a | 117.77 ± 2.36 bc | 105.17 ± 0.06 d | |
| 0–10 cm | F | 13.34 * | 56.10 ** | 1.4 | 1.65 | 0.02 | 19.05 * |
| N | 40.60 ** | 12.29 * | 10.72 * | 26.90 ** | 0.05 | 5.07 | |
| F × N | 1.2 | 33.84 ** | 0.73 | 6.15 | 3.36 | 7.14 | |
| 10–20 cm | F | 3.36 | 20.93 * | 1.93 | 9.22 * | 5.33 | 170.98 ** |
| N | 11.36 * | 23.81 ** | 4.45 | 0.84 | 54.70 ** | 9.51 * | |
| F × N | 4.28 | 11.26 * | 0 | 0.52 | 2.02 | 402.08 ** | |
| Soil Layer (cm) | Treatment | Soil Nitrate Nitrogen NO3− (mg/kg) | Soil Ammonium Nitrogen NH4+ (mg/kg) | ||||
|---|---|---|---|---|---|---|---|
| TS | HS | MS | TS | HS | MS | ||
| 0–10 cm | CFN12 | 1.00 ± 0.01 c | 0.76 ± 0.00 a | 1.18 ± 0.02 b | 20.03 ± 0.33 a | 17.67 ± 0.03 b | 15.45 ± 0.07 b |
| CFN18 | 2.27 ± 0.00 a | 0.63 ± 0.02 a | 0.69 ± 0.00 c | 16.74 ± 0.11 b | 19.85 ± 0.53 a | 17.85 ± 0.11 a | |
| OFN12 | 2.24 ± 0.04 a | 0.41 ± 0.00 b | 0.60 ± 0.00 d | 16.34 ± 0.00 b | 16.24 ± 0.20 b | 15.37 ± 0.13 b | |
| OFN18 | 1.43 ± 0.00 b | 0.50 ± 0.02 b | 1.48 ± 0.01 a | 19.03 ± 0.42 a | 19.69 ± 0.10 a | 17.05 ± 0.52 a | |
| 10–20 cm | CFN12 | 0.82 ± 0.02 a | 0.69 ± 0.01 a | 0.97 ± 0.01 a | 17.52 ± 0.15 ab | 17.49 ± 0.16 a | 16.67 ± 0.12 a |
| CFN18 | 0.60 ± 0.04 bc | 0.56 ± 0.01 a | 0.47 ± 0.01 d | 17.27 ± 0.22 b | 16.10 ± 0.68 a | 16.70 ± 0.31 a | |
| OFN12 | 0.70 ± 0.01 ab | 0.24 ± 0.00 a | 0.66 ± 0.00 c | 17.06 ± 0.50 b | 17.54 ± 0.08 a | 16.53 ± 0.01 a | |
| OFN18 | 0.55 ± 0.01 c | 0.87 ± 0.01 a | 0.87 ± 0.01 b | 19.73 ± 0.64 a | 16.88 ± 0.51 a | 16.52 ± 0.47 a | |
| 0–10 cm | F | 85.71 ** | 410.83 ** | 69.42 ** | 6.49 | 7.62 | 2.6 |
| N | 110.23 ** | 1.98 | 255.01 ** | 1.21 | 95.511 ** | 54.607 ** | |
| F × N | 2306.38 ** | 94.260 ** | 3107.97 ** | 119.832 ** | 4.88 | 1.72 | |
| 10–20 cm | F | 13.53 * | 70.89 ** | 23.45 ** | 5.53 | 0.95 | 0.29 |
| N | 67.70 ** | 858.12 ** | 268.84 ** | 8.059 * | 5.72 | 0 | |
| F × N | 3.41 | 1985.11 ** | 1619.28 ** | 11.738 * | 0.71 | 0 | |
| Soil Layer | Treatment | PC1 | PC2 | Total Points | Rank |
|---|---|---|---|---|---|
| 0–10 cm | CFN12 | −3.5306 | −0.50979 | −4.04039 | 4 |
| CFN18 | −2.44123 | 0.709985 | −1.73125 | 3 | |
| OFN12 | 3.149705 | 2.59166 | 5.741365 | 1 | |
| OFN18 | 2.82213 | −2.79186 | 0.03027 | 2 | |
| 10–20 cm | CFN12 | −4.27673 | 1.25186 | −3.02487 | 3 |
| CFN18 | 0.0382 | −3.49288 | −3.45468 | 4 | |
| OFN12 | 2.494955 | 2.32264 | 4.817595 | 1 | |
| OFN18 | 1.743565 | −0.08163 | 1.66194 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Yang, Y.; Chen, Y.; Yu, S.; Huang, X.; Jiang, M.; Zhang, Z.; Huang, L. Optimizing Effects of Organic Farming and Moderately Low Nitrogen Levels on Soil Carbon and Nitrogen Pools. Agronomy 2025, 15, 1561. https://doi.org/10.3390/agronomy15071561
Wang G, Yang Y, Chen Y, Yu S, Huang X, Jiang M, Zhang Z, Huang L. Optimizing Effects of Organic Farming and Moderately Low Nitrogen Levels on Soil Carbon and Nitrogen Pools. Agronomy. 2025; 15(7):1561. https://doi.org/10.3390/agronomy15071561
Chicago/Turabian StyleWang, Guanghua, Yu Yang, Yuqi Chen, Shilong Yu, Xiaomin Huang, Min Jiang, Zujian Zhang, and Lifen Huang. 2025. "Optimizing Effects of Organic Farming and Moderately Low Nitrogen Levels on Soil Carbon and Nitrogen Pools" Agronomy 15, no. 7: 1561. https://doi.org/10.3390/agronomy15071561
APA StyleWang, G., Yang, Y., Chen, Y., Yu, S., Huang, X., Jiang, M., Zhang, Z., & Huang, L. (2025). Optimizing Effects of Organic Farming and Moderately Low Nitrogen Levels on Soil Carbon and Nitrogen Pools. Agronomy, 15(7), 1561. https://doi.org/10.3390/agronomy15071561






