Impact of Organic Fertilizer Substitution on Soil Microbial Communities and Cotton Yield in Xinjiang
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Soil Sampling and Analysis
2.4. DNA Extraction and High-Throughput Sequencing
2.5. Bioinformatics Analysis
2.6. Cotton Yield Measurement
2.7. Statistical Analysis
2.8. Data Availability
3. Results
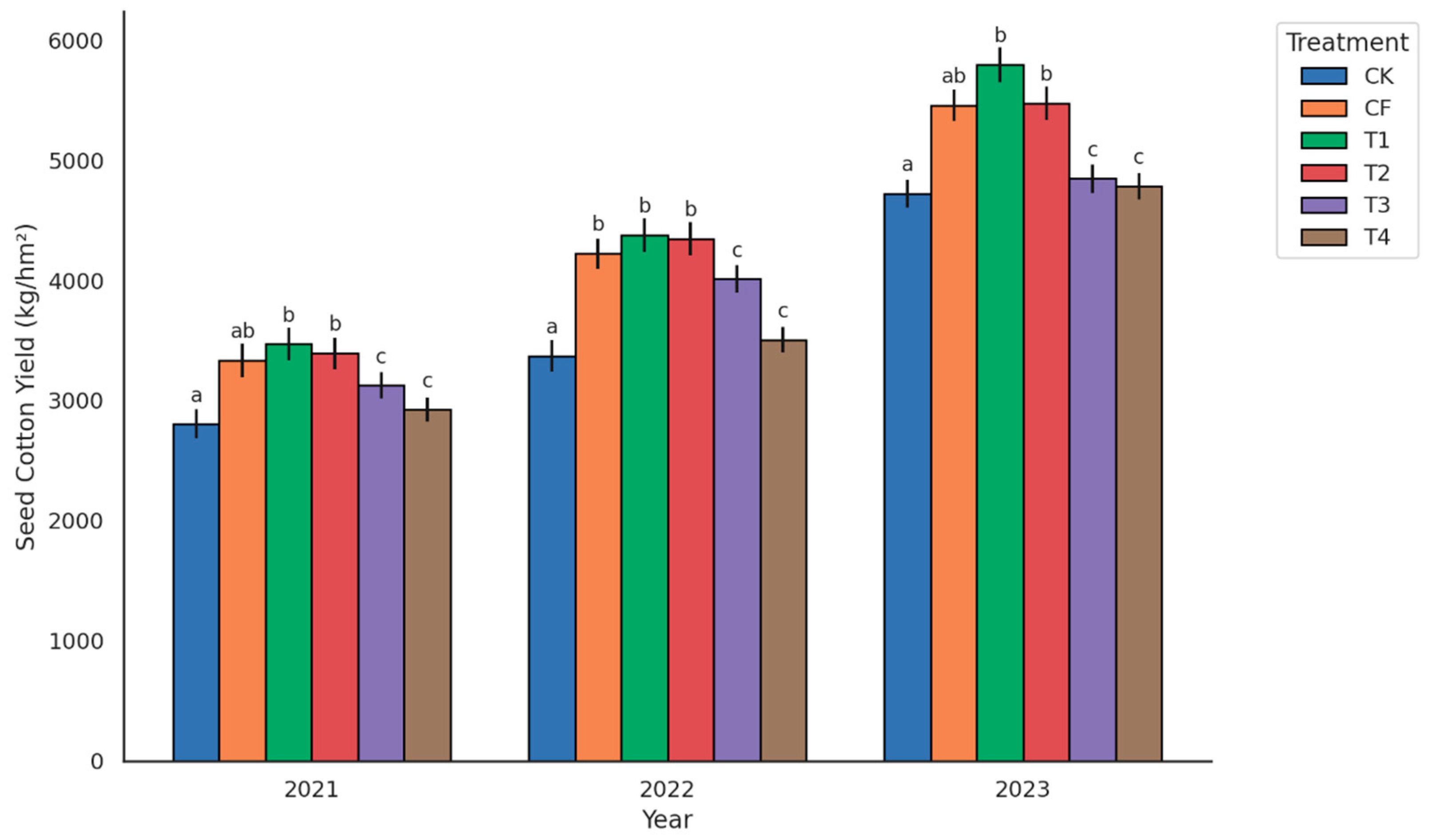
3.1. Cotton Yield Response to Organic Fertilizer Substitution
3.2. Soil Bacterial Community Analysis
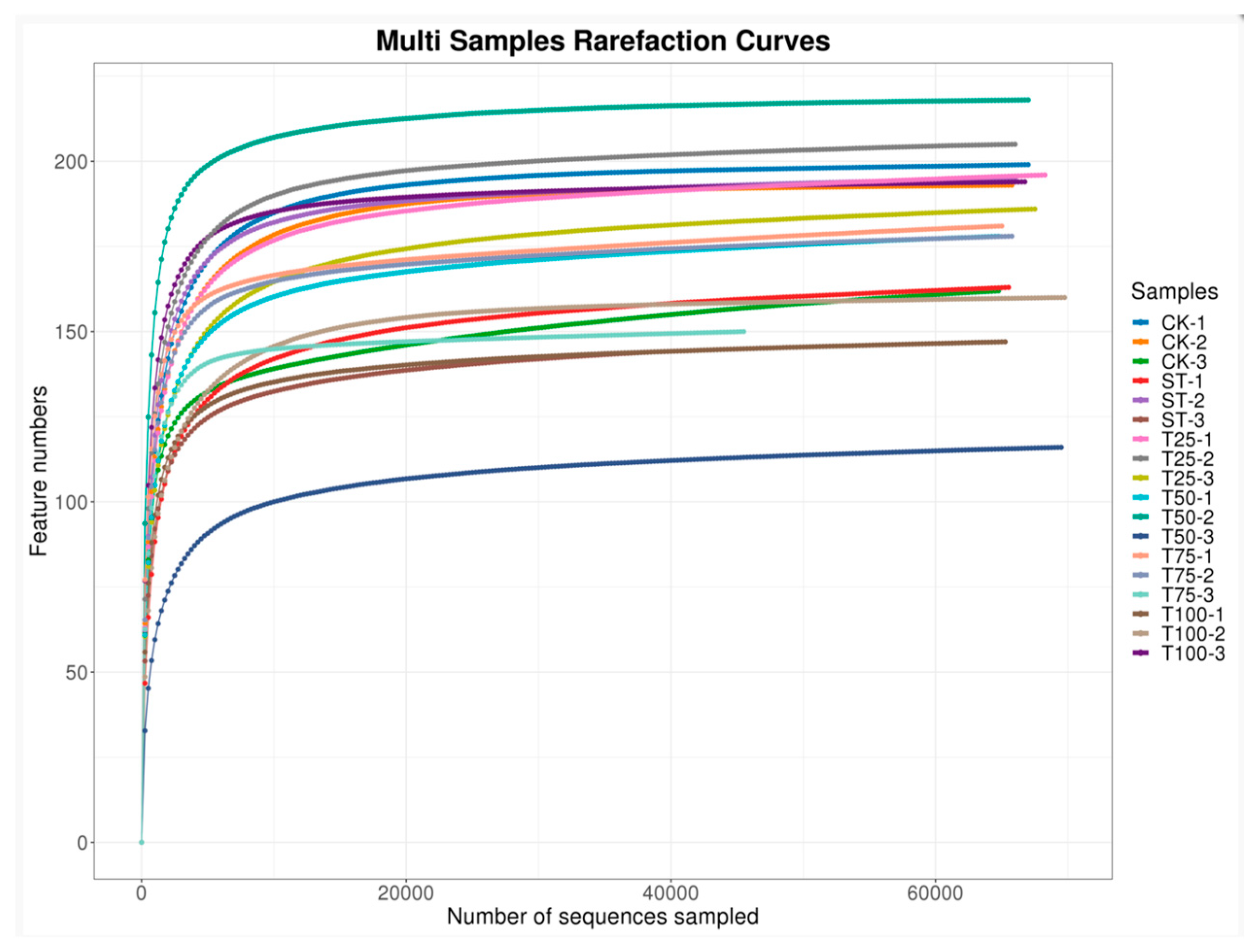
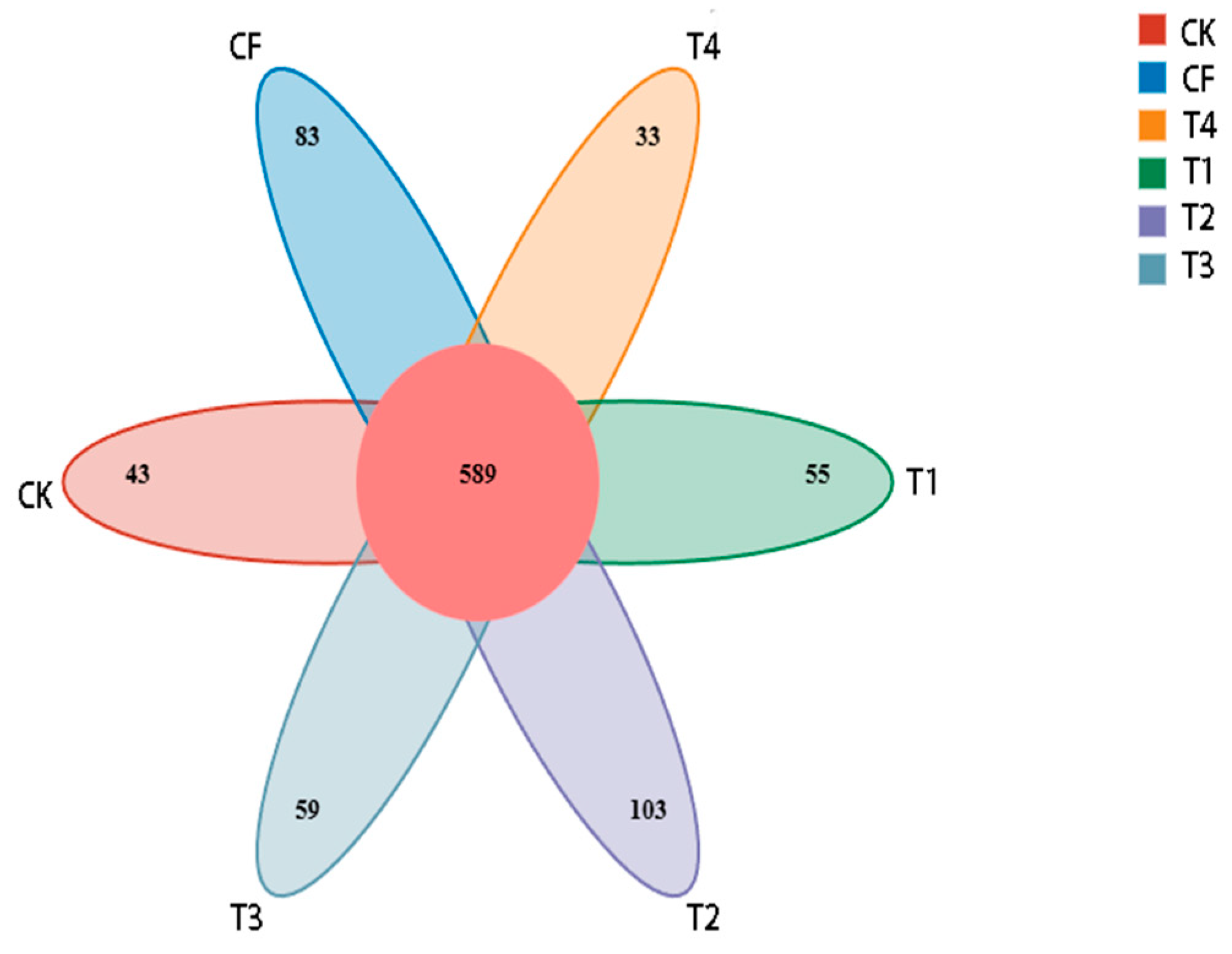
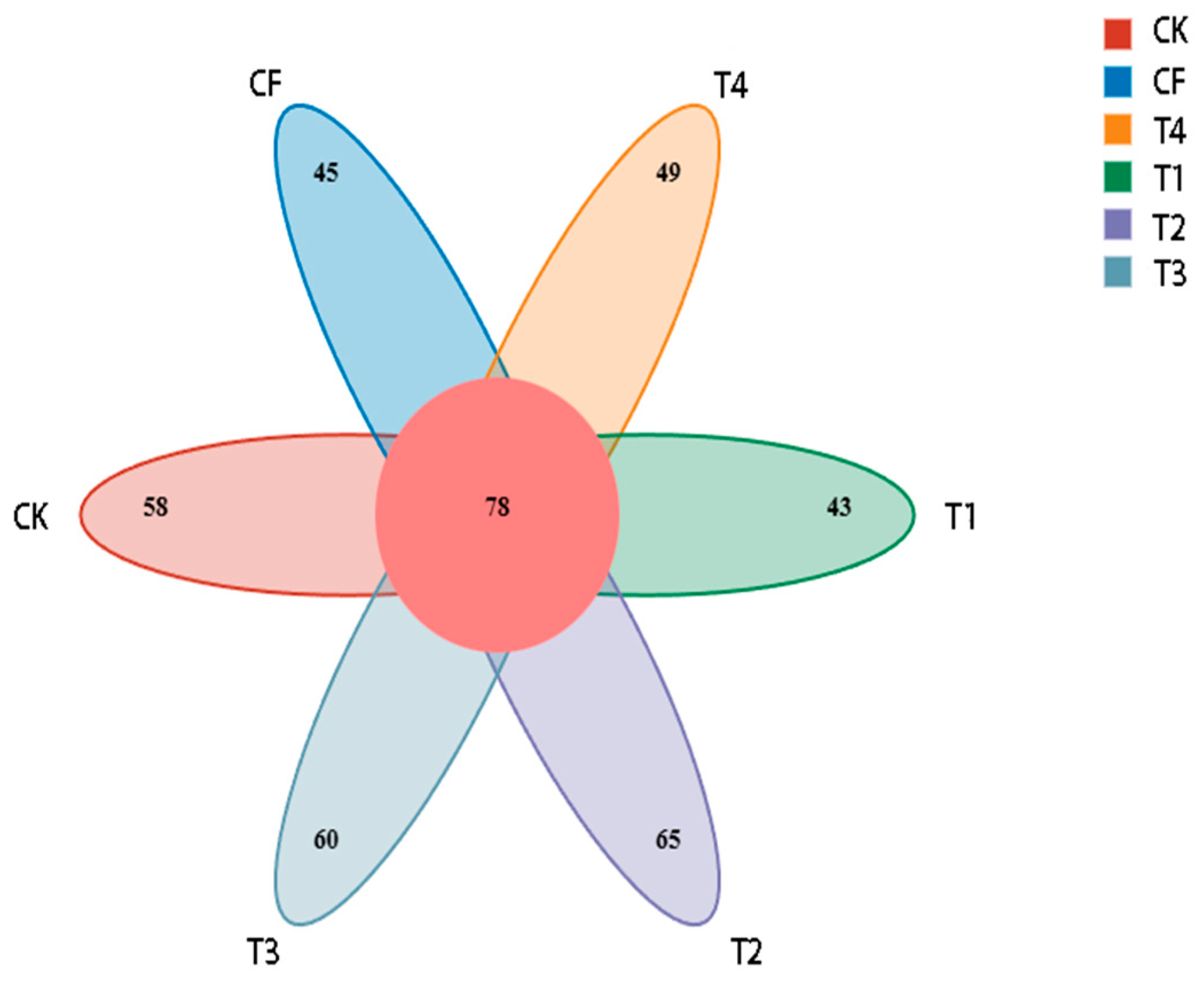
3.2.1. Sequencing Statistics and Bacterial OTUs
3.2.2. Bacterial Alpha Diversity
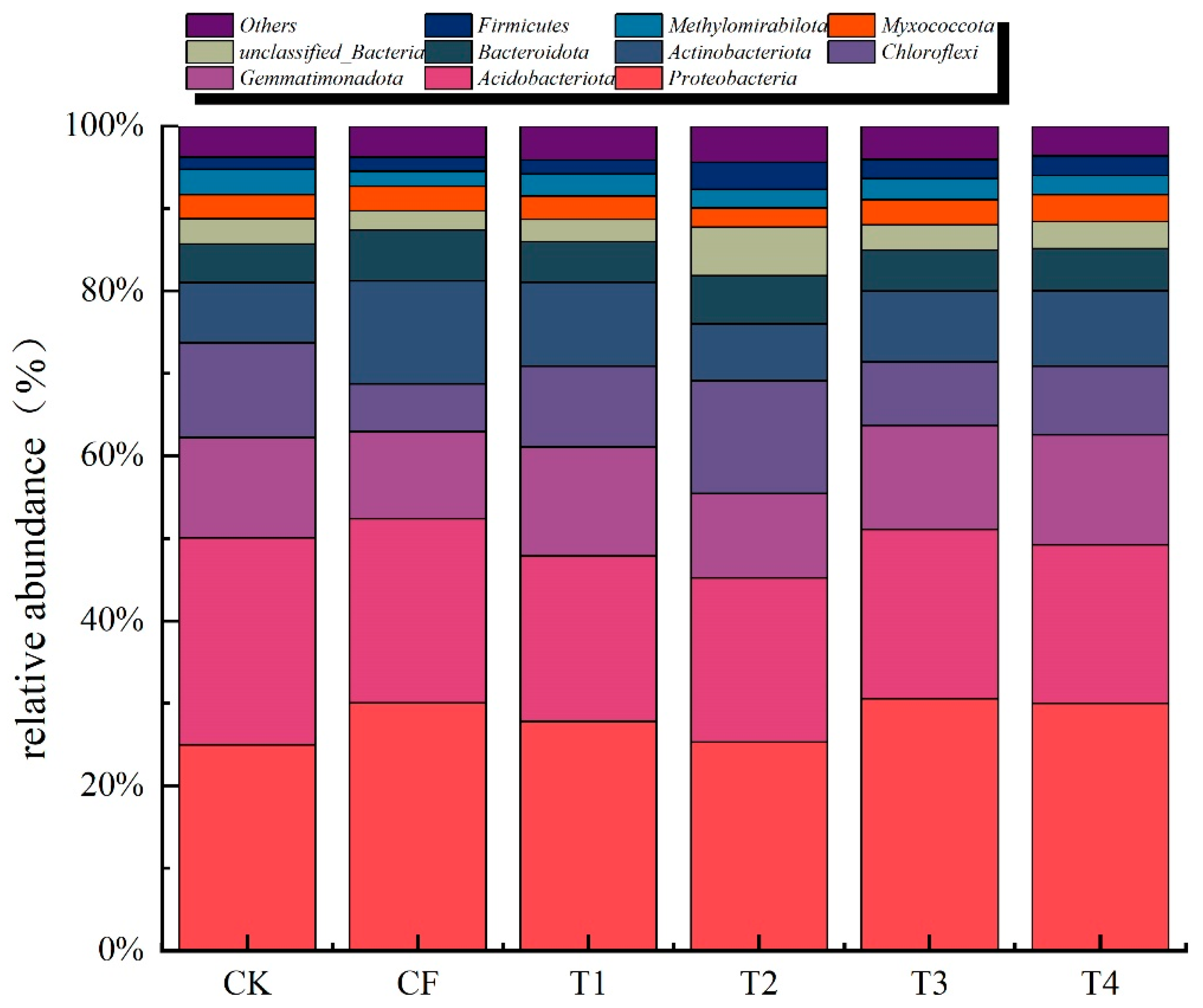
3.2.3. Bacterial Community Composition at the Phylum Level
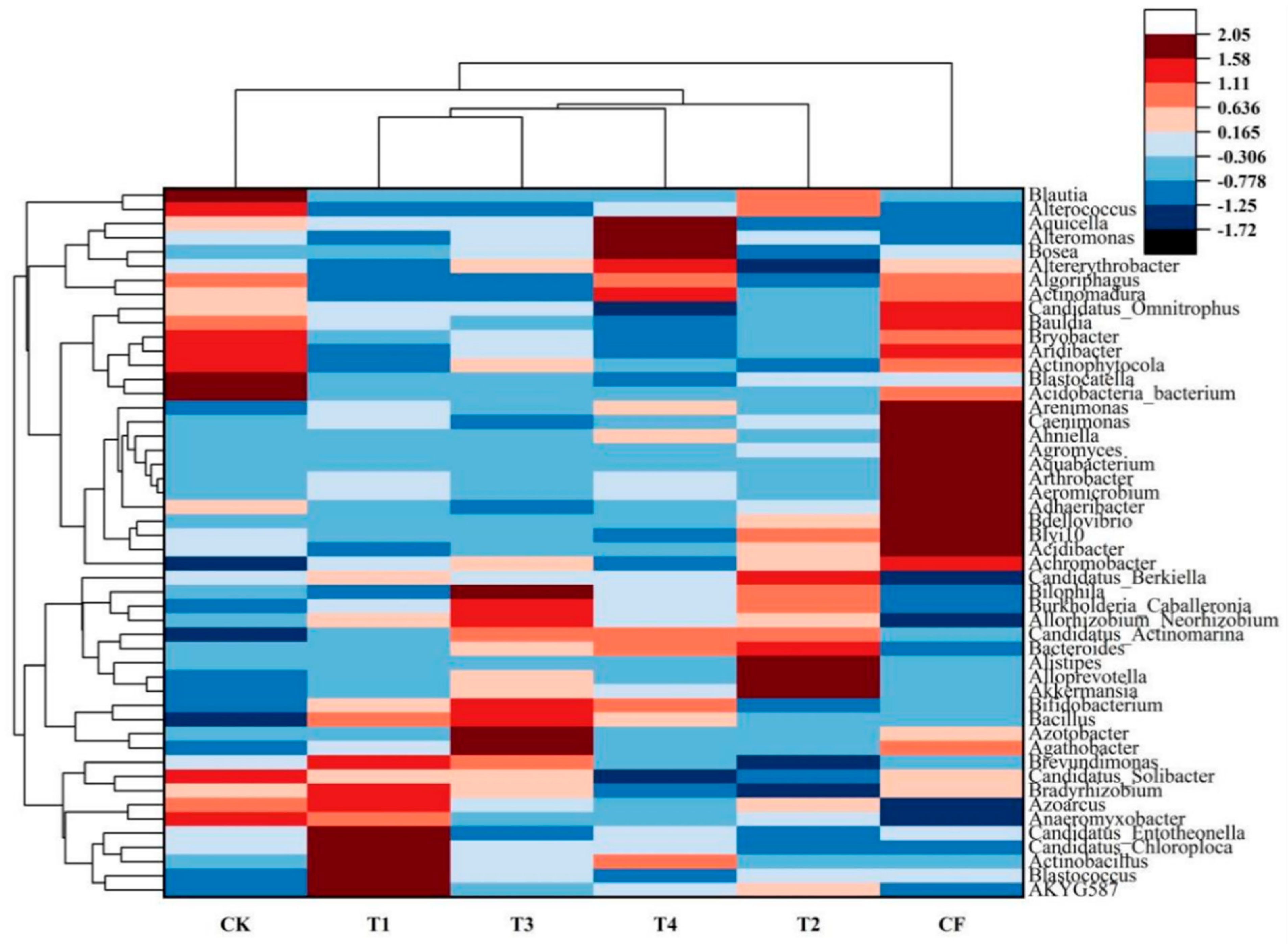
3.2.4. Bacterial Community Composition at the Genus Level
3.2.5. Bacterial Differential Abundance Analysis
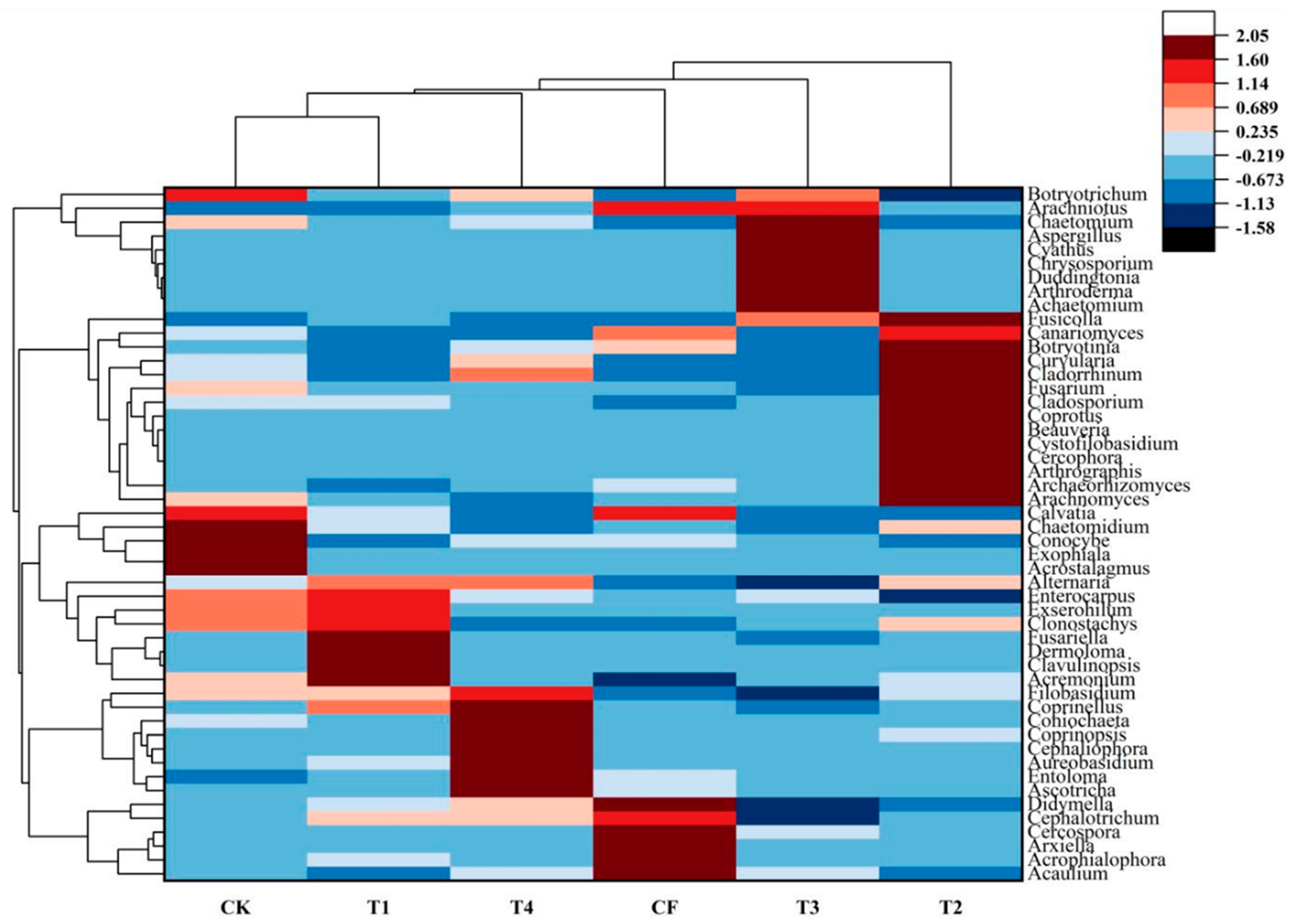
3.3. Soil Fungal Community Analysis
3.3.1. Fungal OTUs and Species Richness
3.3.2. Fungal Alpha Diversity
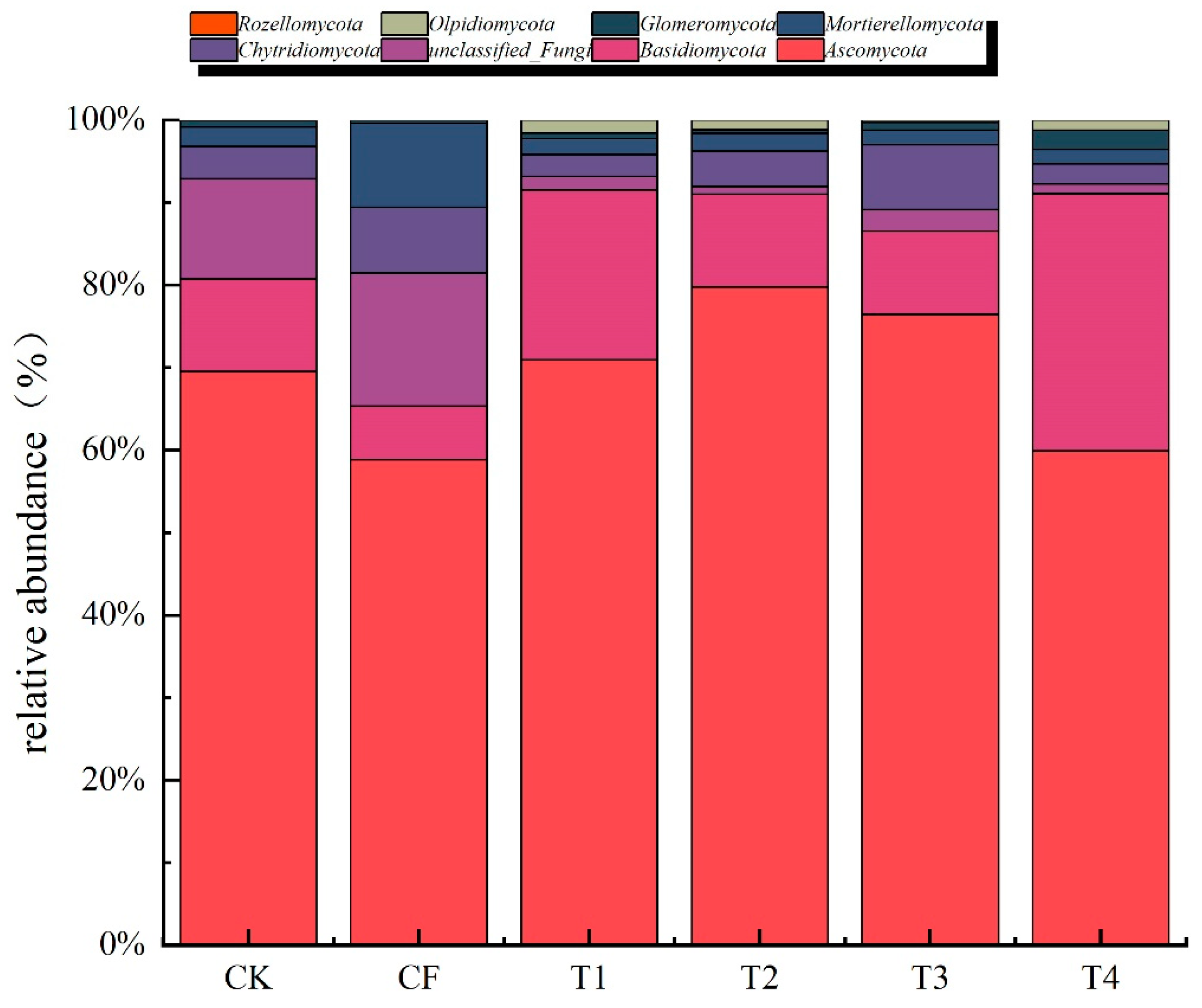
3.3.3. Fungal Community Composition at the Phylum Level
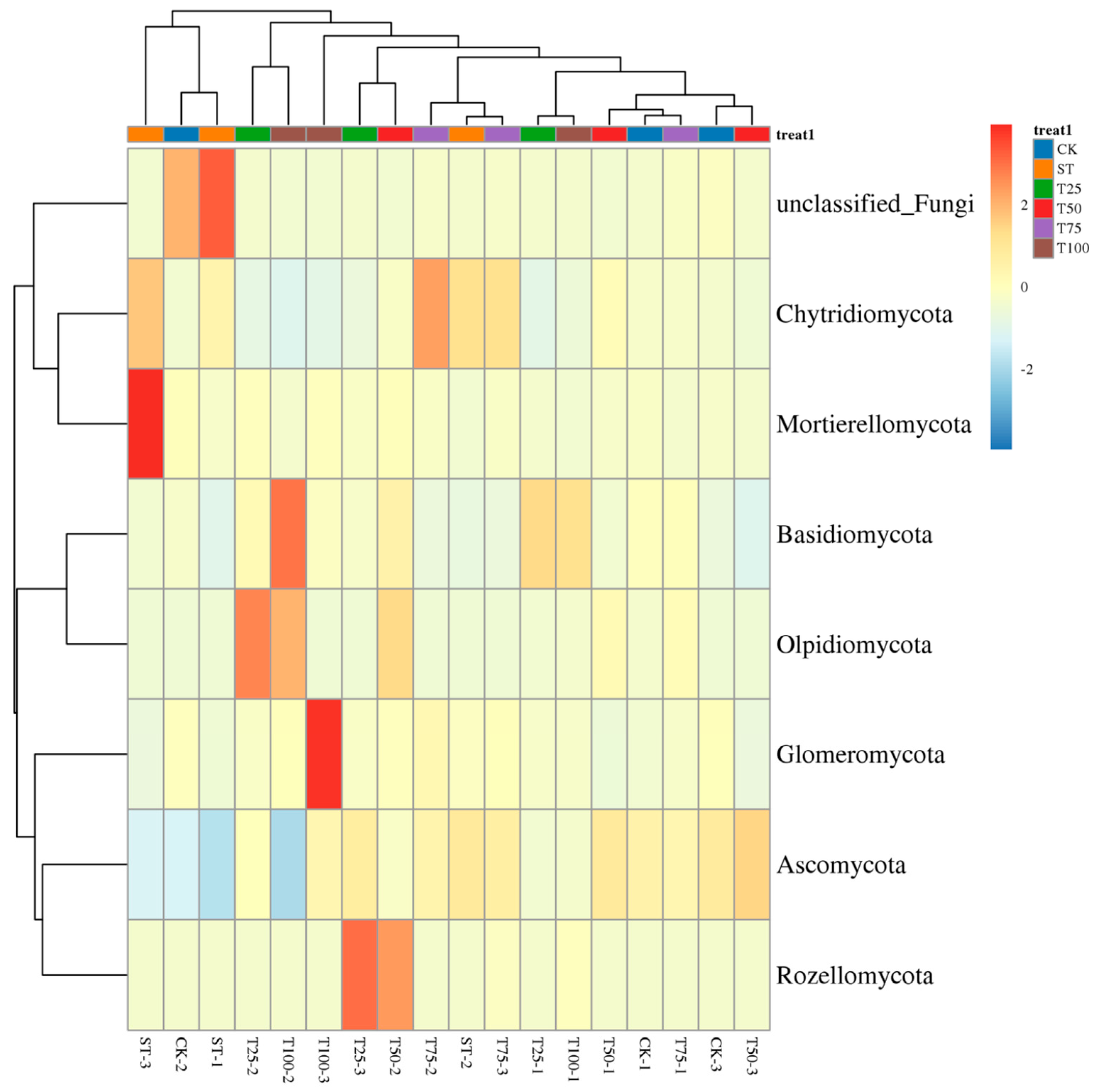
3.3.4. Fungal Community Composition at the Genus Level
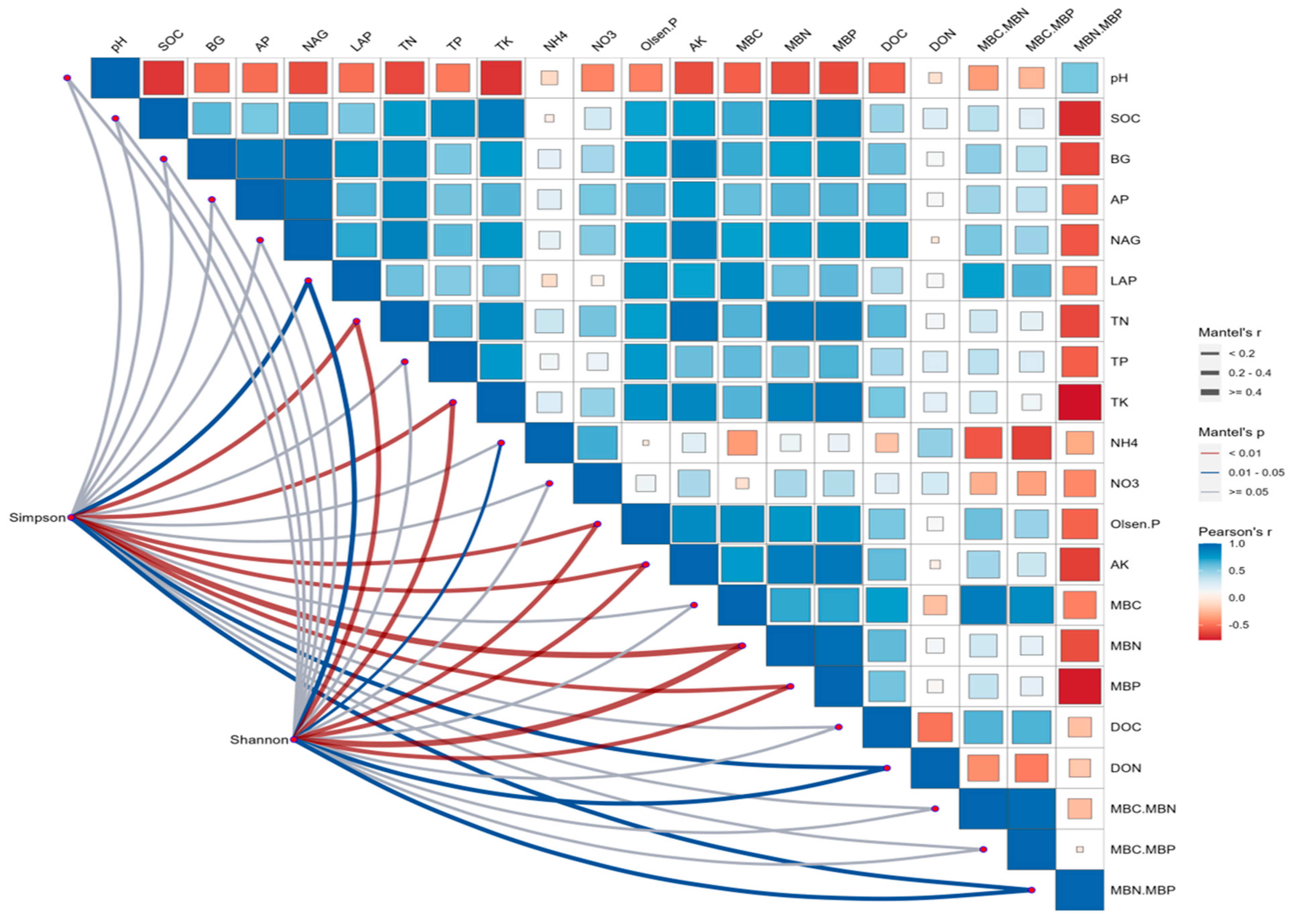
3.4. Microbial-Yield Relationship Analysis
4. Discussion
4.1. Effects of Organic Fertilizer Substitution on Cotton Yield
4.2. Effects of Organic Fertilizer Substitution on Bacterial Community Structure
4.2.1. Analysis of Differentially Abundant Bacterial Taxa
4.2.2. Comparison with Global Soil Microbiome Studies
4.2.3. Ecosystem Services Provided by Enriched Bacterial Groups
- (1)
- Nutrient Cycling Enhancement: The increased abundance of Proteobacteria and Actinobacteriota enhances phosphatase and urease activities by 25–40% compared to chemical fertilizer treatments [36]. These enzymes are critical for phosphorus and nitrogen mineralization, improving nutrient availability for plants.
- (2)
- Soil Structure Improvement: Actinobacteriota and certain Proteobacteria produce extracellular polysaccharides that act as soil-binding agents, improving aggregate stability by 15–25% [37]. This enhances water infiltration and reduces erosion risk.
- (3)
- Plant Disease Suppression: The enrichment of Streptomyces and other antibiotic-producing bacteria provides natural biocontrol services. These bacteria can reduce soil-borne pathogen populations by 30–50% through antibiotic production and competitive exclusion [38].
- (4)
- Carbon Sequestration: Enhanced microbial biomass and activity in organic treatments increase soil organic carbon storage by 12–18% over three years, contributing to climate change mitigation [39].
4.3. Effects of Organic Fertilizer Substitution on Fungal Community Structure
4.3.1. Analysis of Differentially Abundant Fungal Taxa
4.3.2. Comparison with Global Fungal Diversity Studies
4.3.3. Ecosystem Services Provided by Enriched Fungal Groups
- (1)
- Enhanced Nutrient Acquisition: AMF networks can extend the effective root surface area by 100–1000-fold, dramatically improving nutrient and water uptake efficiency [47]. This may explain the superior cotton performance despite reduced chemical fertilizer inputs.
- (2)
- Soil Structure Stabilization: Fungal hyphae act as a biological “glue,” binding soil particles and improving aggregate stability by 20–40% [48]. This enhances soil porosity and water infiltration capacity.
- (3)
- Biological Disease Control: The enrichment of Trichoderma and the reduction in pathogenic fungi provides natural disease suppression services worth an estimated USD 50–100/ha annually in reduced fungicide applications [49].
- (4)
- Organic Matter Decomposition: Enhanced saprotrophic fungal activity accelerates the decomposition of crop residues and organic amendments, improving nutrient cycling efficiency by 25–35% [50].
4.4. Relationship Between Microbial Community Structure and Cotton Yield
4.4.1. Microbial–Yield Correlations and Mechanisms
4.4.2. Optimal Microbiome Balance for Cotton Production
5. Conclusions
5.1. Microbial Community Responses
5.2. Cotton Productivity and Optimal Management Strategy
5.3. Ecosystem Services and Sustainability Implications
5.4. Scientific and Practical Contributions
5.5. Future Research Directions
- Functional Genomics Approach: Investigate the functional aspects of enriched microbial communities through metagenomics and transcriptomics to understand the mechanisms underlying improved soil health and plant productivity.
- Genotype-Specific Responses: Conduct comparative studies across different cotton genotypes to optimize organic fertilizer substitution strategies for specific varieties and breeding programs. Understanding how root architecture, mycorrhizal associations, and nutrient transporter gene expression influence responses to organic inputs could enhance the effectiveness of these practices.
- Long-term Sustainability Assessment: Establish long-term monitoring studies (10+ years) to evaluate the sustainability of organic substitution practices and their effects on soil quality, crop productivity, and environmental impacts over extended periods. This includes assessments of soil organic matter accumulation, nutrient cycling efficiency, and greenhouse gas emissions.
- Regional Adaptation Studies: Expand research to different agroecological zones to validate the generalizability of optimal substitution rates and develop region-specific recommendations for diverse soil types and climatic conditions.
- Integrated Management Systems: Investigate the interaction between organic fertilizer substitution and other sustainable practices such as cover cropping, conservation tillage, and integrated pest management to develop holistic agroecological systems.
- Economic and Environmental Life Cycle Analysis: Conduct comprehensive economic and environmental assessments to quantify the full benefits and costs of organic fertilizer substitution, including impacts on farm profitability, the carbon footprint, and ecosystem service provisioning.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ernawati, N.M.L.; Ngawit, I.K.; Farida, N. Effectiveness of organic wastes and forages to increase soil fertility status and crop yield in dry lands. J. Degrad. Min. Lands Manag. 2014, 1, 165–174. [Google Scholar] [CrossRef][Green Version]
- Enke, L.; Changrong, Y.; Xurong, M.; Wenqing, H.; Bing, S.H.; Linping, D.; Qin, L.; Shuang, L.; Tinglu, F. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Subardja, V.O.; Anas, I.; Widyastuti, R. Utilization of organic fertilizer to increase paddy growth and productivity using System of Rice Intensification (SRI) method in saline soil. J. Degrad. Min. Lands Manag. 2016, 3, 543–549. [Google Scholar] [CrossRef][Green Version]
- Bolyen, E.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; Bai, Y.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Iqbal, A.; Gui, H.; Wang, S.; Zhang, H.; Wang, X.; Dong, Q.; Zheng, C.; Pang, N.; Ullah, I.; Song, M.; et al. Influence of Partial Substitution of Chemical Fertilizer Through Organic Manure on Cotton Yield and Soil Fertility in Yangtze River Regions of China. Commun. Soil Sci. Plant Anal. 2024, 55, 2312–2321. [Google Scholar] [CrossRef]
- Wang, N.; Zhan, J.; Feng, K.; Qi, J.; Nan, H. Higher yield sustainability and soil quality by reducing chemical fertilizer with organic fertilizer application under a single-cotton cropping system. Front. Plant Sci. 2024, 15, 1494667. [Google Scholar] [CrossRef]
- Shi, X.; Hao, X.; Shi, F.; Li, N.; Tian, Y.; Han, P.; Wang, J.; Liu, P.; Luo, H. Improving cotton productivity and nutrient use efficiency by partially replacing chemical fertilizers with organic liquid fertilizer under mulched drip irrigation. Ind. Crop. Prod. 2024, 216, 118731. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Q.; Wei, K.; Zhao, X.; Tao, W.; Sun, Y.; Su, L.; Deng, M. Comprehensive assessment of combined inorganic and organic fertilization strategies on cotton cultivation: Implications for sustainable agriculture. J. Sci. Food Agric. 2024, 104, 8456–8468. [Google Scholar] [CrossRef]
- Ali, A.; Ghani, M.I.; Li, Y.; Ding, H.; Meng, H.; Cheng, Z. Hiseq Base Molecular Characterization of Soil Microbial Community, Diversity Structure, and Predictive Functional Profiling in Continuous Cucumber Planted Soil Affected by Diverse Cropping Systems in an Intensive Greenhouse Region of Northern China. Int. J. Mol. Sci. 2019, 20, 2619. [Google Scholar] [CrossRef]
- Wang, D.; Felice, M.L.; Scow, K.M. Impacts and interactions of biochar and biosolids on agricultural soil microbial communities during dry and wet-dry cycles. Appl. Soil Ecol. 2020, 152, 103570. [Google Scholar] [CrossRef]
- Liu, C.; Deng, N.; He, Z.; Tang, W.; Wang, X.; Chen, L.; Wang, R.; Chen, Y. Effects of Camellia oleifera clone selection on soil nutrient and microbial community structure. Agron. J. 2021, 113, 200–209. [Google Scholar] [CrossRef]
- Berek, A.K. Exploring the potential roles of biochars on land degradation mitigation. J. Degrad. Min. Lands Manag. 2014, 1, 149–158. [Google Scholar] [CrossRef]
- Supriyadi, S.; Sudaryanto, R.; Winarno, J.; Hartati, S.; Jamil, I.S. The quantitative soil quality assessment for tobacco plant in Sindoro mountainous zone. J. Degrad. Min. Lands Manag. 2014, 1, 105–110. [Google Scholar] [CrossRef][Green Version]
- Muddarisna, N.; Prijono, S. The potential of Arachis pintoi biomass to improve quality of soil continuously used for cassava cropping. J. Degrad. Min. Lands Manag. 2014, 1, 87–92. [Google Scholar] [CrossRef][Green Version]
- Shi, X.; Hao, X.; Khan, A.; Li, N.; Li, J.; Shi, F.; Tian, Y.; Nepal, J.; Wang, J.; Luo, H. Increase in cotton yield through improved leaf physiological functioning under the soil condition of reduced chemical fertilization compensated by the enhanced organic liquid fertilization. Front. Plant Sci. 2023, 14, 1225939. [Google Scholar] [CrossRef]
- Shi, Y.; Niu, X.; Chen, B.; Pu, S.; Ma, H.; Li, P.; Feng, G.; Ma, X. Chemical fertilizer reduction combined with organic fertilizer affects the soil microbial community and diversity and yield of cotton. Front. Microbiol. 2023, 14, 1295722. [Google Scholar] [CrossRef]
- Duan, N.; Li, L.; Liang, X.; Fine, A.; Zhuang, J.; Radosevich, M.; Schaeffer, S.M. Variation in Bacterial Community Structure Under Long-Term Fertilization, Tillage, and Cover Cropping in Continuous Cotton Production. Front. Microbiol. 2022, 13, 847005. [Google Scholar] [CrossRef]
- Shu, X.; Liu, W.; Huang, H.; Ye, Q.; Zhu, S.; Peng, Z.; Li, Y.; Deng, L.; Yang, Z.; Chen, H.; et al. Meta-Analysis of Organic Fertilization Effects on Soil Bacterial Diversity and Community Composition in Agroecosystems. Plants 2023, 12, 3801. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Li, W.; Ali, A.; Chen, J.; Sun, M.; Gao, Z.; Yang, Z. Moderate organic fertilizer substitution for partial chemical fertilizer improved soil microbial carbon source utilization and bacterial community composition in rain-fed wheat fields: Current year. Front. Microbiol. 2023, 14, 1190052. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Li, Y.; Han, X.; Li, B.; Zhang, X.; Li, Q.; Liang, W. Organic substitutions improve soil quality and maize yield through increasing soil microbial diversity. J. Clean. Prod. 2022, 347, 131323. [Google Scholar] [CrossRef]
- Kumar, Y.S.S.; Reddy, P.V.R.; Babu, D.V.; Nayak, S.B.; Vishnuvardhan, K.M.; Rao, E.S.V.N.; Raghavendra, T. Effect of fourteen years of long term organic and inorganic fertilization on productivity, soil quality and grain quality of rice (Oryza sativa L.). Indian J. Agric. Res. 2023, 57, 178–183. [Google Scholar] [CrossRef]
- Fang, J.; Deng, Y.; Che, R.; Han, C.; Zhong, W. Bacterial community composition in soils covered by different vegetation types in the Yancheng tidal marsh. Environ. Sci. Pollut. Res. 2020, 27, 21517–21532. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chang, Z.; Hu, X.; Li, Y.; Duan, C.; Yang, L.; Wang, H.; Li, T. Combined Application of Chemical and Organic Fertilizers Promoted Soil Carbon Sequestration and Bacterial Community Diversity in Dryland Wheat Fields. Land 2024, 13, 1296. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Sun, W.; Li, Z.; Lei, J.; Liu, X. Bacterial Communities of Forest Soils along Different Elevations: Diversity, Structure, and Functional Composition with Potential Impacts on CO2 Emission. Microorganisms 2022, 10, 766. [Google Scholar] [CrossRef]
- Vargas, L.K.; Da Costa, P.B.; Beneduzi, A.; Lisboa, B.B.; Passaglia, L.M.P.; Granada, C.E. Soil fertility level is the main modulator of prokaryotic communities in a meta-analysis of 197 soil samples from the Americas and Europe. Appl. Soil Ecol. 2023, 186, 104811. [Google Scholar] [CrossRef]
- Mujakic, I.; Andrei, A.; Shabarova, T.; Fecskeova, L.K.; Salcher, M.M.; Piwosz, K.; Ghai, R.; Koblizek, M. Common Presence of Phototrophic Gemmatimonadota in Temperate Freshwater Lakes. Msystems 2021, 6, e01241-20. [Google Scholar] [CrossRef]
- Qin, F.; Rao, D.; Yu, H.; Han, Y.; Pan, G.; Hu, Z.; Teng, Y.; Lyu, F.; Yan, P.; Yang, H.; et al. Reductive soil disinfestation to improve soil properties in long-term tobacco cultivation. Arch. Für Acker-Und Pflanzenbau Und Bodenkd. 2023, 69, 3284–3299. [Google Scholar] [CrossRef]
- Du, J.; Wang, Z.; Hu, L.; Wang, L.; Fang, J.; Liu, R. Comparative Genomics Reveal Distinct Environment Preference and Functional Adaptation Among Lineages of Gemmatimonadota. Microorganisms 2024, 12, 2198. [Google Scholar] [CrossRef]
- Araujo, R.; Gupta, V.V.S.R.; Reith, F.; Bissett, A.; Mele, P.; Franco, C.M.M. Biogeography and emerging significance of Actinobacteria in Australia and Northern Antarctica soils. Soil Biol. Biochem. 2020, 146, 107805. [Google Scholar] [CrossRef]
- Mokni-Tlili, S.; Mehri, I.; Ghorbel, M.; Hassen, W.; Hassen, A.; Jedidi, N.; Hamdi, H. Community-level genetic profiles of actinomycetales in long-term biowaste-amended soils. Arch. Microbiol. 2020, 202, 2607–2617. [Google Scholar] [CrossRef]
- Schrempf, H. Actinobacteria within soils: Capacities for mutualism, symbiosis and pathogenesis. FEMS Microbiol. Lett. 2013, 342, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Jiang, L.; Yang, Y.; Shi, J.; Guan, X.; Sun, T.; Zhao, H.; Wang, Y.; Liu, Y. Effect of Mild Organic Substitution on Soil Quality and Microbial Community. Agronomy 2024, 14, 888. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity-A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Ferreira, N.G.C.; Morgado, R.G.; Amaro, A.; Machado, A.L.; Soares, A.M.V.M.; Loureiro, S. The effects of temperature, soil moisture and UV radiation on biomarkers and energy reserves of the isopod Porcellionides pruinosus. Appl. Soil Ecol. 2016, 107, 224–236. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, D.; Saleem, M.; Wang, B.; Hu, S.; Delgado Baquerizo, M.; Bai, Y. Rare soil microbial taxa regulate the negative effects of land degradation drivers on soil organic matter decomposition. J. Appl. Ecol. 2021, 58, 1658–1669. [Google Scholar] [CrossRef]
- Li, K.; Xing, X.; Wang, S.; Liao, R.; Hassan, M.U.; Aamer, M.; Barbanti, L.; Wen, T.; Xu, H. Organic fertilisation enhances network complexity among bacteria, fungi, and protists by improving organic matter and phosphorus in acidic agricultural soils. Eur. J. Soil Biol. 2024, 122, 103649. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying enzymes in filamentous basidiomycetes-ecological, functional and phylogenetic review. J. Basic Microb. 2010, 50, 5–20. [Google Scholar] [CrossRef]
- Nuutinen, V.; Butt, K.R.; Hyväluoma, J.; Ketoja, E.; Mikola, J. Soil faunal and structural responses to the settlement of a semi-sedentary earthworm Lumbricus terrestris in an arable clay field. Soil Biol. Biochem. 2017, 115, 285–296. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, B.R.; Kim, J.; Lee, Y.; Nam, H.S.; Lee, T.K. Enhanced Soil Fertility and Carbon Dynamics in Organic Farming Systems: The Role of Arbuscular Mycorrhizal Fungal Abundance. J. Fungi 2024, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Pertile, G.; Panek, J.; Gryta, A.; Oszust, K.; Lipiec, J.; Usowicz, B. Mycobiome Composition and Diversity under the Long-Term Application of Spent Mushroom Substrate and Chicken Manure. Agronomy 2021, 11, 410. [Google Scholar] [CrossRef]
- Arun, A.; Eyini, M. Comparative studies on lignin and polycyclic aromatic hydrocarbons degradation by basidiomycetes fungi. Bioresour. Technol. 2011, 102, 8063–8070. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Walochnik, J.; Venditti, D.; Steinmann, J.; Müller, K.; Michel, R. Microsporidia-like parasites of amoebae belong to the early fungal lineage Rozellomycota. Parasitol. Res. 2014, 113, 1909–1918. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Xiang, X.; Liu, J.; Zhang, J.; Li, D.; Xu, C.; Kuzyakov, Y. Divergence in fungal abundance and community structure between soils under long-term mineral and organic fertilization. Soil Till. Res. 2020, 196, 104491. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Angulo, V.; Bleichrodt, R.J.; Dijksterhuis, J.; Erktan, A.; Hefting, M.M.; Kraak, B.; Kowalchuk, G.A. Enhancement of soil aggregation and physical properties through fungal amendments under varying moisture conditions. Environ. Microbiol. 2024, 26, e16627. [Google Scholar] [CrossRef]
- Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; González-López, Ó.; Gutiérrez, S.; Casquero, P.A. Influence of Fungicide Application and Vine Age on Trichoderma Diversity as Source of Biological Control Agents. Agronomy 2021, 11, 446. [Google Scholar] [CrossRef]
- Dasgupta, D.; Richardson, A.E.; Camuy-Vélez, L.A.; Kirkby, C.; Kirkegaard, J.A.; Banerjee, S. Microbial dynamics during in-situ organic matter decomposition reveals the importance of keystone taxa in the core microbiome. Appl. Soil Ecol. 2024, 199, 105396. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Scotti, R.; D’Agostino, N.; Zaccardelli, M.; Tucci, M. Phyto-Friendly Soil Bacteria and Fungi Provide Beneficial Outcomes in the Host Plant by Differently Modulating Its Responses through (In)Direct Mechanisms. Plants 2022, 11, 2672. [Google Scholar] [CrossRef] [PubMed]
- Parris, S.; Lovell, J.T.; Ding, F.; Zhang, Z.; Olvey, J.; Olvey, M.; Schmutz, J.; Grimwood, J.; Sreedasyam, A.; Kumar, S.; et al. Polyploidy-mediated variations in glutamate receptor proteins linked to Fusarium wilt resistance in upland cotton. Plant J. Cell Mol. Biol. 2025, 122, e70125. [Google Scholar] [CrossRef] [PubMed]
- Harindintwali, J.D.; Zhou, J.; Muhoza, B.; Wang, F.; Herzberger, A.; Yu, X. Integrated eco-strategies towards sustainable carbon and nitrogen cycling in agriculture. J. Environ. Manag. 2021, 293, 112856. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Tang, Z.; Wang, J.; Zhang, Y. Long-term organic fertilization reshapes the communities of bacteria and fungi and enhances the activities of C- and P-cycling enzymes in calcareous alluvial soil. Appl. Soil Ecol. 2024, 194, 105204. [Google Scholar] [CrossRef]
| Treatment | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|
| CK | 20 b | 42 b | 109 bc | 176 bc | 244 cd | 254 c |
| CF | 20 ab | 44 ab | 112 abc | 184 ab | 274 a | 291 a |
| T1 | 22 a | 44 ab | 114 a | 186 a | 257 bc | 271 b |
| T2 | 21 ab | 42 b | 108 c | 172 c | 241 d | 259 c |
| T3 | 20 ab | 45 ab | 112 ab | 186 a | 264 ab | 276 b |
| T4 | 21 ab | 45 a | 113 a | 189 a | 268 ab | 289 a |
| Treatment | Coverage | Chao 1 | Ace | Simpson | Shannon | PD Whole Tree |
|---|---|---|---|---|---|---|
| CK | 100% | 749.65 ± 17.71 c | 749.72 ± 17.70 b | 0.9963 ± 0.0004 b | 8.65 ± 0.06 c | 55.33 ± 2.62 b |
| CF | 99.99% | 873.61 ± 41.69 a | 875.59 ± 43.14 a | 0.9972 ± 0.0004 a | 9.12 ± 0.09 a | 57.52 ± 2.56 ab |
| T1 | 100% | 919.89 ± 37.51 a | 919.65 ± 37.81 a | 0.9971 ± 0.0004 a | 9.20 ± 0.09 a | 59.74 ± 2.91 a |
| T2 | 99.99% | 802.15 ± 30.14 b | 802.21 ± 30.19 b | 0.9965 ± 0.0005 ab | 8.92 ± 0.14 b | 55.00 ± 0.98 b |
| T3 | 100% | 926.11 ± 19.36 a | 926.18 ± 19.45 a | 0.9973 ± 0.0004 a | 9.19 ± 0.11 a | 59.34 ± 1.09 a |
| T4 | 99.99% | 924.58 ± 18.36 a | 924.56 ± 18.62 a | 0.9973 ± 0.0001 a | 9.19 ± 0.03 a | 60.39 ± 0.56 a |
| Treatment | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|
| CK | 7 b | 18 ab | 34 ab | 57 b | 88 ab | 106 a |
| CF | 7 b | 18 b | 34 ab | 55 b | 81 c | 96 cd |
| T1 | 7 a | 18 b | 33 b | 55 b | 87 abc | 105 ab |
| T2 | 7 a | 16 c | 36 a | 65 a | 93 a | 108 a |
| T3 | 8 a | 18 ab | 33 b | 56 b | 82 bc | 99 bc |
| T4 | 8 a | 19 a | 36 a | 56 b | 93 a | 90 d |
| Treatment | Coverage | Chao 1 | Ace | Simpson | Shannon | PD Whole Tree |
|---|---|---|---|---|---|---|
| CK | 99.99% | 196.03 ± 8.01 ab | 194.53 ± 5.14 ab | 0.9110 ± 0.02 a | 5.07 ± 0.04 b | 45.26 ± 1.52 a |
| CF | 99.98% | 175.15 ± 4.85 c | 174.02 ± 5.33 c | 0.8182 ± 0.03 b | 4.53 ± 0.10 c | 43.62 ± 2.09 ab |
| T1 | 99.99% | 199.69 ± 9.61 a | 198.50 ± 6.32 a | 0.9203 ± 0.03 a | 5.35 ± 0.23 ab | 44.10 ± 1.47 ab |
| T2 | 99.99% | 183.56 ± 9.03 bc | 185.08 ± 9.18 b | 0.9344 ± 0.04 a | 5.10 ± 0.10 b | 45.40 ± 2.17 a |
| T3 | 99.99% | 179.56 ± 5.19 c | 184.99 ± 2.33 b | 0.9475 ± 0.02 a | 5.30 ± 0.17 ab | 44.42 ± 1.15 ab |
| T4 | 99.99% | 172.74 ± 6.78 c | 163.55 ± 5.07 c | 0.9300 ± 0.03 a | 5.58 ± 0.24 a | 41.84 ± 1.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudurezike, A.; Linxin, F.; Yan, Z.; Yibati, H. Impact of Organic Fertilizer Substitution on Soil Microbial Communities and Cotton Yield in Xinjiang. Agronomy 2025, 15, 1540. https://doi.org/10.3390/agronomy15071540
Abudurezike A, Linxin F, Yan Z, Yibati H. Impact of Organic Fertilizer Substitution on Soil Microbial Communities and Cotton Yield in Xinjiang. Agronomy. 2025; 15(7):1540. https://doi.org/10.3390/agronomy15071540
Chicago/Turabian StyleAbudurezike, Abudukeyoumu, Fan Linxin, Zhang Yan, and Halihashi Yibati. 2025. "Impact of Organic Fertilizer Substitution on Soil Microbial Communities and Cotton Yield in Xinjiang" Agronomy 15, no. 7: 1540. https://doi.org/10.3390/agronomy15071540
APA StyleAbudurezike, A., Linxin, F., Yan, Z., & Yibati, H. (2025). Impact of Organic Fertilizer Substitution on Soil Microbial Communities and Cotton Yield in Xinjiang. Agronomy, 15(7), 1540. https://doi.org/10.3390/agronomy15071540








