Vvmrp1, Vvmt1, and Vvmt2 Co-Expression Improves Cadmium Tolerance and Reduces Cadmium Accumulation in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Vectors, and Chemicals
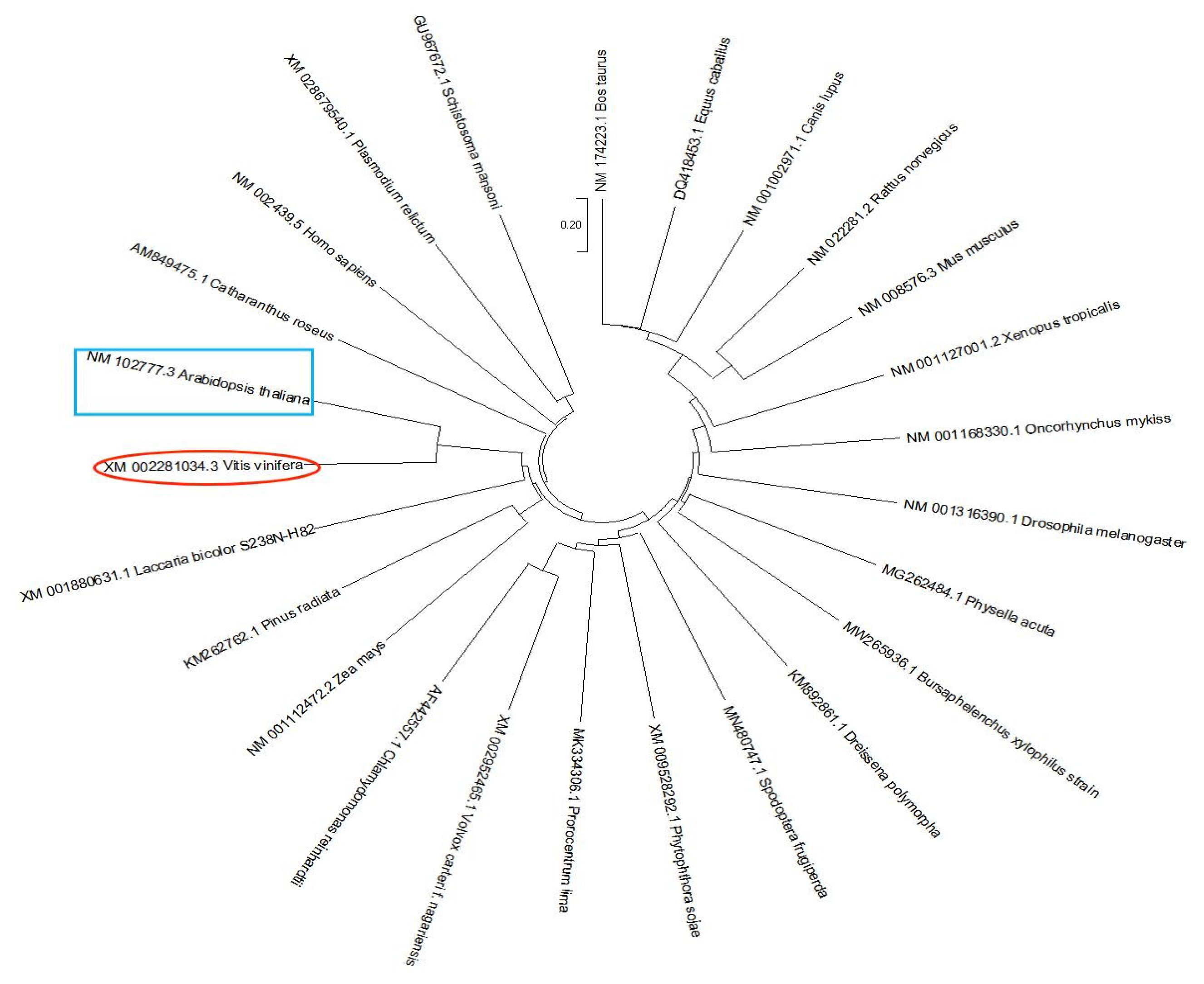
2.2. Vvmrp1 Phylogeny
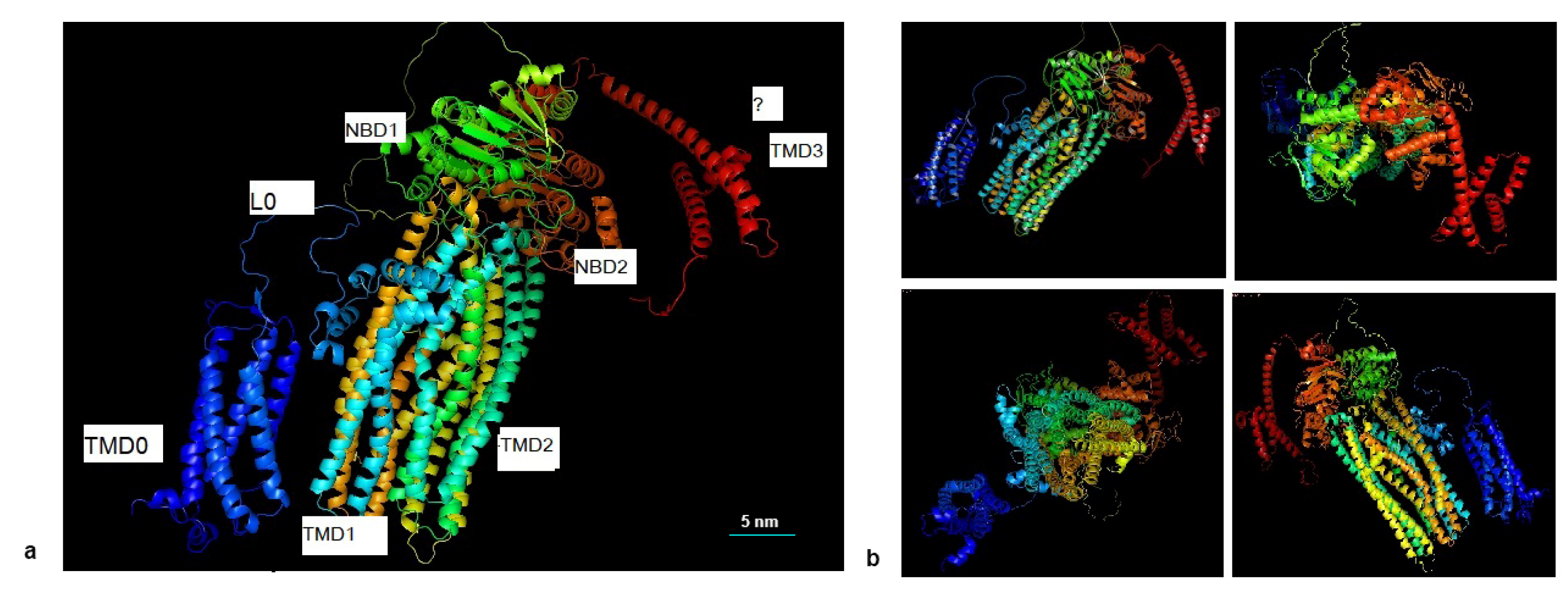
2.3. Stereo View of the Structure of VvMRP1
2.4. Design and Chemical Synthesis of Vvmrp1, Vvmt1, and Vvmt2 Genes of Vitis vinifera
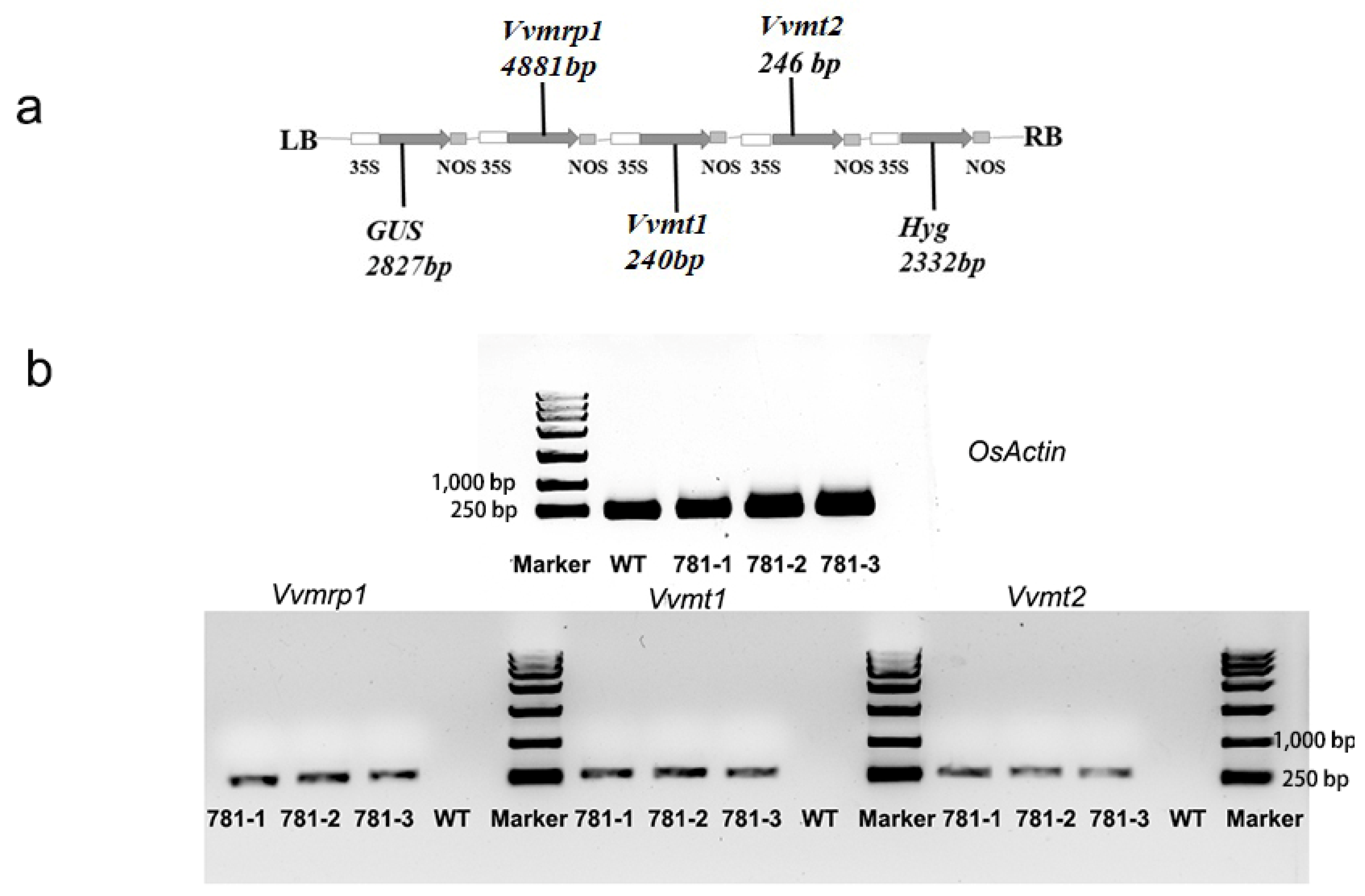
2.5. Plant Expression Vector Construction and Plant Transformation
2.6. RT-PCR Detection of Transgenic Plants
2.7. Tolerance of Transgenic Rice Overexpressing Vvmrp1, Vvmt1, and Vvmt2
2.8. Statistical Analysis
3. Results
3.1. Vvmrp1 Gene Phylogeny
3.2. The Tertiary Structure of Vvmrp1
3.3. Chemical Synthesis of Vvmrp1, Vvmt1, and Vvmt2
3.4. Construction of Triple-Gene Expression Vectors and Generation of Transgenic Rice Plants
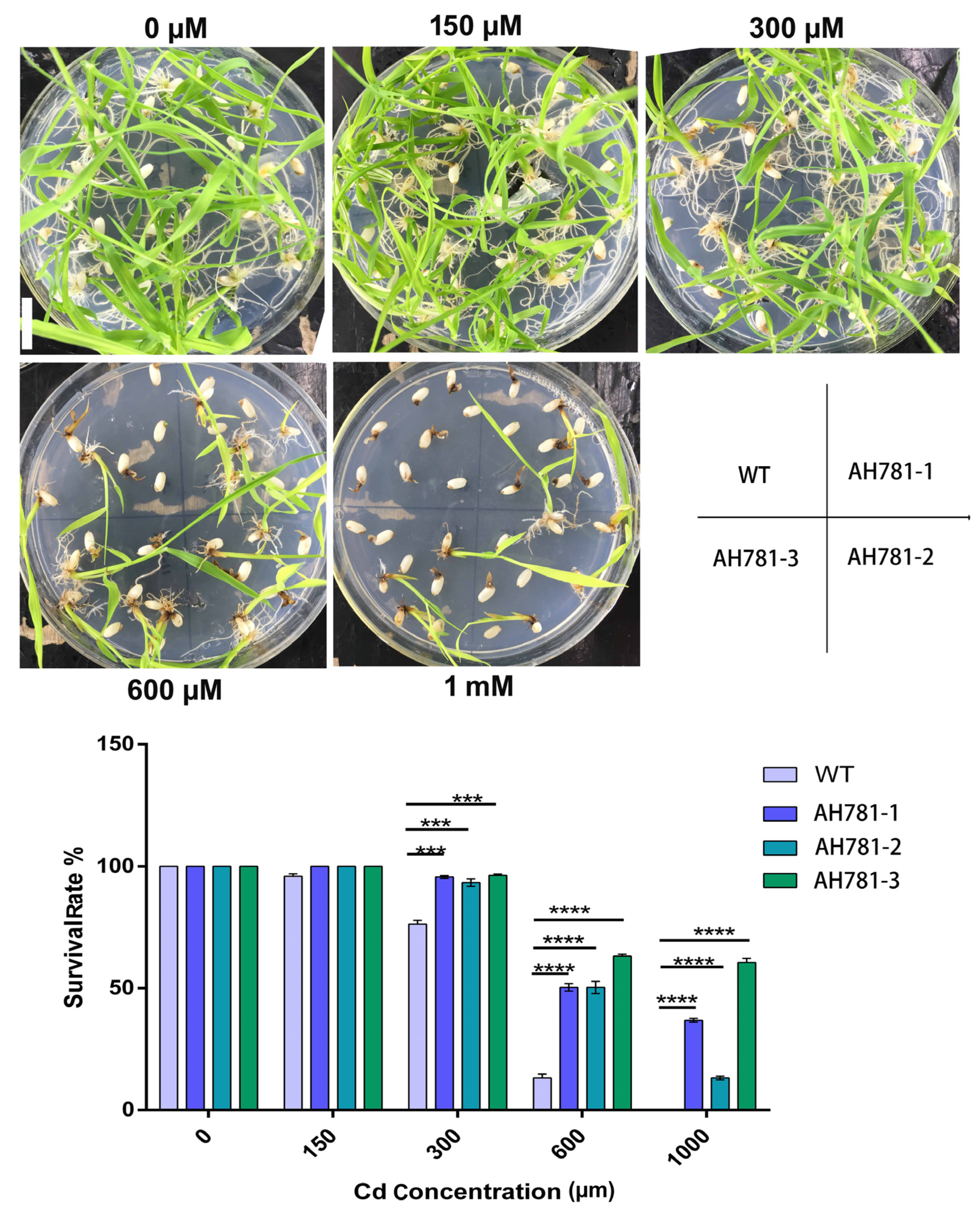
3.5. Germination of Transgenic Rice on Cd Medium
3.6. Cadmium Tolerance of Transgenic Rice Seedlings
3.7. Phenotypic Divergence Between Transgenic and WT Plants Under Cd Stress Across the Full Life Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cd | cadmium |
| GSH | glutathione |
| ABC | ATP-binding cassette transporter |
| WHO | World Health Organization |
| MRP | multidrug resistance-associated protein |
| MT | metallothionein |
| WT | wild type |
| PCR | polymerase chain reaction |
| ROS | reactive oxygen species |
References
- Xu, H.; Huang, Y.; Xiong, X.; Zhu, H.; Lin, J.; Shi, J.; Tang, C.; Xu, J. Changes in soil Cd contents and microbial communities following Cd-containing straw return. Environ. Pollut. 2023, 330, 121753. [Google Scholar] [CrossRef] [PubMed]
- He, J.Q.; Liu, D.H.; Deng, L.; Chang, H.; Qin, H.; Yin, Z. Bioavailability and exposure assessment of cadmium in farmland soil: A review. Asian J. Ecol. 2017, 12, 69–82. Available online: https://doi.org/10.7524/AJE.1673-5897.20161019001 (accessed on 10 October 2024).
- Hou, D.; O’connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Wang, T.; Liao, W.; Wu, Z.; Wu, M.; Song, Z.; Li, Y.; Luo, P. Cadmium polypeptide mitigates Cd toxicity in rice via reducing oxidative stress and regulating pectin modification. Plant Cell Rep. 2024, 43, 163. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wu, J.; Li, T.-T.; Shi, P.; Ma, Q.; Di, D.W. An overview of the mechanisms through which plants regulate ROS homeostasis under cadmium stress. Antioxidants 2024, 13, 1174. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Carne, G.; Makowski, D.; Carrillo, S.; Guérin, T.; Jitaru, P.; Reninger, J.-C.; Rivière, G.; Bemrah, N. Probabilistic determination of a maximum acceptable level of contaminant to reduce the risk of overexposure for a novel or emerging food: The case of cadmium in edible seaweed in the French population. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2022, 39, 1439–1452. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Chen, J.; Wei, J.; Liu, H.; Sui, F.; Li, C.; Zhao, P. OsAMT1.1 knockout-induced decrease in cadmium absorption and accumulation by rice related to cadmium absorption-related gene downregulation. Ecotoxicol. Environ. Saf. 2024, 288, 117377. [Google Scholar] [CrossRef]
- Mills, R.F.; Krijger, G.C.; Baccarini, P.J.; Hall, J.; Williams, L.E. Functional expression of AtHMA4, a P1B-type ATPase of the Zn/Co/Cd/Pb subclass. Plant J. 2003, 35, 164–176. [Google Scholar] [CrossRef]
- Mani, A.; Sankaranarayanan, K. In Silico Analysis of natural resistance-associated macrophage protein (NRAMP) family of transporters in rice. Protein J. 2018, 37, 237–247. [Google Scholar] [CrossRef]
- Yu, Y.; Rong, K.; Sui, X.; Zhang, L.; Zhang, M.; Hu, H.; Jia, J.; Wu, J.; Li, C. Analysis of NRAMP genes in the Triticeae reveals that TaNRAMP5 positively regulates cadmium (Cd) tolerance in wheat (Triticum aestivum). Plant Physiol. Biochem. 2025, 219, 109321. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.; Zhang, L.; Chen, C.; He, H.; Wang, W.; Zeng, X.; Shao, Y. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017, 7, 14438. [Google Scholar] [CrossRef]
- Li, R.; Yang, Y.; Cao, H.; Peng, X.; Yu, Q.; He, L.; Chen, J.; Xiang, L.; Liu, W. Heterologous expression of the tobacco metallothionein gene NtMT2F confers enhanced tolerance to Cd stress in Escherichia coli and Arabidopsis thaliana. Plant Physiol. Biochem. 2023, 195, 247–255. [Google Scholar] [CrossRef]
- Fu, S.; Lu, Y.; Zhang, X.; Yang, G.; Chao, D.; Wang, Z.; Shi, M.; Chen, J.; Chao, D.-Y.; Li, R.; et al. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J. Exp. Bot. 2019, 70, 5909–5918. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Teo, J.; Tian, D.; Yin, Z. Genetic engineering low-arsenic and low-cadmium rice grain. J. Exp. Bot. 2024, 75, 2143–2155. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Lai, D.; He, M.; Zhang, X.; Zhang, W.; Ji, M.; Zhu, Y.; Wang, Y.; Liu, L.; et al. RsPDR8, a member of ABCG subfamily, plays a positive role in regulating cadmium efflux and tolerance in radish (Raphanus sativus L.). Plant Physiol. Biochem. 2023, 205, 108149. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Zhang, W.; Ji, M.; Dong, J.; Lai, D.; Yu, W.; Zhang, X.; Zhu, Y.; Wang, Y.; et al. RsWRKY75 promotes ROS scavenging and cadmium efflux via activating the transcription of RsAPX1 and RsPDR8 in radish (Raphanus sativus L.). Plant Cell Rep. 2025, 44, 65. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.; Timsina, J.; Johnson, D.E.; Gathala, M.K.; Krupnik, T.J. Multi-year weed community dynamics and rice yields as influenced by tillage, crop establishment, and weed control: Implications for rice-maize rotations in the eastern Gangetic plains. Crop Prot. 2020, 138, 105334. [Google Scholar] [CrossRef]
- Jiang, P.; Zhong, X.; Zhang, X.; You, S.; Liu, J.; Yu, G. Effect of Mn on Cd2+ uptake by protoplasts of the Cd/Mn hyperaccumulator Celosia argentea Linn. differs by treatment method. Plant Physiol. Biochem. 2024, 214, 108925. [Google Scholar] [CrossRef]
- Bickers, S.C.; Benlekbir, S.; Rubinstein, J.L.; Kanelis, V. Structure of Ycf1p reveals the transmembrane domain TMD0 and the regulatory region of ABCC transporters. Proc. Natl. Acad. Sci. USA 2021, 118, e2025853118. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, J.; Chen, M.; Yin, H.; Miao, P.; Bai, P.; Yin, J. The use of mrp1-deficient (Danio rerio) zebrafish embryos to investigate the role of Mrp1 in the toxicity of cadmium chloride and benzo[a]pyrene. Aquat. Toxicol. 2017, 186, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Frelet-Barrand, A.; Kolukisaoglu, H.Ü.; Plaza, S.; Rüffer, M.; Azevedo, L.; Hörtensteiner, S.; Marinova, K.; Weder, B.; Schulz, B.; Klein, M. Comparative mutant analysis of Arabidopsis ABCC-type ABC transporters: AtMRP2 contributes to detoxification, vacuolar organic anion transport and chlorophyll degradation. Plant Cell Physiol. 2008, 49, 557–569. [Google Scholar] [CrossRef]
- Khandelwal, N.K.; Wasi, M.; Nair, R.; Gupta, M.; Kumar, M.; Mondal, A.K.; Gaur, N.A.; Prasad, R. Vacuolar sequestration of azoles, a novel strategy of azole Antifungal resistance conserved across pathogenic and nonpathogenic teast. Antimicrob. Agents Chemother. 2019, 63, e01347-18. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Yang, Y.; Wang, Z.; Guan, W.; Liu, Y.; Yu, H.; Zou, L. The cadmium tolerance enhancement through regulating glutathione conferred by vacuolar compartmentalization in Aspergillus sydowii. Chemosphere 2024, 352, 141500. [Google Scholar] [CrossRef]
- Zientara, K.; Wawrzyńska, A.; Łukomska, J.; López-Moya, J.R.; Liszewska, F.; Assunção, A.G.; Aarts, M.G.; Sirko, A. Activity of the AtMRP3 promoter in transgenic Arabidopsis thaliana and Nicotiana tabacum plants is increased by cadmium, nickel, arsenic, cobalt and lead but not by zinc and iron. J. Biotechnol. 2009, 139, 258–263. [Google Scholar] [CrossRef]
- Gaillard, S.; Jacquet, H.; Vavasseur, A.; Leonhardt, N.; Forestier, C. AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and up-regulated by cadmium in Arabidopsis thaliana. BMC Plant Biol. 2008, 8, 22. [Google Scholar] [CrossRef]
- Bhati, K.K.; Alok, A.; Kumar, A.; Kaur, J.; Tiwari, S.; Pandey, A.K. Silencing of ABCC13 transporter in wheat reveals its involvement in grain development, phytic acid accumulation and lateral root formation. J. Exp. Bot. 2016, 67, 4379–4389. [Google Scholar] [CrossRef]
- Feng, T.; He, X.; Zhuo, R.; Qiao, G.; Han, X.; Qiu, W.; Chi, L.; Zhang, D.; Liu, M. Identification and functional characterization of ABCC transporters for Cd tolerance and accumulation in Sedum alfredii Hance. Sci. Rep. 2020, 10, 20928. [Google Scholar] [CrossRef]
- Pirzadeh, S.; Shahpiri, A. Functional characterization of a type 2 metallothionein isoform (OsMTI-2b) from rice. Int. J. Biol. Macromol. 2016, 88, 491–496. [Google Scholar] [CrossRef]
- García-Risco, M.; González, A.; Calatayud, S.; Lopez-Jaramillo, F.J.; Pedrini-Martha, V.; Albalat, R.; Dallinger, R.; Dominguez-Vera, J.M.; Palacios, Ò.; Capdevila, M. Metal-dependent glycosylation in recombinant metallothioneins. Chem. Commun. 2022, 58, 13755–13758. [Google Scholar] [CrossRef] [PubMed]
- Pakdee, O.; Songnuan, W.; Panvisavas, N.; Pokethitiyook, P.; Yokthongwattana, K.; Meetam, M. Functional characterization of metallothionein-like genes from Physcomitrella patens: Expression profiling, yeast heterologous expression, and disruption of PpMT1.2a gene. Planta 2019, 250, 427–443. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Huang, Y.; Wang, X.; Ni, H.; Ma, Q.; Ran, H.; Cai, J.; Lin, X.; Luo, T.; Wu, C.; et al. Ketogenic diet time-dependently prevents NAFLD through upregulating the expression of antioxidant protein metallothionein-2. Clin. Nutr. 2024, 43, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, S.; Yang, Y.; Ding, Z.; Wang, Q.; Zhao, J.; Liu, X.; Chu, X.; Tian, J.; Wu, N.; et al. Identification of cadmium-binding proteins from rice (Oryza sativa L.). Int. J. Biol. Macromol. 2018, 119, 597–603. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Wang, Y.J.; Hu, M.M.; Cui, X.M.; Lou, Y.H.; Zhuge, Y.P. Exogenous NO mediated the detoxification pathway of tomato seedlings under different stress of Cu and Cd. Ying Yong Sheng Tai Xue Bao 2018, 29, 4199–4207. (In English) [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Li, Q.; Chen, Z.; Hou, X.; Zhao, C.; Guo, Q. IlNRAMP5 is required for cadmium accumulation and the growth in Iris lactea under cadmium exposures. Int. J. Biol. Macromol. 2023, 253 Pt 4, 127103. [Google Scholar] [CrossRef]
- Wang, K.; Yan, T.Z.; Xu, S.L.; Yan, X.; Zhou, Q.F.; Zhao, X.H.; Li, Y.F.; Wu, Z.X.; Qin, P.; Fu, C.J.; et al. Validating a segment on chromosome 7 of japonica for establishing low-cadmium accumulating indica rice variety. Sci. Rep. 2021, 11, 6053. [Google Scholar] [CrossRef]
- Tian, S.; Liang, S.; Qiao, K.; Wang, F.; Zhang, Y.; Chai, T. Co-expression of multiple heavy metal transporters changes the translocation, accumulation, and potential oxidative stress of Cd and Zn in rice (Oryza sativa). J. Hazard. Mater. 2019, 380, 120853. [Google Scholar] [CrossRef]
- Wang, K.; Ma, J.Y.; Li, M.Y.; Qin, Y.S.; Bao, X.C.; Wang, C.C.; Cui, D.L.; Xiang, P.; Ma, L.Q. Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells: Oxidative stress, cell ccle arrest and apoptosis. Sci. Total Environ. 2021, 756, 143951. [Google Scholar] [CrossRef]
- Farhat, Y.A.; Kim, S.H.; Seyfferth, A.L.; Zhang, L.; Neumann, R.B. Altered arsenic availability, uptake, and allocation in rice under elevated temperature. Sci. Total Environ. 2021, 763, 143049. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, Y.; Yu, X.; Liu, J.; Yang, L.; Gao, Y.; Ke, G.; Zhou, M.; Mu, B.; Xiao, S.; et al. Overexpression of two CDPKs from wild Chinese grapevine enhances powdery mildew resistance in Vitis vinifera and Arabidopsis. New Phytol. 2021, 230, 2029–2046. [Google Scholar] [CrossRef]
- Song, W.Y.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.; Ma, J.F. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA. 2014, 111, 15699–156704. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.H.; Xiong, A.S.; Yao, Q.H. A direct and efficient PAGE-mediated overlap extension PCR method for gene multiple-site mutagenesis. Appl. Microbiol. Biotechnol. 2006, 73, 234–240. [Google Scholar] [CrossRef]
- Tian, Y.S.; Xu, J.; Xiong, A.S.; Zhao, W.; Fu, X.Y.; Peng, R.H.; Yao, Q.H. Improvement of glyphosate resistance through concurrent mutations in three amino acids of the Ochrobactrum 5-enopyruvylshikimate-3-phosphate synthase. Appl. Environ. Microbiol. 2011, 77, 8409–8414. [Google Scholar] [CrossRef]
- Xiong, A.S.; Yao, Q.H.; Peng, R.H.; Duan, H.; Li, X.; Fan, H.Q.; Cheng, Z.M.; Li, Y. PCR-based accurate synthesis of long DNA sequences. Nat. Protoc. 2006, 1, 791–797. [Google Scholar] [CrossRef]
- Meng, C.; Wang, P.; Hao, Z.; Gao, Z.; Li, Q.; Gao, H.; Liu, Y.; Li, Q.; Wang, Q.; Feng, F. Ecological and health risk assessment of heavy metals in soil and Chinese herbal medicines. Environ. Geochem. Health 2022, 44, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Bakos, E.; Hegedüs, T.; Holló, Z.; Welker, E.; Tusnády, G.E.; Zaman, G.J.; Flens, M.J.; Váradi, A.; Sarkadi, B. Membrane topology and glycosylation of the human multidrug resistance-associated protein. J. Biol. Chem. 1996, 271, 12322–12326. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Fu, Y.; Xie, H.; Song, S.; Qiu, M.; Wen, J.; Chen, M.; Chen, G.; Tian, Y.; et al. Mutation at Different Sites of Metal Transporter Gene OsNramp5 Affects Cd Accumulation and Related Agronomic Traits in Rice (Oryza sativa L.). Front. Plant Sci. 2019, 10, 1081. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Sun, D.; Yang, Z.M. OsPDR20 is an ABCG metal transporter regulating cadmium accumulation in rice. J. Environ. Sci. 2024, 136, 21–34. [Google Scholar] [CrossRef]
- Yang, G.; Fu, S.; Huang, J.; Li, L.; Long, Y.; Wei, Q.; Wang, Z.; Chen, Z.; Xia, J. The tonoplast-localized transporter OsABCC9 is involved in cadmium tolerance and accumulation in rice. Plant Sci. 2021, 307, 110894. [Google Scholar] [CrossRef] [PubMed]
- Scoccianti, V.; Crinelli, R.; Tirillini, B.; Mancinelli, V.; Speranza, A. Uptake and toxicity of Cr (III) in celery seedlings. Chemosphere 2006, 64, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Tóth, T.; Zsiros, O.; Kis, M.; Garab, G.; Kovács, L. Cadmium exerts its toxic effects on photosynthesis via a cascade mechanism in the cyanobacterium, Synechocystis PCC 6803. Plant Cell. Environ. 2012, 35, 2075–2086. [Google Scholar] [CrossRef]
- Wang, S.; Wufuer, R.; Duo, J.; Li, W.; Pan, X. Cadmium Caused Different Toxicity to Photosystem I and Photosystem II of Freshwater Unicellular Algae Chlorella pyrenoidosa (Chlorophyta). Toxics 2022, 10, 352. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Li, N.; Zhou, C.; Feng, H.; Yang, J.; Han, X. Cadmium toxicity reduction in rice (Oryza sativa L.) through iron addition during primary reaction of photosynthesis. Ecotoxicol. Environ. Saf. 2020, 200, 110746. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, S.; He, L.; Ashraf, M.F.; Ihtisham, M.; Warraich, E.A.; Tang, X. Molybdenum-induced regulation of antioxidant defense-mitigated cadmium stress in aromatic rice and improved crop growth, yield, and quality traits. Antioxidants 2021, 10, 838. [Google Scholar] [CrossRef]
- Kulsum, P.G.P.S.; Khanam, R.; Das, S.; Nayak, A.K.; Tack, F.M.G.; Meers, E.; Vithanage, M.; Shahid, M.; Kumar, A.; Chakraborty, S.; et al. A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ. Res. 2023, 220, 115098. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef]
- Kim, D.Y.; Bovet, L.; Kushnir, S.; Noh, E.W.; Martinoia, E.; Lee, Y. AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiol. 2006, 140, 922–932. [Google Scholar] [CrossRef]
- Sekhar, B.; Priyanka, B.; Reddy, V.D.; Rao, K.V. Metallothionein 1 (CcMT1) of pigeonpea (Cajanus cajan L.) confers enhanced tolerance to copper and cadmium in Escherichia coli and Arabidopsis thaliana. Environ. Exp. Bot. 2011, 72, 131–139. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, T.; Cheng, J.-S.; Yi, Y.-J.; Han, J.-J.; Cheng, H.-L.; Li, Q.; Tang, N.; Liang, M.-X. Heterologous expression of the metallothionein PpMT2 gene from Physcomitrella patens confers enhanced tolerance to heavy metal stress on transgenic Arabidopsis plants. Plant Growth Regul. 2020, 90, 63–72. [Google Scholar] [CrossRef]
- Nakayasu, M.; Ohno, K.; Takamatsu, K.; Aoki, Y.; Yamazaki, S.; Takase, H.; Shoji, T.; Yazaki, K.; Sugiyama, A. Tomato roots secrete tomatine to modulate the bacterial assemblage of the rhizosphere. Plant Physiol. 2021, 186, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xin, J.P.; Tian, R.N. Research progress in the mitigative effects of rhizosphere microorganisms on heavy metal stress in plants and their mechanisms. Biotechnol. Bull. 2022, 38, 213. Available online: https://link.cnki.net/doi/10.13560/j.cnki.biotech.bull.1985.2021-0811 (accessed on 4 March 2025).
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. Rev. Environ. Contam. Toxicol. 2017, 241, 73–137. [Google Scholar] [CrossRef]
- Ou, C.; Cheng, W.; Wang, Z.; Yao, X.; Yang, S. Exogenous melatonin enhances Cd stress tolerance in Platycladusorientalis seedlings by improving mineral nutrient uptake and oxidative stress. Ecotoxicol. Environ. Saf. 2023, 252, 114619. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, G. Differences between barley cultivars in seedling growth and in uptake of cadmium and nutrients under various Cd levels. Ying Yong Sheng Tai Xue Bao. 2002, 13, 1595–1599. (In Chinese) [Google Scholar] [PubMed]
- Krężel, A.; Maret, W. The Bioinorganic Chemistry of Mammalian Metallothioneins. Chem. Rev. 2021, 121, 14594–14648. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Kozyreva, T.; Zovo, K.; Palumaa, P. Affinity gradients drive copper to cellular destinations. Nature 2010, 465, 645–648. [Google Scholar] [CrossRef]
- Whillier, S.; Raftos, J.E.; Chapman, B.; Kuchel, P.W. Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Rep. 2009, 14, 115–124. [Google Scholar] [CrossRef]
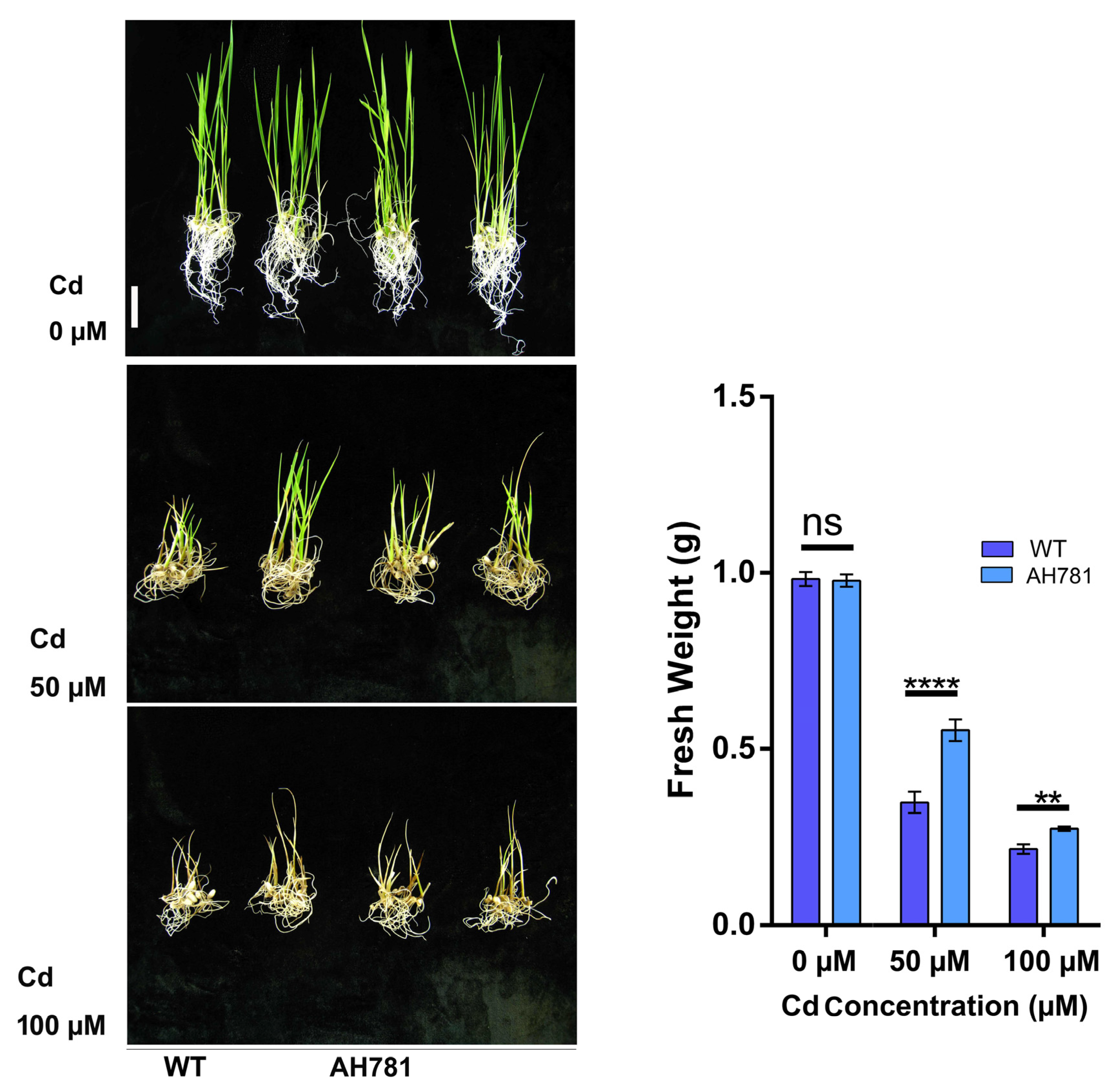
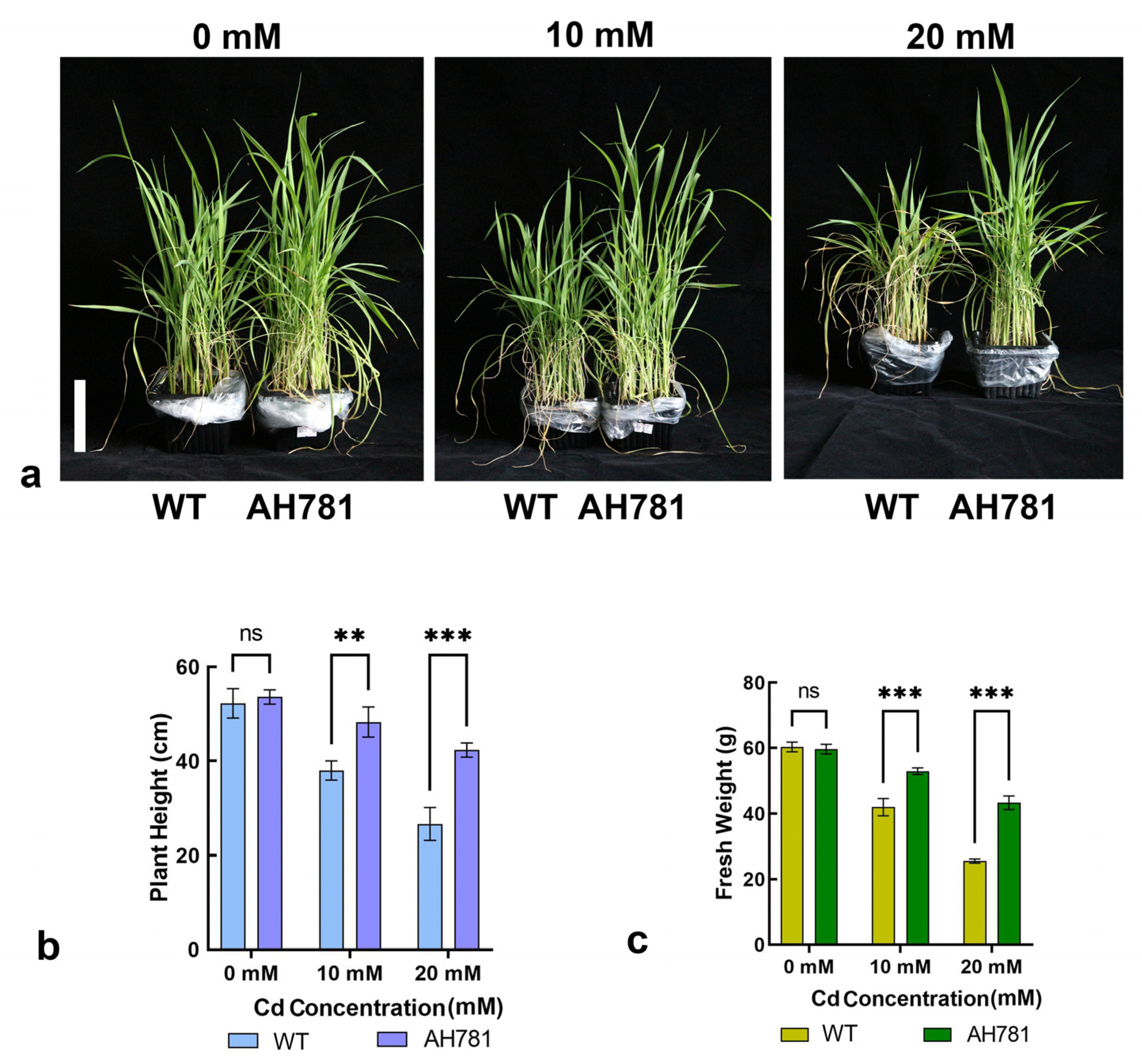
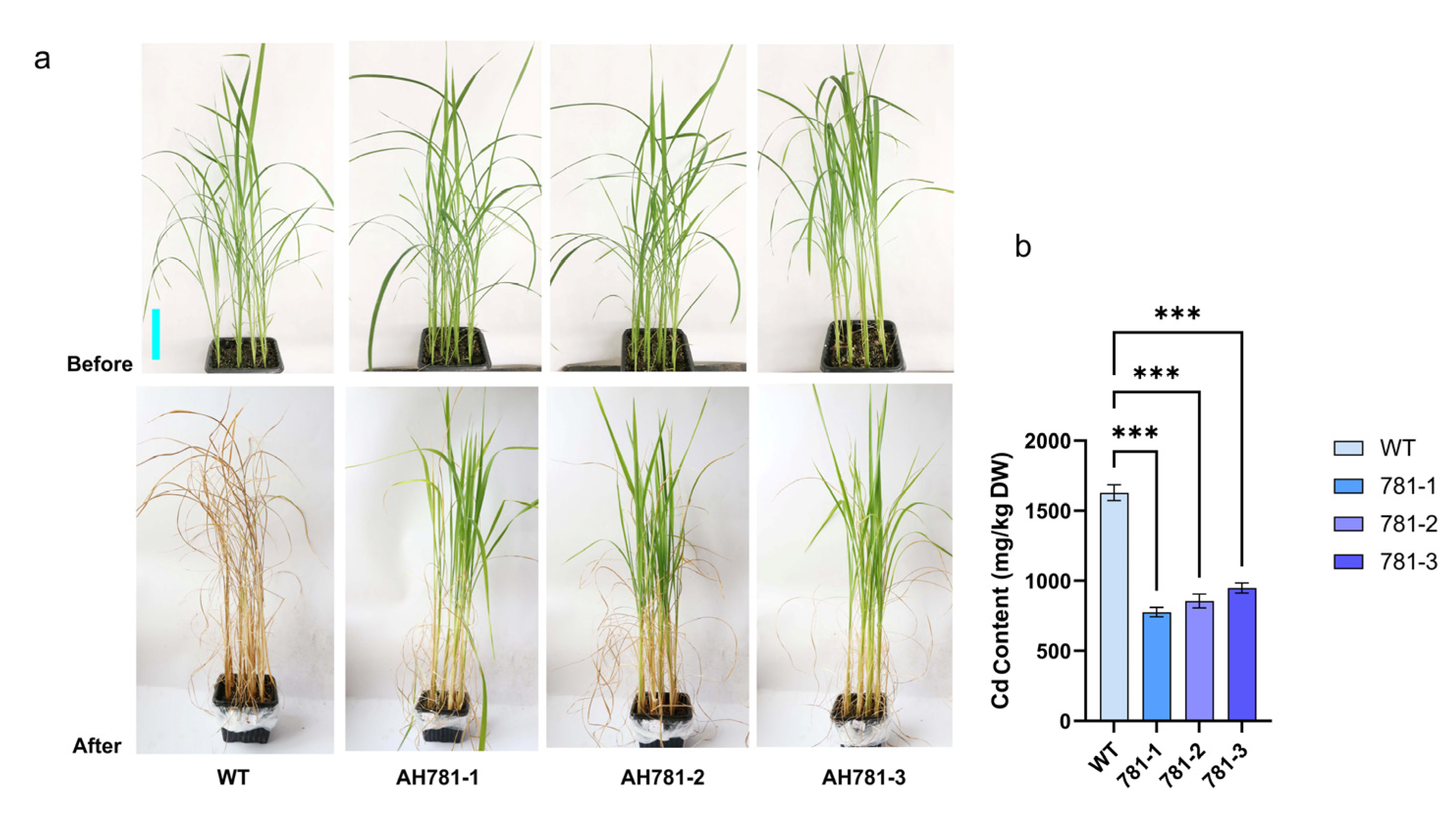
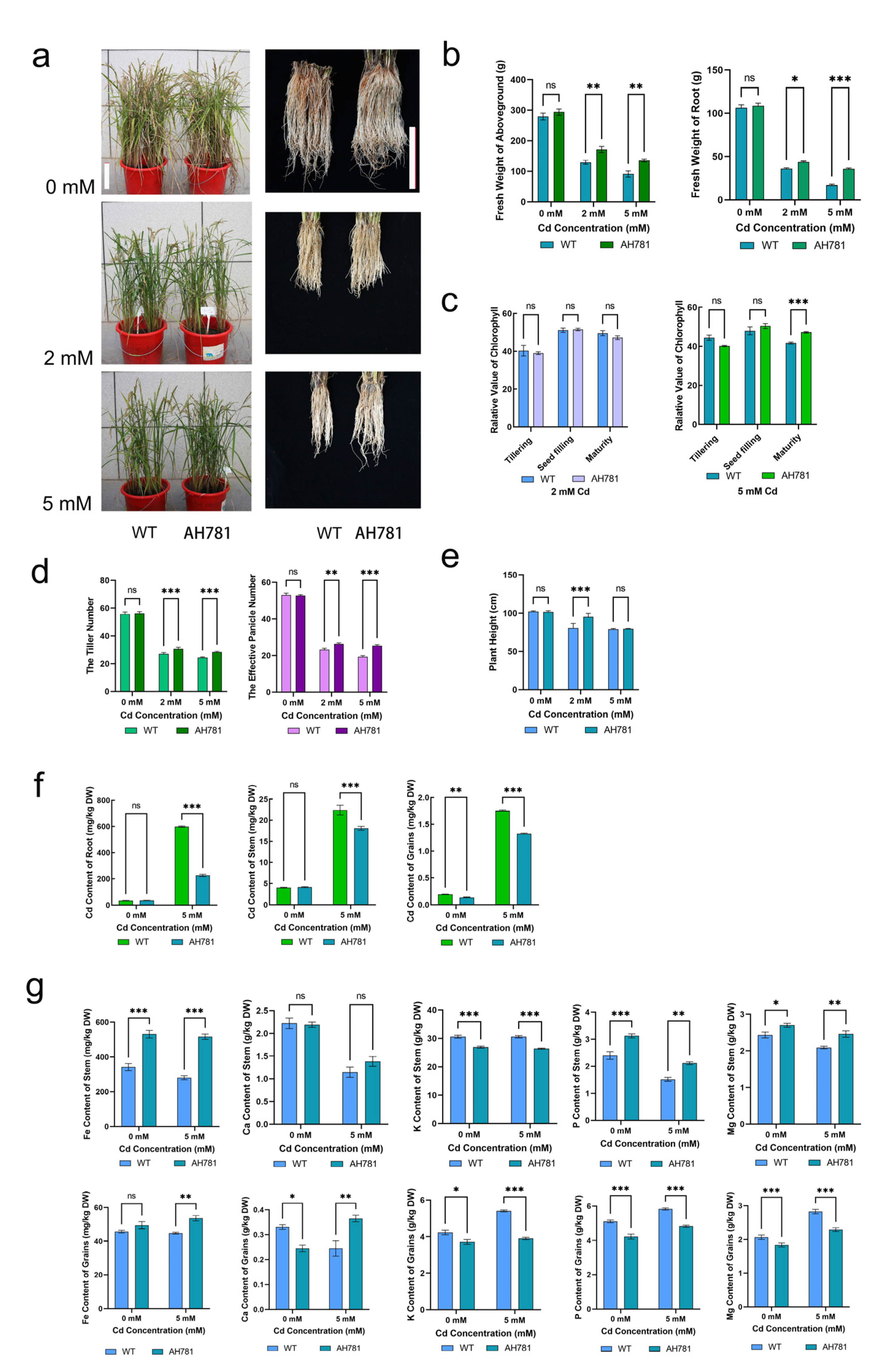
| Cd Concentration (mM) | Organs | Cd Content of AH781 (mg/kg DW) | Cd Content of WT (mg/kg DW) | Rate (%) |
|---|---|---|---|---|
| 0 | root | 0 a | 5 ± 1 b | 0 |
| shoot | 0 a | 0 a | 0 | |
| 10 | root | 1019 ± 15.3 a | 1962 ± 60.5 b | 51.9 |
| shoot | 88 ± 6.2 a | 156 ± 15.32 b | 56.4 | |
| 20 | root | 1977 ± 35.67 a | 3015 ± 70.87 b | 65 |
| shoot | 483 ± 25.43 a | 628 ± 35.25 b | 77.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Wang, Y.; Qian, C.; Yao, Q.; Liu, Q. Vvmrp1, Vvmt1, and Vvmt2 Co-Expression Improves Cadmium Tolerance and Reduces Cadmium Accumulation in Rice. Agronomy 2025, 15, 1493. https://doi.org/10.3390/agronomy15061493
Han H, Wang Y, Qian C, Yao Q, Liu Q. Vvmrp1, Vvmt1, and Vvmt2 Co-Expression Improves Cadmium Tolerance and Reduces Cadmium Accumulation in Rice. Agronomy. 2025; 15(6):1493. https://doi.org/10.3390/agronomy15061493
Chicago/Turabian StyleHan, Hongjuan, Yu Wang, Cen Qian, Quanhong Yao, and Qiaoquan Liu. 2025. "Vvmrp1, Vvmt1, and Vvmt2 Co-Expression Improves Cadmium Tolerance and Reduces Cadmium Accumulation in Rice" Agronomy 15, no. 6: 1493. https://doi.org/10.3390/agronomy15061493
APA StyleHan, H., Wang, Y., Qian, C., Yao, Q., & Liu, Q. (2025). Vvmrp1, Vvmt1, and Vvmt2 Co-Expression Improves Cadmium Tolerance and Reduces Cadmium Accumulation in Rice. Agronomy, 15(6), 1493. https://doi.org/10.3390/agronomy15061493






