Spatiotemporal Regulation of Starch–Sugar Metabolism by Potassium Enhances Carbon Partitioning and Processing Quality in Potatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Plant
2.2. Test Plants Obtained
2.3. Experimental Treatment
2.4. Sampling
2.5. Assessment of Phenotypic and Physiological Traits
2.5.1. Morphological Traits
2.5.2. Physiological Indices
- Note: All reagents in the test are from China National Pharmaceutical Group Co., Ltd. (Beijing, China).
2.5.3. Data Analysis
3. Results
3.1. Effects of Different K+ Concentrations on Potato Plant Growth
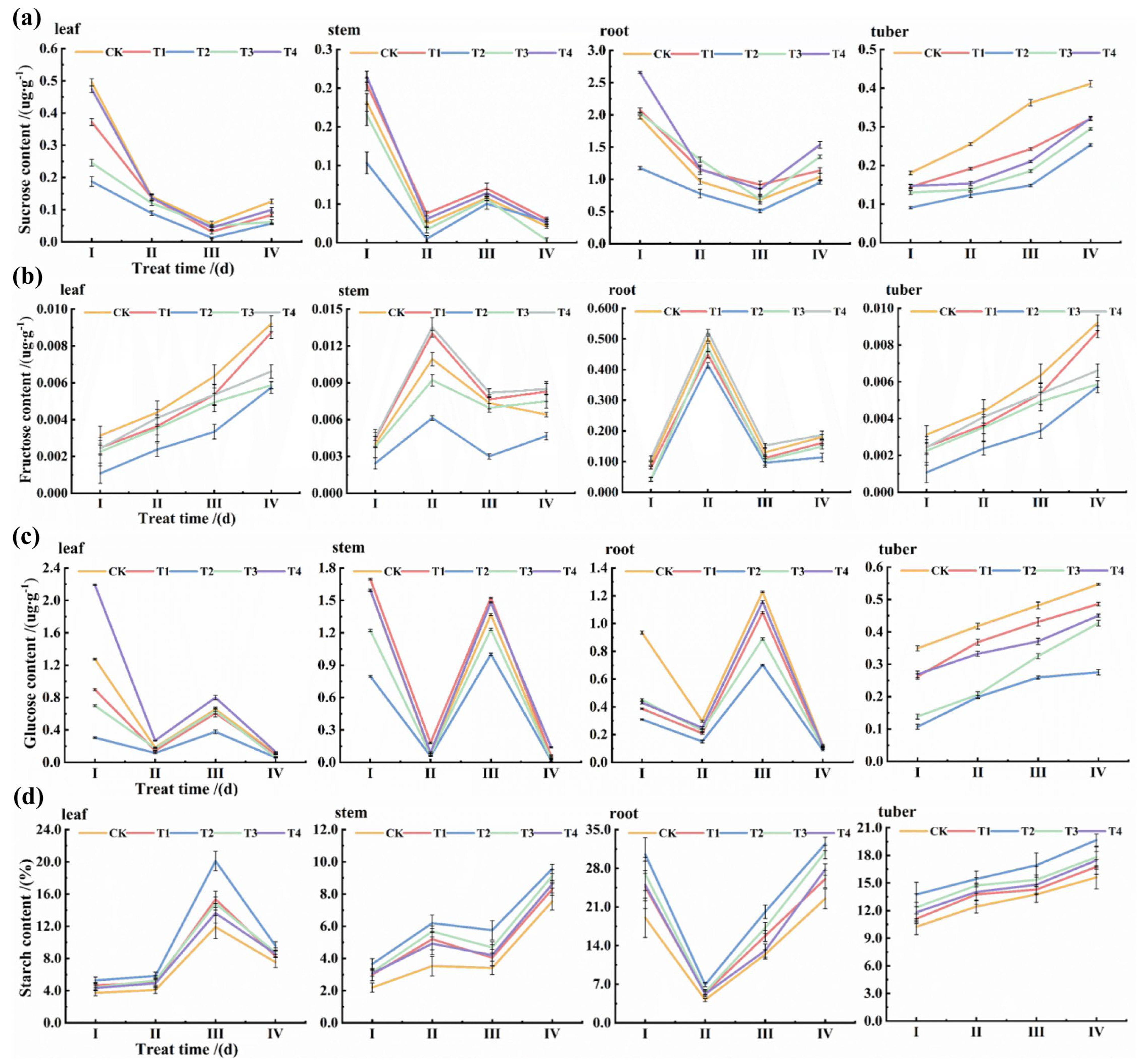
3.2. Effects of Different K+ Concentrations on Sucrose, Fructose, Glucose, and Starch Contents of Various Organs
3.3. Dynamics of Sucrose, Fructose, Glucose, and Starch Contents
3.4. Trends of Starch and Sugar Metabolism and Forms of Carbon Storage Under Different Treatments, Periods, and Tissue Sites
3.5. Trends in Starch–Sugar Metabolism and Forms of Carbon Presence in Different Treatments, Periods, and Tissue Sites
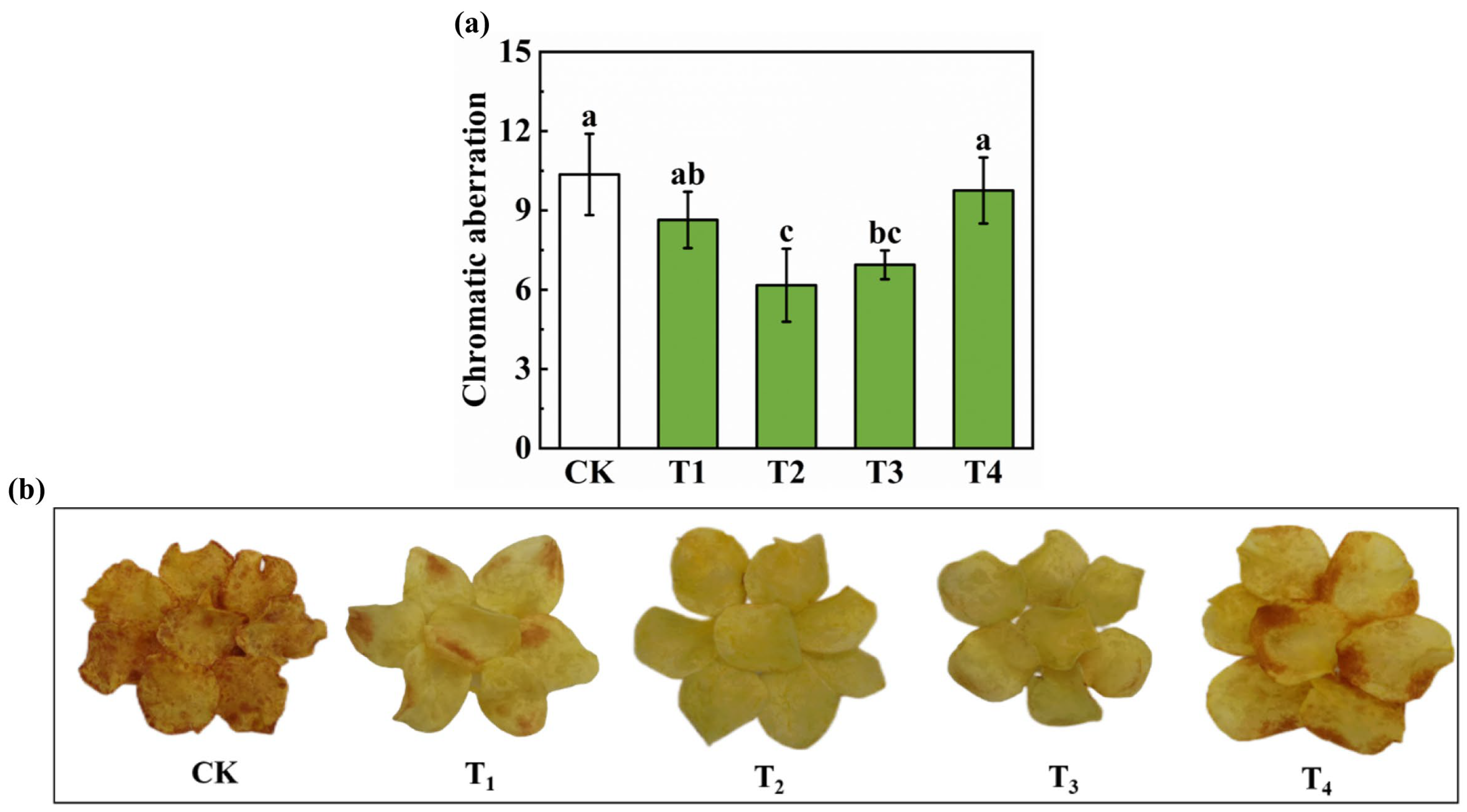
3.6. Effects of Different Potassium Treatments on Potato Yield Components and Quality of Fried Potato Chips
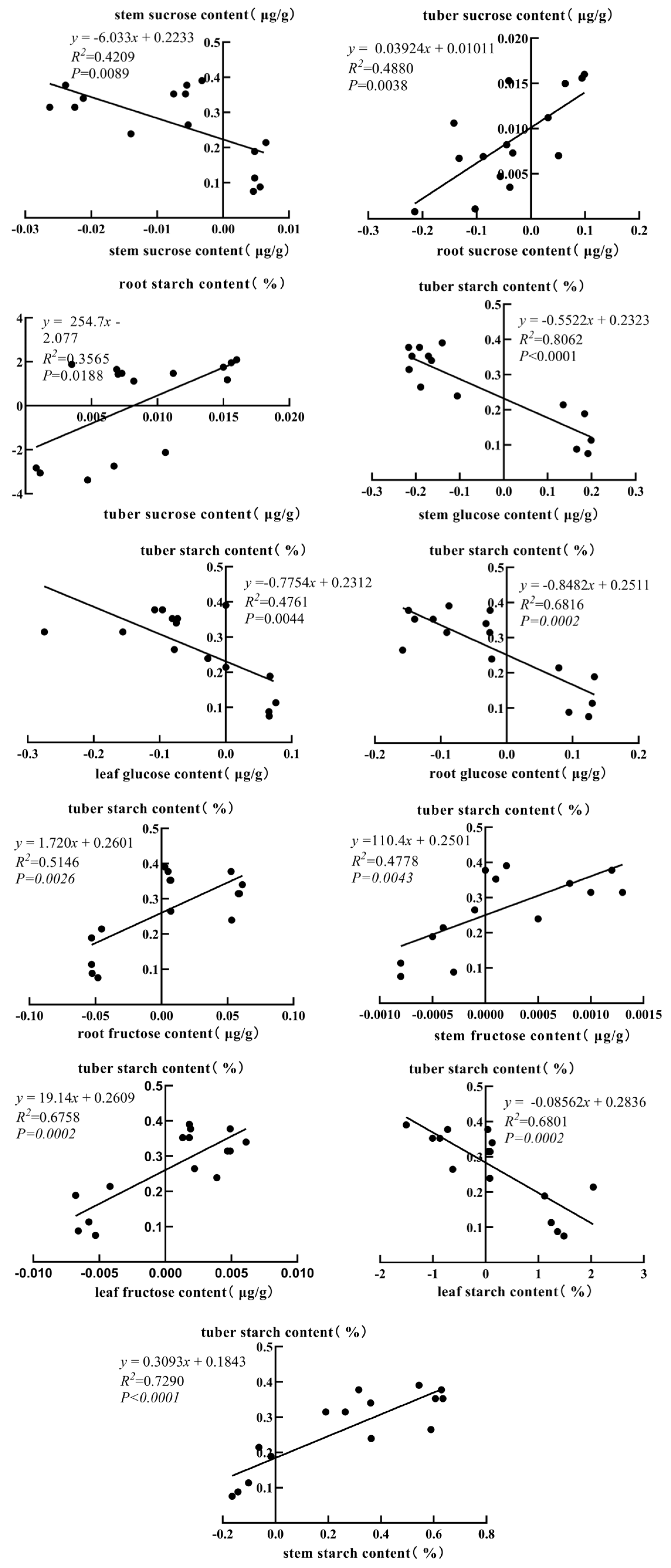
3.7. Relationships Between Starch and Sugar Contents and Potato Plant Growth and Yield
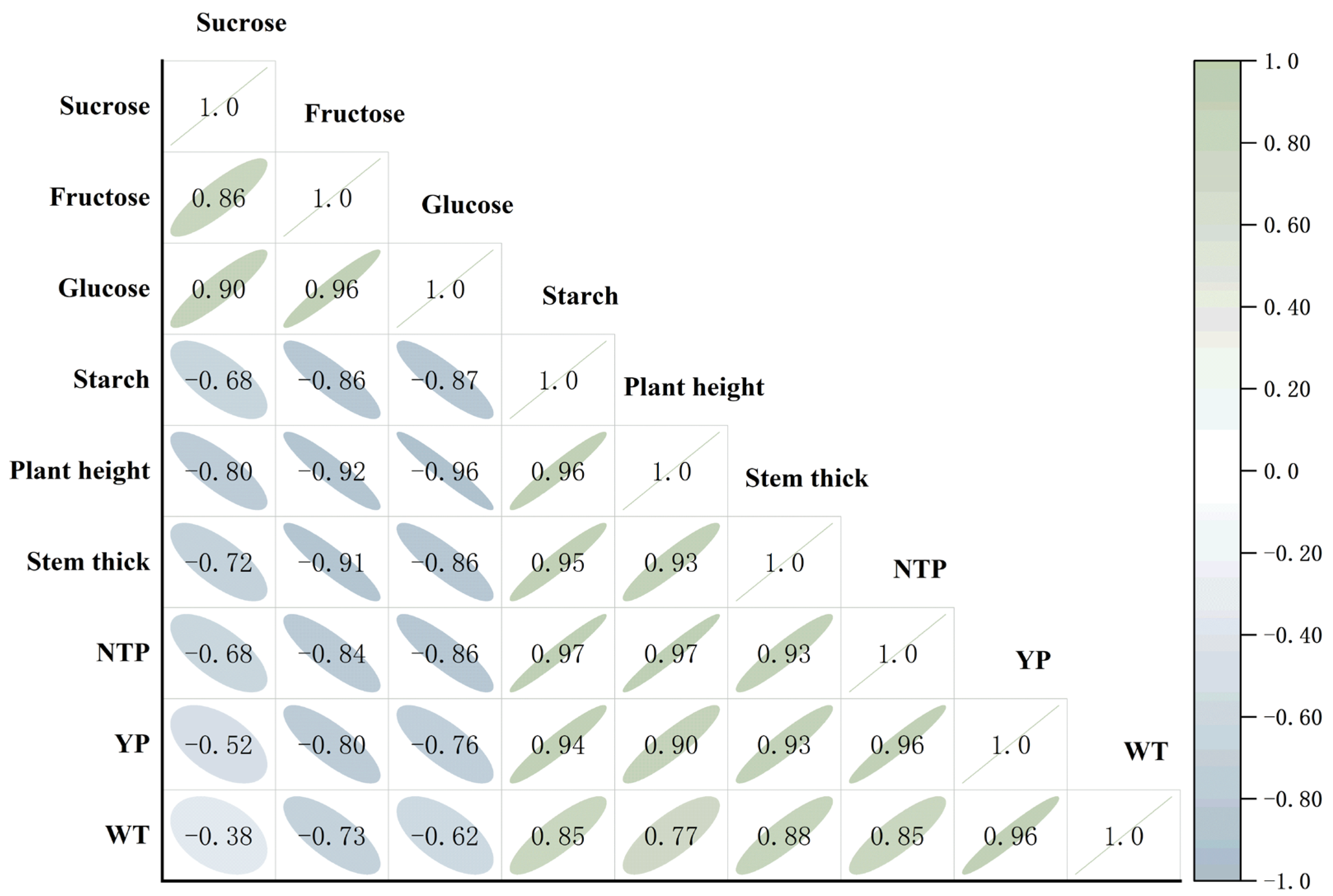
3.8. Correlation Analysis of Starch–Sugar Metabolic Rate Among Different Plant Tissues
4. Discussion
4.1. Effects of Potassium on Potato Plant Growth and Tuber Yield
4.2. Effects of Carbohydrate Composition, Content, and Metabolic Regulation on Potato Tuber Quality and Processing Performance
4.3. Effect of Potassium on the Dynamics of Starch–Sugar Metabolism and Key Transformation Processes in Tubers
4.4. Carbohydrate Transport and Partitioning Characteristics of Potato Tissues
4.5. Future Research Directions
5. Conclusions
- Effect of potassium on potato growth and tuber quality. The appropriate potassium concentration (23.5 mmol/L) promoted potato growth and increased tuber yield, and simultaneously increased starch accumulation and decreased the reducing sugar content within the tubers, thus improving processing quality.
- Dynamic changes in starch and sugar contents. Starch and sugar contents showed significant changes at different developmental stages and in various organs (leaves, stems, roots, and tubers), with more allocation of carbon to starch synthesis under the 23.5 mmol/L potassium concentration treatment and a high and stable rate of starch accumulation in the tubers.
- Correlations in starch–sugar metabolism rates among tissues. Further correlation analyses showed that the rates of starch–sugar metabolism were closely correlated among the tissues, suggesting a synergistic role of the tissues in the overall regulation of carbon metabolism.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazadul, I.M.; Sauda, N.; Afroz, N.; Nasir, U.M.; Nurul, A.M.; Mushfiqur, R.M.; Hasan, T.M.M.; Mohammed, A.A.; Ahmed, G.; Sharif, A. Dry Matter, Starch Content, Reducing Sugar, Color and Crispiness Are Key Parameters of Potatoes Required for Chip Processing. Horticulturae 2022, 8, 362. [Google Scholar] [CrossRef]
- Šimková, D.; Lachman, J.; Hamouz, K.; Vokál, B. Effect of cultivar, location and year on total starch, amylose, phosphorus content and starch grain size of high starch potato cultivars for food and industrial processing. Food Chem. 2013, 141, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Torabian, S.; Farhangi-Abriz, S.; Qin, R.; Noulas, C.; Loka, D.A. Potassium: A Vital Macronutrient in Potato Production—A Review. Agronomy 2021, 11, 543. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, Y.; Guo, T.; Zhang, P.; He, P.; Johnston, A.; Shcherbakov, A. Potassium management in potato production in Northwest region of China. Field Crops Res. 2015, 174, 48–54. [Google Scholar] [CrossRef]
- Neilson, J.; Lagüe, M.; Thomson, S.; Aurousseau, F.; Murphy, A.M.; Bizimungu, B.; Deveaux, V.; Bègue, Y.; Jacobs, J.M.E.; Tai, H.H. Gene expression profiles predictive of cold-induced sweetening in potato. Funct. Integr. Genom. 2017, 1, 459–476. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, Z.; Xia, H.; Sheng, M.; Liu, M.; Pan, S.; Li, Z.; Liu, J. Potassium Fertilization Stimulates Sucrose-to-Starch Conversion and Root Formation in Sweet Potato (Ipomoea batatas (L.) Lam.). Int. J. Mol. Sci. 2021, 22, 4826. [Google Scholar] [CrossRef]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Zhao, Y.; Fu, Y.; Yan, B.; Wan, X.; Cheng, G.; Zhang, W. Effects of different phosphorus and potassium supply on the root architecture, phosphorus and potassium uptake, and utilization efficiency of hydroponic rice. Sci. Rep. 2024, 14, 21178. [Google Scholar] [CrossRef]
- Shah, I.H.; Wu, J.H.; Li, X.Y.; Hameed, M.K.; Manzoor, M.A.; Li, P.L.; Zhang, Y.D.; Niu, Q.L.; Chang, L.Y. Exploring the role of nitrogen and potassium in photosynthesis implications for sugar: Accumulation and translocation in horticultural crops. Sci. Hortic. 2024, 327, 112832. [Google Scholar] [CrossRef]
- Huang, L.; Tan, H.; Zhang, C.; Li, Q.; Liu, Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Kang, S.; Chen, J. SUGAR Model-Assisted Analysis of Carbon Allocation and Transformation in Tomato Fruit Under Different Water Along with Potassium Conditions. Front. Plant Sci. 2020, 11, 712. [Google Scholar] [CrossRef]
- Martineau, E.; Domec, J.C.; Bosc, A.; Dannoura, M.; Gibon, Y.; Bénard, C.; Jordan-Meille, L. The role of potassium on maize leaf carbon exportation under drought condition. Acta Physiol. Plant. 2017, 39, 219. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Tan, M.; Zhang, C.; Jiao, J.; Wu, P.; Feng, K.; Li, L. NnNF-YB1 induced by the potassium fertilizer enhances starch synthesis in rhizomes of Nelumbo nucifera. Ind. Crops Prod. 2023, 203, 117197. [Google Scholar] [CrossRef]
- Shi, L.; Wang, J.; Liu, Y.; Ma, C.; Guo, S.; Lin, S.; Wang, J. Transcriptome analysis of genes involved in starch biosynthesis in developing Chinese chestnut (Castanea mollissima Blume) seed kernels. Sci. Rep. 2021, 11, 3570. [Google Scholar] [CrossRef]
- Li, Y.; Yin, M.; Li, L.; Zheng, J.; Yuan, X.; Wen, Y. Optimized potassium application rate increases foxtail millet grain yield by improving photosynthetic carbohydrate metabolism. Front. Plant Sci. 2022, 13, 1044065. [Google Scholar] [CrossRef]
- Wilmer, L.; Pawelzik, E.; Naumann, M. Comparison of the Effects of Potassium Sulphate and Potassium Chloride Fertilisation on Quality Parameters, Including Volatile Compounds, of Potato Tubers After Harvest and Storage. Front. Plant Sci. 2022, 13, 920212. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, Q.; Zhang, H.; Li, M.; Song, B.; Zhao, Z. Effects of potassium fertilization on potato starch physicochemical properties. Int. J. Biol. Macromol. 2018, 117, 467–472. [Google Scholar] [CrossRef]
- Ali, M.M.E.; Petropoulos, S.A.; Abdelfattah, D.; Elbagory, M.; Mohamed, M.H. Plant Growth, Yield and Quality of Potato Crop in Relation to Potassium Fertilization. Agronomy 2021, 11, 675. [Google Scholar] [CrossRef]
- Sheng, W.; Wei, C. Screening methods for cereal grains with different starch components: A mini review. J. Cereal Sci. 2022, 108, 103557. [Google Scholar] [CrossRef]
- Liu, K.; Xv, J.; Si, C.; Shi, Q.; Ding, Z.; Tang, X.; Xv, M.; Shi, Y.; Liu, J. Effect of potassium fertilization on storage root number, yield, and appearance quality of sweet potato (Ipomoea batatas L.). Front. Plant Sci. 2023, 14, 1298739. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Yun, Z.; Ya, L.; Xin, W.; Meng, K.; Hui, Y.; Dai, M.; Qiang, L. Quantifying cultivation technique and growth dynamics of purple-fleshed sweetpotato (Ipomoea batatas L.) in China. Field Crop Res. 2018, 227, 41–48. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, L.; Peng, C.; Li, H.; Li, X.; Zhang, M. Banana plant counting and morphological parameters measurement based on terrestrial laser scanning. Plant Methods 2022, 18, 66. [Google Scholar] [CrossRef]
- Meng, Z.; Duan, A.; Chen, D.; Dassanayake, K.B.; Wang, X.; Liu, Z.; Liu, H.; Gao, S. Suitable indicators using stem diameter variation-derived indices to monitor the water status of greenhouse tomato plants. PLoS ONE 2017, 12, e0171423. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, A.; Chen, X.; Jin, R.; Li, H.; Tang, Z. The Effect of Potassium Deficiency on Growth and Physiology in Sweetpotato [Ipomoea batatas (L.) Lam.] during Early Growth. HortScience 2017, 52, 1020–1028. [Google Scholar] [CrossRef]
- Shi, B.; Guo, X.; Liu, H.; Jiang, K.; Liu, L.; Yan, N.; Farag, M.A.; Liu, L. Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chem. 2024, 438, 137994. [Google Scholar] [CrossRef]
- Clasen, B.M.; Stoddard, T.J.; Luo, S.; Demorest, Z.L.; Li, J.; Cedrone, F.; Tibebu, R.; Davison, S.; Ray, E.E.; Daulhac, A. Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol. J. 2016, 14, 169–176. [Google Scholar] [CrossRef]
- Ji, S.; Grimm, B.; Wang, P. Chloroplast SRP43 and SRP54 independently promote thermostability and membrane binding of light-dependent protochlorophyllide oxidoreductases. Plant J. 2023, 115, 1583–1598. [Google Scholar] [CrossRef]
- Bhattarai, B. In Effect of Potassium on Quality and Yield of Potato Tubers—A Review. Int. J. Agric. Environ. Food Sci. 2018, 3, 7–12. [Google Scholar] [CrossRef]
- Sandaña, P.; Soratto, R.P.; Silva, J.C.A.; Valenzuela, A.; Parecido, R.J.; Fernandes, A.M.; Ciampitti, I.A. Critical potassium dilution curve for potato crops. Field Crops Res. 2024, 316, 109492. [Google Scholar] [CrossRef]
- Lal, M.K.; Tiwari, R.K.; Kumar, R.; Naga, K.C.; Kumar, A.; Singh, B.; Raigond, P.; Dutt, S.; Chourasia, K.N.; Kumar, D.; et al. Effect of potato apical leaf curl disease on glycemic index and resistant starch of potato (Solanum tuberosum L.) tubers. Food Chem. 2021, 359, 129939. [Google Scholar] [CrossRef]
- Tu, B.; Liu, C.; Tian, B.; Zhang, Q.; Liu, X.; Herbert, S.J. Reduced abscisic acid content is responsible for enhanced sucrose accumulation by potassium nutrition in vegetable soybean seeds. J. Plant Res. 2017, 130, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Zhang, X.; Wang, C.; Zhou, C. Effects of Different Water and Potassium Supply on Glucose, Fructose, and Sucrose Carbon Allocation in Tomato Fruit. Plant Growth Regul. 2022, 41, 684–700. [Google Scholar] [CrossRef]
- Liu, T.; Kawochar, M.A.; Liu, S.; Cheng, Y.; Begum, S.; Wang, E.; Zhou, T.; Liu, T.; Cai, X.; Song, B. Suppression of the tonoplast sugar transporter, StTST3.1, affects transitory starch turnover and plant growth in potato. Plant J. 2023, 113, 342–356. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wen, G.; Li, G.; Li, Z.; Zhang, R.; Ma, S.; Zhou, J.; Xie, C. Mapping QTL underlying tuber starch content and plant maturity in tetraploid potato. Crop J. 2019, 7, 261–272. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Dong, J.; Sun, Y.; Fu, H. Split Application of Potassium Reduces Rice Chalkiness by Regulating Starch Accumulation Process Under High Temperatures. Agronomy 2025, 15, 116. [Google Scholar] [CrossRef]
- Benito, P.; Bellón, J.; Porcel, R.; Yenush, L.; Mulet, J.M. The Biostimulant, Potassium Humate Ameliorates Abiotic Stress in Arabidopsis thaliana by Increasing Starch Availability. Int. J. Mol. Sci. 2023, 24, 12140. [Google Scholar] [CrossRef]
- Peng, L.; Xiao, H.; Li, R.; Zeng, Y.; Gu, M.; Moran, N.; Yu, L.; Xu, G. Potassium transporter OsHAK18 mediates potassium and sodium circulation and sugar translocation in rice. Plant Physiol. 2023, 193, 2003–2020. [Google Scholar] [CrossRef]
- Shankar, A.; Singh, A.; Kanwar, P.; Srivastava, A.K.; Pandey, A.; Suprasanna, P.; Kapoor, S.; Pandey, G.K. Gene expression analysis of rice seedling under potassium deprivation reveals major changes in metabolism and signaling components. PLoS ONE 2013, 8, e70321. [Google Scholar] [CrossRef]
- Shah, I.H.; Manzoor, M.A.; Azam, M.; Jinhui, W.; Li, X.; Rehman, A.; Li, P.; Zhang, Y.; Niu, Q.; Chang, L. Comprehensive characterization and expression profiling of sucrose phosphate synthase (SPS) and sucrose synthase (SUS) family in Cucumis melo under the application of nitrogen and potassium. BMC Plant Biol. 2025, 25, 285. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Sajid, M.; Ullah, R.; Haya, S.; Shahab, M. Dose Optimization of Potassium (K) for Yield and Quality Increment of Strawberry (Fragaria ×ananassa Duch) Chandler. Exp. Agric. 2014, 4, 1526–1535. [Google Scholar] [CrossRef]
- Fan, W.; Gao, H.; Zhang, L.; Mao, D.; Li, Y.; Zhang, L.; Li, J.; Zhao, X.; Hou, H. Genome-wide identification and expression profiling of MST, SUT and SWEET transporters in Dendrobium catenatum. BMC Genom. 2024, 25, 1213. [Google Scholar] [CrossRef]
- Xinru, B.; Yunke, Z.; Guangcun, L.; Lu, L. Regulation of storage organ formation by long-distance tuberigen signals in potato. Hortic. Res. 2025, 12, uhae360. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Bergonzi, S.; Oortwijn, M.; Sonnewald, S.; Du, M.; Visser, R.G.F.; Sonnewald, U.; Bachem, C.W.B. Source-Sink Regulation Is Mediated by Interaction of an FT Homolog with a SWEET Protein in Potato. Curr. Biol. 2019, 29, 1178–1186. [Google Scholar] [CrossRef]

| Treatment | NH4NO3 (g·L−1) | KNO3 (g·L−1) |
|---|---|---|
| CK | 240.18 | 0.00 |
| T1 | 202.60 | 47.50 |
| T2 | 146.20 | 118.75 |
| T3 | 127.40 | 285.00 |
| T4 | 179.64 | 380.00 |
| Sucrose (µg/g) | Fructose (µg/g) | Glucose (µg/g) | Starch (%) | |
|---|---|---|---|---|
| Treatments (W) | ||||
| CK | 0.44 ± 0.01 b | 0.07 ± 0.00 b | 0.61 ± 0.00 b | 9.63 ± 0.57 d |
| T1 | 0.45 ± 0.01 b | 0.06 ± 0.00 c | 0.53 ± 0.00 c | 11.35 ± 0.32 c |
| T2 | 0.29 ± 0.00 c | 0.05 ± 0.00 e | 0.30 ± 0.00 e | 13.86 ± 0.19 a |
| T3 | 0.43 ± 0.01 b | 0.06 ± 0.00 d | 0.43 ± 0.00 d | 12.30 ± 0.32 b |
| T4 | 0.51 ± 0.00 a | 0.07 ± 0.00 a | 0.63 ± 0.00 a | 11.34 ± 0.36 c |
| Stages (S) | ||||
| I | 0.66 ± 0.00 a | 0.03 ± 0.00 d | 0.80 ± 0.00 b | 11.15 ± 0.46 c |
| II | 0.35 ± 0.01 c | 0.14 ± 0.00 a | 0.20 ± 0.00 c | 7.41 ± 0.17 d |
| III | 0.27 ± 0.01 d | 0.04 ± 0.00 c | 0.83 ± 0.01 a | 12.58 ± 0.12 b |
| IV | 0.41 ± 0.00 b | 0.05 ± 0.00 b | 0.17 ± 0.00 d | 15.64 ± 0.14 a |
| Parts (p) | ||||
| leaf | 0.15 ± 0.00 c | 0.03 ± 0.00 b | 0.49 ± 0.00 b | 8.30 ± 0.16 c |
| stem | 0.07 ± 0.00 d | 0.01 ± 0.00 c | 0.71 ± 0.00 a | 5.29 ± 0.11 d |
| root | 1.25 ± 0.02 a | 0.21 ± 0.00 a | 0.46 ± 0.00 c | 18.59 ± 0.27 a |
| tuber | 0.22 ± 0.00 b | 0.01 ± 0.00 c | 0.34 ± 0.00 d | 14.60 ± 0.17 b |
| W × S | 318.61 ** | 75.12 ** | 967.60 ** | 8.88 ** |
| W × P | 96.71 ** | 6.05 ** | 1421.76 ** | 2.93 ** |
| S × P | 1684.37 ** | 3813.62 ** | 15079.85 ** | 341.03 ** |
| W × S × P | 53.51 ** | 4.77 ** | 692.99 ** | 2.37 ** |
| P | S | T | P1 | P2 | P3 | ΔS | ΔP1 | ΔP2 | ΔP3 |
|---|---|---|---|---|---|---|---|---|---|
| Leaf | SI | CK | 2.07 | 0.38 | 41.24 | - | - | - | |
| T1 | 3.64 ** | 0.41 ns | 54.85 * | - | - | - | |||
| T2 | 10.50 ** | 0.60 ** | 29.45 * | - | - | - | |||
| T3 | 4.60 ** | 0.35 ns | 57.03 * | - | - | - | |||
| T4 | 1.61 * | 0.21 * | 97.05 ** | - | - | - | |||
| SII | CK | 10.47 | 0.56 | 2.83 | S(II-I) | 8.40 | 0.19 | −38.41 | |
| T1 | 14.90 * | 0.70 * | 2.87 ns | 11.26 * | 0.29 ** | −51.98 * | |||
| T2 | 24.06 ** | 0.58 ns | 3.11 * | 13.56 ** | −0.02 ** | −26.34 * | |||
| T3 | 15.11 * | 0.52 ns | 3.18 * | 10.51 * | 0.18 ns | −53.85 * | |||
| T4 | 10.66 ns | 0.42 * | 4.84 ** | 9.05 ns | 0.21 * | −92.22 ** | |||
| SIII | CK | 16.36 | 0.08 | 37.21 | S(III-II) | 5.89 | −0.48 | 34.38 | |
| T1 | 23.53 * | 0.05 * | 44.21 * | 8.64 * | −0.65 * | 41.34 * | |||
| T2 | 50.40 ** | 0.03 ** | 45.52 * | 26.35 ** | −0.54 * | 42.41 * | |||
| T3 | 21.44 * | 0.08 ns | 70.55 ** | 6.33 * | −0.45 ns | 67.37 ** | |||
| T4 | 15.86 ns | 0.05 * | 53.80 ** | 5.20 * | −0.37 * | 48.97 * | |||
| SIV | CK | 28.13 | 0.88 | 3.33 | S(IV-III) | 11.76 | 0.80 | −33.87 | |
| T1 | 40.89 * | 0.70 * | 4.29 * | 17.35 * | 0.65 * | −39.92 * | |||
| T2 | 69.15 ** | 0.71 * | 2.86 * | 18.75 ** | 0.67 * | −42.66 * | |||
| T3 | 58.93 ** | 0.71 * | 2.99 * | 37.49 ** | 0.64 * | −67.56 ** | |||
| T4 | 33.40 * | 0.63 * | 4.54 * | 17.54 * | 0.58 * | −49.27 * | |||
| Steam | SI | CK | 1.23 | 0.11 | 398.22 | - | - | - | |
| T1 | 1.57 * | 0.12 ns | 386.37 ns | - | - | - | |||
| T2 | 4.04 ** | 0.13 * | 327.50 * | - | - | - | |||
| T3 | 2.27 ** | 0.14 * | 324.65 * | - | - | - | |||
| T4 | 1.69 * | 0.13 * | 353.73 * | - | - | - | |||
| SII | CK | 30.14 | 0.26 | 7.50 | S(II-I) | 28.90 | 0.15 | −390.71 | |
| T1 | 22.37 * | 0.20 * | 13.95 ** | 20.80 * | 0.07 ** | −372.42 ns | |||
| T2 | 90.87 ** | 0.08 ** | 9.26 * | 86.83 ** | −0.05 ** | −318.24 * | |||
| T3 | 59.26 ** | 0.22 * | 7.55 ns | 56.99 ** | 0.08 * | −317.10 * | |||
| T4 | 36.65 * | 0.30 * | 6.65 ns | 34.96 * | 0.16 ns | −347.08 * | |||
| SIII | CK | 2.38 | 0.04 | 186.27 | S(III-II) | −27.76 | −0.22 | 178.77 | |
| T1 | 2.53 ns | 0.05 * | 199.18 ns | −19.84 * | −0.15 * | 185.23 ns | |||
| T2 | 5.45 ** | 0.05 * | 333.13 ** | −85.42 ** | −0.03 ** | 323.87 ** | |||
| T3 | 3.62 * | 0.05 * | 177.01 ns | −55.64 ** | −0.17 * | 169.46 ns | |||
| T4 | 2.71 * | 0.04 ns | 181.02 ns | −33.95 * | −0.25 * | 174.37 ns | |||
| SIV | CK | 104.72 | 0.42 | 6.94 | S(IV-III) | 102.33 | 0.37 | −179.33 | |
| T1 | 85.55 * | 0.45 ns | 7.06 ns | 83.02 | 0.41 * | −192.12 ns | |||
| T2 | 185.11 ** | 1.15 ** | 4.15 * | 179.65 | 1.10 ** | −328.98 ** | |||
| T3 | 219.21 ** | 0.09 ** | 4.11 * | 215.59 | 0.04 ** | −172.90 ns | |||
| T4 | 49.64 ** | 0.17 ** | 16.43 ** | 46.93 | 0.13 ** | −164.58 ns | |||
| Root | SI | CK | 6.37 | 1.91 | 9.96 | - | - | - | |
| T1 | 9.68 ** | 4.45 ** | 4.83 ** | - | - | - | |||
| T2 | 20.03 ** | 3.34 ** | 7.35 * | - | - | - | |||
| T3 | 10.71 ** | 4.12 ** | 10.54 ns | - | - | - | |||
| T4 | 7.82 * | 4.92 ** | 3.88 ** | - | - | - | |||
| SII | CK | 2.36 | 1.21 | 0.59 | S(II-I) | −4.01 | −0.70 | −9.36 | |
| T1 | 2.99 * | 1.75 * | 0.47 ns | −6.70 ** | −2.70 ** | −4.36 ** | |||
| T2 | 5.14 ** | 1.38 * | 0.36 * | −14.88 ** | −1.96 ** | −6.99 * | |||
| T3 | 2.79 * | 1.86 * | 0.49 * | −7.92 ** | −2.25 ** | −10.06 ns | |||
| T4 | 2.71 * | 1.50 * | 0.48 * | −5.11 * | −3.42 ** | −3.40 ** | |||
| SIII | CK | 6.10 | 0.50 | 9.44 | S(III-II) | 3.74 | −0.71 | 8.85 | |
| T1 | 7.48 * | 0.77 ** | 9.70ns | 4.49 * | −0.98 * | 9.23 ns | |||
| T2 | 15.40 ** | 0.64 * | 7.29 ** | 10.26 ** | −0.74 ns | 6.93 * | |||
| T3 | 10.22 ** | 0.69 * | 8.54 ns | 7.43 ** | −1.17 ** | 8.05 ns | |||
| T4 | 6.08 ns | 0.65 * | 7.59 ** | 3.37 * | −0.85 * | 7.12 * | |||
| SIV | CK | 16.74 | 3.43 | 0.69 | S(IV-III) | 10.64 | 2.92 | −8.76 | |
| T1 | 18.63 * | 4.32 * | 0.64 ns | 11.16 ns | 3.55 * | −9.06 ns | |||
| T2 | 28.15 ** | 4.71 * | 0.77 ** | 12.75 * | 4.07 * | −6.52 * | |||
| T3 | 19.26 * | 5.25 ** | 0.72 ns | 9.04 * | 4.55 ** | −7.82 * | |||
| T4 | 15.12 * | 5.10 ** | 0.61 ** | 9.04 * | 4.46 ** | −6.98 * | |||
| Tuber | SI | CK | 19.19 | 0.51 | 111.60 | - | - | - | |
| T1 | 27.15 * | 0.55 ns | 106.41 ns | - | - | - | |||
| T2 | 69.28 ** | 0.84 ** | 99.00 * | - | - | - | |||
| T3 | 45.64 ** | 0.92 ** | 61.49 * | - | - | - | |||
| T4 | 28.22 * | 0.54 ns | 110.46 ns | - | - | - | |||
| SII | CK | 18.36 | 0.61 | 95.09 | S(II-I) | −0.83 | 0.09 | −16.52 | |
| T1 | 24.42 * | 0.52 * | 101.14 ns | −2.73 ** | −0.03 ** | −5.27 ** | |||
| T2 | 47.50 ** | 0.62 ns | 83.68 * | −21.79 ** | −0.22 ** | −15.33 ns | |||
| T3 | 42.45 ** | 0.66 ns | 58.53 * | −3.19 ** | −0.26 ** | −2.96 ** | |||
| T4 | 28.66 ** | 0.46 * | 81.19 * | 0.44 ** | −0.09 ** | −29.28 ** | |||
| SIII | CK | 16.18 | 0.74 | 75.83 | S(III-II) | −2.18 | 0.14 | −19.26 | |
| T1 | 21.05 * | 0.56 * | 80.41 ns | −3.37 ** | 0.04 ** | −20.73 ns | |||
| T2 | 41.20 ** | 0.56 * | 77.70 ns | −6.29 ** | −0.05 ** | −5.98 ** | |||
| T3 | 29.76 ** | 0.56 * | 65.89 * | −12.69 ** | −0.09 ** | 7.36 ** | |||
| T4 | 25.27 ** | 0.56 * | 69.19 ns | −3.39 ** | 0.10 * | −12.00 * | |||
| SIV | CK | 16.13 | 0.74 | 59.38 | S(IV-III) | −0.05 | 0.00 | −16.45 | |
| T1 | 20.54 * | 0.65 * | 55.67 ns | −0.51 ** | 0.09 ** | −24.74 ** | |||
| T2 | 36.82 ** | 0.90 * | 47.89 * | −4.38 ** | 0.34 ** | −29.81 ** | |||
| T3 | 24.48 ** | 0.68 ns | 72.74 * | −5.27 ** | 0.12 ** | 6.85 ** | |||
| T4 | 22.42 * | 0.71 ns | 67.97 * | −2.86 ** | 0.15 ** | −1.22 ** |
| S | T | P | Sucrose | Fructose | Glucose | Starch | P | Sucrose | Fructose | Glucose | Starch |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S(II-I) | CK | Leaf | −50 × 10−3 | 4.89 × 10−3 | −0.16 | 0.05 | Root | −140 × 10−3 | 60 × 10−3 | −0.09 | −2.13 |
| T1 | −30 × 10−3 * | 4.88 × 10−3 ns | −0.11 * | 0.04 * | −130 × 10−3 ns | 50 × 10−3 * | −0.03 ** | −2.74 ** | |||
| T2 | −10 × 10−3 ** | 3.86 × 10−3 ns | −0.03 ** | 0.08 ** | −60 × 10−3 ** | 50 × 10−3 * | −0.02 ** | −3.38 ** | |||
| T3 | −20 × 10−3 ** | 10 × 10−3 ** | −0.08 ** | 0.12 ** | −100 × 10−3 * | 60 × 10−3 ns | −0.03 ** | −3.06 ** | |||
| T4 | −50 × 10−3 ns | 4.71 × 10−3 ns | −0.27 ** | 0.09 ** | −210 × 10−3 ** | 60 × 10−3 ns | −0.03 ** | −2.82 ** | |||
| S(III-II) | CK | −10 × 10−3 | −10 × 10−3 | 0.07 | 1.12 | −40 × 10−3 | −50 × 10−3 | 0.13 | 1.18 | ||
| T1 | −20 × 10−3 ** | −10 × 10−3 ns | 0.07 ns | 1.48 * | −30 × 10−3 * | −50 × 10−3 ns | 0.12 ns | 1.48 * | |||
| T2 | −10 × 10−3 ns | −4.16 × 10−3 ** | 0.00 ** | 2.04 ** | −40 × 10−3 ns | −50 × 10−3 ns | 0.08 * | 1.89 * | |||
| T3 | −10 × 10−3 ns | −10 × 10−3 ns | 0.07 ns | 1.36 * | −90 × 10−3 ** | −50 × 10−3 ns | 0.09 * | 1.66 * | |||
| T4 | −10 × 10−3 ns | −10 × 10−3 ns | 0.08 * | 1.24 * | −40 × 10−3 ns | −50 × 10−3 ns | 0.13 ns | 1.12 * | |||
| S(IV-III) | CK | 10 × 10−3 ns | 2.19 × 10−3 | −0.08 | −0.62 | 50 × 10−3 | 10 × 10−3 | −0.16 | 1.44 | ||
| T1 | 10 × 10−3 ns | 1.26 × 10−3 * | −0.07 * | −1.01 ** | 30 × 10−3 * | 10 × 10−3 ns | −0.14 * | 1.47 ns | |||
| T2 | 10 × 10−3 ns | 1.82 × 10−3 * | 0.00** | −1.51 ** | 60 × 10−3 * | 2.50 × 10−3** | −0.09 ** | 1.76 * | |||
| T3 | 1.74 × 10−3 ** | 1.82 × 10−3 * | −0.08 ns | −0.87 * | 90 × 10−3 ** | 10 × 10−3 ns | −0.11 ** | 1.97 * | |||
| T4 | 10 × 10−3 ns | 1.93 × 10−3 * | −0.10 * | −0.72 * | 100 × 10−3 ** | 4.91 × 10−3** | −0.15 ns | 2.10 * | |||
| S(II-I) | CK | Stem | −20 × 10−3 | 0.99 × 10−3 | −0.22 | 0.19 | Tuber | 10 × 10−3 | 18.1 × 10−3 | 0.01 | 0.31 |
| T1 | −20 × 10−3 ns | 1.23 × 10−3 ** | −0.22 ns | 0.32 ** | 10 × 10−3 ns | 16.8 × 10−3 * | 0.02 ** | 0.38 * | |||
| T2 | −10 × 10−3 ** | 52.9 × 10−3 * | −0.11 ** | 0.36 ** | 4.74 × 10−3 ** | 18.5 × 10−3 ns | 0.01 ns | 0.24 * | |||
| T3 | −20 × 10−3 ns | 77.9 × 10−3 * | −0.16 * | 0.36 ** | 1.11 × 10−3 ** | 18.1 × 10−3 ns | 0.01 ns | 0.34 * | |||
| T4 | −30 × 10−3 ** | 1.29 × 10−3 ** | −0.22 ns | 0.26 * | 0.79 × 10−3 ** | 23.7 × 10−3 ns | 0.01 ns | 0.31 ns | |||
| S(III-II) | CK | 4.80 × 10−3 | −51.2 × 10−3 | 0.18 | −0.02 | 20 × 10−3 | 28.0 × 10−3 | 0.01 | 0.19 | ||
| T1 | 4.60 × 10−3 ns | −76.6 × 10−3 ** | 0.19 ns | −0.16 ** | 10 × 10−3 ** | 2.45 × 10−3 * | 0.01 ns | 0.08 ** | |||
| T2 | 10 × 10−3 ** | −44.8 × 10−3 * | 0.13 * | −0.06 ** | 3.48 × 10−3 ** | 13.8 × 10−3 ** | 0.01 ns | 0.21 * | |||
| T3 | 10 × 10−3 ** | −32.3 × 10−3 * | 0.17 ns | −0.14 ** | 10 × 10−3 ** | 20.2 × 10−3 * | 0.02** | 0.09 * | |||
| T4 | 4.77 × 10−3 ns | −76.2 × 10−3 * | 0.20 * | −0.10 ** | 10 × 10−3 ** | 18.1 × 10−3 * | 0.01 ns | 0.11 * | |||
| S(IV-III) | CK | −10 × 10−3 | −13.3 × 10−3 | −0.19 | 0.59 | 10 × 10−3 | 40.9 × 10−3 | 0.01 | 0.26 | ||
| T1 | −10 × 10−3 ns | 904 × 10−3 ** | −0.21 * | 0.61 ns | 10 × 10−3 ns | 48.2 × 10−3 * | 0.01 ns | 0.35 * | |||
| T2 | −3.25 × 10−3 ** | 23.7 × 10−3 ** | −0.14 * | 0.54 ns | 10 × 10−3 ns | 34.4 × 10−3 * | 0.00 ** | 0.39 ** | |||
| T3 | −10 × 10−3 ns | 775 × 10−3 ** | −0.17 * | 0.63 ns | 20 × 10−3 ** | 13.3 × 10−3 ** | 0.01 ns | 0.35 * | |||
| T4 | −10 × 10−3 ns | 430 × 10−3 ** | −0.19 ns | 0.63 ns | 20 × 10−3 ** | 18.1 × 10−3 ** | 0.01 ns | 0.38 * |
| T | Number of Tubers Per Plant/n | Weight of Individual Tuber/g | Yield Per Plant/g |
| CK | 2.75 ± 0.17 e | 0.31 ± 0.06 c | 0.89 ± 0.09 d |
| T1 | 4.47 ± 0.17 d | 2.34 ± 0.08 b | 7.46 ± 0.19 c |
| T2 | 7.77 ± 0.23 a | 2.87 ± 0.11 a | 12.17 ± 0.19 a |
| T3 | 6.06 ± 0.18 b | 2.13 ± 0.12 b | 9.19 ± 0.19 b |
| T4 | 4.98 ± 0.12 c | 2.12 ± 0.24 b | 7.64 ± 0.23 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-L.; Feng, S.-L.; Guo, R.; Yang, H.-Y.; Cheng, L.-X.; Yu, B.; Liu, J. Spatiotemporal Regulation of Starch–Sugar Metabolism by Potassium Enhances Carbon Partitioning and Processing Quality in Potatoes. Agronomy 2025, 15, 1481. https://doi.org/10.3390/agronomy15061481
Li J-L, Feng S-L, Guo R, Yang H-Y, Cheng L-X, Yu B, Liu J. Spatiotemporal Regulation of Starch–Sugar Metabolism by Potassium Enhances Carbon Partitioning and Processing Quality in Potatoes. Agronomy. 2025; 15(6):1481. https://doi.org/10.3390/agronomy15061481
Chicago/Turabian StyleLi, Jin-Li, Shu-Lei Feng, Rong Guo, Hong-Yu Yang, Li-Xiang Cheng, Bin Yu, and Juan Liu. 2025. "Spatiotemporal Regulation of Starch–Sugar Metabolism by Potassium Enhances Carbon Partitioning and Processing Quality in Potatoes" Agronomy 15, no. 6: 1481. https://doi.org/10.3390/agronomy15061481
APA StyleLi, J.-L., Feng, S.-L., Guo, R., Yang, H.-Y., Cheng, L.-X., Yu, B., & Liu, J. (2025). Spatiotemporal Regulation of Starch–Sugar Metabolism by Potassium Enhances Carbon Partitioning and Processing Quality in Potatoes. Agronomy, 15(6), 1481. https://doi.org/10.3390/agronomy15061481






