Exogenous Cytokinins Regulate Nitrogen Metabolism in Soybean Under Low Phosphorus Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Cytokinins Treatment
2.2. Plant Materials and Sampling
2.3. Measurement and Calculation Methods
2.4. Nodule Nitrogenase Activity Detection
2.5. Soluble Protein Content Detection
2.6. Free Amino Acid Detection
3. Results
3.1. The Effect of Exogenous Cytokinin on Nitrogen Accumulation in Soybean Under Low P Stress
3.2. The Effect of Exogenous Cytokinin on 15N Abundance in Soybean Under Low P Stress
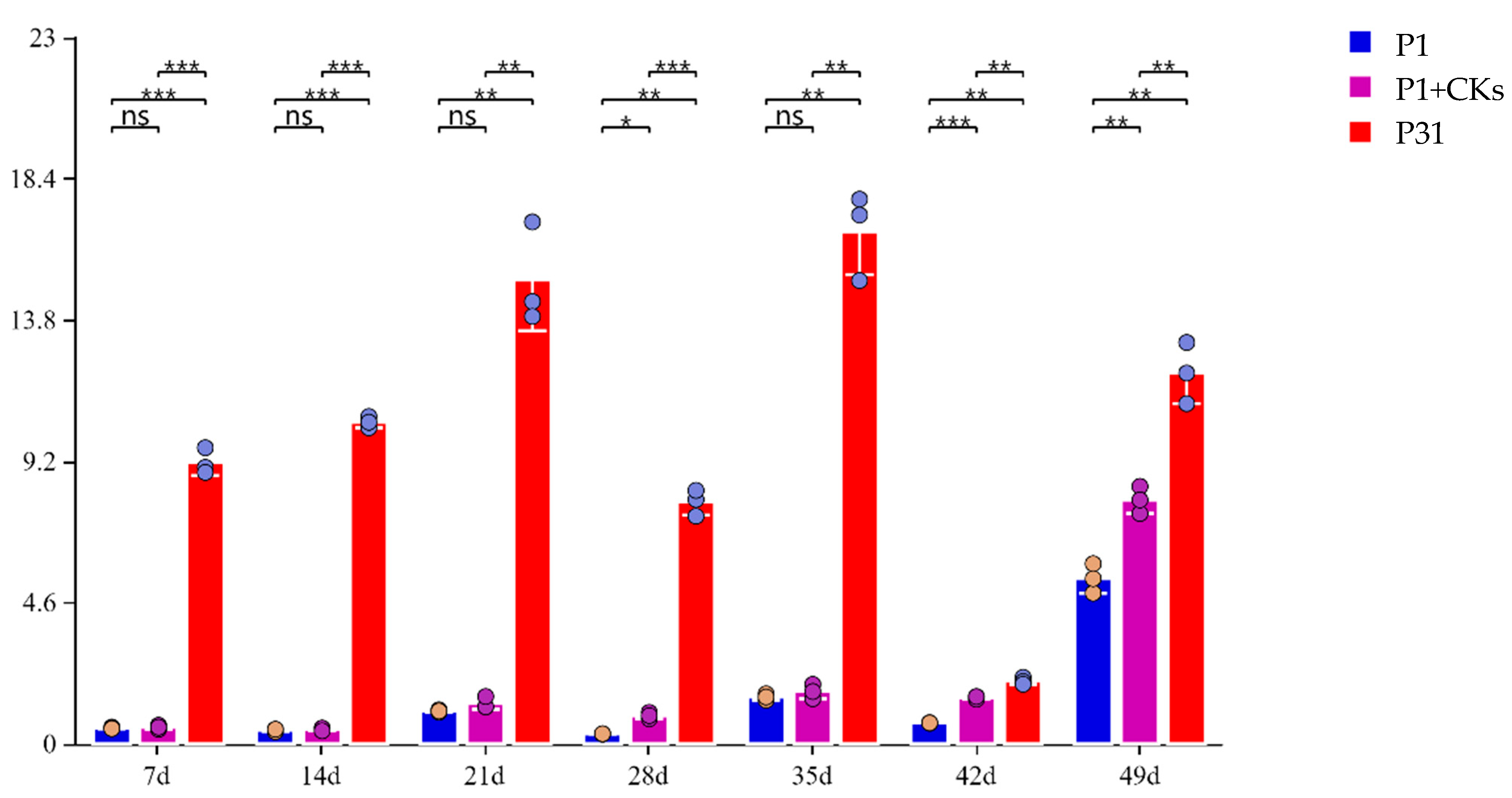
3.3. The Effect of Exogenous Cytokinin on Nodule Nitrogen Fixation Accumulation in Soybean Under Low P Stress
3.4. The Effect of Exogenous Cytokinin on Nitrogenase Activity in Soybean Under Low P Stress
3.5. The Effect of Exogenous Cytokinin on Soluble Protein in Soybean Nodules Under Low P Stress
3.6. The Effect of Exogenous Cytokinin on Free Amino Acid in Soybean Nodules Under Low P Stress
4. Discussion
5. Conclusions
- (1)
- Exogenous cytokinin regulated soybean nitrogen metabolism by increasing nitrogen accumulation, nitrogen fixation accumulation, nitrogen fixation rate (at 35 days), and nitrogenase activity (ARA and SNA) in soybeans under low P stress.
- (2)
- Exogenous cytokinin enhanced the absorption of fertilizer nitrogen to alleviate the inhibition of low P stress on soybean early growth and development.
- (3)
- Exogenous cytokinin significantly increased the soluble protein content in nodules to regulate nitrogen metabolism under low P stress, while the changes in free amino acids in nodules were not obvious.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, S.L.; Li, R.; Chen, H.F.; Zhang, C.J.; Chen, L.M.; Hao, Q.N.; Chen, S.L.; Shan, Z.H.; Yang, Z.L.; Zhang, X.J.; et al. RNA-Seq analysis of nodule development at five different developmental stages of soybean (Glycine max) inoculated with Bradyrhizobium japonicum strain 113-2. Sci. Rep. 2017, 7, 42248. [Google Scholar] [CrossRef]
- Guo, B.F.; Sun, L.P.; Jiang, S.Q.; Ren, H.L.; Sun, R.J.; Wei, Z.Y.; Hong, H.L.; Luan, X.Y.; Wang, J.; Wang, X.B.; et al. Correction to: Soybean genetic resources contributing to sustainable protein production. Theor. Appl. Genet. 2022, 135, 4123. [Google Scholar] [CrossRef]
- Zhao, M.L.; Guo, R.; Li, M.X.; Liu, Y.; Wang, X.X.; Fu, H.; Wang, S.Y.; Liu, X.Y.; Shi, L.X. Physiological characteristics and metabolomics reveal the tolerance mechanism to low nitrogen in Glycine soja leaves. Physiol. Plant. 2019, 168, 819–834. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Kong, Y.; Li, X.; Li, W.; Du, H.; Zhang, C. GmPAP12 Is Required for nodule development and nitrogen fixation under phosphorus starvation in soybean. Front. Plant Sci. 2020, 11, 450. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Yang, Z.; Du, H.; Xing, X.; Li, W.; Kong, Y.; Li, X.; Zhang, C. A small heat shock protein, GmHSP17.9, from nodule confers symbiotic nitrogen fixation and seed yield in soybean. Plant Biotechnol. J. 2022, 20, 103–115. [Google Scholar] [CrossRef]
- Song, J.; Liu, Y.; Cai, W.; Zhou, S.; Fan, X.; Hu, H.; Ren, L.; Xue, Y. Unregulated GmAGL82 due to phosphorus deficiency positively regulates root nodule growth in soybean. Int. J. Mol. Sci. 2024, 25, 1802. [Google Scholar] [CrossRef]
- Saikia, S.P.; Jain, V. Biological nitrogen fixation with non-legumes: An achievable target or a dogma. Curr. Sci. 2007, 92, 317–322. [Google Scholar]
- Sur, S.; Bothra, A.K.; Sen, A. Symbiotic nitrogen fixation-A bioinformatics perspective. Biotechnology 2010, 9, 257–273. [Google Scholar] [CrossRef]
- Zhao, L.F.; Xu, Y.J.; Lai, X.H. Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz. J. Microbiol. 2017, 308, 269–278. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphate acquisition. Annu. Rev. Plant Phys. 1999, 50, 665–693. [Google Scholar] [CrossRef]
- Ham, B.K.; Chen, J.Y.; Yan, Y.; Lucas, W.J. Insights into plant phosphate sensing and signaling. Curr. Opin. Biotech. 2018, 49, 1–9. [Google Scholar] [CrossRef]
- Zhu, S.N.; Chen, Z.J.; Xie, B.X.; Guo, Q.; Chen, M.H.; Liang, C.Y.; Bai, Z.L.; Wang, X.R.; Wang, H.C.; Liao, H.; et al. A phosphate starvation responsive malate dehydrogenase, GmMDH12 mediates malate synthesis and nodule size in soybean (Glycine max). Environ. Exp. Bot. 2021, 189, 104560. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cai, Y.P.; Yao, W.W.; Chen, L.; Hou, W.S. The soybean NUCLEAR FACTOR-Y C4 and α-EXPANSIN 7 module influences phosphorus uptake by regulating root morphology. Plant Physiol. 2025, 197, kiae478. [Google Scholar] [CrossRef]
- Makoudi, B.; Kabbadj, A.; Mouradi, M.; Amenc, L.; Domergue, O.; Blair, M.; Drevon, J.J.; Ghoulam, C. Phosphorus deficiency increases nodule phytase activity of faba bean-rhizobia symbiosis. Acta Physiol. Plant. 2018, 40, 63. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.T.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Taliman, N.; Dong, Q.; Echigo, K.; Raboy, V.; Saneoka, H. Effect of phosphorus fertilization on the growth, photosynthesis, nitrogen fixation, mineral accumulation, seed yield, and seed quality of a soybean low-phytate line. Plants 2019, 8, 119. [Google Scholar] [CrossRef]
- Wang, S.Q.; Han, X.Z.; Qiao, Y.F.; Yan, J.; Li, X.H. Nodule growth, nodulation and nitrogen fixation in soybean (Glycine max L.) as affected by P deficiency stress. Soybean Sci. 2009, 28, 1000–1003. [Google Scholar]
- Lu, M.Y.; Cheng, Z.Y.; Zhang, X.M.; Huang, P.H.; Fan, C.M.; Yu, G.L.; Chen, F.L.; Xu, K.; Chen, Q.S.; Miao, Y.C.; et al. Spatial divergence of PHR-PHT1 modules maintains phosphorus homeostasis in soybean nodules. Plant Physiol. 2020, 184, 01209–02019. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liang, Q.; Lyu, X.; Li, S.; Gong, Z.; Dong, S.; Yan, C.; Ma, C. Regulation of phosphorus supply on nodulation and nitrogen fixation in soybean plants with dual-root systems. Agronomy 2021, 11, 2354. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, X.L. Responses of nitrogen metabolism pathways to low-phosphorus stress: Decrease in nitrogen accumulation and alterations in protein metabolism in soybeans. Agronomy 2025, 15, 836. [Google Scholar] [CrossRef]
- Miller, C.O.; Skoog, F.; Okomura, F.S. Isolation, structure and synthesis of kinetin, a substance promoting cell division. J. Am. Chem. Soc. 1956, 78, 1375–1380. [Google Scholar] [CrossRef]
- Wu, Y.F. Physiological Mechanism of Exogenous Cytokinin on Drought Stress Mitigation Effect of Meng Yuan Cerasus humilis; Inner Mongolia Agricultural University: Hohhot, China, 2022. [Google Scholar]
- Li, Y.; Zhao, J.H.; Li, J.R.; Qiao, B.C.; Liu, Z.X.; Gao, F.; Yang, D.Q.; Li, X.D. Effects of exogenous 6-ba on root growth and pod yield of flooded peanut at different growth stages. Sci. Agric. Sin. 2020, 53, 3665–3678. [Google Scholar] [CrossRef]
- Verslues, P.E. ABA and cytokinins: Challenge and opportunity for plant stress research. Plant Mol. Biol. 2016, 91, 629–640. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Gracheva, N.V.; Grishenkova, N.N.; Dukhovskis, P.V.; Brazaitite, A.A. Cytokinin-like growth regulators mitigate toxic action of zinc and nickel ions on maize seedling. Russ. J. Plant Physl. 2007, 54, 381–387. [Google Scholar] [CrossRef]
- Hong, J.P.; Kim, W.T. Isolation and functional characterization of the Ca-DREBLP1 gene encoding a dehydration-responsive element binding-factor-like protein 1 in hot pepper (Capsicum annuum L. cv. Pukang). Planta 2005, 220, 875–888. [Google Scholar] [CrossRef]
- Kulaeva, O.N.; Prokoptseva, O.S. Recent advances in the study of mechanisms of action of phytohormones. Biochemistry 2004, 69, 233–247. [Google Scholar] [CrossRef]
- Kempster, R.; Barat, M.; Bishop, L.; Rufino, M.; Borras, L.; Dodd, L.C. Genotype and cytokinin effects on soybean yield and biological nitrogen fixation across soil tempera. Ann. Appl. Biol. 2021, 178, 341–354. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. Int. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Yao, Y.B.; Cheng, L.L.; Liu, X.L.; Xue, Y.G.; Tang, X.F.; Cao, D.; He, W.J.; Luan, X.Y. The Effect of Exogenous Cytokinin on Photosynthetic Products Accumulation and Enzyme Activity in Soybean Nodules under Phosphorus Stress. Soybean Sci. 2025, 44, 103–110. Available online: https://link.cnki.net/urlid/23.1227.S.20250423.1435.010 (accessed on 23 April 2025).
- Gremaud, M.F.; Harper, J.E. Selection and initial characterization of partially nitrate tolerant nodulation mutants of soybean. Plant Physiol. 1989, 89, 169–173. [Google Scholar] [CrossRef]
- Chen, H.; Di, W.; Yao, Y.B.; Gong, Z.P.; Ma, C.M. Study on nitrogenase activity and nitrogen fixation of different soybean varieties. J. Nucl. Agric. Sci. 2013, 3, 379–383. [Google Scholar]
- GB/T 8314-2013; Determination of Total Free Amino Acids in Tea. China Standard Press: Beijing, China, 2013.
- Yang, W.B.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Hoyerová, K.; Hošek, P. New insights into the metabolism and role of cytokinin N-glucosides in plants. Front. Plant Sci. 2020, 11, 741. [Google Scholar] [CrossRef]
- Osugi, A.; Sakakibara, H. Q&A: How do plants respond to cytokinins and what is their importance? BMC Biol. 2015, 13, 102. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Yao, Y.B.; Wu, D.T.; Gong, Z.P.; Zhao, J.K.; Ma, C.M. Variation of nitrogen accumulation and yield in response to phosphorus nutrition of soybean (Glycine max L. Merr.). J. Plant Nutrition 2018, 41, 1138–1147. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Kariali, E.; Mohapatra, P.K. Hormonal regulation of tiller dynamics in di erentially-tillering rice cultivars. Plant Growth Regul. 2007, 53, 215–223. [Google Scholar] [CrossRef]
- Zatloukal, M.; Plihalova, L.; Klaskova, J.; Spichal, L.; Koprna, R.; Dolezal, K. Substituted 6-anilino-9-heterocyclylpurine Derivatives for Inhibition of Plant Stress. U.S. Patent EP3233860A1, 25 October 2017. [Google Scholar]
- Alharby, H.; Alzahrani, Y.M.; Rady, M. Seeds pretreatment with zeatins or maize grain-derived organic biostimulant improved hormonal contents, polyamine gene expression, and salinity and drought tolerance of wheat. Int. J. Agric. Biol. 2020, 24, 714–724. [Google Scholar] [CrossRef]
- Yaronskaya, E.; Vershilovskaya, I.; Poers, Y.; Alawady, A.E.; Averina, N.; Grimm, B. Cytokinin effects on tetrapyrrole biosynthesis and photosynthetic activity in barley seedlings. Planta 2006, 224, 700–709. [Google Scholar] [CrossRef]
- Werner, T.; Holst, K.; Pörs, Y.; Guivar´ch, A.; Mustroph, A.; Chriqui, D.; Grimm, B.; Schm¨ulling, T. Cytokinin defiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot. 2008, 59, 2659–2672. [Google Scholar] [CrossRef]
- Aswathi, K.P.R.; Kalaji, H.M.; Puthur, J.T. Seed priming of plants aiding in drought stress tolerance and faster recovery: A review. Plant Growth Regul. 2022, 97, 235–253. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Biostimulant priming in oryza sativa: A novel approach to reprogram the functional biology under nutrient-deficient soil. Cereal Res. Commun. 2022, 50, 45–52. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmulling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Bertell, G.; Eliasson, L. Cytokinin effects on root growth and possible interactions with ethylene and indole-3-acetic acid. Physiol. Plantarum 1992, 84, 255–261. [Google Scholar] [CrossRef]
- Karunadasa, S.S.; Kurepa, J.; Shull, T.E.; Smalle, J.A. Cytokinin-induced protein synthesis suppresses growth and osmotic stress tolerance. New Phytol. 2020, 227, 50–64. [Google Scholar] [CrossRef]
- Klambt, D. Cytokinin effects on protein synthesis of in vitro systems of higher plants. Plant Cell Physiol. 1976, 17, 73–76. [Google Scholar] [CrossRef]
- Muren, R.C.; Fosket, D.E. Cytokinin-mediated translational control of protein synthesis in cultured cells of Glycine max. J. Exp. Bot. 1977, 28, 775–784. [Google Scholar] [CrossRef]
- Tepfer, D.A.; Fosket, D.E. Hormone-mediated translational control of protein synthesis in cultured cells of Glycine max. Dev. Biol. 1978, 62, 486–497. [Google Scholar] [CrossRef]
- Szweykowska, A.; Gwozdz, E.; Spychała, M. The cytokinin control of protein synthesis in plants. In Metabolism and Molecular Activities of Cytokinins; Guern, J., Peaud-Leno€ el, C., Eds.; Springer: Berlin, Germany, 1981; pp. 212–217. [Google Scholar]
- Černý, M.; Kuklová, A.; Hoehenwarter, W.; Fragner, L.; Novák, O.; Rotková, G.; Jedelsky, P.L.; Žáková, K.; Šmehilová, M.; Strnad, M.; et al. Proteome and metabolome profiling of cytokinin action in Arabidopsis identifying both distinct and similar responses to cytokinin down- and up-regulation. J. Exp. Bot. 2013, 64, 4193–4206. [Google Scholar] [CrossRef]
- Venturi, V.; Keel, C. Signaling in the rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Moormann, J.; Heinemann, B.; Hildebrandt, T.M. News about amino acid metabolism in plant-microbe interactions. Trends Biochem. Sci. 2022, 47, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Pollard, A.M.; Höfler, C.; Poschet, G.; Wirtz, M.; Hell, R.; Sourjik, V. Relation between chemotaxis and consumption of amino acids in bacteria. Mol. Microbiol. 2015, 96, 1272–1282. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araujo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Planchet, E.; Limami, A.M. Amino Acid Synthesis under Abiotic Stress. In Amino Acids in Higher Plants; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2015; pp. 262–276. [Google Scholar] [CrossRef]
- Saad, S.; Van, H.C.; Joachim, S.; Lam-Son Phan, T. Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef]
- Medhi, A.K.; Dhar, S.; Roy, A. Effect of different growth regulators and phosphorus levels on nodulation, yield and quality components in green gram. Indian J. Plant Physiol. 2014, 19, 74–78. [Google Scholar] [CrossRef]
- Ban, Y.J.; Song, Y.H.; Kim, J.Y.; Cha, J.Y.; Ali, I.; Baiseitova, A.; Shah, A.B.; Kim, W.Y.; Park, K.H. A Significant Change in Free Amino Acids of Soybean (Glycine max L. Merr) through Ethylene Application. Molecules 2021, 26, 1128. [Google Scholar] [CrossRef] [PubMed]



| Treatments | Aboveground | Root | Nodule | |
|---|---|---|---|---|
| 7 days | P1 | 27.88 ± 0.72 c | 12.91 ± 0.13 b | 1.21 ± 0.05 c |
| P1 + CKs | 34.19 ± 0.26 b | 15.19 ± 0.18 a | 1.73 ± 0.01 b | |
| P31 | 53.20 ± 0.67 a | 11.31 ± 0.10 c | 7.10 ± 0.11 a | |
| 14 days | P1 | 38.73 ± 0.75 c | 13.33 ± 0.11 b | 2.04 ± 0.02 b |
| P1 + CKs | 45.28 ± 0.52 b | 14.83 ± 0.91 b | 2.05 ± 0.01 b | |
| P31 | 91.60 ± 0.36 a | 16.89 ± 0.03 a | 9.84 ± 0.20 a | |
| 21 days | P1 | 47.37 ± 0.93 c | 18.03 ± 0.59 b | 1.95 ± 0.13 b |
| P1 + CKs | 54.06 ± 0.42 b | 18.23 ± 0.04 b | 2.15 ± 0.06 b | |
| P31 | 184.36 ± 1.71 a | 41.45 ± 0.24 a | 25.73 ± 0.26 a | |
| 28 days | P1 | 69.09 ± 1.43 c | 22.70 ± 0.78 c | 2.45 ± 0.11 c |
| P1 + CKs | 86.85 ± 2.33 b | 31.67 ± 0.99 b | 4.07 ± 0.09 b | |
| P31 | 287.09 ± 3.39 a | 71.45 ± 0.56 a | 29.23 ± 0.07 a | |
| 35 days | P1 | 100.11 ± 1.68 c | 32.13 ± 0.66 c | 3.17 ± 0.05 c |
| P1 + CKs | 135.33 ± 0.43 b | 48.15 ± 0.79 b | 7.29 ± 0.07 b | |
| P31 | 439.45 ± 8.42 a | 91.36 ± 0.29 a | 36.62 ± 0.14 a | |
| 42 days | P1 | 181.05 ± 0.35 c | 55.86 ± 0.98 c | 4.81 ± 0.12 c |
| P1 + CKs | 206.69 ± 2.97 b | 68.45 ± 0.47 b | 9.59 ± 0.13 b | |
| P31 | 444.68 ± 1.43 a | 114.85 ± 3.59 a | 35.52 ± 0.51 a | |
| 49 days | P1 | 286.90 ± 2.34 b | 90.27 ± 1.17 b | 8.40 ± 0.07 c |
| P1 + CKs | 290.20 ± 6.87 b | 109.47 ± 0.93 a | 10.18 ± 0.11 b | |
| P31 | 565.46 ± 4.49 a | 112.84 ± 2.11 a | 36.37 ± 0.31 a | |
| Treatments | Aboveground | Root | Nodule | |
|---|---|---|---|---|
| 7 days | P1 | 1.90 ± 0.00 b | 2.07 ± 0.01 b | 0.96 ± 0.01 b |
| P1 + CKs | 2.03 ± 0.00 a | 2.17 ± 0.02 a | 1.18 ± 0.01 a | |
| P31 | 1.93 ± 0.02 b | 1.80 ± 0.03 c | 0.99 ± 0.00 b | |
| 14 days | P1 | 2.12 ± 0.00 a | 2.27 ± 0.01 a | 1.09 ± 0.00 b |
| P1 + CKs | 2.14 ± 0.04 a | 2.29 ± 0.04 a | 1.18 ± 0.02 a | |
| P31 | 1.92 ± 0.03 b | 2.13 ± 0.07 a | 0.70 ± 0.02 c | |
| 21 days | P1 | 2.30 ± 0.02 a | 2.26 ± 0.03 a | 1.11 ± 0.01 b |
| P1 + CKs | 2.29 ± 0.03 a | 2.29 ± 0.01 a | 1.20 ± 0.00 a | |
| P31 | 1.31 ± 0.01 b | 1.74 ± 0.00 b | 0.47 ± 0.01 c | |
| 28 days | P1 | 2.37 ± 0.01 b | 2.37 ± 0.01 b | 1.17 ± 0.02 a |
| P1 + CKs | 2.49 ± 0.01 a | 2.51 ± 0.00 a | 1.17 ± 0.02 a | |
| P31 | 1.36 ± 0.02 c | 1.80 ± 0.00 c | 0.52 ± 0.01 b | |
| 35 days | P1 | 2.44 ± 0.00 a | 2.44 ± 0.02 a | 1.20 ± 0.00 a |
| P1 + CKs | 2.24 ± 0.04 b | 2.33 ± 0.03 b | 1.02 ± 0.03 b | |
| P31 | 1.40 ± 0.00 c | 1.87 ± 0.01 c | 0.58 ± 0.03 c | |
| 42 days | P1 | 2.43 ± 0.05 a | 2.55 ± 0.02 a | 0.94 ± 0.03 a |
| P1 + CKs | 2.20 ± 0.00 b | 2.36 ± 0.00 b | 0.76 ± 0.02 b | |
| P31 | 1.48 ± 0.00 c | 1.87 ± 0.00 c | 0.54 ± 0.00 c | |
| 49 days | P1 | 2.29 ± 0.05 a | 2.45 ± 0.04 a | 0.83 ± 0.05 a |
| P1 + CKs | 2.07 ± 0.02 b | 2.28 ± 0.01 b | 0.72 ± 0.05 a | |
| P31 | 1.45 ± 0.02 c | 1.86 ± 0.02 c | 0.49 ± 0.02 b | |
| Treatments | Aboveground | Root | Nodule | |
|---|---|---|---|---|
| 7 days | P1 | 11.00 ± 0.20 b | 4.52 ± 0.04 a | 0.84 ± 0.04 c |
| P1 + CKs | 11.91 ± 0.11 b | 4.83 ± 0.19 a | 1.10 ± 0.01 b | |
| P31 | 20.39 ± 0.17 a | 4.87 ± 0.06 a | 4.98 ± 0.10 a | |
| 14 days | P1 | 12.27 ± 0.41 b | 3.90 ± 0.01 b | 1.32 ± 0.00 b |
| P1 + CKs | 14.05 ± 0.11 b | 4.16 ± 0.17 b | 1.27 ± 0.02 b | |
| P31 | 35.84 ± 0.19 a | 5.62 ± 0.15 a | 7.71 ± 0.10 a | |
| 21 days | P1 | 13.02 ± 0.14 b | 5.17 ± 0.00 b | 1.24 ± 0.03 b |
| P1 + CKs | 14.75 ± 0.33 b | 5.05 ± 0.11 b | 1.32 ± 0.03 b | |
| P31 | 107.13 ± 1.03 a | 18.56 ± 0.57 a | 21.89 ± 0.31 a | |
| 28 days | P1 | 17.55 ± 0.02 b | 5.72 ± 0.16 c | 1.55 ± 0.08 c |
| P1 + CKs | 18.66 ± 0.09 b | 6.53 ± 0.32 b | 2.55 ± 0.01 b | |
| P31 | 159.88 ± 0.09 a | 30.13 ± 0.03 a | 24.52 ± 0.02 a | |
| 35 days | P1 | 23.08 ± 0.17 c | 7.63 ± 0.12 c | 1.99 ± 0.04 c |
| P1 + CKs | 39.67 ± 0.21 b | 12.95 ± 0.07 b | 5.16 ± 0.14 b | |
| P31 | 241.14 ± 0.41 a | 37.42 ± 0.09 a | 29.87 ± 0.24 a | |
| 42 days | P1 | 42.27 ± 0.31 c | 10.61 ± 0.16 c | 3.47 ± 0.04 c |
| P1 + CKs | 65.80 ± 0.33 b | 17.36 ± 0.15 b | 7.38 ± 0.16 b | |
| P31 | 233.83 ± 1.45 a | 44.40 ± 0.18 a | 29.49 ± 0.35 a | |
| 49 days | P1 | 80.04 ± 0.55 c | 20.41 ± 0.54 c | 6.29 ± 0.18 c |
| P1 + CKs | 100.74 ± 0.68 b | 31.20 ± 0.96 b | 8.00 ± 0.15 b | |
| P31 | 291.50 ± 4.58 a | 46.70 ± 0.04 a | 30.88 ± 0.44 a | |
| Treatments | Aboveground | Root | Nodule | |
|---|---|---|---|---|
| 7 days | P1 | 39.90 ± 0.23 a | 34.71 ± 0.46 b | 69.45 ± 0.36 a |
| P1 + CKs | 35.73 ± 0.11 b | 31.42 ± 0.82 c | 62.71 ± 0.50 b | |
| P31 | 39.13 ± 0.32 a | 42.98 ± 1.02 a | 68.85 ± 0.21 a | |
| 14 days | P1 | 32.64 ± 0.33 b | 28.10 ± 0.57 b | 65.57 ± 0.31 b |
| P1 + CKs | 32.25 ± 0.40 b | 27.41 ± 0.29 b | 62.48 ± 0.81 c | |
| P31 | 39.19 ± 1.00 a | 32.76 ± 0.28 a | 77.84 ± 0.68 a | |
| 21 days | P1 | 27.48 ± 0.88 b | 28.65 ± 0.98 b | 64.89 ± 0.56 b |
| P1 + CKs | 27.63 ± 0.96 b | 27.55 ± 0.55 b | 61.86 ± 0.16 c | |
| P31 | 58.49 ± 0.36 a | 45.08 ± 0.17 a | 85.10 ± 0.33 a | |
| 28 days | P1 | 25.27 ± 0.63 b | 24.97 ± 0.53 b | 63.12 ± 0.69 b |
| P1 + CKs | 21.42 ± 0.56 c | 20.59 ± 0.31 c | 63.05 ± 0.73 b | |
| P31 | 56.73 ± 0.91 a | 42.77 ± 0.18 a | 83.70 ± 0.37 a | |
| 35 days | P1 | 22.97 ± 0.14 c | 22.94 ± 0.79 c | 61.83 ± 0.18 c |
| P1 + CKs | 28.86 ± 0.25 b | 26.01 ± 0.15 b | 67.43 ± 0.99 b | |
| P31 | 55.54 ± 0.06 a | 40.85 ± 0.37 a | 81.59 ± 0.96 a | |
| 42 days | P1 | 23.10 ± 0.70 c | 19.33 ± 0.79 c | 70.08 ± 0.99 c |
| P1 + CKs | 30.24 ± 0.23 b | 25.08 ± 0.05 b | 75.85 ± 0.71 b | |
| P31 | 53.00 ± 0.24 a | 40.54 ± 0.14 a | 82.97 ± 0.17 a | |
| 49 days | P1 | 27.55 ± 0.79 c | 22.43 ± 0.27 c | 73.74 ± 0.82 b |
| P1 + CKs | 34.34 ± 0.88 b | 27.84 ± 0.61 b | 76.97 ± 0.69 b | |
| P31 | 53.78 ± 0.90 a | 41.08 ± 0.76 a | 84.38 ± 0.68 a | |
| Treatments | Number (per Plant) | Weight (g/Plant) | |
|---|---|---|---|
| 7 days | P1 | 0.03 ± 0.00 c | 30.33 ± 0.29 c |
| P1 + CKs | 0.04 ± 0.00 b | 35.33 ± 0.29 b | |
| P31 | 0.14 ± 0.00 a | 74.50 ± 0.67 a | |
| 14 days | P1 | 0.05 ± 0.00 b | 28.58 ± 0.06 c |
| P1 + CKs | 0.05 ± 0.00 b | 39.92 ± 0.24 b | |
| P31 | 0.20 ± 0.00 a | 64.92 ± 0.87 a | |
| 21 days | P1 | 0.05 ± 0.00 b | 28.50 ± 0.07 c |
| P1 + CKs | 0.06 ± 0.00 b | 42.42 ± 0.21 b | |
| P31 | 0.55 ± 0.01 a | 87.83 ± 0.46 a | |
| 28 days | P1 | 0.07 ± 0.00 c | 34.75 ± 0.34 c |
| P1 + CKs | 0.12 ± 0.00 b | 54.08 ± 0.78 b | |
| P31 | 0.67 ± 0.00 a | 97.00 ± 0.78 a | |
| 35 days | P1 | 0.09 ± 0.00 c | 30.25 ± 0.21 c |
| P1 + CKs | 0.18 ± 0.00 b | 91.50 ± 0.96 b | |
| P31 | 0.87 ± 0.00 a | 136.17 ± 1.54 a | |
| 42 days | P1 | 0.12 ± 0.00 c | 40.83 ± 0.29 c |
| P1 + CKs | 0.23 ± 0.00 b | 81.00 ± 0.09 b | |
| P31 | 0.81 ± 0.01 a | 225.08 ± 1.43 a | |
| 49 days | P1 | 0.22 ± 0.00 c | 71.75 ± 2.55 c |
| P1 + CKs | 0.36 ± 0.02 b | 109.92 ± 1.05 b | |
| P31 | 0.91 ± 0.01 a | 184.08 ± 1.78 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Xue, Y.; Yan, J.; Tang, X.; Cao, D.; He, W.; Luan, X.; Liu, Q.; Zhu, Z.; Liu, X. Exogenous Cytokinins Regulate Nitrogen Metabolism in Soybean Under Low Phosphorus Stress. Agronomy 2025, 15, 1459. https://doi.org/10.3390/agronomy15061459
Yao Y, Xue Y, Yan J, Tang X, Cao D, He W, Luan X, Liu Q, Zhu Z, Liu X. Exogenous Cytokinins Regulate Nitrogen Metabolism in Soybean Under Low Phosphorus Stress. Agronomy. 2025; 15(6):1459. https://doi.org/10.3390/agronomy15061459
Chicago/Turabian StyleYao, Yubo, Yongguo Xue, Jun Yan, Xiaofei Tang, Dan Cao, Wenjin He, Xiaoyan Luan, Qi Liu, Zifei Zhu, and Xinlei Liu. 2025. "Exogenous Cytokinins Regulate Nitrogen Metabolism in Soybean Under Low Phosphorus Stress" Agronomy 15, no. 6: 1459. https://doi.org/10.3390/agronomy15061459
APA StyleYao, Y., Xue, Y., Yan, J., Tang, X., Cao, D., He, W., Luan, X., Liu, Q., Zhu, Z., & Liu, X. (2025). Exogenous Cytokinins Regulate Nitrogen Metabolism in Soybean Under Low Phosphorus Stress. Agronomy, 15(6), 1459. https://doi.org/10.3390/agronomy15061459







