Dynamic Effects of Sodium Selenite on the Rhizospheric Microenvironment, Growth, and Antioxidative Responses of Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Sampling
2.4. Analysis Methods
2.4.1. Se Speciation in Soil Solution
2.4.2. Se Fractionation in Soil
- (1)
- Soluble Se (SOL-Se): 10 mL of deionized water was added to 2 g soil in a 15 mL polycarbonate centrifuge tube. After shaking for 2 h at 200 cpm, the mixture centrifuged for 30 min at 5000 rpm and filtered through a 0.45 µm filter. The supernatant was separated from the residue and provided an estimate of soluble Se. The remaining precipitate was used for the next step of extraction.
- (2)
- Exchangeable Se (EX-Se): 10 mL of 0.1 M KH2PO4/K2HPO4 (pH 7.0) was added to the above tube and shaken for 2 h, and the sampling procedure was repeated.
- (3)
- Organic matter-bound Se (OM-Se): 10 mL of 0.1 M NaOH was added to the remaining soil. The mixture was shaken for 30 min, heated in a water bath at 90 °C for 2 h with intermittent shaking. The suspensions were again centrifuged and filtered, and the supernatant was collected. The sample was rinsed once with 10 mL of deionized water and shaken for 30 min. After centrifugation and filtering, the supernatants were combined.
2.4.3. Se Concentrations in Plant
2.4.4. Determination of Se Concentration
2.4.5. Assay of Enzyme Activities and Lipid Peroxidation
2.5. Data Analysis
3. Results
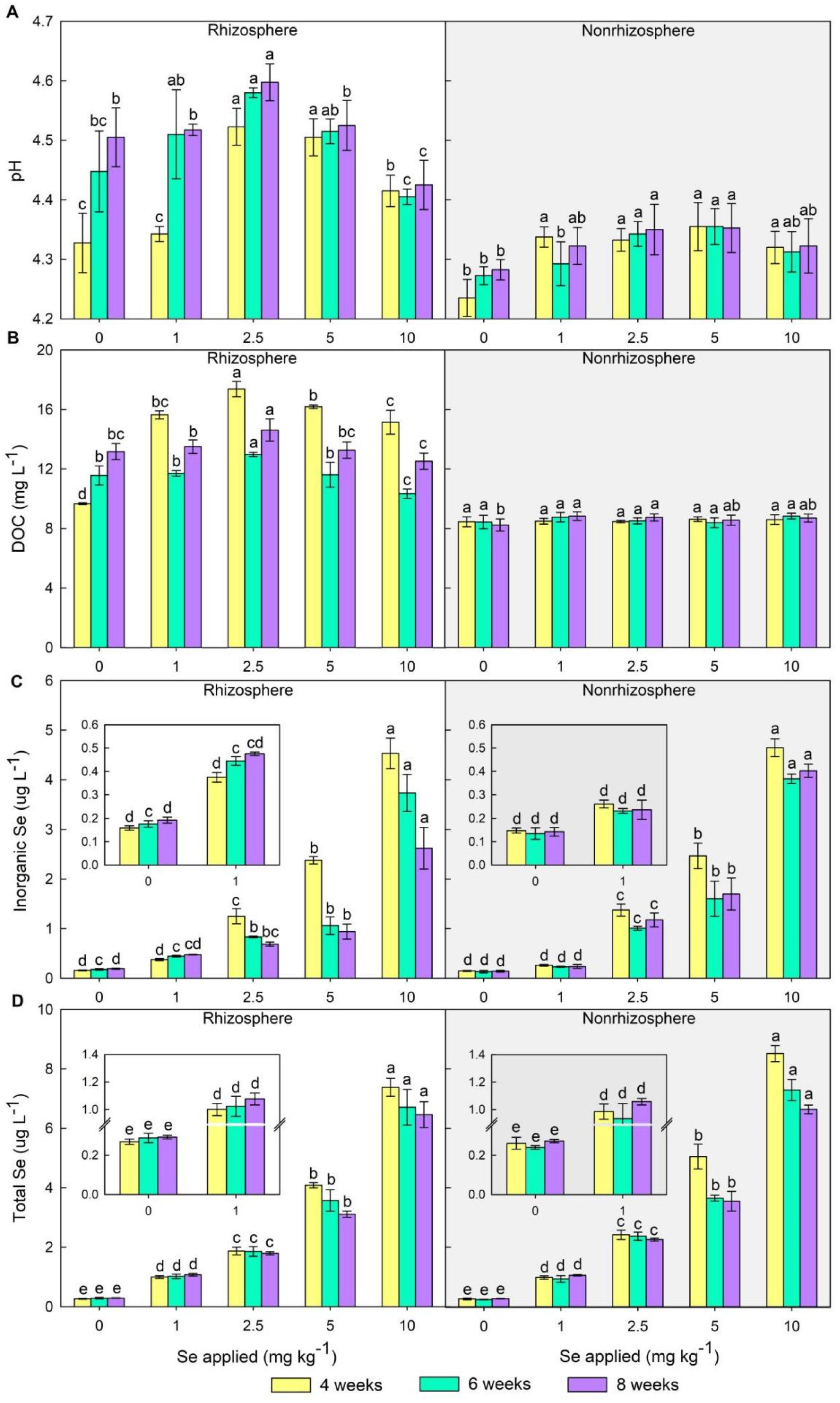
3.1. Changes of Chemical Properties in Soil Solution
3.1.1. pH and DOC
3.1.2. Inorganic Se and Total Se
3.2. Changes of Se Fractions in Soil
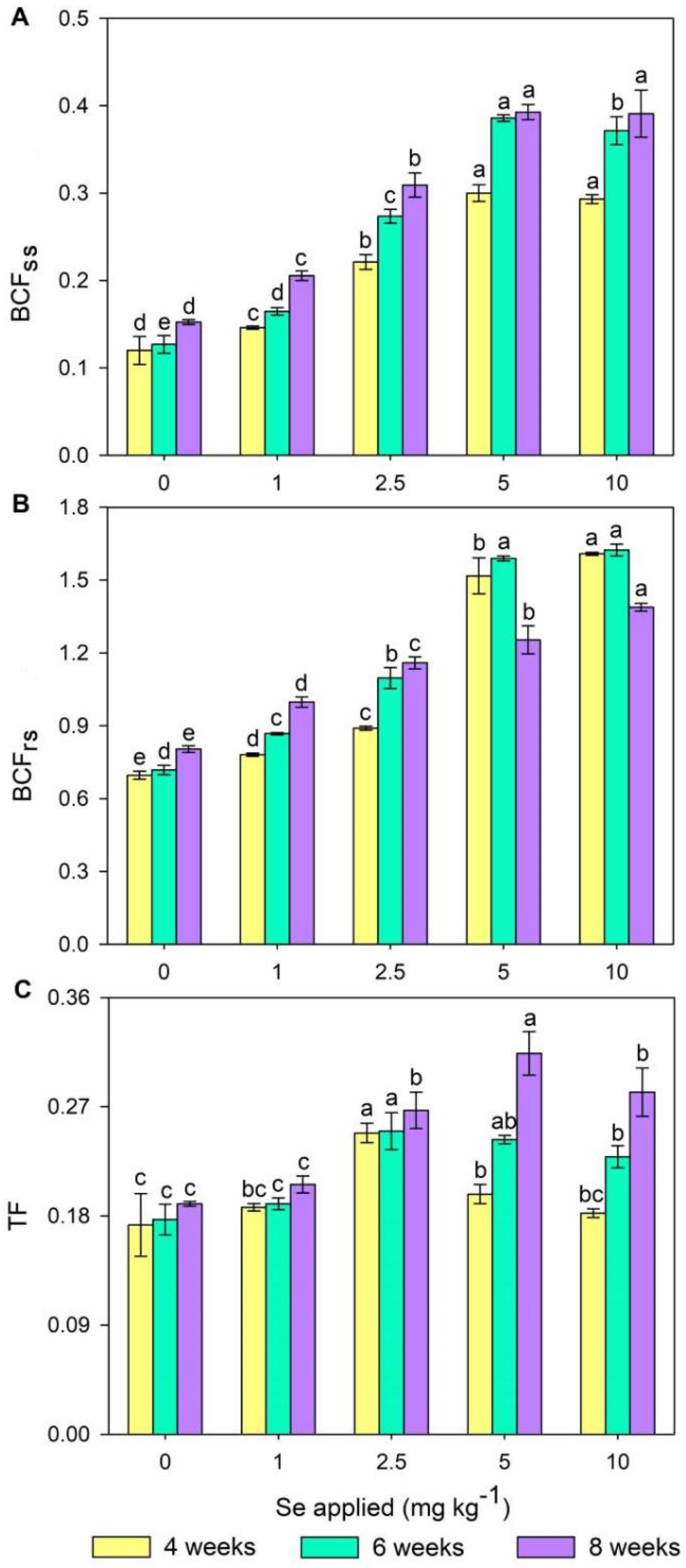
3.3. Biomass, Se Accumulation, and Translocation in Wheat Plants
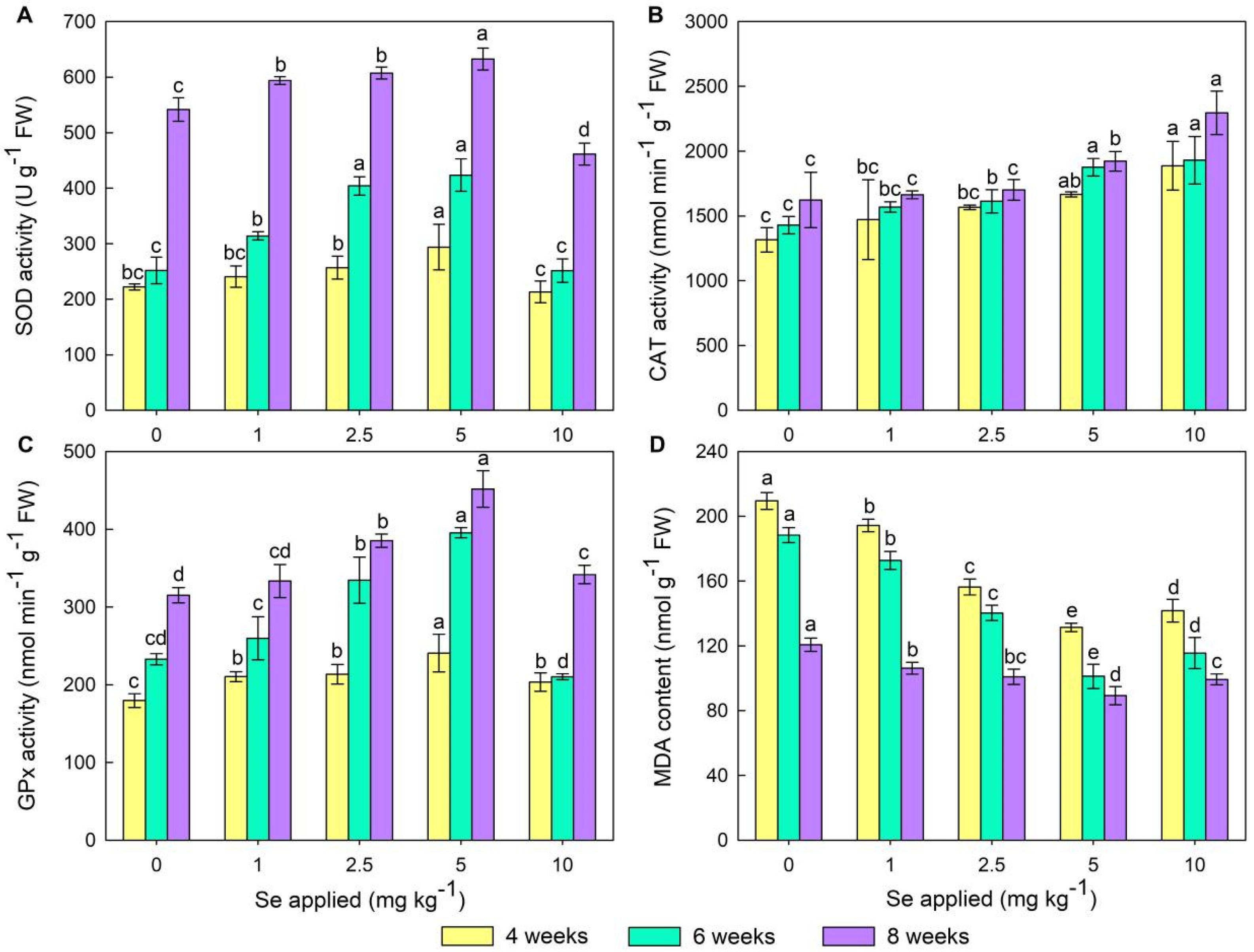
3.4. Physiological Indexes Analysis in Wheat Leaves
4. Discussion
4.1. Effects of Se Treatments on Soil Se Availability
4.2. Effects of Se Treatments on Wheat Growth and Se Absorption
4.3. Effects of Se Treatments on the Antioxidant System and MDA in Wheat Leaves
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and Its Supplementation in Cardiovascular Disease—What Do We Know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The argument for increasing selenium intake. Proc. Nutr. Soc. 2002, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Whanger, P.D. Selenium and its relationship to cancer: An update. Br. J. Nutr. 2004, 91, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of Selenium Deficiency and Toxicity Worldwide: Affected Areas, Selenium-Related Health Issues, and Case Studies. In Selenium in Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 209–230. [Google Scholar]
- Tan, C.; Nancharaiah, Y.V.; Hullebusch, E.V.; Lens, P.N.L. Selenium: Environmental Significance, Pollution, and Biological Treatment Technologies. In Anaerobic Treatment of Mine Wastewater for the Removal of Selenate and its Co-Contaminants; CRC Press: London, UK, 2016; pp. 886–907. [Google Scholar]
- White, R.R.; Hardaway, C.J.; Richert, J.C.; Sneddon, J. Selenium–Lead Interactions in Crawfish (Procambrus clarkii) in a Controlled Laboratory Environment. Microchem. J. 2012, 102, 91–114. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Li, Y.; Yu, J.; Yang, L.; Feng, F.; Chen, Z. Speciation, Distribution, and Bioavailability of Soil Selenium in the Tibetan Plateau Kashin–Beck Disease Area—A Case Study in Songpan County, Sichuan Province, China. Biol. Trace Elem. Res. 2013, 156, 367–375. [Google Scholar] [CrossRef]
- Liu, N.; Wang, M.; Zhou, F.; Zhai, H.; Qi, M.; Liu, Y.; Liang, D. Selenium Bioavailability in Soil-Wheat System and Its Dominant Influential Factors: A Field Study in Shaanxi Province, China. Sci. Total Environ. 2021, 770, 144664. [Google Scholar] [CrossRef]
- Dai, Z.; Imtiaz, M.; Rizwan, M.; Yuan, Y.; Huang, H.; Tu, S. Dynamics of Selenium Uptake, Speciation, and Antioxidant Response in Rice at Different Panicle Initiation Stages. Sci. Total Environ. 2019, 691, 827–834. [Google Scholar] [CrossRef]
- Li, Y.; Ju, S.; Huang, J.; Wu, H.; Lin, Z.; Wang, Y.; Zhang, B. Novel Multifunctional Natural Selenium Supplement Development, In Vitro and In Vivo Analysis, and Risk-Benefit Assessment: Selenium-Enriched Chicory as a Case Study. J. Clean. Prod. 2023, 382, 135273. [Google Scholar] [CrossRef]
- Ding, M.; Kong, Y.; Liu, J.; Li, H.; Li, S.; Yang, Y.; Zhang, C.; Xiao, C.; Rehman, M.; Maqbool, Z.; et al. Evaluation of the Ameliorative Role of Soil Amendments and Selenium on Morphophysiological Traits, Oxidative Stress, and Quality Attributes of Wheat (Triticum aestivum L.) under Varying Drought Stress Conditions. J. Hazard. Mater. Adv. 2025, 18, 100693. [Google Scholar] [CrossRef]
- Yan, G.; Wu, L.; Hou, M.; Jia, S.; Jiang, L.; Zhang, D. Effects of Selenium Application on Wheat Yield and Grain Selenium Content: A Global Meta-Analysis. Field Crops Res. 2024, 307, 109266. [Google Scholar] [CrossRef]
- Peng, Z.; Sun, H.; Guo, Y.; Chen, Y.; Yin, X. Combining Depth and Rate of Selenium Fertilizer Basal Application to Improve Selenium Content and Yield in Sweet Maize. Agronomy 2025, 15, 775. [Google Scholar] [CrossRef]
- Geng, W.; Zhao, Y.; Mao, Z.; Wang, X.; Wu, N.; Xu, X. The Effects of Combined Use of Black Soldier Fly Larvae Frass Fertilizer with Exogenous Selenium on Rice Growth and Accumulation of Heavy Metals. J. Soil. Sci. Plant Nutr. 2022, 22, 5133–5143. [Google Scholar] [CrossRef]
- Cheng, C.; Li, Q.; Yi, Y.; Yang, H.; Coldea, T.E.; Zhao, H. Selenium Biofortification during Barley (Hordeum vulgare L.) Germination: Comparative Analysis of Selenate, Selenite, and Selenomethionine on Se-Protein Accumulation and Phenolic Acid Profile. Food Chem. 2025, 485, 144548. [Google Scholar] [CrossRef]
- Sadak, M.S.; Bakhoum, G.S. Selenium-Induced Modulations in Growth, Productivity, and Physiochemical Responses to Water Deficiency in Quinoa (Chenopodium quinoa) Grown in Sandy Soil. Biocatal. Agric. Biotechnol. 2022, 44, 102449. [Google Scholar] [CrossRef]
- Gorni, P.H.; Rodrigues, C.; Spera, K.D.; Correia, R.F.C.C.; Mendes, N.A.C.; Reis, A.R. Selenium Fertilization Enhances Carotenoid and Antioxidant Metabolism to Scavenge ROS and Increase Yield of Maize Plants Under Drought Stress. Plant Physiol. Biochem. 2025, 221, 109675. [Google Scholar] [CrossRef]
- Araujo, M.A.; Melo, A.A.R.; Silva, V.M.; Reis, A.R. Selenium Enhances ROS Scavenging Systems and Sugar Metabolism Increasing Growth of Sugarcane Plants. Plant Physiol. Biochem. 2023, 201, 107798. [Google Scholar] [CrossRef]
- Kamali-Andani, N.; Fallah, S.; Peralta-Videa, J.R.; Golkar, P.A. Comprehensive Study of Selenium and Cerium Oxide Nanoparticles on Mung Bean: Individual and Synergistic Effect on Photosynthesis Pigments, Antioxidants, and Dry Matter Accumulation. Sci. Total Environ. 2022, 830, 154837. [Google Scholar] [CrossRef]
- Consortium, T.I.W.G.S. A Chromosome-Based Draft Sequence of the Hexaploid Bread Wheat (Triticum aestivum) Genome. Science 2014, 345, 1251788. [Google Scholar]
- Liu, H.; Yang, Y.E.; Wang, Z.H.; Li, F.C.; Li, K.Y.; Yang, N.; Wang, S.; Wang, H.; He, G.; Dai, J. Selenium Content in Wheat Grains from Different Wheat Regions of China and Its Regulation. Chin. Agric. Sci. 2016, 49, 14. [Google Scholar]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N.; Bukhari, M.A. Supplemental Selenium Improves Wheat Grain Yield and Quality Through Alterations in Biochemical Processes Under Normal and Water Deficit Conditions. Food Chem. 2015, 175, 350–357. [Google Scholar] [CrossRef]
- Di, X.; Qin, X.; Zhao, L.; Liang, X.; Xu, Y.; Sun, Y.; Huang, Q. Selenium Distribution, Translocation and Speciation in Wheat (Triticum aestivum L.) After Foliar Spraying Selenite and Selenate. Food Chem. 2023, 400, 134077. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yu, F.; Huang, X.; Qin, H.; Zhu, Z. Effects of Microorganisms on Soil Selenium and Its Uptake by Pak Choi in Selenium-Enriched Lateritic Red Soil. Ecotoxicol. Environ. Saf. 2023, 257, 114927. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, R.; Wu, B.; Zhang, C.; Wang, J.; Lyu, L.; Wu, F. Influence of Arbuscular Mycorrhizal Fungi on Selenium Uptake by Winter Wheat Depends on the Level of Selenate Spiked in Soil. Chemosphere 2022, 291, 132813. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of Root-Mediated pH Changes in the Rhizosphere and Their Responses to Environmental Constraints: A Review. Plant Soil. 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, L.; Li, J.; Zhang, C.; Lyu, L.; Man, Y.B.; Wu, F. Influence of Exogenous Selenomethionine and Selenocystine on Uptake and Accumulation of Se in Winter Wheat (Triticum aestivum L. cv. Xinong 979). Environ. Sci. Pollut. Res. 2023, 30, 23887–23897. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction with Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Kikkert, J.; Hale, B.; Berkelaar, E. Selenium Accumulation in Durum Wheat and Spring Canola as a Function of Amending Soils with Selenite, Selenate and/or Sulphate. Plant Soil. 2013, 372, 629–641. [Google Scholar] [CrossRef]
- Huang, Y.X.; Zhao, L.J.; Keller, A.A. Interactions, Transformations, and Bioavailability of Nano-Copper Exposed to Root Exudates. Environ. Sci. Technol. 2017, 51, 9774–9783. [Google Scholar] [CrossRef]
- Yang, X.; Römheld, V.; Marschner, H. Effect of Bicarbonate and Root Zone Temperature on Uptake of Zn, Fe, Mn, and Cu by Different Rice Cultivars (Oryza sativa L.) Grown in Calcareous Soil. Plant Soil. 1993, 155, 441–444. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W.; Zhang, H.; Nurmi, J.; Štipek, K.; Fischerova, Z.; Stingeder, G. Rhizosphere Characteristics of the Arsenic Hyperaccumulator Pteris vittata L. and Monitoring of Phytoremoval Efficiency. Environ. Sci. Technol. 2003, 37, 5008–5014. [Google Scholar] [CrossRef]
- Weiss, J.V.; Emerson, D.; Megonigal, J.P. Rhizosphere Iron (III) Deposition and Reduction in a Juncus effusus L.-Dominated Wetland. Soil. Sci. Soc. Am. J. 2005, 69, 1861–1870. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, H.; Yang, L.; Ye, B.; Wang, W. Dynamic Changes of Rhizosphere Properties and Antioxidant Enzyme Responses of Wheat Plants (Triticum aestivum L.) Grown in Mercury-Contaminated Soils. Chemosphere 2013, 93, 972–977. [Google Scholar] [CrossRef]
- Carvalho, G.S.; Oliveira, J.R.; Curi, N.; Schulze, D.G.; Marques, J.J. Selenium and Mercury in Brazilian Cerrado Soils and Their Relationships with Physical and Chemical Soil Characteristics. Chemosphere 2019, 218, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Awasthi, M.K.; Xing, W.; Liu, R.; Bao, H.; Wang, X.; Wu, F. Arbuscular Mycorrhizal Fungi Increase the Bioavailability and Wheat (Triticum aestivum L.) Uptake of Selenium in Soil. Ind. Crops Prod. 2020, 150, 112383. [Google Scholar] [CrossRef]
- Matos, R.P.; Lima, V.M.P.; Windmöller, C.C.; Nascentes, C.C. Correlation Between the Natural Levels of Selenium and Soil Physicochemical Characteristics from the Jequitinhonha Valley (MG), Brazil. J. Geochem. Explor. 2017, 172, 195–202. [Google Scholar] [CrossRef]
- Wang, D.; Xue, M.Y.; Wang, Y.K.; Zhou, D.Z.; Tang, L.; Cao, S.Y.; Wei, Y.H.; Yang, C.; Liang, D.L. Effects of Straw Amendment on Selenium Aging in Soils: Mechanism and Influential Factors. Sci. Total Environ. 2019, 657, 871–881. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yang, L.; Li, H. Dynamics of Rhizosphere Properties and Antioxidative Responses in Wheat (Triticum aestivum L.) Under Cadmium Stress. Ecotoxicol. Environ. Saf. 2014, 102, 55–61. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, Y.; Zhang, Q.; Zeng, C.; Deng, X.; Yuan, H.; Zeng, Q. Field-Scale Differences in Rhizosphere Micro-Characteristics of Cichorium intybus, Ixeris polycephala, Sunflower, and Sedum alfredii in the Phytoremediation of Cd-Contaminated Soil. Ecotoxicol. Environ. Saf. 2023, 262, 115137. [Google Scholar] [CrossRef]
- Li, J.; Peng, Q.; Liang, D.; Liang, S.; Chen, J.; Sun, H.; Lei, P. Effects of Aging on the Fraction Distribution and Bioavailability of Selenium in Three Different Soils. Chemosphere 2016, 144, 2351–2359. [Google Scholar] [CrossRef]
- Zeng, T.; Khaliq, M.A.; Li, H.; Jayasuriya, P.; Guo, J.; Li, Y.; Wang, G. Assessment of Cd Availability in Rice Cultivation (Oryza sativa): Effects of Amendments and the Spatiotemporal Chemical Changes in the Rhizosphere and Bulk Soil. Ecotoxicol. Environ. Saf. 2020, 196, 110490. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; Van Vark, W. Soil Analysis Procedures Using 0.01 M Calcium Chloride as Extraction Reagent. Commun. Soil. Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Weng, L.; Vega, F.A.; Supriatin, S.; Bussink, W.; Riemsdijk, W.H.V. Speciation of Se and DOC in Soil Solution and Their Relation to Se Bioavailability. Environ. Sci. Technol. 2011, 45, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kulp, T.R.; Pratt, L.M. Speciation and Weathering of Selenium in Upper Cretaceous Chalk and Shale from South Dakota and Wyoming, USA. Geochim. Cosmochim. Acta. 2004, 68, 3687–3701. [Google Scholar] [CrossRef]
- GB5009.93-2017; National Standard for Food Safety-Determination of Selenium in Food. National Health and Family Planning Commission of the People’s Republic of China State Food and Drug Administration: Beijing, Chian, 2017.
- Chen, Q.; Shi, W.; Wang, X. Selenium Speciation and Distribution Characteristics in the Rhizosphere Soil of Rice (Oryza sativa L.) Seedlings. Commun. Soil. Sci. Plant Anal. 2010, 41, 1411–1425. [Google Scholar] [CrossRef]
- Wang, H.Y.; Yang, L.F.; Chen, T.; Liu, Y.; Shi, X.Y.; Li, L.; Li, T.L. Occurrence Forms of Selenium and Zinc in Typical Wheat-Growing Areas of Shanxi Province. Chin. J. Ecol. 2025, 44, 912–919. [Google Scholar]
- Sun, X.; Yi, Q.; Tang, S.H.; Li, P.; Fu, H.T.; Wu, Y.P.; Zhang, M. Research Progress on Selenium Nutrition in Rice. Chin. J. Soil. Sci. 2023, 54, 223–231. (In Chinese) [Google Scholar]
- Gustafsson, J.P.; Persson, I.; Oromieh, A.G.; van Schaik, J.W.; Sjostedt, C.; Kleja, D.B. Chromium (III) Complexation to Natural Organic Matter: Mechanisms and Modeling. Environ. Sci. Technol. 2014, 48, 1753–1761. [Google Scholar] [CrossRef]
- Shen, Y.C.; Zhou, J. The Occurrence and Migration Transformation of Selenium in Soil. Anhui Geol. 2011, 21, 186–191. (In Chinese) [Google Scholar]
- Ma, Y.Z. Differences in Selenium Content, Distribution, and Bioavailability Between Paddy and Dryland Soils in Typical Selenium Rich Areas of Ziyang and Their Causal Analysis. Master’s Thesis, Northwest A&F University, Xianyang, China, 2022. (In Chinese). [Google Scholar]
- Hageman, S.P.; van der Weijden, R.D.; Weijma, J.; Buisman, C.J. Microbiological Selenate to Selenite Conversion for Selenium Removal. Water Res. 2013, 47, 2118–2128. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Wang, Q.; Luo, Z.; Yu, Y.; Jiang, R.F. Effects of Root Iron Plaque on Selenite and Selenate Dynamics in Rhizosphere and Uptake by Rice (Oryza sativa). Plant Soil. 2015, 388, 255–266. [Google Scholar] [CrossRef]
- Yan, Z.X.; Li, Y.; Peng, S.Y.; Wei, L.; Zhang, B.; Deng, X.Y.; Zhong, M.; Cheng, X. Cadmium Biosorption and Mechanism Investigation Using Two Cadmium-Tolerant Microorganisms Isolated from Rhizosphere Soil of Rice. J. Hazard. Mater. 2024, 470, 134134. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Yang, X.; Weston, L.A. The Role of Root Exudates and Allelochemicals in the Rhizosphere. Plant Soil. 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Bravin, M.N.; Martí, A.L.; Clairotte, M.; Hinsinger, P. Rhizosphere Alkalisation—A Major Driver of Copper Bioavailability Over a Broad pH Range in an Acidic, Copper-Contaminated Soil. Plant Soil. 2009, 318, 257–268. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, X.M.; Li, W.J.; Tu, Y.L.; Deng, H.; Du, J.W. Deciphering Heavy Metal Adsorption Capacity of Soil Based on Its Physicochemical Properties and Adsorption Reaction Time Using Machine Learning. J. Environ. Chem. Eng. 2025, 13, 116913. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N. Selenium (Se) Regulates Seedling Growth in Wheat Under Drought Stress. Adv. Chem. 2014, 2014, 670–675. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Garcia-Sanchez, F. Selenium Impedes Cadmium and Arsenic Toxicity in Potato by Modulating Carbohydrate and Nitrogen Metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Cipriano, P.E.; Júnior, M.S.; Souza, R.R.D.; Silva, D.F.D.; Silva, R.F.D.; Silva, M.L.D.S.; Faquin, V.; Guilherme, L.R.G. Selenium Inorganic Sources Applied to Soil: Effects on Gas Exchange and Anatomical Changes of Radishes. S. Afr. J. Bot. 2024, 170, 17. [Google Scholar] [CrossRef]
- Mendes Araujo, A.; Lima Lessa, J.H.D.; Chanavat Gustavo, L.; Curi, N.; Lopes, G. How Sulfate Content and Soil Depth Affect the Adsorption/Desorption of Selenate and Selenite in Tropical Soils? Rev. Bras. Cienc. Solo. 2020, 44, e0200084. [Google Scholar]
- Vila, P.A.; Faquin, V.; Vila, F.W.; Kachinski, W.D.; Carvalho, G.S.; Guilherme, L.R.G. Phosphorus and Sulfur in a Tropical Soil and Their Effects on Growth and Selenium Accumulation in Leucaena leucocephala (Lam.) de Wit. Environ. Sci. Pollut. Res. 2020, 27, 44060–44072. [Google Scholar] [CrossRef]
- Saeed, K.; Hussain, M.A.; Abdalla, M.A.; Mühling, K.H. Selenium Increases the Capacity of Antioxidative Defense and Their Accompanying Metal Cofactors in Maize Under Sulfate Salinity. Plant Stress. 2025, 16, 100816. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of Selenium Supplementation on Growth and Selenium Accumulation on Spinach (Spinacia oleracea L.) Plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F.; Reis, A.R. Physiological, Biochemical, and Ultrastructural Characterization of Selenium Toxicity in Cowpea Plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Li, H. Uptake, Translocation and Biotransformation of Selenium Nanoparticles in Rice Seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Guerrero, B.; Llugany, M.; Palacios, O.; Valiente, M. Dual Effects of Different Selenium Species on Wheat. Plant Physiol. Biochem. 2014, 83, 300–307. [Google Scholar] [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium Uptake, Translocation and Speciation in Wheat Supplied with Selenate or Selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Zhang, N. Effects of Exogenous Selenium with Different Valence States on Selenium Uptake and Translocation in Wheat. Master’s Thesis, Shihezi University, Shihezi, China, 2016. (In Chinese). [Google Scholar]
- Fu, D.D. Effects of Different Exogenous Selenium on Selenium Absorption, Distribution, and Translocation in Winter Wheat. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2011. (In Chinese). [Google Scholar]
- Li, Y.; Xiao, Y.; Hao, J.; Fan, S.; Dong, R.; Zeng, H.; Han, Y. Effects of Selenate and Selenite on Selenium Accumulation and Speciation in Lettuce. Plant Physiol. Biochem. 2022, 192, 162–171. [Google Scholar] [CrossRef]
- Kang, Y.; Qin, H.; Wang, G.; Lei, B.; Yang, X.; Zhong, M. Selenium Nanoparticles Mitigate Cadmium Stress in Tomato Through Enhanced Accumulation and Transport of Sulfate/Selenite and Polyamines. J. Agric. Food Chem. 2024, 72, 1473–1486. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous Chlorogenic Acid Alleviates Oxidative Stress in Apple Leaves by Enhancing Antioxidant Capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Brown, K.; Arthur, J.R. Selenium, Selenoproteins and Human Health: A Review. Public. Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, S.H.B.; Chu, L.Y.; Lu, Z.H.; Kang, C.M. Primary Antioxidant Free Radical Scavenging and Redox Signaling Pathways in Higher Plant Cells. Int. J. Biol. Sci. 2007, 4, 8. [Google Scholar] [CrossRef]
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Jia, W. Selenium in Soil-Plant System: Transport, Detoxification and Bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- Huang, G.X.; Ding, C.F.; Li, Y.S.; Zhang, T.L.; Wang, X.X. Selenium Enhances Iron Plaque Formation by Elevating the Radial Oxygen Loss of Roots to Reduce Cadmium Accumulation in Rice (Oryza sativa L.). J. Hazard. Mater. 2020, 398, 122860. [Google Scholar] [CrossRef]
- Somagattu, P.; Chinnannan, K.; Yammanuru, H.; Reddy, U.K.; Nimmakayala, P. Selenium Dynamics in Plants: Uptake, Transport, Toxicity, and Sustainable Management Strategies. Sci. Total Environ. 2024, 949, 175033. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Xu, Y.M.; Liu, Y.Y.; Qin, X.; Rong, H.; Liang, X.F. Selenium Application Alters Soil Cadmium Bioavailability and Reduces Its Accumulation in Rice Grown in Cd-Contaminated Soil. Environ. Sci. Pollut. Res. 2018, 25, 31175–31182. [Google Scholar] [CrossRef]
- Molnár, Á.; Kolbert, Z.; Kéri, K.; Feigl, G.; Ördög, A.; Szőllősi, R.; Erdei, L. Selenite-Induced Nitro-Oxidative Stress Processes in Arabidopsis thaliana and Brassica juncea. Ecotoxicol. Environ. Saf. 2018, 148, 664–674. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-Metal-Induced Reactive Oxygen Species: Phytotoxicity and Physicochemical Changes in Plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 20–74. [Google Scholar] [CrossRef]
- Alshaal, T.; Szabolcsy, É.D.; Fári, M.; Veres, S.; Kaszás, L.; Kovács, Z.; Eissa, F.; Elhawat, N. Agricultural Sustainability and the Challenges of Selenium Nanoparticles (SeNPs): Their Role in Supporting the Environmental Economy. Plant Stress. 2025, 16, 100846. [Google Scholar] [CrossRef]
- Zhai, H.; Xue, M.; Du, Z.; Wang, D.; Zhou, F.; Feng, P.; Liang, D.L. Leaching Behaviors and Chemical Fraction Distribution of Exogenous Selenium in Three Agricultural Soils Through Simulated Rainfall. Ecotoxicol. Environ. Saf. 2019, 173, 393–400. [Google Scholar] [CrossRef]
| Time | Se Added (mg Se kg−1) | SOL-Se | EX-Se | OM-Se | |||
|---|---|---|---|---|---|---|---|
| Content (µg kg−1) | Ratio (%) | Content (mg kg−1) | Ratio (%) | Content (mg kg−1) | Ratio (%) | ||
| 4 weeks | 0 | 1.81 ± 0.04 e | 0.83 | 0.02 ± 0.00 e | 7.15 | 0.03 ± 0.00 e | 14.67 |
| 1 | 7.56 ± 0.13 d | 0.86 | 0.09 ± 0.01 d | 9.87 | 0.15 ± 0.00 d | 16.88 | |
| 2.5 | 20.80 ± 0.97 c | 1.01 | 0.23 ± 0.01 c | 11.08 | 0.61 ± 0.01 c | 29.46 | |
| 5 | 44.87 ± 3.48 b | 1.07 | 0.49 ± 0.03 b | 11.77 | 1.38 ± 0.05 b | 32.83 | |
| 10 | 94.65 ± 2.84 a | 1.09 | 1.14 ± 0.04 a | 13.20 | 3.65 ± 0.09 a | 42.17 | |
| 6 weeks | 0 | 1.82 ± 0.03 e | 0.86 | 0.01 ± 0.00 e | 7.05 | 0.03 ± 0.00 e | 13.90 |
| 1 | 7.35 ± 0.16 d | 0.86 | 0.08 ± 0.00 d | 9.44 | 0.14 ± 0.01 d | 16.64 | |
| 2.5 | 20.03 ± 0.29 c | 0.98 | 0.20 ± 0.01 c | 10.00 | 0.57 ± 0.01 c | 27.83 | |
| 5 | 44.50 ± 3.18 b | 1.07 | 0.48 ± 0.03 b | 11.55 | 1.36 ± 0.04 b | 32.76 | |
| 10 | 92.63 ± 6.08 a | 1.08 | 1.08 ± 0.07 a | 12.58 | 3.51 ± 0.03 a | 40.96 | |
| 8 weeks | 0 | 1.83 ± 0.03 e | 0.89 | 0.01 ± 0.00 e | 6.65 | 0.03 ± 0.00 e | 13.94 |
| 1 | 7.30 ± 0.29 d | 0.86 | 0.08 ± 0.00 d | 9.34 | 0.14 ± 0.01 d | 16.10 | |
| 2.5 | 19.62 ± 0.25 c | 0.99 | 0.20 ± 0.01 c | 10.34 | 0.53 ± 0.02 c | 26.83 | |
| 5 | 41.65 ± 0.90 b | 1.03 | 0.46 ± 0.02 b | 11.43 | 1.31 ± 0.07 b | 32.22 | |
| 10 | 91.82 ± 2.27 a | 1.08 | 1.02 ± 0.05 a | 11.98 | 3.32 ± 0.09 a | 38.86 | |
| Time | Se Added (mg Se kg−1) | SOL-Se | EX-Se | OM-Se | |||
|---|---|---|---|---|---|---|---|
| Content (µg kg−1) | Ratio (%) | Content (mg kg−1) | Ratio (%) | Content (mg kg−1) | Ratio (%) | ||
| 4 weeks | 0 | 1.59 ± 0.08 e | 0.71 | 0.01 ± 0.00 e | 6.57 | 0.03 ± 0.00 e | 14.28 |
| 1 | 8.51 ± 0.20 d | 0.95 | 0.08 ± 0.01 d | 9.43 | 0.15 ± 0.00 d | 16.44 | |
| 2.5 | 24.02 ± 1.53 c | 1.16 | 0.23 ± 0.02 c | 11.20 | 0.62 ± 0.01 c | 29.86 | |
| 5 | 51.08 ± 2.39 b | 1.16 | 0.52 ± 0.01 b | 11.76 | 1.49 ± 0.01 b | 33.81 | |
| 10 | 106.66 ± 1.58 a | 1.21 | 1.15 ± 0.03 a | 13.02 | 3.72 ± 0.04 a | 42.18 | |
| 6 weeks | 0 | 1.59 ± 0.08 e | 0.73 | 0.01 ± 0.00 e | 6.39 | 0.03 ± 0.00 e | 13.39 |
| 1 | 8.14 ± 0.37 d | 0.95 | 0.08 ± 0.00 d | 9.44 | 0.14 ± 0.00 d | 16.19 | |
| 2.5 | 22.02 ± 1.16 c | 1.07 | 0.22 ± 0.00 c | 10.69 | 0.60 ± 0.01 c | 29.28 | |
| 5 | 48.43 ± 3.80 b | 1.13 | 0.50 ± 0.02 b | 11.54 | 1.41 ± 0.06 b | 32.74 | |
| 10 | 98.29 ± 5.03 a | 1.13 | 1.12 ± 0.04 a | 12.86 | 3.64 ± 0.08 a | 41.74 | |
| 8 weeks | 0 | 1.62 ± 0.02 e | 0.77 | 0.01 ± 0.00 e | 6.37 | 0.02 ± 0.00 e | 11.53 |
| 1 | 8.88 ± 0.26 d | 1.05 | 0.08 ± 0.00 d | 9.20 | 0.13 ± 0.01 d | 15.84 | |
| 2.5 | 19.65 ± 0.89 c | 0.96 | 0.21 ± 0.01 c | 10.48 | 0.57 ± 0.01 c | 28.22 | |
| 5 | 46.37 ± 2.12 b | 1.08 | 0.47 ± 0.02 b | 10.89 | 1.39 ± 0.05 b | 32.37 | |
| 10 | 97.60 ± 3.76 a | 1.12 | 1.09 ± 0.01 a | 12.51 | 3.52 ± 0.06 a | 40.52 | |
| Parameters | Se Added (mg Se kg−1) | WAP4 | WAP6 | WAP8 | |||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | ||
| Dry weight | 0 | 1.10 ± 0.10 a | 0.50 ± 0.06 a | 1.34 ± 0.11 c | 0.50 ± 0.04 bc | 1.40 ± 0.09 b | 0.54 ± 0.04 b |
| 1 | 1.17 ± 0.18 a | 0.54 ± 0.03 a | 1.40 ± 0.04 bc | 0.55 ± 0.04 ab | 1.40 ± 0.12 b | 0.56 ± 0.04 b | |
| 2.5 | 1.28 ± 0.03 a | 0.55 ± 0.03 a | 1.50 ± 0.07 ab | 0.59 ± 0.01 a | 1.43 ± 0.07 b | 0.58 ± 0.05 b | |
| 5 | 1.15 ± 0.10 a | 0.57 ± 0.05 a | 1.57 ± 0.08 a | 0.61 ± 0.06 a | 1.80 ± 0.12 a | 0.71 ± 0.09 a | |
| 10 | 1.15 ± 0.09 a | 0.51 ± 0.07 a | 1.31 ± 0.08 c | 0.46 ± 0.08 c | 1.24 ± 0.06 c | 0.47 ± 0.08 b | |
| Se content | 0 | 0.03 ± 0.01 e | 0.16 ± 0.01 e | 0.03 ± 0.01 e | 0.16 ± 0.01 e | 0.03 ± 0.01 e | 0.18 ± 0.01 e |
| 1 | 0.15 ± 0.01 d | 0.81 ± 0.01 d | 0.17 ± 0.01 d | 0.90 ± 0.01 d | 0.21 ± 0.01 d | 1.04 ± 0.02 d | |
| 2.5 | 0.47 ± 0.02 c | 1.89 ± 0.02 c | 0.58 ± 0.02 c | 2.33 ± 0.09 c | 0.66 ± 0.03 c | 2.47 ± 0.05 c | |
| 5 | 1.34 ± 0.04 b | 6.76 ± 0.33 b | 1.72 ± 0.02 b | 7.08 ± 0.05 b | 1.75 ± 0.04 b | 5.58 ± 0.26 b | |
| 10 | 2.81 ± 0.05 a | 15.43 ± 0.06 a | 3.57 ± 0.15 a | 15.59 ± 0.23 a | 3.75 ± 0.26 a | 13.33 ± 0.16 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, F.; Zhang, H.; Zhang, F.; Zhu, X.; Li, H.; Xu, Y. Dynamic Effects of Sodium Selenite on the Rhizospheric Microenvironment, Growth, and Antioxidative Responses of Wheat (Triticum aestivum L.). Agronomy 2025, 15, 1427. https://doi.org/10.3390/agronomy15061427
Qin F, Zhang H, Zhang F, Zhu X, Li H, Xu Y. Dynamic Effects of Sodium Selenite on the Rhizospheric Microenvironment, Growth, and Antioxidative Responses of Wheat (Triticum aestivum L.). Agronomy. 2025; 15(6):1427. https://doi.org/10.3390/agronomy15061427
Chicago/Turabian StyleQin, Fang, Han Zhang, Feiyan Zhang, Xiangrui Zhu, Hongji Li, and Yuefeng Xu. 2025. "Dynamic Effects of Sodium Selenite on the Rhizospheric Microenvironment, Growth, and Antioxidative Responses of Wheat (Triticum aestivum L.)" Agronomy 15, no. 6: 1427. https://doi.org/10.3390/agronomy15061427
APA StyleQin, F., Zhang, H., Zhang, F., Zhu, X., Li, H., & Xu, Y. (2025). Dynamic Effects of Sodium Selenite on the Rhizospheric Microenvironment, Growth, and Antioxidative Responses of Wheat (Triticum aestivum L.). Agronomy, 15(6), 1427. https://doi.org/10.3390/agronomy15061427






