The Contribution of Microbial- and Plant-Derived Carbon to Soil Organic Carbon Fractions and Stability Under Manure Application Combined with Straw Incorporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Sample Collection
2.3. SOC Mineralization Rate
2.4. SOC Fractions
2.5. Microbial Necromass Carbon
2.6. Lignin Phenols
2.7. Soil Enzyme Activity and Phospholipid Fatty Acid
2.8. Statistical Analysis
3. Results
3.1. SOC Fraction and Stability
3.2. Microbial Necromass and Lignin Phenols
3.3. Enzyme Activity and Microbial Community Composition
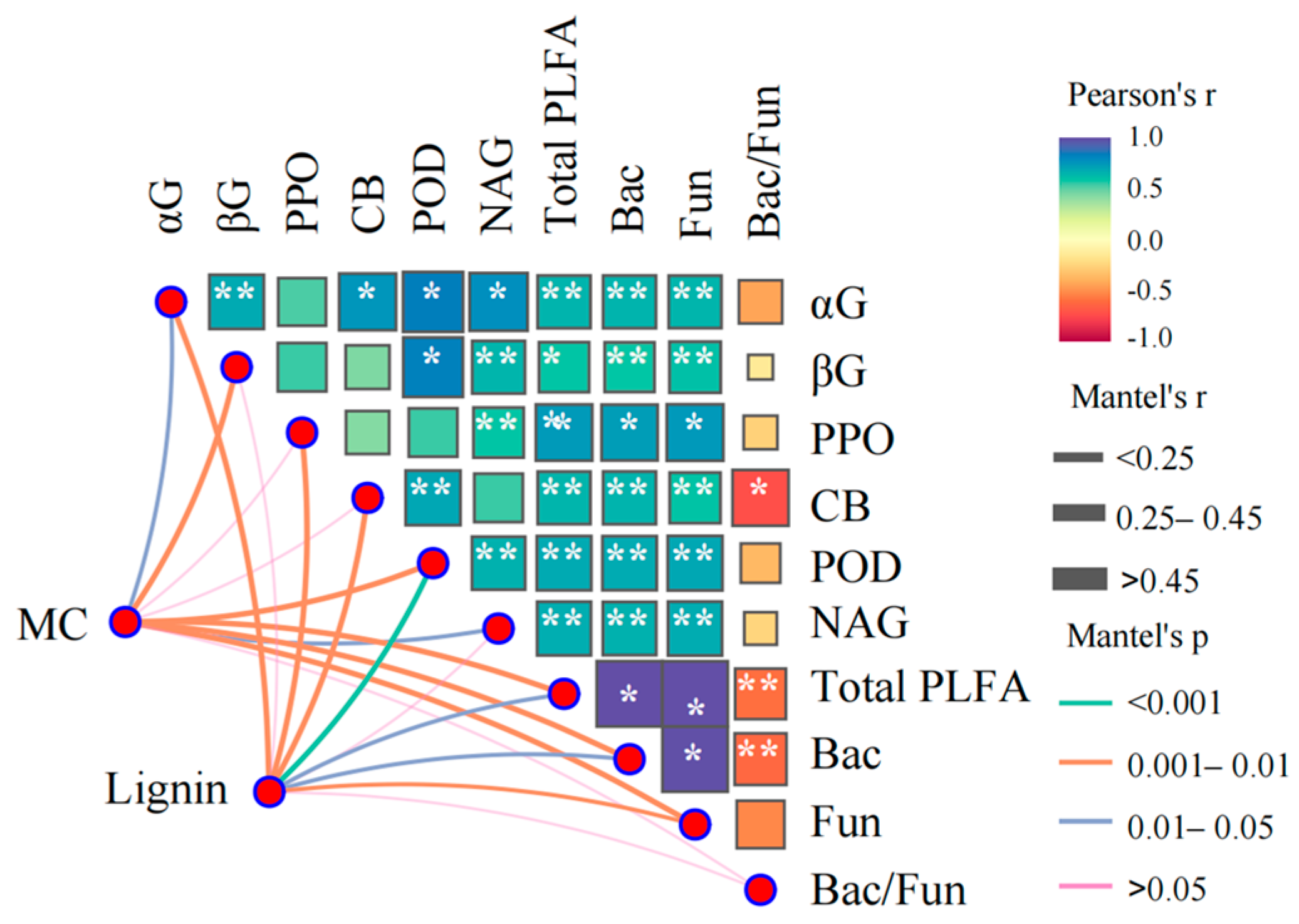
3.4. Effects of Enzyme and Microorganism on Microbial Necromass and Lignin Phenols
3.5. Effects of Microbial Necromass and Lignin Phenols on SOC Fraction
4. Discussion
4.1. Effect of Straw Returning and Manure on Microbial Necromass and Lignin Phenol
4.2. Effect of Microbial Necromass and Lignin Phenol on SOC Fraction
4.3. Effect of Microbial Necromass and Lignin Phenol on SOC Mineraliation Rates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Chapter one—Mineral-organic associations: Formation, properties, and relevance in soil environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral-organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Huang, X.L.; Jia, Z.X.; Jiao, X.Y.; Wang, J.L.; Huang, X.F. Long-term manure applications to increase carbon sequestration and macroaggregate-stabilized carbon. Soil Biol. Biochem. 2022, 174, 108827. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhou, J.; Wang, J.; Wang, L.; Yuan, J.; Xu, C.; Dong, Y.; Chen, Y.; Ai, Y.; et al. Combined application of chemical fertilizer and organic amendment improved soil quality in a wheat-sweet potato rotation system. Agronomy 2024, 14, 2160. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Cao, Y.F.; Ding, J.Z.; Li, J.; Xin, Z.M.; Ren, S.; Wang, T. Necromass-derived soil organic carbon and its drivers at the global scale. Soil Biol. Biochem. 2023, 181, 109025. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.Y.; Liu, J.J.; Liu, J.Y.; Han, L.; Wang, X.; Liu, H.Y.; Xu, M.P.; Yang, G.H.; Ren, C.J.; et al. The contribution of microbial necromass carbon to soil organic carbon in soil aggregates. Appl. Soil Ecol. 2023, 190, 104985. [Google Scholar] [CrossRef]
- Simpson, A.J.; Simpson, M.J.; Smith, E.; Kelleher, B.P. Microbially derived inputs to soil organic matter: Are current estimates too low? Environ. Sci. Technol. 2007, 41, 8070–8076. [Google Scholar] [CrossRef]
- Zhou, T.R.; Fan, J.B.; Zhang, L.X.; Lv, Q.L.; Wang, T.H.; Meng, Y.S.; Hu, H.; Gao, H.X.; Wang, J.; Ren, X.Q.; et al. The accumulation of plant- and microbial-derived carbon and its contribution to soil organic carbon in reclaimed saline-sodic farmland. Appl. Soil Ecol. 2024, 202, 105558. [Google Scholar] [CrossRef]
- Ma, T.; Yang, Z.Y.; Shi, B.W.; Gao, W.J.; Li, Y.F.; Zhu, J.X.; He, J.S. Phosphorus supply suppressed microbial necromass but stimulated plant lignin phenols accumulation in soils of alpine grassland on the Tibetan Plateau. Geoderma 2023, 431, 116376. [Google Scholar] [CrossRef]
- Lei, K.; Dai, W.; Wang, J.; Li, Z.; Cheng, Y.; Jiang, Y.; Yin, W.; Wang, X.; Song, X.; Tang, Q. Biochar and straw amendments over a decade divergently alter soil organic carbon accumulation pathways. Agronomy 2024, 14, 2176. [Google Scholar] [CrossRef]
- Yu, Y.L.; Zhu, N.X.; Ren, Y.; Dong, M.H.; Sun, G.F.; Virk, A.L.; Li, F.M.; Yang, H.S.; Kan, Z.R. Effects of crop rotation on plant- and microbial-derived carbon within particulate and mineral fractions in paddy soils. Agric. Ecosyst. Environ. 2025, 380, 109398. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.J.; Wang, J.; Yu, Q.; Wei, C.Z.; Jiang, L.C.; Huang, J.H.; Zhang, Y.H.; Jiang, Y.; Zhang, H.Y.; et al. Increase in mineral-associated organic carbon does not offset the decrease in particulate organic carbon under long-term nitrogen enrichment in a steppe ecosystem. Soil Biol. Biochem. 2025, 202, 109695. [Google Scholar] [CrossRef]
- Meng, X.T.; Zhang, X.C.; Li, Y.N.; Jiao, Y.P.; Fan, L.C.; Jiang, Y.J.; Qu, C.Y.; Filimonenko, E.; Jiang, Y.H.; Tian, X.H.; et al. Nitrogen fertilizer builds soil organic carbon under straw return mainly via microbial necromass formation. Soil Biol. Biochem. 2024, 188, 109223. [Google Scholar] [CrossRef]
- Li, J.N.; Zhao, J.; Liao, X.H.; Hu, P.L.; Wang, W.Y.; Ling, Q.M.; Xie, L.; Xiao, J.; Zhang, W.; Wang, K.L. Pathways of soil organic carbon accumulation are related to microbial life history strategies in fertilized agroecosystems. Sci. Total Environ. 2024, 927, 172191. [Google Scholar] [CrossRef]
- Huang, J.; Ebach, M.C.; Triantafilis, J. Cladistic analysis of Chinese soil taxonomy. Geoderma Reg. 2017, 10, 11–20. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Feng, X.; Nielsen, L.L.; Simpson, M.J. Responses of soil organic matter and microorganisms to freeze-thaw cycles. Soil Biol. Biochem. 2007, 39, 2027–2037. [Google Scholar] [CrossRef]
- Gao, Q.Q.; Wang, L.H.; Fang, Y.Y.; Gao, Y.; Ma, L.X.; Wang, X.; Li, Y.Y.; Wu, X.P.; Du, Z.L. Conservation agriculture boosts topsoil organic matter by restoring free lipids and lignin phenols biomarkers in distinct fractions. Soil Till. Res. 2025, 248, 106463. [Google Scholar] [CrossRef]
- Wen, Y.; Tang, Y.; Wen, J.; Wang, Q.; Bai, L.; Wang, Y.; Su, S.; Wu, C.; Lv, J.; Zeng, X. Variation of intra-aggregate organic carbon affects aggregate formation and stability during organic manure fertilization in a fluvo-aquic soil. Soil Use Manage. 2021, 37, 151–163. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Yang, L. Continuous straw returning enhances the carbon sequestration potential of soil aggregates by altering the quality and stability of organic carbon. J. Environ. Manag. 2024, 358, 120903. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chang, Y.; Sokol, N.W.; van Groenigen, K.J.; Bradford, M.A.; Ji, D.C.; Crowther, T.W.; Liang, C.; Luo, Y.Q.; Kuzyakov, Y.; Wang, J.K.; et al. A stoichiometric approach to estimate sources of mineral-associated soil organic matter. Glob. Change Biol. 2024, 30, 17092. [Google Scholar] [CrossRef]
- Xu, Y.N.; Yu, Y.L.; Sheng, J.; Wang, Y.K.; Yang, H.S.; Li, F.M.; Liu, S.P.; Kan, Z.R. Long-term residue returning increased subsoil carbon quality in a rice-wheat cropping system. J. Environ. Manag. 2024, 360, 121088. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Dong, L.B.; Fan, M.C.; Shangguan, Z.P. Long-term vegetation restoration promotes lignin phenol preservation and microbial anabolism in forest plantations: Implications for soil organic carbon dynamics. Sci. Total Environ. 2024, 928, 172635. [Google Scholar] [CrossRef]
- Yang, S.S.; Sun, J.Y.; Wang, C.; Li, S.Y.; Li, Z.B.; Luo, W.; Wei, G.H.; Chen, W.M. Residue quality drives SOC sequestration by altering microbial taxonomic composition and ecophysiological function in desert ecosystem. Environ. Res. 2024, 250, 118518. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bol, R.; An, T.T.; Liu, Y.C.; Xu, Y.D.; Li, S.Y.; Wang, J.K. Divergent accumulation of microbial necromass and plant lignin phenol induced by adding maize straw to fertilized soils. Soil Till. Res. 2024, 243, 106177. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Li, J.; Zhou, F.; Liang, X.; Zhu, X.; He, H.; Zhang, X. Complementation between microbial necromass and plant debris governs the longterm build-up of the soil organic carbon pool in conservation agriculture. Soil Biol. Biochem. 2023, 178, 108963. [Google Scholar] [CrossRef]
- Wang, N.; Cui, Y.; Zhou, Y.; Liu, P.X.; Wang, M.S.; Sun, H.H.; Huang, Y.B.; Wang, S. Changes in the glucose concentration affect the formation of humic-like substances in polyphenol-maillard reactions involving gibbsite. Molecules 2024, 29, 2115. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.R.; An, S.S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Li, Z.; Duan, X.; Guo, X.B.; Gao, W.; Li, Y.; Zhou, P.; Zhu, Q.H.; O’Donnell, A.G.; Dai, K.; Wu, J.S. Microbial metabolic capacity regulates the accrual of mineral-associated organic carbon in subtropical paddy soils. Soil Biol. Biochem. 2024, 195, 109457. [Google Scholar] [CrossRef]
- Zhang, X.W.; Wang, H.N.; Liu, Y.; Dong, H.L. Effect of clay mineral on bacteria-virus interactions and the fate of microbial biomass carbon. Geochim. Et Cosmochim. Acta 2025, 394, 383–392. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant-or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Mikutta, R.; Turner, S.; Schippers, A.; Gentsch, N.; Meyer-Stüve, S.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Eger, A.; Hempel, G.; et al. Microbial and abiotic controls on mineral-associated organic matter in soil profiles along an ecosystem gradient. Sci. Rep. 2019, 9, 10294. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Zhang, X.L.; Zhang, Z.W.; Wang, R.F.; Feng, C.; Xie, Y.X.; Chen, S.J.; Liu, T.Q. Microbial inoculants addition increases microbial necromass but decreases plant lignin contribution to soil organic carbon in rice paddies. Soil Till. Res. 2025, 250, 106529. [Google Scholar] [CrossRef]
- Keller, A.B.; Borer, E.T.; Collins, S.L.; DeLancey, L.C.; Fay, P.A.; Hofmockel, K.S.; Leakey, A.D.B.; Mayes, M.A.; Seabloom, E.W.; Walter, C.A.; et al. Soil carbon stocks in temperate grasslands differ strongly across sites but are insensitive to decade-long fertilization. Glob. Change Biol. 2022, 28, 1659–1677. [Google Scholar] [CrossRef]
- Underwood, T.R.; Bourg, I.C.; Rosso, K.M. Mineral-associated organic matter is heterogeneous and structured by hydrophobic, charged, and polar interactions. Proc. Natl. Acad. Sci. USA 2024, 121, e2413216121. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, J.; Zhang, L.P.; Sun, G.F.; Zheng, J.C. Organic fertilizer substitution increased soil organic carbon through the association of microbial necromass C with iron oxides. Soil Till. Res. 2025, 248, 106402. [Google Scholar] [CrossRef]
- Huang, X.; Jia, Z.; Guo, J.; Li, T.; Sun, D.; Meng, H.; Yu, G.; He, X.; Ran, W.; Zhang, S.; et al. Ten-year long-term organic fertilization enhances carbon sequestration and calcium-mediated stabilization of aggregate-associated organic carbon in a reclaimed Cambisol. Geoderma 2019, 355, 113880. [Google Scholar] [CrossRef]
- Huang, X.L.; Li, Y.Y.; Zhang, D.D.; Zhao, Y.; Wang, Y.; Liu, Q.X.; Dong, E.W.; Wang, J.S.; Jiao, X.Y. Long-term organic fertilization combined with deep ploughing enhances carbon sequestration in a rainfed sorghum-maize rotation system. Geoderma 2024, 442, 116778. [Google Scholar] [CrossRef]
- Yang, J.J.; Li, A.Y.; Yang, Y.F.; Li, G.H.; Zhang, F. Soil organic carbon stability under natural and anthropogenic-induced perturbations. Earth Sci. Rev. 2020, 205, 103199. [Google Scholar] [CrossRef]
- Dong, S.; Li, R.; Zhou, K.; Wei, Y.; Li, J.; Cheng, M.; Chen, P.; Hu, X. Response of humification process to fungal inoculant in corn straw composting with two different kinds of nitrogen sources. Sci. Total Environ. 2024, 946, 174461. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.Y.; Wu, Y.; Wang, S.Y.; Li, Y.R.; Zhao, J.H.; Huang, F.L.; Wu, J.Q. The reverse function of lignin-degrading enzymes: The polymerization ability to promote the formation of humic substances in domesticated composting. Bioresour. Technol. 2023, 380, 129059. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae1, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Chen, X.H.; Huang, C.Q.; Tan, W.F. Arbuscular mycorrhizal hyphal networks and glomalin-related soil protein jointly promote soil aggregation and alter aggregate hierarchy in Calcaric Regosol. Geoderma 2024, 452, 117096. [Google Scholar] [CrossRef]
- Zhu, S.; Dai, G.; Ma, T.; Chen, L.; Chen, D.; Lv, X.; Wang, X.; Zhu, J.; Zhang, Y.; Bai, Y.; et al. Distribution of lignin phenols in comparison with plant-derived lipids in the alpine versus temperate grassland soils. Plant Soil 2019, 439, 325–338. [Google Scholar] [CrossRef]
- Li, Z.; Wei, X.M.; Zhu, Z.K.; Fang, Y.Y.; Yuan, H.Z.; Li, Y.H.; Zhu, Q.H.; Guo, X.B.; Wu, J.S.; Kuzyakov, Y.; et al. Organic fertilizers incorporation increased microbial necromass accumulation more than mineral fertilization in paddy soil via altering microbial traits. Appl. Soil Ecol. 2024, 193, 105137. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lei, L.; Xiao, W.F.; Yang, X.; Horwath, W.R.; Liao, Y.L.; Yang, H.B.; Jian, Z.J.; Zeng, L.X. Vetch cover crops increase particulate organic carbon in citrus orchard by increasing lignin phenols. Appl. Soil Ecol. 2025, 207, 105921. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef] [PubMed]






| Treatments | αG (µmol·d−1·g−1) | βG (µmol·d−1·g−1) | PPO (µmol·d−1·g−1) | CB (µmol·d−1·g−1) | NAG (µmol·d−1·g−1) | POD (µmol·d−1·g−1) |
|---|---|---|---|---|---|---|
| CF | 15.3 ± 1.2 c | 67.0 ± 5.9 b | 312.7 ± 12.5 b | 67.6 ± 10.5 b | 6.1 ± 2.7 b | 73.81 ± 8.97 c |
| CM | 21.7 ± 0.5 b | 95.8 ± 10.5 a | 318.1 ± 29.9 b | 78.8 ± 3.2 ab | 13.2 ± 1.9 a | 81.29 ± 7.95 bc |
| CS | 24.7 ± 2.8 ab | 103.1 ± 12.9 a | 341.5 ± 45.3 ab | 75.1 ± 4.6 b | 13.9 ± 1.4 a | 90.92 ± 6.92 ab |
| CMS | 26.6 ± 2.1 a | 99.7 ± 10.1 a | 391.9 ± 14.3 a | 87.8 ± 2.7 a | 15.7 ± 1.4 a | 97.52 ± 4.41 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Liu, X.; Yang, N.; Li, Y.; Zhang, J. The Contribution of Microbial- and Plant-Derived Carbon to Soil Organic Carbon Fractions and Stability Under Manure Application Combined with Straw Incorporation. Agronomy 2025, 15, 1424. https://doi.org/10.3390/agronomy15061424
Wen Y, Liu X, Yang N, Li Y, Zhang J. The Contribution of Microbial- and Plant-Derived Carbon to Soil Organic Carbon Fractions and Stability Under Manure Application Combined with Straw Incorporation. Agronomy. 2025; 15(6):1424. https://doi.org/10.3390/agronomy15061424
Chicago/Turabian StyleWen, Yunjie, Xian Liu, Na Yang, Yongping Li, and Jiancheng Zhang. 2025. "The Contribution of Microbial- and Plant-Derived Carbon to Soil Organic Carbon Fractions and Stability Under Manure Application Combined with Straw Incorporation" Agronomy 15, no. 6: 1424. https://doi.org/10.3390/agronomy15061424
APA StyleWen, Y., Liu, X., Yang, N., Li, Y., & Zhang, J. (2025). The Contribution of Microbial- and Plant-Derived Carbon to Soil Organic Carbon Fractions and Stability Under Manure Application Combined with Straw Incorporation. Agronomy, 15(6), 1424. https://doi.org/10.3390/agronomy15061424





