Phosphorus Utilization Efficiency Among Corn Era Hybrids Released over Seventy-Five Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Selection, Experimental Conditions, and Phosphorus Treatments
2.2. Parameter Measurements, Harvesting, Sample Preparation, and Analysis
2.3. Statistical Analysis
3. Results
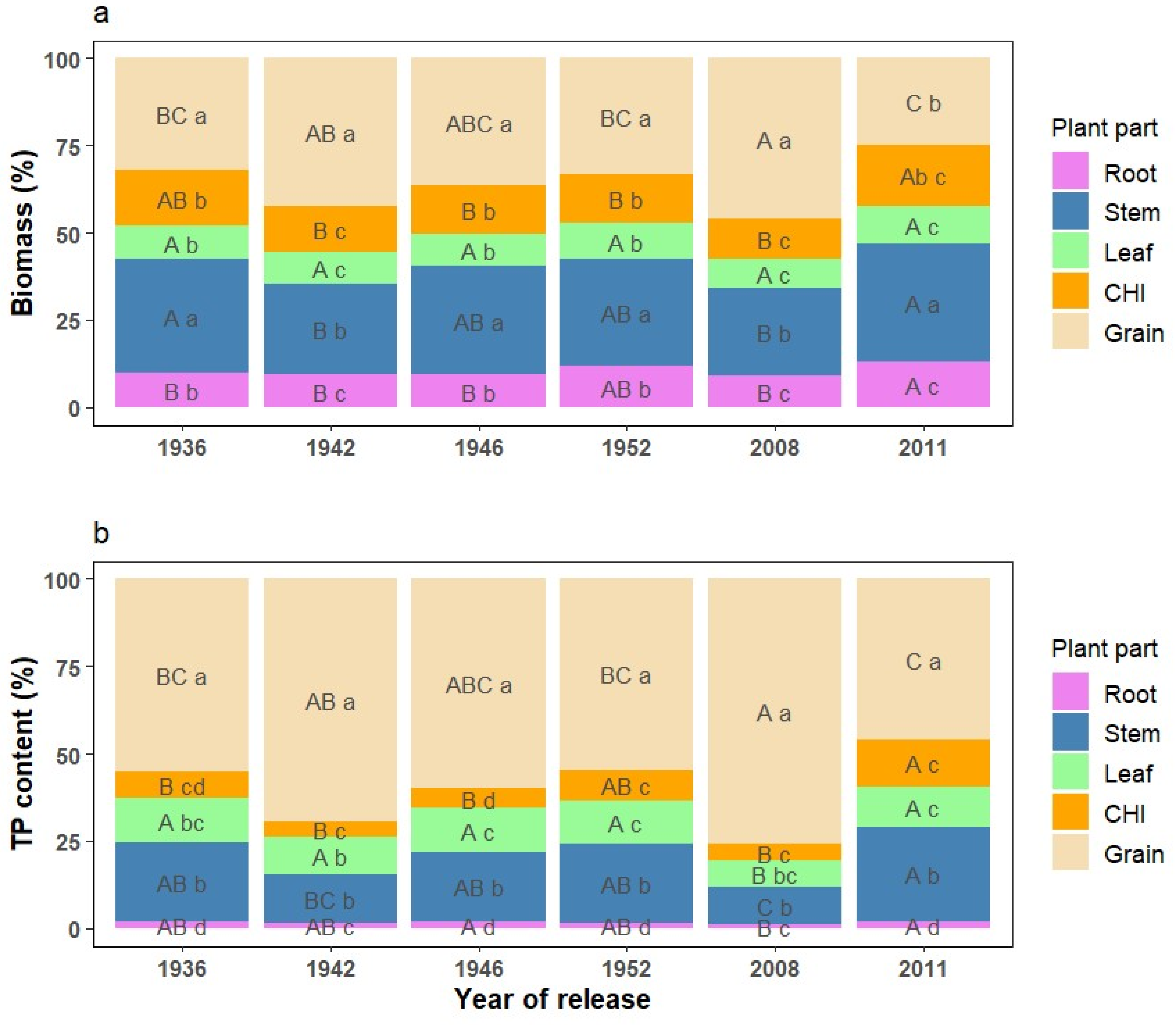
3.1. Corn Biomass Production
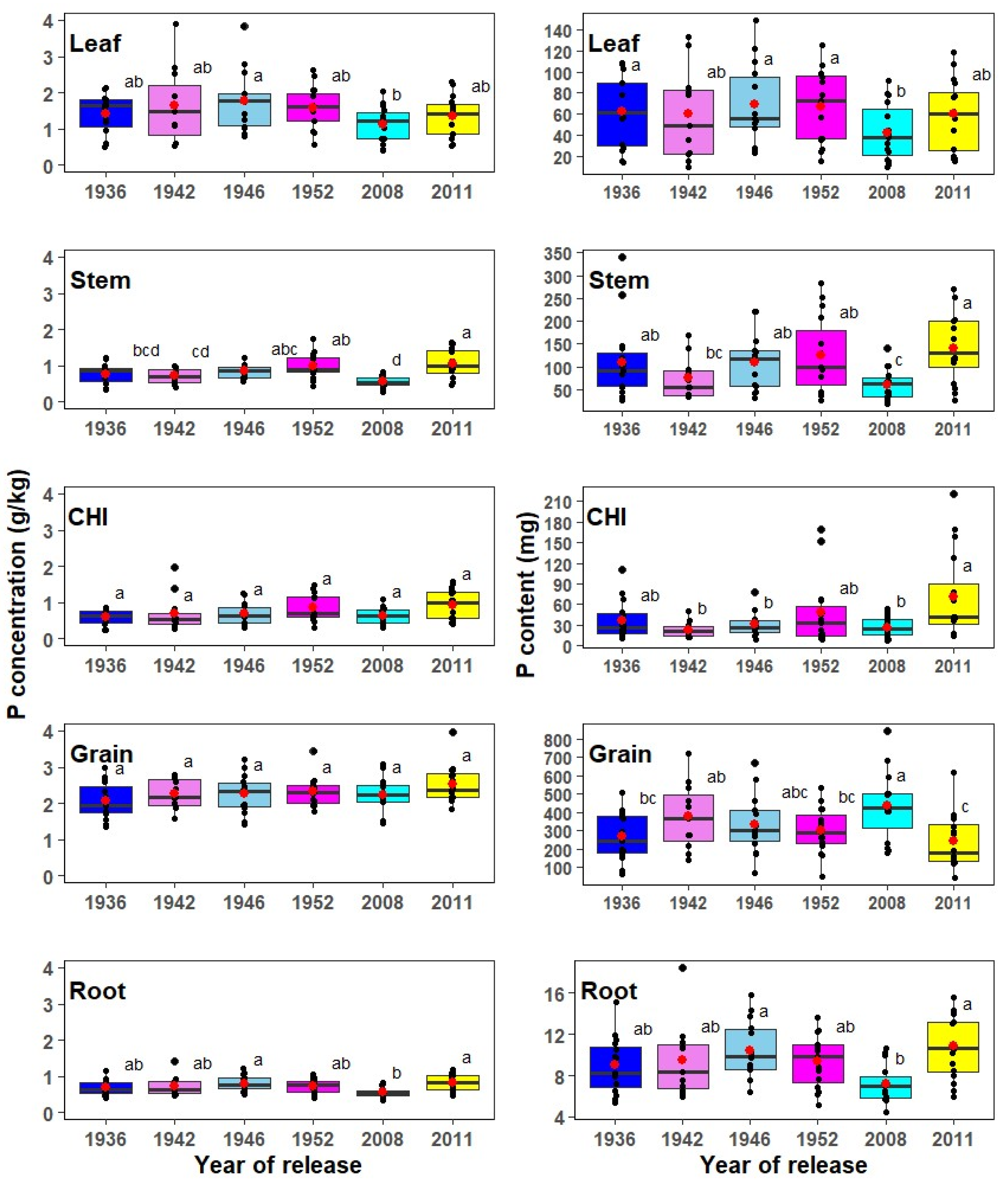
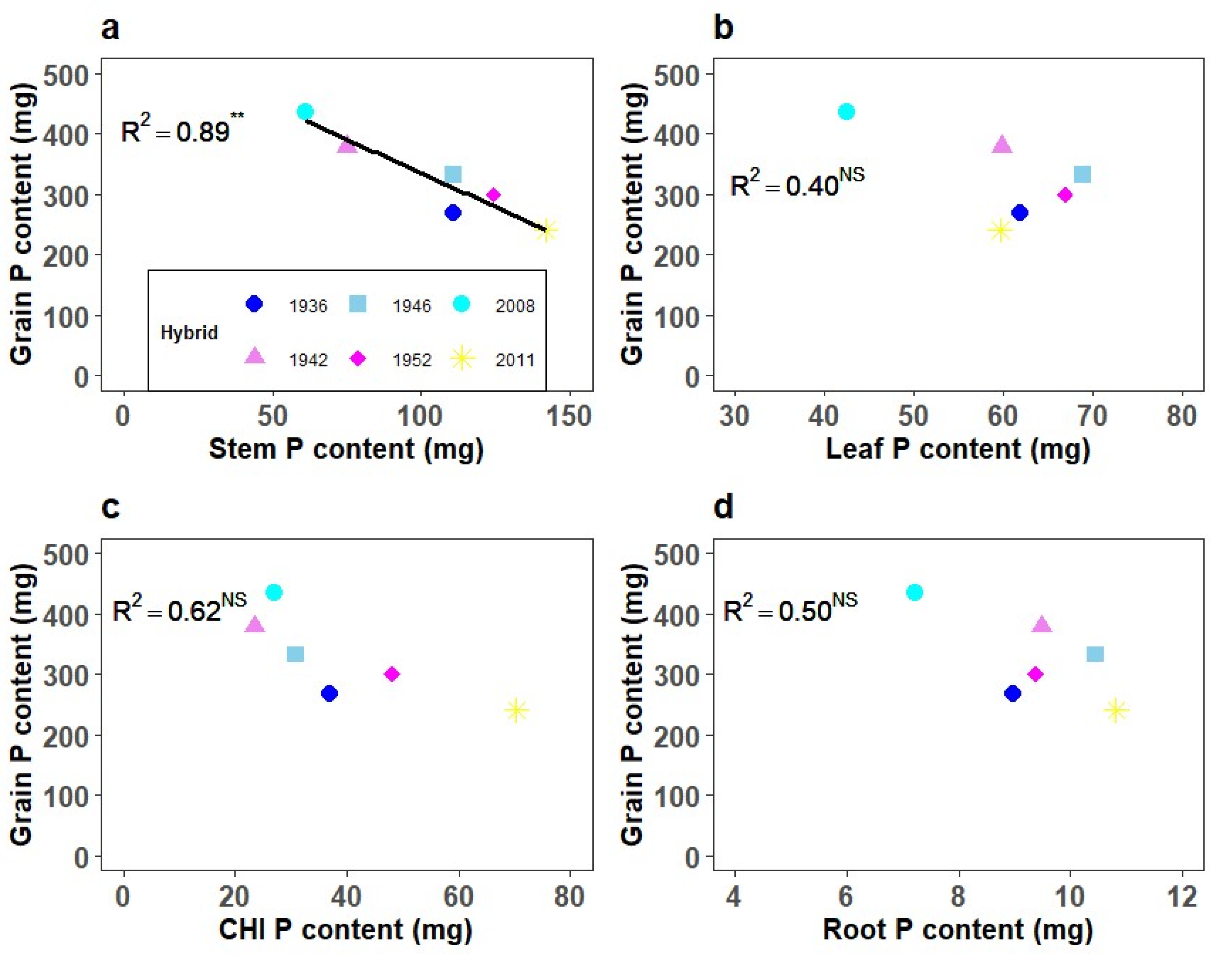
3.2. Phosphorus Concentration and Content in Corn Plant Parts
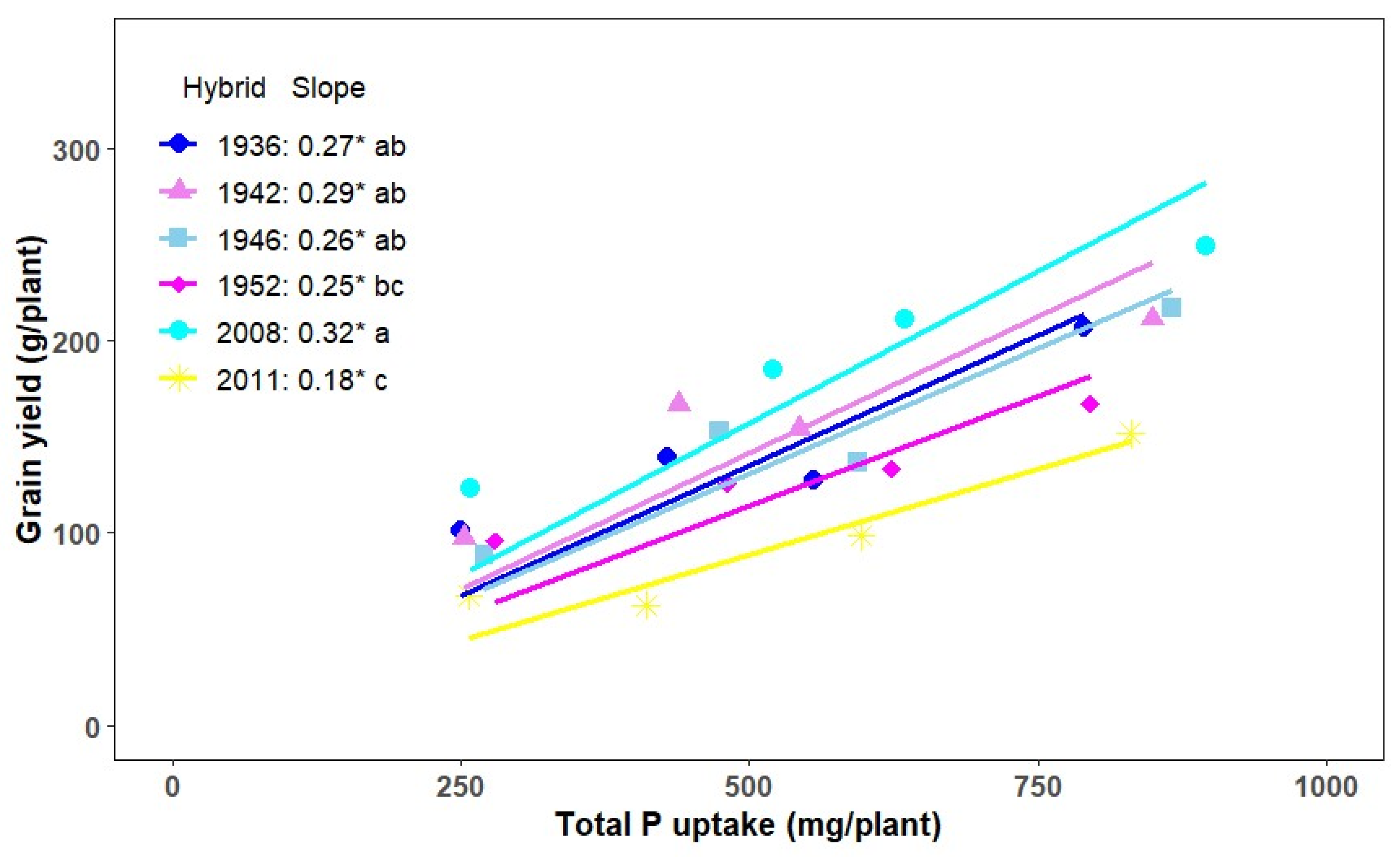
3.3. Phosphorus Utilization Efficiency
4. Discussion
4.1. Hybrid Biomass and Partitioning
4.2. Plant Phosphorus Concentration, Uptake, and Partitioning
4.3. Phosphorus Utilization Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pieters, A.J.; Paul, M.J.; Lawlor, D.W. Low sink demand limits photosynthesis under Pi deficiency. J. Exp. Bot 2001, 52, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Rychter, A.M.; Rao, I.M.; Rao, I.M. Role of Phosphorus in Photosynthetic Carbon Metabolism; Taylor & Francis Group: Abingdon, UK, 2005. [Google Scholar]
- Cordell, D.; Drangert, J.O.; White, S. The Story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic p in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes That Control How Soil Ph Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Chardon, W.J.; Bechmann, M.E.; Gascuel-Odoux, C.; Hofman, G.; Kronvang, B.; Rubæk, G.H.; Ulén, B.; Dorioz, J.M. Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Sci. Total Environ. 2014, 468–469, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, W.; Chen, J.; Bruijnzeel, L.A.; Mao, Z.; Yang, X.; Cardinael, R.; Meng, F.R.; Sidle, R.C.; Seitz, S.; et al. Reductions in water, soil and nutrient losses and pesticide pollution in agroforestry practices: A review of evidence and processes. Plant Soil 2020, 453, 45–86. [Google Scholar] [CrossRef]
- Vandamme, E.; Rose, T.; Saito, K.; Jeong, K.; Wissuwa, M. Integration of P acquisition efficiency, P utilization efficiency and low grain P concentrations into P-efficient rice genotypes for specific target environments. Nutr. Cycl. Agroecosyst. 2016, 104, 413–427. [Google Scholar] [CrossRef]
- Itoh, S.; Barber, S.A. Phosphorus uptake by six plant species as related to root hairs. Agron. J. 1983, 75, 457–461. [Google Scholar] [CrossRef]
- Smith, J.S.C. Genetic variability within U.S. hybrid maize: Multivariate analysis of isozyme data. Crop Sci. 1984, 24, 1041–1046. [Google Scholar] [CrossRef]
- Wu, Q.P.; Chen, F.J.; Chen, Y.L.; Yuan, L.X.; Zhang, F.S.; Mi, G.H. Root growth in response to nitrogen supply in Chinese maize hybrids released between 1973 and 2009. Sci. China Life Sci. 2011, 54, 642–650. [Google Scholar] [CrossRef]
- Rose, T.J.; Rose, M.T.; Pariasca-Tanaka, J.; Heuer, S.; Wissuwa, M. The frustration with utilization: Why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Front. Plant Sci. 2011, 2, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, J.; Liu, Z.; Chen, Z.; Ren, W.; Gong, X.; Wang, L.; Cai, H.; Pan, Q.; Yuan, L.; et al. Breeding for high-yield and nitrogen use efficiency in maize: Lessons from comparison between Chinese and US cultivars. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 2021; Volume 166, pp. 251–275. ISBN 9780128245873. [Google Scholar]
- Ciampitti, I.A.; Lemaire, G. From use efficiency to effective use of nitrogen: A dilemma for maize breeding improvement. Sci. Total Environ. 2022, 826, 154125. [Google Scholar] [CrossRef] [PubMed]
- Woli, K.P.; Boyer, M.J.; Elmore, R.W.; Sawyer, J.E.; Abendroth, L.J.; Barker, D.W. Corn era hybrid response to nitrogen fertilization. Agron. J. 2016, 108, 473–486. [Google Scholar] [CrossRef]
- Haase, C.; Wentzel, S.; Schmidt, R.; Joergensen, R.G. Changes in P fractions after long-term application of biogas slurry to soils under organic farming. Org. Agric. 2016, 6, 297–306. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Physiological perspectives of changes over time in maize yield dependency on nitrogen uptake and associated nitrogen efficiencies: A review. Field Crops Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Grain nitrogen source changes over time in maize: A review. Crop Sci. 2013, 53, 366–377. [Google Scholar] [CrossRef]
- Echarte, L.; Rothstein, S.; Tollenaar, M. The Response of leaf photosynthesis and dry matter accumulation to nitrogen supply in an older and a newer maize hybrid. Crop Sci. 2008, 48, 656–665. [Google Scholar] [CrossRef]
- Emmett, B.D.; Buckley, D.H.; Smith, M.E.; Drinkwater, L.E. Eighty years of maize breeding alters plant nitrogen acquisition but not rhizosphere bacterial community composition. Plant Soil 2018, 431, 53–69. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Ochoa-Cadavid, I. Phosphorus acquisition and internal utilization efficiency among maize landraces from the Central Mexican Highlands. Field Crops Res. 2014, 156, 123–134. [Google Scholar] [CrossRef]
- Haegele, J.W.; Cook, K.A.; Nichols, D.M.; Below, F.E. Changes in nitrogen use traits associated with genetic improvement for grain yield of maize hybrids released in different decades. Crop Sci. 2013, 53, 1256–1268. [Google Scholar] [CrossRef]
- Bender, R.R.; Haegele, J.W.; Ruffo, M.L.; Below, F.R. Modern corn hybrids’ nutrient uptake patterns. Better Crop. 2013, 97, 7–10. [Google Scholar]
- Woli, K.P.; Sawyer, J.E.; Boyer, M.J.; Abendroth, L.J.; Elmore, R.W. Corn era hybrid macronutrient and dry matter accumulation in plant components. Agron. J. 2018, 110, 1648–1658. [Google Scholar] [CrossRef]
- Olmedo Pico, L.B.; Zhang, C.; Vyn, T.J. The central role of ear nitrogen uptake in maize endosperm cell and kernel weight determination during the lag period. Field Crops Res. 2021, 273, 108285. [Google Scholar] [CrossRef]
- Ampong, K.; Penn, C.J.; Camberato, J.J. The timing of phosphorus availability to corn: What growth stages are most critical for maximizing yield ? Agronomy 2024, 14, 2731. [Google Scholar] [CrossRef]
- Schleuss, P.M.; Widdig, M.; Heintz-Buschart, A.; Kirkman, K.; Spohn, M. Interactions of nitrogen and phosphorus cycling promote p acquisition and explain synergistic plant-growth responses. Ecology 2020, 101, e03003. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Ray, K.; Banerjee, H.; Dutta, S.; Sarkar, S.; Murrell, T.S.; Singh, V.K.; Majumdar, K. Macronutrient management effects on nutrient accumulation, partitioning, remobilization, and yield of hybrid maize cultivars. Front. Plant Sci. 2020, 11, 1307. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Xu, Z.; Zhang, J.; Shen, J.; Cheng, L. Phosphorus partitioning contribute to phosphorus use efficiency during grain filling in Zea mays. Front. Plant Sci. 2023, 14, 1223532. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J.; Wiethorn, M.A. How much phosphorus uptake is required for achieving maximum maize grain yield? Part 1: Luxury consumption and implications for yield. Agronomy 2023, 13, 95. [Google Scholar] [CrossRef]
- Woli, K.P.; Sawyer, J.E.; Boyer, M.J.; Abendroth, L.J.; Elmore, R.W. Corn era hybrid dry matter and macronutrient accumulation across development stages. Agron. J. 2017, 109, 751–761. [Google Scholar] [CrossRef]
- Chen, K.; Camberato, J.J.; Tuinstra, M.R.; Kumudini, S.V.; Tollenaar, M.; Vyn, T.J. Genetic Improvement in density and nitrogen stress tolerance traits over 38 years of commercial maize hybrid release. Field Crops Res. 2016, 196, 438–451. [Google Scholar] [CrossRef]
- Wiethorn, M.; Penn, C.; Camberato, J. A research method for semi-automated large-scale cultivation of maize to full maturity in an artificial environment. Agronomy 2021, 11, 1898. [Google Scholar] [CrossRef]
- McKay Fletcher, D.M.; Ruiz, S.; Dias, T.; Petroselli, C.; Roose, T. Linking root structure to functionality: The impact of root system architecture on citrate-enhanced phosphate uptake. New Phytol. 2020, 227, 376–391. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Braun, D.M. Phloem Loading and Unloading of Sucrose: What a long, strange trip from source to sink. Annu. Rev. Plant Biol. 2022, 73, 553–584. [Google Scholar] [CrossRef]
- Wang, C.; Ning, P. Post-silking phosphorus recycling and carbon partitioning in maize under low to high phosphorus inputs and their effects on grain yield. Front. Plant Sci. 2019, 10, 784. [Google Scholar] [CrossRef]
- Chen, K.; Kumudini, S.V.; Tollenaar, M.; Vyn, T.J. Plant biomass and nitrogen partitioning changes between silking and maturity in newer versus older maize hybrids. Field Crops Res. 2015, 183, 315–328. [Google Scholar] [CrossRef]
- Mueller, S.M.; Messina, C.D.; Vyn, T.J. Simultaneous gains in grain yield and nitrogen efficiency over 70 years of maize genetic improvement. Sci. Rep. 2019, 9, 9095. [Google Scholar] [CrossRef]
- Duvick, D.N. The contribution of breeding to yield advances in maize (Zea mays L.). Adv. Agron. 2005, 86, 83–145. [Google Scholar] [CrossRef]
- Chen, K.; Camberato, J.J.; Vyn, T.J. Maize grain yield and kernel component relationships to morphophysiological traits in commercial hybrids separated by four decades. Crop Sci. 2017, 57, 1641–1657. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? a source-sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef] [PubMed]
- Olmedo Pico, L.B.; Vyn, T.J. Dry matter gains in maize kernels are dependent on their nitrogen accumulation rates and duration during grain filling. Plants 2021, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Gavrichkova, O.; Kuzyakov, Y. Respiration costs associated with nitrate reduction as estimated by 14co2 pulse labeling of corn at various growth stages. Plant Soil 2010, 329, 433–445. [Google Scholar] [CrossRef]
- Farrar, J.F.; Jones, D.L. The control of carbon acquisition by roots. New Phytol. 2000, 147, 43–53. [Google Scholar] [CrossRef]
- Wang, F.; Rose, T.; Jeong, K.; Kretzschmar, T.; Wissuwa, M. The knowns and unknowns of phosphorus loading into grains, and implications for phosphorus efficiency in cropping systems. J. Exp. Bot. 2016, 67, 1221–1229. [Google Scholar] [CrossRef]
- DeBruin, J.L.; Schussler, J.R.; Mo, H.; Cooper, M. Grain yield and nitrogen accumulation in maize hybrids released during 1934 to 2013 in the US Midwest. Crop Sci. 2017, 57, 1431–1446. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780123849052. [Google Scholar]
- Christy, A.L.; Ferrier, J.M. A mathematical treatment of munch’s pressure-flow hypothesis of phloem translocation. Plant Physiol. 1973, 52, 531–538. [Google Scholar] [CrossRef][Green Version]
- Adriano, D.C. Phosphorus-micronutrient interaction effects on crop production. J. Plant Nutr. 1981, 3, 593–613. [Google Scholar] [CrossRef]
- Yan, S.; Wu, Y.; Fan, J.; Zhang, F.; Guo, J.; Zheng, J.; Wu, L.; Lu, J. Quantifying nutrient stoichiometry and radiation use efficiency of two maize cultivars under various water and fertilizer management practices in Northwest China. Agric. Water Manag. 2022, 271, 107772. [Google Scholar] [CrossRef]
| Abbreviation | Definition | Calculation | Unit |
|---|---|---|---|
| PUtE | Change in grain yield (y) over change in total plant P uptake (x) based on linear regression | ∆y/∆x | g/mg |
| gPUtE | Percentage of TP in grain, P harvest index | mg/mg |
| Corn Hybrid | ||||||
|---|---|---|---|---|---|---|
| Parameter | 1936 | 1942 | 1946 | 1952 | 2008 | 2011 |
| Total biomass (g plant−1) | 432 ± 39 a | 383 ± 36 a | 410 ± 40 a | 392 ±38 a | 414 ± 30 a | 380 ± 29 a |
| Kernel rows per ear | 13.9 ± 0.5 a | 14.0 ± 0.5 a | 13.6 ± 0.6 a | 15.2 ± 0.4 a | 14.4 ± 0.4 a | 14.4 ± 0.2 a |
| 100 kernel weight (g) | 27.7 ± 1.0 b | 30.3 ± 1.4 ab | 28.4 ± 1.0 ab | 29.6 ±1.3 ab | 32.3 ± 1.0 a | 20.9 ± 1.0 c |
| Kernel number per plant | 506 ± 68 a | 536 ± 50 a | 543 ± 64 a | 436 ± 40 a | 604 ± 49 a | 446 ± 58 a |
| Grain yield (g plant−1) | 160 ± 22 abc | 187 ± 21 ab | 172 ± 19 abc | 150 ± 16 bc | 219 ± 16 a | 109 ± 17 c |
| Root/shoot ratio | 0.11 ± 0.01 b | 0.10 ± 0.01 b | 0.11 ± 0.01 b | 0.13 ± 0.01 ab | 0.10 ± 0.01 b | 0.16 ± 0.01 a |
| TP content (mg plant−1) | 487 ± 53 b | 547 ± 80 ab | 553 ± 65 ab | 549 ± 53 ab | 573 ± 66 a | 524 ± 58 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ampong, K.; Penn, C.J.; Camberato, J.; Quinn, D.; Williams, M. Phosphorus Utilization Efficiency Among Corn Era Hybrids Released over Seventy-Five Years. Agronomy 2025, 15, 1407. https://doi.org/10.3390/agronomy15061407
Ampong K, Penn CJ, Camberato J, Quinn D, Williams M. Phosphorus Utilization Efficiency Among Corn Era Hybrids Released over Seventy-Five Years. Agronomy. 2025; 15(6):1407. https://doi.org/10.3390/agronomy15061407
Chicago/Turabian StyleAmpong, Kwame, Chad J. Penn, James Camberato, Daniel Quinn, and Mark Williams. 2025. "Phosphorus Utilization Efficiency Among Corn Era Hybrids Released over Seventy-Five Years" Agronomy 15, no. 6: 1407. https://doi.org/10.3390/agronomy15061407
APA StyleAmpong, K., Penn, C. J., Camberato, J., Quinn, D., & Williams, M. (2025). Phosphorus Utilization Efficiency Among Corn Era Hybrids Released over Seventy-Five Years. Agronomy, 15(6), 1407. https://doi.org/10.3390/agronomy15061407






