Influence of Nitrogen Addition Levels on N2O Flux and Yield of Spring Wheat in the Loess Plateau
Abstract
1. Introduction
2. Methods
2.1. Study Site
2.2. Experimental Design for N Addition in Spring Wheat
2.3. Sampling and Analysis of N2O Gas
2.4. Spring Wheat Yield and Soil Sample Collection and Analysis
2.5. Statistical Analysis
3. Results
3.1. Changes in Soil Characteristics and Spring Wheat Yield Under Different N Addition Levels
3.2. Variation in N2O Flux in Spring Wheat Soil Under Different N Addition Levels
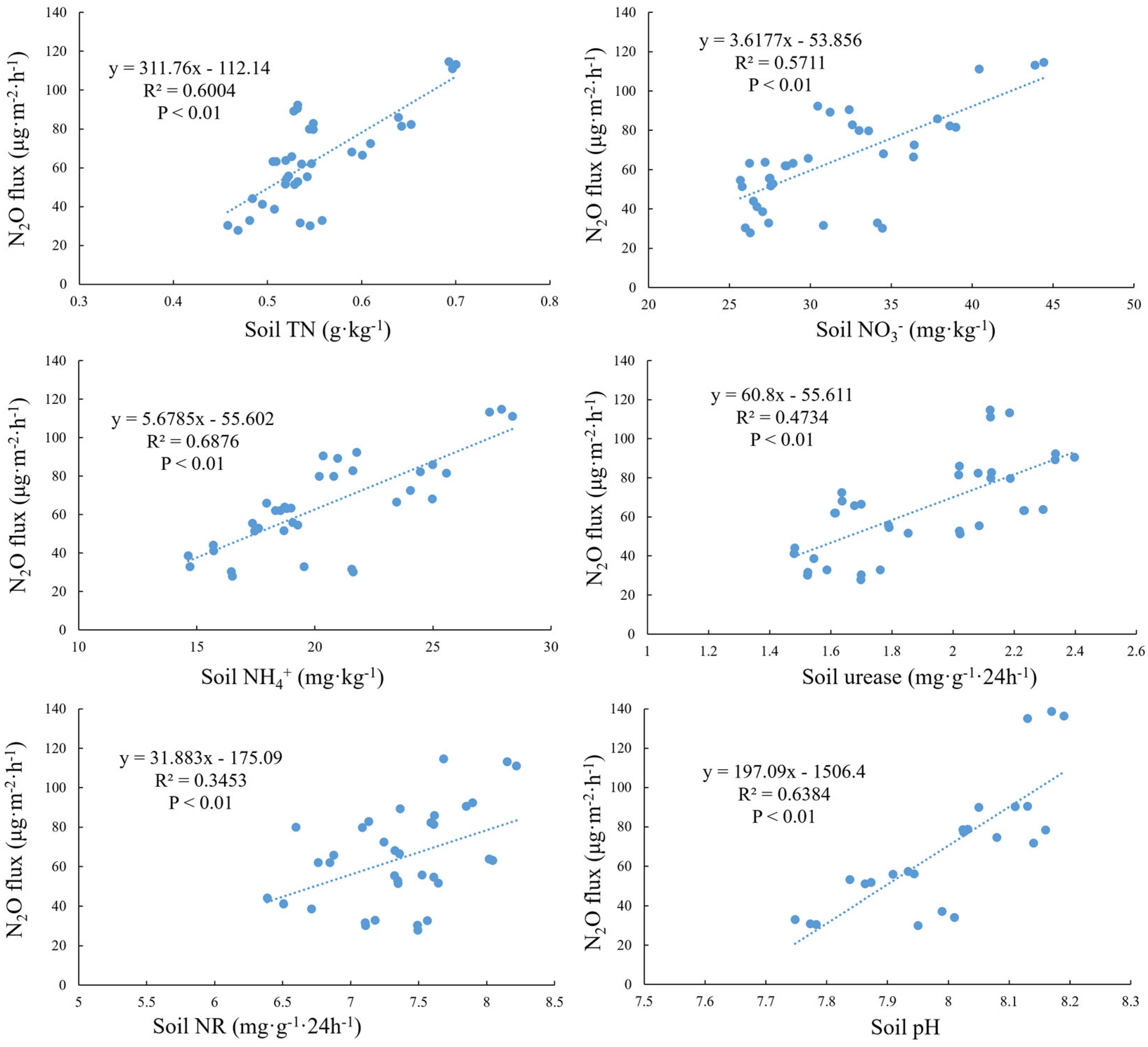
3.3. Correlation Analysis Between Soil Characteristics and N2O Flux
4. Discussion
4.1. Effects of Different N Addition Levels on Soil Characteristics in Spring Wheat
4.2. Effects of Different N Addition Levels on N2O Flux in Spring Wheat
4.3. Effects of Different N Addition Levels on Spring Wheat Yield and the Environment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voigt, C.; Lamprecht, R.E.; Marushchak, M.E.; Lind, S.E.; Novakovskiy, A.; Aurela, M.; Biasi, C. Warming of subarctic tundra increases emissions of all three important greenhouse gases–carbon dioxide, methane, and nitrous oxide. Glob. Change Biol. 2017, 23, 3121–3138. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Yao, Y. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yu, Z.; Zhang, J.; Cao, P.; Tian, H.; Nevison, C. Century-long changes and drivers of soil nitrous oxide (N2O) emissions across the contiguous United States. Glob. Change Biol. 2022, 28, 2505–2524. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, F.; Ciais, P.; Davidson, E.A.; Tubiello, F.N.; Niu, X.; Zhu, D. Global mapping of crop-specific emission factors highlights hotspots of nitrous oxide mitigation. Nat. Food 2021, 2, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Wang, T.; Song, X.; Zhao, C.; Rees, R.M.; Liu, Z.; Siddique, K.H. Reducing N2O emissions with enhanced efficiency nitrogen fertilizers (EENFs) in a high-yielding spring maize system. Environ. Pollut. 2021, 273, 116422. [Google Scholar] [CrossRef]
- Ma, L.; Shan, J.; Yan, X. Nitrite behavior accounts for the nitrous oxide peaks following fertilization in a fluvo-aquic soil. Biol. Fertil. Soils 2015, 51, 563–572. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, X.; Hao, T.; Zeng, M.; Shen, J.; Zhang, F.; de Vries, W. Cropland acidification increases risk of yield losses and food insecurity in China. Environ. Pollut. 2020, 256, 113145. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Li, L.; Coulter, J.A.; Xie, J.; Gopalakrishnan, S.; Zhang, R.; Khan, S. Long-term nitrogen fertilization impacts on soil bacteria, grain yield and nitrogen use efficiency of wheat in semiarid loess plateau, China. Agronomy 2020, 10, 1175. [Google Scholar] [CrossRef]
- Xu, C.; Han, X.; Ru, S.; Cardenas, L.; Rees, R.M.; Wu, D.; Meng, F. Crop straw incorporation interacts with N fertilizer on N2O emissions in an intensively cropped farmland. Geoderma 2019, 341, 129–137. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, B.; Shen, J.; Zhu, X.; Yi, W.; Li, Y.; Wu, J. Contrasting effects of straw and straw-derived biochar applications on soil carbon accumulation and nitrogen use efficiency in double-rice cropping systems. Agric. Ecosyst. Environ. 2021, 311, 107286. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Y.; Gao, X.; Liu, J.; Wang, Z.; Gao, M. Nitrous oxide emission and the related denitrifier community: A short-term response to organic manure substituting chemical fertilizer. Ecotoxicol. Environ. Saf. 2020, 192, 110291. [Google Scholar] [CrossRef]
- Heil, J.; Liu, S.; Vereecken, H.; Brueggemann, N. Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties. Soil Biol. Biochem. 2015, 84, 107–115. [Google Scholar] [CrossRef]
- Rue, K.; Rusevova, K.; Biles, C.L.; Huling, S.G. Abiotic hydroxylamine nitrification involving manganese-and iron-bearing minerals. Sci. Total Environ. 2018, 644, 567–575. [Google Scholar] [CrossRef]
- Duan, P.; Zhou, J.; Feng, L.; Jansen-Willems, A.B.; Xiong, Z. Pathways and controls of N2O production in greenhouse vegetable production soils. Biol. Fertil. Soils 2019, 55, 285–297. [Google Scholar] [CrossRef]
- Gan, X.; Hu, H.; Fu, Q.; Zhu, J. Nitrate reduction coupling with As (III) oxidation in neutral As-contaminated paddy soil preserves nitrogen, reduces N2O emissions and alleviates As toxicity. Sci. Total Environ. 2024, 912, 169360. [Google Scholar] [CrossRef]
- Pan, H.; Ying, S.; Liu, H.; Zeng, L.; Zhang, Q.; Liu, Y.; Di, H. Microbial pathways for nitrous oxide emissions from sheep urine and dung in a typical steppe grassland. Biol. Fertil. Soils 2018, 54, 717–730. [Google Scholar] [CrossRef]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
- Maharjan, B.; Venterea, R.T. Nitrite intensity explains N management effects on N2O emissions in maize. Soil Biol. Biochem. 2013, 66, 229–238. [Google Scholar] [CrossRef]
- Zuccarini, P.; Sardans, J.; Asensio, L.; Peñuelas, J. Altered activities of extracellular soil enzymes by the interacting global environmental changes. Glob. Change Biol. 2023, 29, 2067–2091. [Google Scholar] [CrossRef]
- Han, P.; Tang, X.; Koch, H.; Dong, X.; Hou, L.; Wang, D.; Shi, G. Unveiling unique microbial nitrogen cycling and nitrification driver in coastal Antarctica. Nat. Commun. 2024, 15, 3143. [Google Scholar] [CrossRef] [PubMed]
- Swify, S.; Mažeika, R.; Baltrusaitis, J.; Drapanauskaitė, D.; Barčauskaitė, K. Modified urea fertilizers and their effects on improving nitrogen use efficiency (NUE). Sustainability 2023, 16, 188. [Google Scholar] [CrossRef]
- Hoang, H.G.; Thuy, B.T.P.; Lin, C.; Vo, D.V.N.; Tran, H.T.; Bahari, M.B.; Vu, C.T. The nitrogen cycle and mitigation strategies for nitrogen loss during organic waste composting: A review. Chemosphere 2022, 300, 134514. [Google Scholar] [CrossRef]
- Hou, S.; Dang, H.; Huang, T.; Huang, Q.; Li, C.; Li, X.; Wang, Z. Targeting high nutrient efficiency to reduce fertilizer input in wheat production of China. Field Crops Res. 2023, 292, 108809. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Bi, X.; Feng, B.; Wang, Z.; Wang, F.; Liu, K. Nitrogen fertilizer management on wheat yield and nitrogen utilization. J. Plant Nutr. 2022, 45, 1953–1960. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, F. Effect of reduced subsequent N supply and organic amendment on soil bacterial community in a wheat-maize rotation system with over-fertilization. Appl. Soil Ecol. 2023, 181, 104659. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, D.; Wang, H.; Cui, R.; Khoshnevisan, B.; Guo, S.; Liu, H. Shallow groundwater fluctuation: An ignored soil N loss pathway from cropland. Sci. Total Environ. 2022, 828, 154554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, Y.; Yang, J.; Zhou, Z.; Gunina, A. Effects of nitrogen fertilization on the rhizosphere priming. Plant Soil 2021, 462, 489–503. [Google Scholar] [CrossRef]
- Aryal, B.; Gurung, R.; Camargo, A.F.; Fongaro, G.; Treichel, H.; Mainali, B.; Puadel, S.R. Nitrous oxide emission in altered nitrogen cycle and implications for climate change. Environ. Pollut. 2022, 314, 120272. [Google Scholar] [CrossRef]
- Linton, N.F.; Machado, P.V.F.; Deen, B.; Wagner-Riddle, C.; Dunfield, K.E. Long-term diverse rotation alters nitrogen cycling bacterial groups and nitrous oxide emissions after nitrogen fertilization. Soil Biol. Biochem. 2020, 149, 107917. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Abalos, D.; Luo, Y.; Hui, D.; Hungate, B.A.; Chen, J. Stimulation of ammonia oxidizer and denitrifier abundances by nitrogen loading: Poor predictability for increased soil N2O emission. Glob. Change Biol. 2022, 28, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Owens, J.; Beres, B.; Li, Y.; Hao, X. Nitrous oxide emissions with enhanced efficiency and conventional urea fertilizers in winter wheat. Nutr. Cycl. Agroecosystems 2021, 119, 307–322. [Google Scholar] [CrossRef]
- Kim, G.W.; Kim, P.J.; Khan, M.I.; Lee, S.J. Effect of rice planting on nitrous oxide (N2O) emission under different levels of nitrogen fertilization. Agronomy 2021, 11, 217. [Google Scholar] [CrossRef]
- Lan, T.; Li, M.; Han, Y.; Deng, O.; Tang, X.; Luo, L.; Gao, X. How are annual CH4, N2O, and NO emissions from rice–wheat system affected by nitrogen fertilizer rate and type? Appl. Soil Ecol. 2020, 150, 103469. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Brüggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Glob. Change Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Luo, S.; Bu, L.; Chen, X.; Yue, S.; Li, S. Response of nitrous oxide emission to soil mulching and nitrogen fertilization in semi-arid farmland. Agric. Ecosyst. Environ. 2014, 188, 20–28. [Google Scholar] [CrossRef]
- Ma, B.L.; Wu, T.Y.; Tremblay, N.; Deen, W.; Morrison, M.J.; McLaughlin, N.B.; Stewart, G. Nitrous oxide fluxes from corn fields: On-farm assessment of the amount and timing of nitrogen fertilizer. Glob. Change Biol. 2010, 16, 156–170. [Google Scholar] [CrossRef]
- Yuan, J.; Sadiq, M.; Rahim, N.; Li, G.; Yan, L.; Wu, J.; Xu, G. Tillage strategy and nitrogen fertilization methods influences on selected soil quality indicators and spring wheat yield under semi-arid environmental conditions of the loess plateau, China. Appl. Sci. 2022, 12, 1101. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, D.; Liu, M.; Hafeez, M.B.; Wen, P.; Wang, X.; Li, J. Optimized fertilizer recommendation method for nitrate residue control in a wheat–maize double cropping system in dryland farming. Field Crops Res. 2021, 271, 108258. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Wang, G.; Qian, X.; Wang, W.; Xu, Y.; Yang, J. Reducing environmental risk by improving crop management practices at high crop yield levels. Field Crops Res. 2021, 265, 108123. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Sun, Y.; Liu, P.; Zhang, Q.; Wang, X.; Li, J. Long-term effects of optimized fertilization, tillage and crop rotation on soil fertility, crop yield and economic profit on the Loess Plateau. Eur. J. Agron. 2023, 143, 126731. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, P.; Tong, Y.; Norse, D.; Lu, Y.; Powlson, D. Overcoming nitrogen fertilizer over-use through technical and advisory approaches: A case study from Shaanxi Province, northwest China. Agric. Ecosyst. Environ. 2015, 209, 89–99. [Google Scholar] [CrossRef]
- Wang, C.; Qi, Z.; Zhao, J.; Gao, Z.; Zhao, J.; Chen, F.; Chu, Q. Sustainable water and nitrogen optimization to adapt to different temperature variations and rainfall patterns for a trade-off between winter wheat yield and N2O emissions. Sci. Total Environ. 2023, 854, 158822. [Google Scholar] [CrossRef]
- Qiu, L.; Gou, X.; Kong, Y.; Tu, F.; Peng, X.; Xu, L.; Tu, L. Nitrogen addition stimulates N2O emissions via changes in denitrification community composition in a subtropical nitrogen-rich forest. J. Environ. Manag. 2023, 348, 119274. [Google Scholar] [CrossRef]
- Yuan, J.; Yao, Y.; Guan, Y.; Sadiq, M.; Li, J.; Liu, S.; Yan, L. Effects of land use patterns on soil properties and nitrous oxide flux on a semi-arid environmental conditions of Loess Plateau China. Glob. Ecol. Conserv. 2024, 51, e02899. [Google Scholar] [CrossRef]
- Du, M.; Yuan, J.; Zhuo, M.; Sadiq, M.; Wu, J.; Xu, G.; Yan, L. Effects of different land use patterns on soil properties and N2O emissions on a semi-arid Loess Plateau of Central Gansu. Front. Ecol. Evol. 2023, 11, 1128236. [Google Scholar] [CrossRef]
- Alhassan, A.R.M.; Yang, C.; Ma, W.; Li, G. Influence of conservation tillage on Greenhouse gas fluxes and crop productivity in spring-wheat agroecosystems on the Loess Plateau of China. PeerJ 2021, 9, e11064. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, Z.; Mou, H.; Li, J.; Zhang, P.; Jia, Z. Impact of farmland mulching practices on the soil bacterial community structure in the semiarid area of the loess plateau in China. Eur. J. Soil Biol. 2019, 92, 8–15. [Google Scholar] [CrossRef]
- Ma, W.; Alhassan, A.R.M.; Wang, Y.; Li, G.; Wang, H.; Zhao, J. Greenhouse gas emissions as influenced by wetland vegetation degradation along a moisture gradient on the eastern Qinghai-Tibet Plateau of North-West China. Nutr. Cycl. Agroecosystems 2018, 112, 335–354. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G. Effects of nitrogen deposition on N2O emission in a wet meadow on the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2023, 191, 105049. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Wu, J.; Gong, Y.; Wei, X.; Lu, Y. Responses of CH4 flux and microbial diversity to changes in rainfall amount and frequencies in a wet meadow in the Tibetan Plateau. Catena 2021, 202, 105253. [Google Scholar] [CrossRef]
- Guan, Y.S. Soil Enzyme and Research Method 309-313; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analyses, Part 2. Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomly, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Harpole, W.S.; Potts, D.L.; Suding, K.N. Ecosystem responses to water and nitrogen amendment in a California grassland. Glob. Change Biol. 2007, 13, 2341–2348. [Google Scholar] [CrossRef]
- Lu, X.; Hou, E.; Guo, J.; Gilliam, F.S.; Li, J.; Tang, S.; Kuang, Y. Nitrogen addition stimulates soil aggregation and enhances carbon storage in terrestrial ecosystems of China: A meta-analysis. Glob. Change Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.I.; Wang, J.; Li, P.; Pathan, S.I.; Sial, T.A.; Datta, R.; Abdelrahman, H. Variations of soil organic carbon fractions in response to conservative vegetation successions on the Loess Plateau of China. Int. Soil Water Conserv. Res. 2023, 11, 561–571. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Li, Y.; Kong, F.; Xi, M. Inorganic carbon sequestration and its mechanism of coastal saline-alkali wetlands in Jiaozhou Bay, China. Geoderma 2019, 351, 221–234. [Google Scholar] [CrossRef]
- Wong, V.N.; Greene, R.S.B.; Dalal, R.C.; Murphy, B.W. Soil carbon dynamics in saline and sodic soils: A review. Soil Use Manag. 2010, 26, 2–11. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Weligama, C.; Tang, C.; Sale, P.W.G.; Conyers, M.K.; Liu, D.L. Application of nitrogen in NO3− form increases rhizosphere alkalisation in the subsurface soil layers in an acid soil. Plant Soil 2010, 333, 403–416. [Google Scholar] [CrossRef]
- Fujii, K.; Funakawa, S.; Hayakawa, C.; Kosaki, T. Contribution of different proton sources to pedogenetic soil acidification in forested ecosystems in Japan. Geoderma 2008, 144, 478–490. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes: A meta-analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Weng, B.; Xie, X.; Yang, J.; Liu, J.; Lu, H.; Yan, C. Research on the nitrogen cycle in rhizosphere of Kandelia obovata under ammonium and nitrate addition. Mar. Pollut. Bull. 2013, 76, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Gao, Y.; Chen, Q.; Li, Z.; Gao, F.; Meng, Q.; Zhang, M. Blended controlled-release nitrogen fertilizer with straw returning improved soil nitrogen availability, soil microbial community, and root morphology of wheat. Soil Tillage Res. 2021, 212, 105045. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.; Wang, Y.; Ma, Y.; Men, M.; Zheng, Y.; Noulas, C. Response of soil N2O emission and nitrogen utilization to organic matter in the wheat and maize rotation system. Sci. Rep. 2021, 11, 4396. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, J.; Wang, Z.; Chen, L.; Zheng, J. Nitrous oxide and methane emissions from a Chinese wheat–rice cropping system under different tillage practices during the wheat-growing season. Soil Tillage Res. 2015, 146, 261–269. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, Y.; Liu, W. High nitrogen application rate and planting density reduce wheat grain yield by reducing filling rate of inferior grain in middle spikelets. Crop J. 2021, 9, 412–426. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; Zhou, J.; Xu, X.; Zhu, Y. Long-term straw mulching with nitrogen fertilization increases nutrient and microbial determinants of soil quality in a maize–wheat rotation on China’s Loess Plateau. Sci. Total Environ. 2021, 775, 145930. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, H.; Dang, T.; Wu, J.; Liu, W.; Hao, M.; Syers, J.K. Winter wheat grain yield associated with precipitation distribution under long-term nitrogen fertilization in the semiarid Loess Plateau in China. Geoderma 2012, 189, 442–450. [Google Scholar] [CrossRef]
- Kyveryga, P.M.; Blackmer, A.M.; Ellsworth, J.W.; Isla, R. Soil pH effects on nitrification of fall-applied anhydrous ammonia. Soil Sci. Soc. Am. J. 2004, 68, 545–551. [Google Scholar]
- Šimek, M.; Jíšová, L.; Hopkins, D.W. What is the so-called optimum pH for denitrification in soil? Soil Biol. Biochem. 2002, 34, 1227–1234. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Lu, X.; Xu, H.; Chen, D.; Li, Y.; Yakov, K. Long-term phosphorus addition alleviates CO2 and N2O emissions via altering soil microbial functions in secondary rather primary tropical forests. Environ. Pollut. 2023, 323, 121295. [Google Scholar] [CrossRef]
- Abdo, A.I.; Xu, Y.; Shi, D.; Li, J.; Li, H.; El-Sappah, A.H.; Kuzyakov, Y. Nitrogen transformation genes and ammonia emission from soil under biochar and urease inhibitor application. Soil Tillage Res. 2022, 223, 105491. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, J.; Liu, G.; Liu, W.; Ma, L. Differential responses of temperature sensitivity of greenhouse gases emission to seasonal variations in plateau riparian zones. Environ. Pollut. 2024, 353, 124190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, P.; Liu, Y.; Du, W.; Jing, H.; Nie, C. Soil properties and microbial abundance explain variations in N2O fluxes from temperate steppe soil treated with nitrogen and water in Inner Mongolia, China. Appl. Soil Ecol. 2021, 165, 103984. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, B.; Dong, Z.; Liu, Y.; Wang, C.; Ye, C. Soil N2O and NOx emissions are directly linked with N-cycling enzymatic activities. Appl. Soil Ecol. 2019, 139, 15–24. [Google Scholar] [CrossRef]
- Liao, J.; Luo, Q.; Hu, A.; Wan, W.; Tian, D.; Ma, J.; Lu, S. Soil moisture—Atmosphere feedback dominates land N2O nitrification emissions and denitrification reduction. Glob. Change Biol. 2022, 28, 6404–6418. [Google Scholar] [CrossRef]
- Yu, C.; Liu, M.; Song, M.; Xu, X.; Zong, N.; Zhu, J.; Shi, P. Nitrogen enrichment enhances the competition for nitrogen uptake between Stipa purpurea and microorganisms in a tibetan alpine steppe. Plant Soil 2023, 488, 503–516. [Google Scholar] [CrossRef]
- Jones, D.L.; Magthab, E.A.; Gleeson, D.B.; Hill, P.W.; Sánchez-Rodríguez, A.R.; Roberts, P.; Murphy, D.V. Microbial competition for nitrogen and carbon is as intense in the subsoil as in the topsoil. Soil Biol. Biochem. 2018, 117, 72–82. [Google Scholar] [CrossRef]
- Jones, D.L.; Clode, P.L.; Kilburn, M.R.; Stockdale, E.A.; Murphy, D.V. Competition between plant and bacterial cells at the microscale regulates the dynamics of nitrogen acquisition in wheat (Triticum aestivum). New Phytol. 2013, 200, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Duan, M.; Wang, R.; Li, J.; Wei, F.; Chen, J.; Wang, G. Planted forests intensified soil microbial metabolic nitrogen and phosphorus limitation on the Loess Plateau, China. Catena 2022, 211, 105982. [Google Scholar] [CrossRef]
- Geng, F.; Li, K.; Liu, X.; Gong, Y.; Yue, P.; Li, Y.; Han, W. Long-term effects of N deposition on N2O emission in an alpine grassland of Central Asia. Catena 2019, 182, 104100. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Zhao, S.; He, S.; Yang, B.; Wang, N.; Yang, L. Heavy rainfall stimulates more N2O emissions from wheat fields during basal and overwintering fertilization phases. Agric. Ecosyst. Environ. 2024, 376, 109227. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.F.; Zhang, F.S.; Du, F.; Li, L.K. Chemical fertilizer use and efficiency change of main grain crops in China. Plant Nutr. Fertil. Sci. 2010, 16, 1136–1143. [Google Scholar]
- Zhang, Y.; Wang, H.; Lei, Q.; Luo, J.; Lindsey, S.; Zhang, J.; Liu, H. Optimizing the nitrogen application rate for maize and wheat based on yield and environment on the Northern China Plain. Sci. Total Environ. 2018, 618, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X.; Hussain, S.; Yang, L.; Gao, S.; Zhang, P.; Ren, X. Reduced nitrogen rate improves post-anthesis assimilates to grain and ameliorates grain-filling characteristics of winter wheat in dry land. Plant Soil 2024, 499, 91–112. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Vogt, J.; Le, T.B. Warming reduces the increase in N2O emission under nitrogen fertilization in a boreal peatland. Sci. Total Environ. 2019, 664, 72–78. [Google Scholar] [CrossRef]

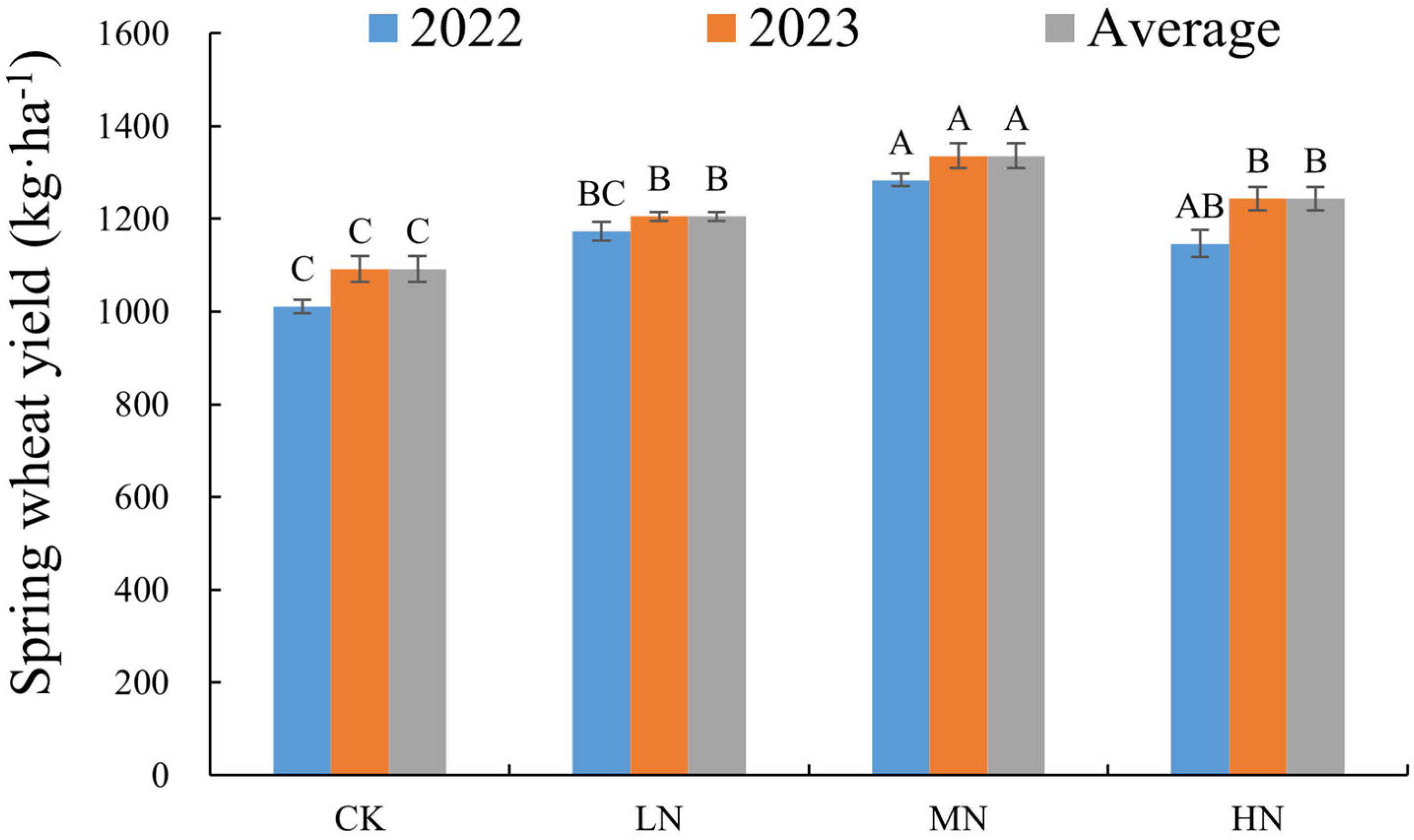
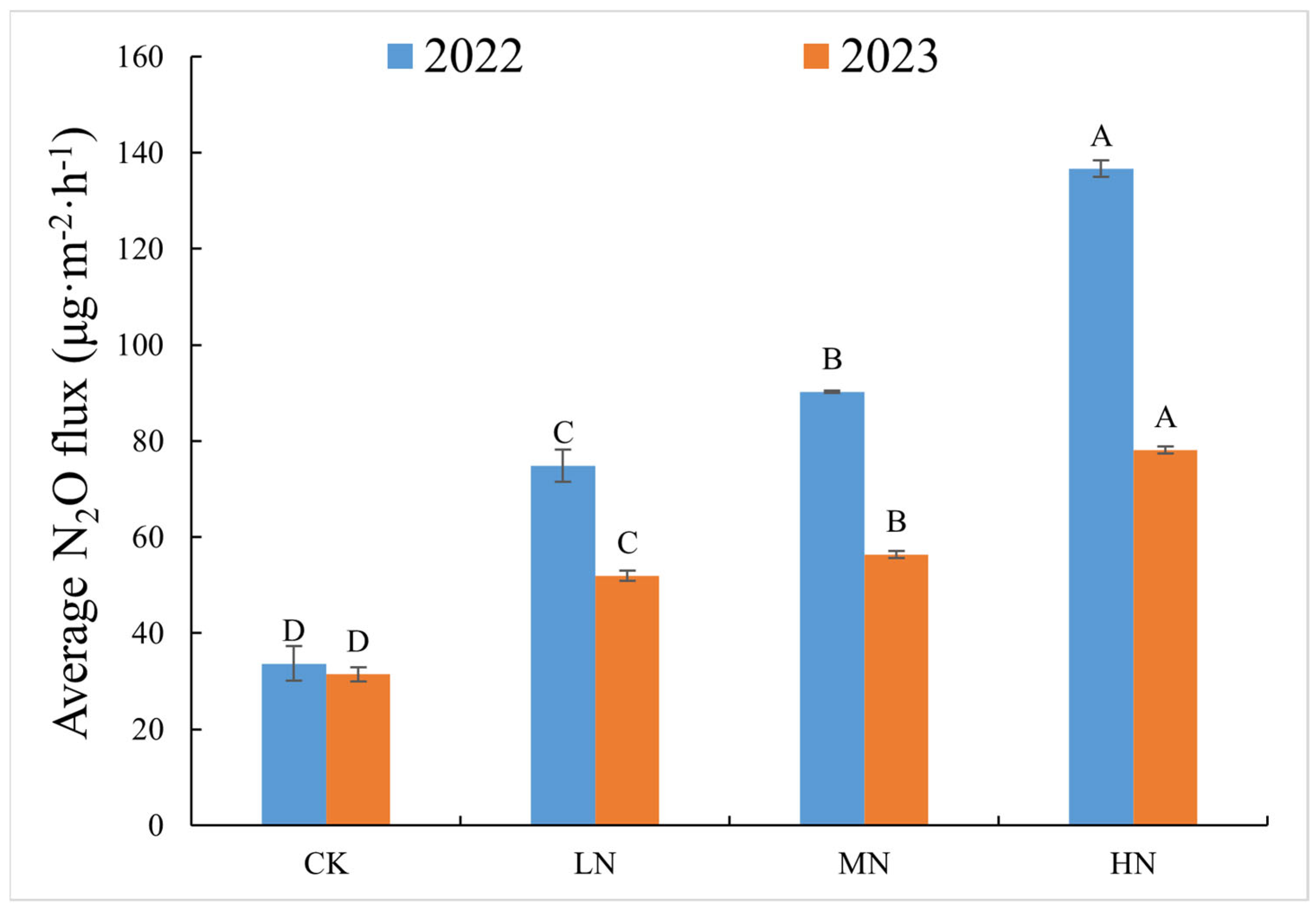
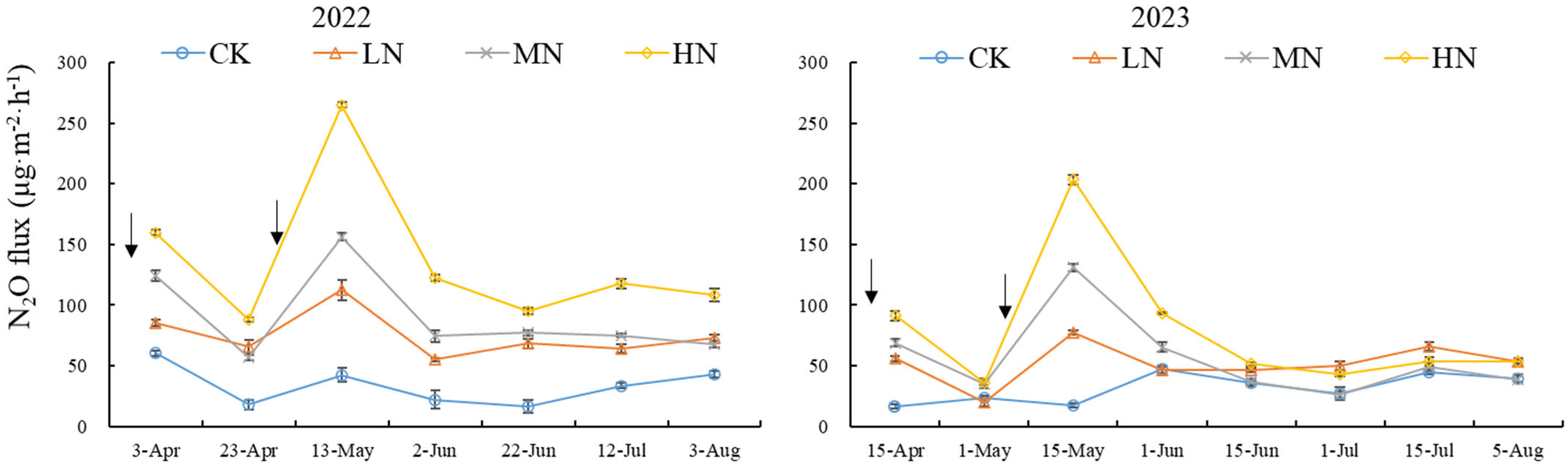
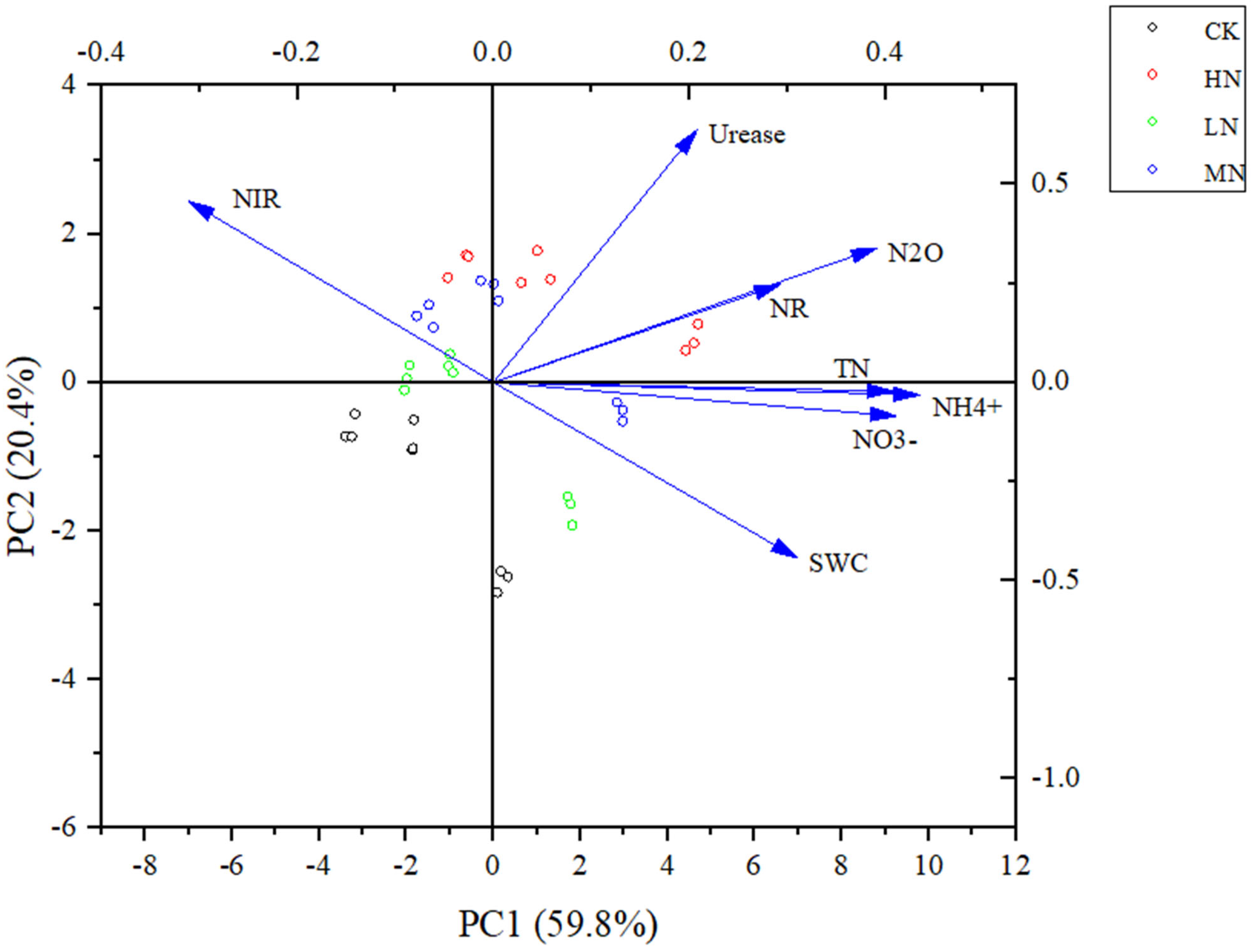
| Treament | SWC (%) | TN (g·kg−1) | NO3− (mg·kg−1) | NH4+ (mg·kg−1) | pH |
|---|---|---|---|---|---|
| CK | 10.71 ± 0.14 A | 0.50 ± 0.01 C | 28.80 ± 0.55 C | 17.38 ± 0.54 C | 7.88 ± 0.01 C |
| LN | 10.06 ± 0.09 B | 0.55 ± 0.00 B | 30.54 ± 0.52 BC | 20.48 ± 0.26 B | 7.99 ± 0.01 B |
| MN | 10.78 ± 0.05 A | 0.56 ± 0.00 B | 30.98 ± 0.55 B | 20.44 ± 0.15 B | 8.01 ± 0.02 B |
| HN | 10.23 ± 0.17 B | 0.59 ± 0.01 A | 35.78 ± 0.65 A | 23.26 ± 0.36 A | 8.10 ± 0.01 A |
| Treament | Season | Urease (mg·g−1) | Nitrate Reductase (mg·g−1) | Nitrite Reductase (mg·g−1) |
|---|---|---|---|---|
| CK | April | 1.55 ± 0.03 D | 7.13 ± 0.02 C | 0.52 ± 0.01 C |
| June | 1.72 ± 0.02 D | 7.52 ± 0.02 B | 0.66 ± 0.01 B | |
| August | 1.50 ± 0.03 D | 6.53 ± 0.09 C | 0.85 ± 0.02 C | |
| Average | 1.60 ± 0.01 D | 7.06 ± 0.05 B | 0.68 ± 0.01 C | |
| LN | April | 1.66 ± 0.02 C | 7.31 ± 0.03 C | 0.55 ± 0.03 B |
| June | 1.81 ± 0.02 C | 7.59 ± 0.03 B | 0.69 ± 0.01 B | |
| August | 1.64 ± 0.03 C | 6.83 ± 0.03 BC | 0.88 ± 0.01 C | |
| Average | 1.70 ± 0.01 C | 7.24 ± 0.03 B | 0.70 ± 0.02 BC | |
| MN | April | 2.04 ± 0.06 B | 7.60 ± 0.01 B | 0.56 ± 0.03 B |
| June | 2.25 ± 0.04 B | 8.03± 0.01 A | 0.70 ± 0.02 B | |
| August | 2.04 ± 0.02 B | 7.34 ± 0.01 A | 0.89 ± 0.02 B | |
| Average | 2.11 ± 0.02 B | 7.55 ± 0.01 A | 0.72 ± 0.01 B | |
| HN | April | 2.14 ± 0.04 A | 8.02 ± 0.17 A | 0.59 ± 0.01 A |
| June | 2.36 ± 0.03 A | 7.70 ± 0.14 B | 0.72 ± 0.01 A | |
| August | 2.15 ± 0.02 A | 6.94 ± 0.05 B | 0.91 ± 0.03 A | |
| Average | 2.21 ± 0.02 A | 7.66 ± 0.11 A | 0.74 ± 0.02 A |
| Source of Variation | 2022 | 2023 | ||||
|---|---|---|---|---|---|---|
| df | F | p | df | F | p | |
| T | 3 | 2472.550 | 0.000 | 3 | 988.924 | 0.000 |
| S | 6 | 748.057 | 0.000 | 7 | 779.576 | 0.000 |
| T × S | 18 | 118.629 | 0.000 | 21 | 250.322 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, J.; Li, G.; Yuan, J. Influence of Nitrogen Addition Levels on N2O Flux and Yield of Spring Wheat in the Loess Plateau. Agronomy 2025, 15, 1377. https://doi.org/10.3390/agronomy15061377
Wang H, Wu J, Li G, Yuan J. Influence of Nitrogen Addition Levels on N2O Flux and Yield of Spring Wheat in the Loess Plateau. Agronomy. 2025; 15(6):1377. https://doi.org/10.3390/agronomy15061377
Chicago/Turabian StyleWang, Haiyan, Jiangqi Wu, Guang Li, and Jianyu Yuan. 2025. "Influence of Nitrogen Addition Levels on N2O Flux and Yield of Spring Wheat in the Loess Plateau" Agronomy 15, no. 6: 1377. https://doi.org/10.3390/agronomy15061377
APA StyleWang, H., Wu, J., Li, G., & Yuan, J. (2025). Influence of Nitrogen Addition Levels on N2O Flux and Yield of Spring Wheat in the Loess Plateau. Agronomy, 15(6), 1377. https://doi.org/10.3390/agronomy15061377





