Abstract
Agriculture is presently facing several ecological concerns related to the upsurging request for premium-value food produced in compliance with natural horticultural tools. The use of natural substances, such as biostimulants, principally protein hydrolysates (PHs), could be useful to maximize overall vegetable plant fitness. However, the mode of application (foliar spray or fertigation) could affect biostimulant efficiency. The current research was conducted to evaluate the effect of a Zea mays-derived PH (Surnan®, SPAA, Pescara, Italy) and its mode of application (foliar spray and/or fertigation) on yield traits, mineral profile, nutritional and functional components, along with NUE of “Florida fortuna” strawberry cultivated under tunnel. The findings showed that the corn-based PH effectively enhanced yield and number of marketable fruits per plant (NMFP) compared with the control (+20.1% and +25.4%, respectively). Fruits from biostimulated plants also showed a higher fruit lightness and ascorbic acid and anthocyanin concentration than fruits from control plots. Furthermore, Surnan® PH increased nitrogen use efficiency (NUE) of strawberry plants. Captivatingly, plants biostimulated via fertigation showed the highest fruit potassium (K) concentration, while those exposed to the foliar spray had the highest fruit phenolic concentration. Generally, our findings recommended that the application of Zea mays-derived PH via foliar spray could be considered a suitable tool to increase functional traits of strawberry grown under tunnel.
1. Introduction
Strawberry (Fragaria × ananassa Duch.) represents an imperative fruit crop cultivated in the north hemisphere [1]. Strawberry fruits enclose functional compounds (e.g., anthocyanins, folate, phenols, ascorbic acid, vitamin E, etc.) deeply connected with healing properties [2]. The strawberry’s global production is over 10,485,454 tons [3]. At the global level, China is the leading producer, followed by the USA and Egypt, while Spain and the Russian Federation are the principal strawberry producers in Europe [3]. Sicily is the primary region in Italy for early strawberry cultivation under protected environments [4], from September to May [5]. However, strawberry cultivation practices under protected structures, such as tunnels and greenhouses, have an elevated ecological collision due to the long productive cycle (8 months) and the noteworthy amounts of plastic materials, nutrients, and pesticides employed [6].
Over the last few years, there has been an encouraging effort in creating and examining innovative agronomic means leading toward vegetable production systems with reduced ecological impact [7,8,9,10]. In this setting, the use of vegetal-derived biostimulants is a technology with a novel and sustainable application potentiality [11]. Manifold studies [12,13,14,15] observed that the use of plant-derived biostimulants may boost crop production and quality of fruiting and leafy green vegetables, even when plants are subjected to abiotic stresses. In this scenario, protein hydrolysates (PHs) are a suggested group of vegetal-derived biostimulants, suitable also for organic farm management [16]. PHs can be supplied through fertigation and/or foliar spray. When applied via foliar spray, PHs are soaked up by the cuticle, epidermal cells, and stomata, and finally attain the foliar mesophyll [17]. There are reports [18,19,20] revealing that PHs provoke crop fitness increment due to hormone-like activities, as well as enhancement of mineral absorption and translocation [21,22]. Since (i) the supply of vegetal-derived PH is a workable strategy for upsurging production and quality of vegetables, (ii) there are conflicting results on the best practices regarding the biostimulant application mode, and (iii) there are no studies regarding the interactive influences between Zea mays-derived PH supply and biostimulant application modes in strawberries, detailed investigations are required. On this basis, we examined the impact of a corn-derived PH biostimulant (SURNAN®) supplied either via foliar spray and/or fertigation on Fragaria × ananassa cv. “Florida fortuna” overall plant fitness. The treatment’s effects were investigated at the agronomical and qualitative levels, focusing on crop yield and fruit nutritional and functional features.
2. Materials and Methods
2.1. Trial Field and Vegetal Material
The experiment was carried out through the fall-spring seasons (2023–2024) in an experimental field of the SAAF Department, located in Marsala, Trapani, Italy (37°44′53″ N; 12°32′41″ E). Before transplanting, the irrigation system was installed and the soil (Sicilian fertile land known as “sciare”) was mulched with a polyethylene film (0.05 mm). Strawberry plants (Fragaria × ananassa “Florida fortuna”) were displaced at a density of 8 plants m−2 in polyethylene multi-tunnels. During the growth cycle, strawberry plants were fertigated (200 kg N ha−1, 150 kg P2O5 ha−1, 300 kg K2O ha−1, and 60 kg iron chelate ha−1). The doses were estimated contemplating the crop need, the predicted yield, and the soil nutrient content. All farming procedures for strawberries grown in Mediterranean climatic conditions (protected cultivation) were followed [23].
2.2. Biostimulant Treatments
Treatments via protein hydrolysate (PHs) supply were performed using Surnan® (SPAA®, Pescara, Italy), a non-microbial biostimulant composed of 2.5% w/w total nitrogen, 8.3% w/w free amino acids (Table S1), and a number of sterols exceeding 900 ppm, obtained from corn gluten protein. Protein hydrolysate treatments started after the first flower emission phase. Two different doses of PH, 0 (control) or 1.5 mL L−1, were supplied via foliar spray, fertigation, or in a combined mode (foliar spray + fertigation). The biostimulant was administered 3 times, at flowering, fruit growth and fruit maturation stages. Control plants received only water.
2.3. Experimental Design and Statistics
The biostimulant application [0 mL L−1 (control) or 1.5 mL L−1] was combined with the 3 application modes (foliar, fertigation, or foliar + fertigation) in a two-factorial experiment, resulting in 6 treatments. For each one, 90 plants were enclosed, leading to a total of 540 plants structured in a randomized complete block design (RCBD). The impacts of biostimulant and the application mode were estimated via a two-way Analysis of variance (ANOVA), setting application mode and biostimulant as the fixed factors. Mean differences were analyzed via Tukey’s test (p < 0.05).
Principal components analysis (PCA) was accomplished to assess the main association between the different application modes and PH supply, verified on the dependent variables evaluated. Principal components (PCs) with eigenvalues superior to 1.0 were selected. Variables were reported in a biplot associated with the decreased number of PCs, and interrelated variables were distinguished. Statistical analyses were run through SPSS software version 21.0 (StatSoft, Inc., Chicago, IL, USA).
2.4. Yield, Nutritional and Functional Traits, Mineral Profile and NUE
Immediately after harvest, yield features were logged on all plants. Fruits were harvested at the full ripening stage (full red color of fruits). Yield was separated into marketable (fruits not suffering from any visible disease or deformity) and unmarketable and expressed as g plant−1. The number of marketable fruits per plant (NMFP) was also logged, and the mean fruit weight of marketable fruits (FWMF) was evaluated and presented as g.
Fruits from the third to the sixth harvest were casually collected and used to perform analytical determination on nutritional and functional features.
To determine the fruit lightness (L*) fruits were determined in the central area of the fruit using a colorimeter (Chroma-meter CR-400, Minolta Corporation, Ltd., Osaka, Japan).
The dry matter percentage (%) was established by drying out 90 g of fresh sample in a ventilated oven (105 °C for 72 h).
To determine fruit firmness, a digital penetrometer was used to test its compressive strength (Trsnc digital penetrometer, Forlì, Italy). The measurement was carried out by using a 3 mm diameter stainless steel cylindrical probe. The fruit compactness values were expressed as Newton (N).
Soluble solids content (CSS) was assessed via a digital refractometer (MTD-045nD, Three-In-One Enterprises Co., Ltd., New Taipei, Taiwan) and expressed as °Brix.
The fruit’s ascorbic acid content was recorded with a refractometer via Ascorbic Acid Test Strip. Briefly, 5 g of fruit pulp was squeezed, and a drop of the filtered juice was applied to the ascorbic acid test strip for the determination; the measurement was repeated twice per sample. The outcomes were shown as mg 100 g−1 fw.
Total polyphenols were estimated as defined by Doumett et al. [24]. Briefly, the determination was accomplished via a spectrophotometric method, using catechin as a reference standard and employing an absorbance of 740 nm. The results were stated as mg of (+)-catechin per 100 g of fresh berries.
The anthocyanin concentration was appraised via the procedure of Rabino and Mancinelli [25]. The pigments were measured by mixing the sample with acidic methanol for 48 h at 4 °C. The mixture was then filtered, and the absorbance was measured at 530 and 657 nm. The formula was employed at the end to assess the results. The outcomes were expressed as mg of Cya-3-glucoside equivalent per 100 g−1 fw.
The fruit nitrogen content (N) was evaluated via the Kjeldahl process. Protein content was estimated by multiplying N concentration by 6.25 and expressed as g 100 g−1 dw. Phosphorus (P) content was determined calorimetrically, as described by Fogg and Wilkinson [26], whereas the potassium (K) and magnesium (Mg) contents of the strawberry fruit were assessed following the method described by Vultaggio et al. [7]. The mineral concentration values were reported as mg 100 g−1 dw.
Nitrogen use efficiency (NUE) was assessed as the ratio of the yield to the application rate of nitrogen, and it is reported as t kg−1.
3. Results
3.1. Crop Productivity and Fruit Quality
For yield traits, ANOVA did not show a significant impact of the B×T interaction (Table 1).

Table 1.
Impact of biostimulant and application mode on yield, number of marketable fruits per plants (NMFP) and mean weight of marketable fruits (MWMF) of strawberry plants.
Not considering the application mode, plants subjected to corn-derived PH had higher marketable yields than control plants, whereas, irrespective of the biostimulant, the application mode did not affect marketable yield. As regards NMFP, ANOVA and means separation revealed that biostimulated plants had higher values compared with plants grown in control plots (Table 1). Averaged over biostimulant, plants subjected to Fol+Fert treatment revealed the highest NMFP. The lowest NMFP was observed in plants exposed to foliar or fertigation treatments (Table 1). Regarding the application mode, control plants had higher MWMF than biostimulated plants. Plants biostimulated via foliar spray or Fol+Fert treatments showed the highest MWMF, while the fertigated plants (Table 1) had the lowest ones.
For SSC, ANOVA did not expose a significant influence of the interaction between the main factors (Figure 1A).
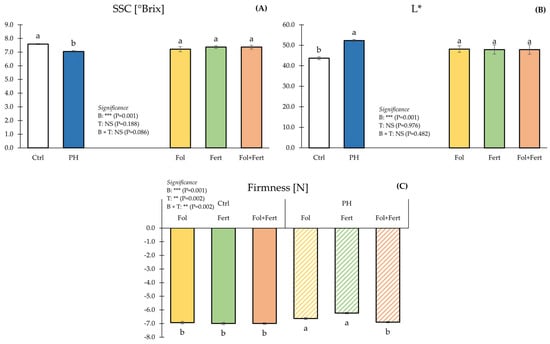
Figure 1.
Impact of biostimulant (B) and application mode (T) on soluble solids content (A), brightness (B) and firmness (C) of strawberry fruits. Data with the same letter are statistically similar agreeing to Tukey test. Results are presented as mean ± SE. Ctrl: untreated; PH: treated with 1.5 mL/L of corn-derived protein hydrolysate; Fol: foliar; Fert: fertigation.
Independently of the application mode, fruit control plants had higher SSC than those from biostimulated plants. Contrariwise, the application mode did not significantly affect SSC (Figure 1A). ANOVA for the L* color coordinate did not highlight a significant impact of the B×T interaction (Figure 1B). Fruits from plants exposed to corn-derived PH showed higher L* values than those from control plots. Inversely, the application mode did not significantly influence the aforesaid parameters (Figure 1B). Combining B and T significantly affected fruit firmness (Figure 1C). Fruits from control plots and those from the PH × Fol+Fert combination had the highest fruit firmness, whereas fruit from the PH × Fol and PH × Fert combinations revealed the lowest firmness (Figure 1C).
3.2. Fuit Dry Matter, Mineral Profile and NUE
For ascorbic acid, ANOVA did not highlight a meaningful effect of the interaction between the fixed factors (Figure 2A).
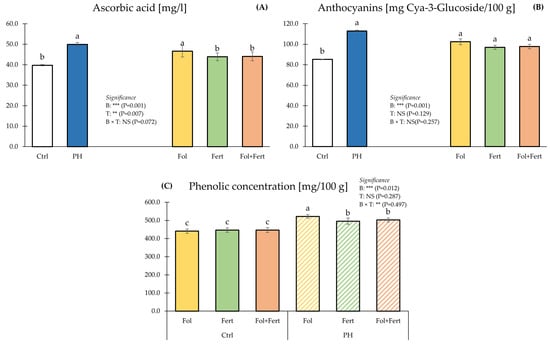
Figure 2.
Impact of biostimulant (B) and application mode (T) on ascorbic acid (A), anthocyanins (B) and phenolic concentration (C) of strawberry fruits. Data with the same letter are statistically similar agreeing to Tukey test. Results are presented as mean ± SE. Ctrl: untreated; PH: treated with 1.5 mL/L of corn-derived protein hydrolysate; Fol: foliar; Fert: fertigation.
Averaged over application modes, fruits from biostimulated plants had a higher ascorbic acid content than those from untreated plots. Irrespective of the biostimulants, plants biostimulated through foliar spray displayed the highest ascorbic acid concentration, while plants biostimulated by the Fert or Fol+Fert mode of application had the lowest fruit ascorbic acid concentration. The ANOVA did not detect a significant B×T interaction (Figure 2B). Strawberry fruits from plants exposed to Zea mays-based PH had a higher anthocyanin content than those from control plants. Contrariwise, the application mode did not significantly impact fruit anthocyanin concentration (Figure 2B). For phenolic concentration, ANOVA showed a significant effect of the interaction between biostimulant and application mode (Figure 2C). Strawberry plants biostimulated via foliar spray had the highest phenolic concentrations, followed by those biostimulated via fertigation or in a combined mode (Fol+Fert). The lowest phenolic concentrations were detected in fruit from control plants (CTR × Fol, CTR × Fert, CTR × Fol+Fert) (Figure 2C).
Combining B and T significantly affected fruit dry matter percentage (Figure 3A).
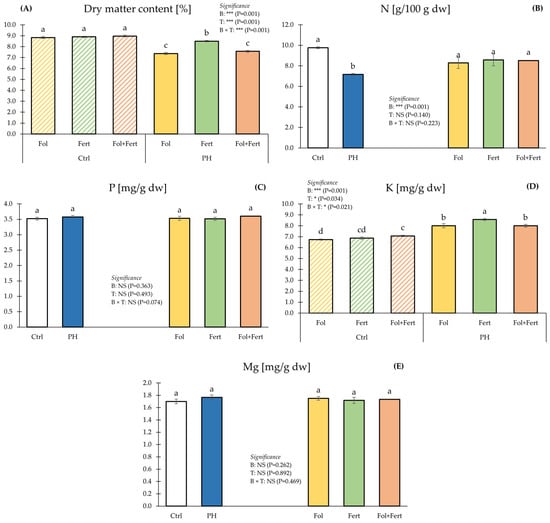
Figure 3.
Impact of biostimulant (B) and application mode (T) on dry matter content (A), nitrogen (B), phosphorous (C), potassium (D) and magnesium (E) of strawberry fruits. Data with the same letter are statistically similar agreeing to Tukey test. Results are presented as mean ± SE. Ctrl: untreated; PH: treated with 1.5 mL/L of corn-derived protein hydrolysate; Fol: foliar; Fert: fertigation.
Strawberry fruits from control plants had the highest dry matter percentage, whereas those from the PH × Fol and PH × Fol+Fert combinations revealed the lowest values. (Figure 3A). Combining B and T significantly did not affect fruit N content (Figure 3B). Averaged over application mode, fruits from control plots had a higher N concentration than those from PH-treated plots. However, the mode of application did not significantly affect N fruit concentration. (Figure 3B). As for fruit phosphorus (P) and magnesium (Mg) concentrations, statistical investigation did not display a significant effect of either the biostimulant or the application mode (Figure 3C,E). Strawberry fruits from plots biostimulated via fertigation had the highest K fruit concentration, followed by those biostimulated via foliar spray or in a combined mode (Fol+fert), which in turn revealed higher values than those from CTR × Fol plots (Figure 3D).
Statistical analysis for NUE did not show a significant effect of the B×T interaction (Figure 4).
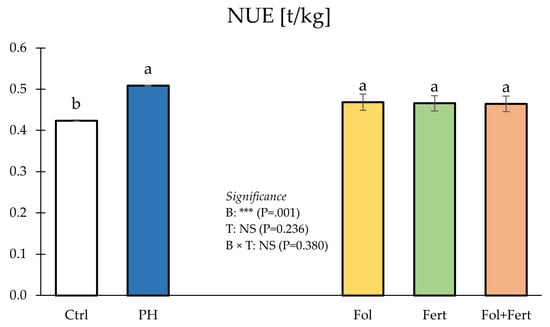
Figure 4.
Impact of biostimulant (B) and application mode (T) on nitrogen use efficiency (NUE) of strawberry plants. Data with the same letter are statistically similar agreeing to Tukey test. Results are presented as mean ± SE. Ctrl: untreated; PH: treated with 1.5 mL/L of corn-derived protein hydrolysate; Fol: foliar; Fert: fertigation.
Irrespective of the application mode (foliar or fertigation or both), Zea mays-derived PH increased NUE compared to the control, whereas, notwithstanding the biostimulant, the application mode did not significantly affect NUE (Figure 4).
3.3. Principal Component Analysis (PCA)
The principal component analysis (PCA) pointed out that the first (PC1) and the second (PC2) components represented 91.7% of the total variance (Figure 5).
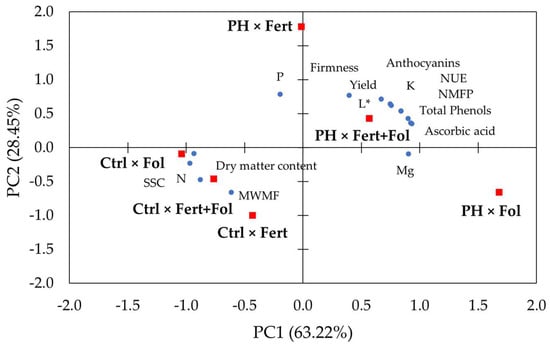
Figure 5.
Loading plot of the principal component analysis (PCA) for strawberry quanti-qualitative features as modulated by biostimulant and application mode. Ctrl: untreated; PH: treated with 1.5 mL/L of corn-derived protein hydrolysate; Fol: foliar; Fert: fertigation; NMFP: number of marketable fruits per plant; MWMF: mean weight of marketable fruits; NUE: nitrogen use efficiency; SSC: soluble solids content.
The PC1 and the PC2 illustrated 63.22% and 28.45% of the total variance, individually (Table 2).

Table 2.
PCA output related to the 2 PCs (PC1 and PC2).
The first component was largely positively associated to yield, NMFP, L*, ascorbic acid, total phenols, anthocyanins, Mg and NUE, and negatively interrelated to SSC, dry matter, and N; PC2 was primarily positively connected to yield, NMFP, L*, firmness, P, K and NUE, and negatively linked to MWMF (Table 2). The PH × Fol is situated in the bottom-right side of the loading plot, the Ctrl × Fol, Ctrl × Fert+Fol, Ctrl × Fert treatments are located in the bottom-left side, PH × Fert+Fol is positioned in the upper-right side, while PH × Fert treatment is sited in the upper-left side (Figure 5).
4. Discussion
The use of biostimulant products has become a popular agronomic practice to increase plant performance in different growing conditions. However, the effects of biostimulants depend on several factors. Among them, the application mode can have a crucial role. Consequently, specific studies on the effect of different application methods on plant performance are required. The aim of the current work was to evaluate the influences of a Zea mays-derived protein hydrolysate, applied via different methods (foliar spray, fertigation, or foliar spray+fertigation), on the quanti-qualitative response and NUE of strawberry plants grown in a tunnel. Results on productive features revealed that, independently of the application mode, biostimulant supply significantly enhanced yield and number of marketable fruits per plant and, concomitantly, reduced the mean weight of marketable fruits. These results are partially in accord with those of Vultaggio et al. [7], who described an increase in woodland strawberry yield and mean weight of marketable fruits when plants were treated with PH. Moreover, outcomes agree with those of Choi et al. [27] who, by studying the influences of PH application on the qualitative and quantitative performances of lettuce and tomato, found an increase in tomato yield and fruit number and an enhancement of shoot yield in lettuce when PH were applied via foliar spray or through the root system. PHs are rich in amino acids and peptides, which enhance plant growth and production [28]. This is ascribed to the fact that amino acids can be easily assimilated by plants and quickly used for protein biosynthesis [29]. Particularly, Surnan® PH contains isoleucine, proline, and valine (Table S1), which function in the increase in plant tolerance to stresses [30,31]. Moreover, corn gluten contains free nutrients [32] that can stimulate the metabolic processes of treated plants, favoring the growth of root systems and the formation of new cells [33], as well as the amino acid tryptophan (Table S1), a precursor of several components, such as auxins [34]. Notable is also the hormone-like activity that PH induces when applied to plants, which in turn contributes to an improvement in plant productive features [35]. Regarding the application mode, our findings revealed that the combined application (Fol+Fert) was the best option for enhancing the number and mean weight of marketable fruits, probably due to the optimization of biostimulant assimilation by plants via the root system and leaves.
Fruits soluble solids content was reduced by PH applications. These data agree with those of Vultaggio et al. [7], who reported a reduction in total soluble solids in plants supplied with PH compared to control plants. However, our outcomes did not agree with those of Colla et al. [36] who described an enhancement in tomato fruit TSS when plants were treated with PH compared to untreated plants. These contrasting results suggest that the modulation of SSC in plants via biostimulant applications may depend on plant species and family. However, we can also hypothesize that, in our research, the use of PH shifted the nutritive balance of plants, favoring the biosynthesis of protein rather than sugars and, consequently, enhancing yield and depressing the production of soluble sugars.
Fruit lightness was promoted by PH treatments. These outcomes are in contrast with those obtained by Rouphael et al. [37] and Carillo et al. [38] on spinach. These differences can be linked to the distinct species employed. In our research, we hypothesize that the positive effects of PH on strawberry fruit lightness might be related to the increase in plant water uptake [39], which in turn influenced fruit hydration, conferring a shinier surface compared with those poorly hydrated. In addition, there is evidence that PH application can influence the biosynthesis of cytokinin and auxin, which in turn regulate the growth and division of fruit epidermis cells [40,41], with positive effects on fruit lightness.
The study revealed a higher fruit firmness in biostimulated plants compared to the control. This outcome is in contrast with that of Soteriou et al. [42] on watermelon but consistent with that of Cardarelli et al. [43] on tomato. The fruit firmness increase reported in our study might be associated with the positive outcome of PH application on cell wall turgidity. In fact, the employment of PH can elicit the biosynthesis of polysaccharides, such as pectin and cellulose, which are fundamental components of cell walls [44]. Moreover, the amino acids comprised in PH are the starting point to produce proteins, enzymes, and other structural components involved in cell wall integrity and plant resistance to mechanical stress [45]. The beneficial effect of PH on firmness could also be linked to the enhanced water uptake efficiency of treated plants, which in turn influenced cell turgidity and fruit firmness [46]. As for the application mode, results underlined that the single application, foliar or via fertigation, is the best option to increase fruit firmness.
In our research, PH application reduced N fruit concentration and increased K values. Similarly, Sabatino et al. [47] observed that the use of a plant PH on lettuce plants significantly decreased N leaf concentration. As testified by Ertani et al. [18] and Malécange et al. [48], plant PH application can enhance nitrate reductase activity, which in turn decreases nitrogen compounds in vegetables. Furthermore, Colla et al. [22] reported that the amino acids contained in PH lead to an accumulation in phloem, which in turn decreases nitrate uptake by roots. Consequently, the decrease in N concentration can be linked to the plant behavior using its nitrogen reserve rather than uptaking it by the root system. As for K, the data overlap with those of Carillo et al. [38] and Rouphael et al. [37] on spinach, who reported significant enhancements of K concentration in plant tissues. These results can be explained via the different compounds comprised in PH. Indeed, according to Colla et al. [36], the presence of signaling molecules, like amino acids and peptides, has a positive effect on nutrient uptake and assimilation. There is also evidence that PH can modify the expression of genes implicated in nutrient transporters across cell membranes [49]. Furthermore, as highlighted by Lucini et al. [50], PH application stimulates the root system growth, increasing nutrient translocation and accumulation in plants. In this regard, our data revealed that fertigation was the optimal mode of application for increasing K concentration in strawberry fruits. Thus, we can speculate that the direct application of biostimulant to the root system had a better effect on root growth and nutrient uptake than the other application modes.
Biostimulant application reduced dry matter content. This decrease can be due to the increase in plant water uptake in biostimulated plants [51]. Captivatingly, among PH-treated plots, fruits from fertigated plants had the highest dry matter content. We can speculate that the higher accumulation of solutes confirmed the results concerning K concentration.
Ascorbic acid, anthocyanins, and phenolic compounds were increased by PH application. Data are in agreement with those of Rouphael et al. [37], who observed an increase in ascorbic acid and total phenols when spinach plants were treated with legume-derived PH. Furthermore, results are coherent with those of Vultaggio et al. [7] on woodland strawberry fruits. The enhancement of secondary metabolites, such as ascorbic acid, anthocyanins, and phenolic compounds, registered in PH-treated plants is connected to the elicitation of the enzymes implied in plant phytochemical homeostasis, to the amelioration of plant nutritional conditions, and to the general modulation of plant secondary metabolism [13,22]. PH also stimulates the biosynthesis of amino acid precursors of many secondary metabolite compounds, such as lysine and methionine [45]. Moreover, considering the aminogram reported in Table S1, Surnan® PH contains phenylalanine, an amino acid involved in the anthocyanin and flavonoid metabolism [52]. Remarkably, foliar application was the most effective in enhancing ascorbic acid and phenolic concentrations, probably due to better and quicker absorption of the biostimulant via the leaves rather than via the root system, as also reported by Fernández and Eichert [17].
In our research, NUE was significantly enhanced by PH supply. Data are consistent with those of Choi et al. [27] on tomato and lettuce. Additionally, outcomes agree with those of Sabatino et al. [47], who registered enhancement of lettuce NUE when plants were treated with PH. As stated by Kumari et al. [53], NUE is the yield obtained per unit of available nitrogen supplied by fertilizer and/or already present in the soil. This parameter is mainly dependent on genotype, environmental characteristics, and agronomic practices. In this regard, it is important to underline that PH can modulate plant NUE via the activation of genes involved in nitrogen uptake and assimilation [54] and, as already described for nitrogen concentration, via the modulation of enzymes like nitrate reductase [48].
5. Conclusions
This study displayed the significance of the interaction between a Zea mays-derived PH supply and different application modes on yield, nutritional and functional traits, and NUE of strawberry. Regardless of the application mode, corn-based PH enhanced plant productivity, fruit lightness, fruit K concentration, functional compounds, such as ascorbic acid and anthocyanins, as well as NUE. Fascinatingly, supplying biostimulant in a combined mode (Fol+Fert), NMFP and fruit firmness were enhanced. Corn-based PH foliar spray (Fol) application increased some fruit functional traits, such as ascorbic acid and polyphenols. Furthermore, when plants were exposed to Zea mays-derived PH via fertigation (Fert), fruit K concentration was increased. Overall, our results indicate that the Zea mays-derived PH is an efficient tool to enhance plant yield and nutritional and functional components in strawberry plants cultivated under tunnels. These findings denote an extended attempt to decrease the environmental effect of the agroecosystems, addressing the existing ecological concerns.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061314/s1, Table S1: Aminogram of Surnan® protein hydrolysate.
Author Contributions
Conceptualization, F.M., L.S. and B.B.C.; methodology, F.M., L.V., L.S. and B.B.C.; software, L.V., P.B., G.N. and E.A.; validation, L.S., G.N., S.L.B. and B.B.C.; formal analysis, F.M., L.V., L.S., P:B., G.N., E.A., G.G.L.P. and B.B.C.; investigation, F.M., L.V., L.S., P.B., E.A., G.G.L.P., S.L.B. and B.B.C.; resources, L.S.; data curation, F.M., L.V., L.S., P.B., G.N., E.A., G.G.L.P. and B.B.C.; writing—original draft preparation, F.M., L.V., L.S. and B.B.C.; writing—review and editing, F.M., L.S., G.N., E.A. and B.B.C.; visualization, L.S. and S.L.B.; supervision, L.S. and S.L.B.; project administration, L.S.; funding acquisition, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SPAA s.r.l., in the framework of the agreement between the SAAF Department—University of Palermo and SPAA s.r.l., grant number CON-0651.
Data Availability Statement
Data of this study are available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Biswas, M.K.; Islam, R.; Hossain, M. Micropropagation and field evaluation of strawberry in Bangladesh. J. Agric. Technol. 2008, 4, 167–182. [Google Scholar]
- Sarıdaş, M.A.; Ağ, E.; Akbaş, F.C.; Akyıldiz, A.; Kargı, S.P. Comparison of superior bred strawberry genotypes with popular cultivars in terms of fruit bioactive compounds during the wide harvest dates. S. Afr. J. Bot. 2022, 147, 142–152. [Google Scholar] [CrossRef]
- FAOSTAT Database. 2023. Available online: https://www.fao.org/faostat/en/#home (accessed on 24 March 2025).
- ISTAT. Available online: https://www.istat.it/ (accessed on 24 February 2025).
- Tesi, R. Colture Protette; Edagricole: Milan, Italy, 2008. [Google Scholar]
- Romero-Gámez, M.; Suárez-Rey, E.M. Environmental footprint of cultivating strawberry in Spain. Int. J. Life Cycle Assess. 2020, 25, 719–732. [Google Scholar] [CrossRef]
- Vultaggio, L.; Allevato, E.; Consentino, B.B.; Bellitto, P.; Napoli, S.; Cannata, C.; Ntatsi, G.; Vasto, S.; Baldassano, S.; La Bella, S.; et al. Joint Action of Trichoderma atroviride and a Vegetal Derived-Protein Hydrolysate Improves Performances of Woodland Strawberry in Italy. Horticulturae 2024, 10, 459. [Google Scholar] [CrossRef]
- Vultaggio, L.; Ciriello, M.; Campana, E.; Bellitto, P.; Consentino, B.B.; Rouphael, Y.; Colla, G.; Mancuso, F.; La Bella, S.; Napoli, S.; et al. Single or Blended Application of Non-Microbial Plant-Based Biostimulants and Trichoderma atroviride as a New Strategy to Enhance Greenhouse Cherry Tomato Performance. Plants 2024, 13, 3048. [Google Scholar] [CrossRef]
- Consentino, B.B.; Vultaggio, L.; Iacuzzi, N.; La Bella, S.; De Pasquale, C.; Rouphael, Y.; Ntatsi, G.; Virga, G.; Sabatino, L. Iodine Biofortification and Seaweed Extract-Based Biostimulant Supply Interactively Drive the Yield, Quality, and Functional Traits in Strawberry Fruits. Plants 2023, 12, 245. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Rotino, G.L.; Palazzolo, E.; Mennella, G.; D’Anna, F. Solanum aethiopicum gr. gilo and Its Interspecific Hybrid with S. melongena as Alternative Rootstocks for Eggplant: Effects on Vigor, Yield, and Fruit Physicochemical Properties of Cultivar ‘Scarlatti’. Agronomy 2019, 9, 223. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition; concept; main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Clément, J.; Delisle-Houde, M.; Nguyen, T.T.A.; Dorais, M.; Tweddell, R.J. Effect of Biostimulants on Leafy Vegetables (Baby Leaf Lettuce and Batavia Lettuce) Exposed to Abiotic or Biotic Stress under Two Different Growing Systems. Agronomy 2023, 13, 879. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.B.; Rouphael, Y.; Baldassano, S.; De Pasquale, C.; Ntatsi, G. Ecklonia maxima-derivate seaweed extract supply as mitigation strategy to alleviate drought stress in chicory plants. Sci. Hortic. 2023, 312, 111856. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activities of two protein hydrolysates on the growth and nitrogen metabolism in maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Kubo, M. Soybean peptide: Novel plant growth promoting peptide from soybean. In Soybean and Nutrition; El-Shemy, R.H., Ed.; Tech Europe Publisher: Giza, Egypt, 2011; pp. 215–230. [Google Scholar]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 96, 28–38. [Google Scholar] [CrossRef]
- Tesi, R. Orticoltura Mediterranea Sostenibile; Pàtron Editore: Bologna, Italy, 2010. [Google Scholar]
- Doumett, S.; Fibbi, D.; Cincinelli, A.; Giordani, E.; Nin, S.; Del Bubba, M. Comparison of Nutritional and Nutraceutical Properties in Cultivated Fruits of Fragaria vesca L. Produced in Italy. Food Res. Int. 2011, 44, 1209–1216. [Google Scholar] [CrossRef]
- Rabino, I.; Mancinelli, A.L. Light, Temperature, and Anthocyanin Production 1. Plant Physiol. 1986, 81, 922–924. [Google Scholar] [CrossRef]
- Fogg, D.N.; Wilkinson, A.N. The colorimetric determination of phosphorus. Analyst 1958, 83, 406–414. [Google Scholar] [CrossRef]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.J. Effects of plant-derived protein hydrolysates on yield, quality, and nitrogen use efficiency of greenhouse grown lettuce and tomato. Agronomy 2022, 12, 1018. [Google Scholar] [CrossRef]
- Martín, M.H.J.; Ángel, M.M.M.; Aarón, S.L.J.; Israel, B.G. Protein hydrolysates as biostimulants of plant growth and development. In Biostimulants: Exploring Sources and Applications; Springer Nature: Singapore, 2022; pp. 141–175. [Google Scholar]
- Heinemann, B.; Hildebrandt, T.M. The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J. Exp. Bot. 2021, 72, 4634–4645. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Joung, J.G.; Fei, Z.; Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef]
- Ji, Y.; Zuo, L.; Wang, F.; Li, D.; Lai, C. Nutritional value of 15 corn gluten meals for growing pigs: Chemical composition, energy content and amino acid digestibility. Arch. Anim. Nutr. 2012, 66, 283–302. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Zhang, Z. Regulation of metabolites by nutrients in plants. In Plant Ionomics: Sensing, Signaling, and Regulation; Wiley: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Tryptophan: A precursor of signaling molecules in higher plants. In Hormones and Plant Response. Plant in Challenging Environments; Springer: Cham, Switzerland, 2021; Volume 2, pp. 273–289. [Google Scholar]
- Pasković, I.; Popović, L.; Pongrac, P.; Polić Pasković, M.; Kos, T.; Jovanov, P.; Franić, M. Protein Hydrolysates—Production, Effects on Plant Metabolism, and Use in Agriculture. Horticulturae 2024, 10, 1041. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Agliassa, C.; Mannino, G.; Molino, D.; Cavalletto, S.; Contartese, V.; Bertea, C.M.; Secchi, F. A new protein hydrolysate-based biostimulant applied by fertigation promotes relief from drought stress in Capsicum annuum L. Plant Physiol. Biochem. 2021, 166, 1076–1086. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino acids biostimulants and protein hydrolysates in agricultural sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Gan, L.; Song, M.; Wang, X.; Yang, N.; Li, H.; Liu, X.; Li, Y. Cytokinins are involved in regulation of tomato pericarp thickness and fruit size. Hortic. Res. 2022, 9, uhab041. [Google Scholar] [CrossRef] [PubMed]
- Soteriou, G.A.; Rouphael, Y.; Emmanouilidou, M.G.; Antoniou, C.; Kyratzis, A.C.; Kyriacou, M.C. Biostimulatory Action of Vegetal Protein Hydrolysate and the Configuration of Fruit Physicochemical Characteristics in Grafted Watermelon. Horticulturae 2021, 7, 313. [Google Scholar] [CrossRef]
- Cardarelli, M.; Ceccarelli, A.V.; El Nakhel, C.; Rouphael, Y.; Salehi, H.; Ganugi, P.; Zhang, L.; Lucini, L.; Pii, Y.; Choi, S.; et al. Foliar applications of a Malvaceae-derived protein hydrolysate and its fractions differentially modulate yield and functional traits of tomato under optimal and suboptimal nitrogen application. J. Sci. Food Agric. 2024, 104, 7603–7616. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. [Google Scholar] [CrossRef]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Amino acids in plants: Regulation and functions in development and stress defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Concepts of Plant Water Relations. In Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2023; pp. 3–23. [Google Scholar]
- Sabatino, L.; Consentino, B.B.; Rouphael, Y.; De Pasquale, C.; Iapichino, G.; D’Anna, F.; La Bella, S. Protein hydrolysates and mo-biofortification interactively modulate plant performance and quality of ‘canasta’lettuce grown in a protected environment. Agronomy 2021, 11, 1023. [Google Scholar] [CrossRef]
- Malécange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant Properties of Protein Hydrolysates: Recent Advances and Future Challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen use efficiency definitions of today and tomorrow. Front. Plant Sci. 2021, 12, 637108. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, V.; Ovadia, R.; Oren-Shamir, M. Phenylalanine in motion: A tale of an essential molecule with many faces. Biotechnol. Adv. 2023, 68, 108246. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, M.; Campana, E.; De Pascale, S.; Rouphael, Y. Implications of Vegetal Protein Hydrolysates for Improving Nitrogen Use Efficiency in Leafy Vegetables. Horticulturae 2024, 10, 132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).