Testing of Saline–Alkali Tolerance in Oat Germplasm Through Integrated Germination and Field Performance Assessments
Abstract
1. Introduction
2. Materials and Methods
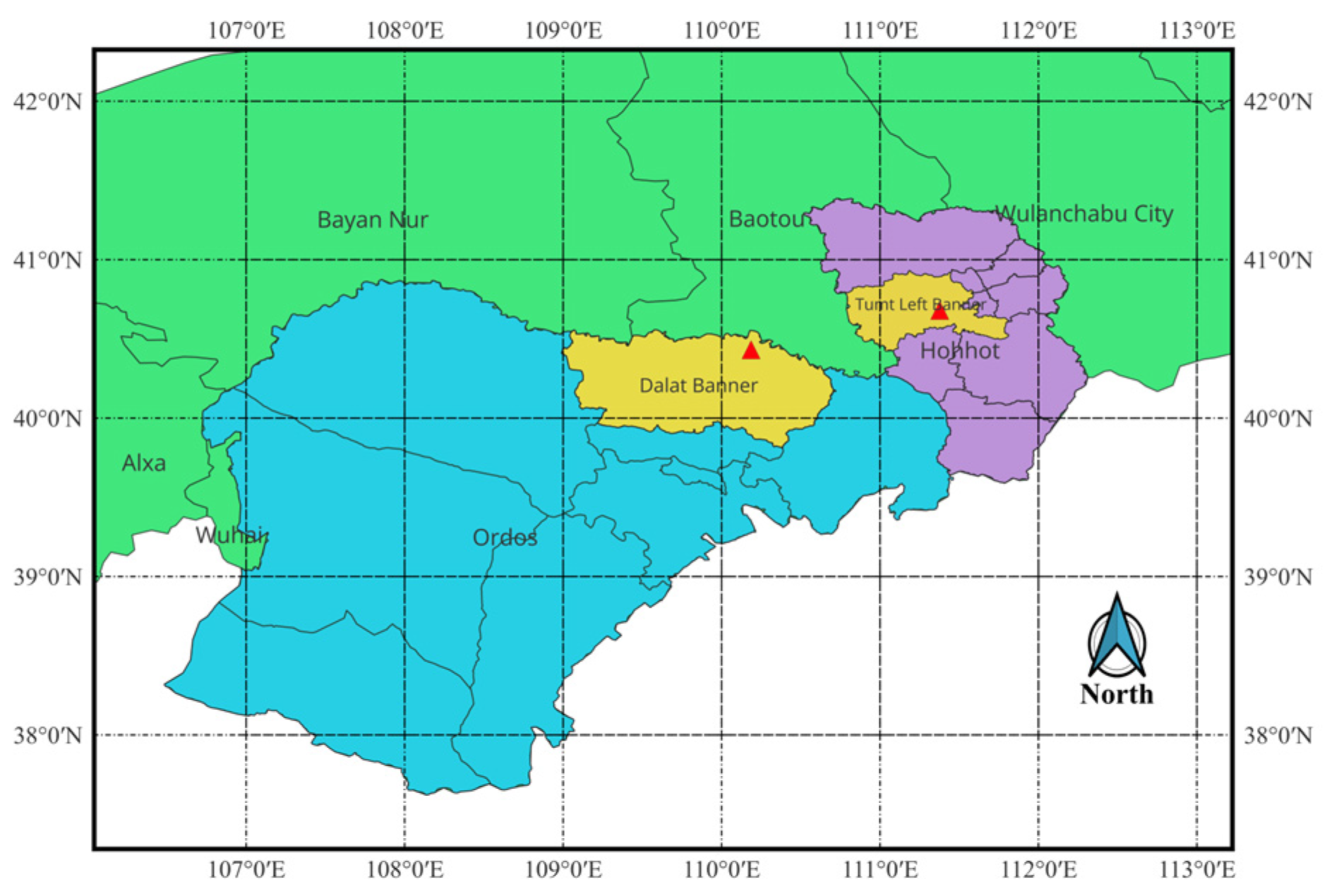
2.1. Experimental Materials
2.2. Experimental Design
2.2.1. Seed Germination and Saline–Alkali Tolerance Screening
2.2.2. Field Identification
2.3. Variables Evaluated
2.3.1. Indicators of Germination Period Measurement
- (a)
- Germination potential (%) = (Number of seeds germinated on day 4/Number of seeds tested for germination) × 100% [13].
- (b)
- Germination rate (%) = (Number of seeds germinated on day 7/Number of seeds tested for germination) × 100% [21].
- (c)
- Bud length refers to the distance from the root–bud junction to the top, with the top referring to the top of the main stem [11].
- (d)
- Root length refers to the distance from the root–bud junction to the bottom, where the bottom refers to the bottom of the main root [11].
2.3.2. Determination of Field Identification Indicators
- (a)
- When oats reached the standard for emergence, the field emergence count was obtained, and the emergence rate was calculated as follows:
- (b)
- Measurement of yield, yield components, and quality: After the oats matured, they were harvested and air–dried. Ten plants were selected from each plot for each germplasm resource. The following traits were measured: plant height, panicle length, number of spikelets, number of grains per panicle, and individual grain weight. Manual threshing was used, and seed and biological yields were measured on a per-plot basis. Ten grams of seed samples were weighed from each germplasm, dried, and ground before being passed through a 1 mm sieve. The samples were then sealed in self–sealing bags for storage and used for grain quality determination. Crude protein and crude fat were measured using the Kjeldahl and Soxhlet extraction methods, respectively. β-glucan was measured using a reagent kit from Shanghai Keshun Biotechnology Co., Ltd. (Shanghai, China).
2.4. Data Processing and Analysis
3. Results
3.1. Analysis of Trait Differences and Relative Values During the Germination Phase of Germplasm Resources
3.2. Principal Component Analysis of Relative Values of Various Traits During the Germination Phase of Germplasm Resources
3.3. Cluster Analysis of Saline–Alkali Tolerance in Germplasm Resources During Germination Phase
3.4. Regression Model Establishment
3.5. Performance of Different Traits in Oat Germplasm Resources from Various Saline–Alkali Soils
3.6. Correlation Analysis of Membership Function Values and D Value of Oat Germplasm Resources
3.7. Principal Component Analysis of Traits in Oat Germplasm Resources
3.8. Cluster Analysis and Comprehensive Evaluation of Oat Germplasm Resources from Different Saline–Alkali Soils
4. Discussion
4.1. Importance of Screening for Saline–Alkali Tolerance During Germination of Oat Germplasm Resources
4.2. Relationship Between Various Oat Germplasm Resources Traits and Saline–Alkali Tolerance Under Natural Conditions
4.3. Significance of Field Evaluation in Breeding Saline–Alkali Tolerant Oat Varieties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiv, P.Y.; Reshu, B.; Harsita, N.; Rakhi, M.; Rajesh, K.S.; Saroj, K.P. Impact of salt stress on growth, productivity and physicochemical properties of plants: A review. Int. J. Chem. Stud. 2019, 7, 1793–1798. [Google Scholar]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Yin, D.J.; Ye, S.J.; Sun, X.Y.; Chen, Q.Y.; Min, T.; Wang, H.X.; Wang, L.M. Integrative analysis of the transcriptome and metabolome reveals genes involved in phenylpropanoid and flavonoid biosynthesis in the trapa bispinosa roxb. Front. Plant Sci. 2022, 13, 913265. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y. How do plants maintain pH and ion homeostasis under saline-alkali stress? Front. Plant Sci. 2023, 14, 1217193. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.M.; Abdul, R.A.; Khalid, M. Rehabilitation of salina ecosystems through cultivation of salt tolerant plants. Pak. J. Bot. 2012, 44, 69–75. [Google Scholar]
- Zhang, B.; Wang, N. Study on the harm of saline alkali land and its improvement technology in China. IOP Conf. Ser. Earth Environ. Sci. 2021, 692, 42053–42058. [Google Scholar] [CrossRef]
- Yu, J.; Li, B.; Song, T.; He, J.; KongLing, Z.; Lian, L.; He, W.; Hai, T.; Huang, X.; Liu, Z.; et al. Integrated physiological and transcriptomic analyses responses to altitude stress in oat (Avena sativa L.). Front. Genet. 2021, 12, 638683. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Zhou, R.; Hu, E.; Peng, X.; Jiang, F.; Wu, Z. An optimized protocol for comprehensive evaluations of salt tolerance in crop germplasm accessions: A case study of Tomato (Solanum lycopersicum L.). Agronomy 2024, 14, 842. [Google Scholar] [CrossRef]
- Sreesaeng, J.; Qiu, C.-W.; Zhang, S.; Shi, S.-H.; Luo, L.; Holford, P.; Wu, F. Identification and characterization of hull-less barley (Hordeum vulgare L.) germplasms for salt tolerance. Plant Growth Regul. 2024, 104, 975–989. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Y.; Wang, Y.; Sun, L.; Bai, W.; Yan, T.; Xu, W.; Huang, R. Screening and identification of sorghum cultivars for alkali tolerance during germination. Sci. Agric. Sin. 2013, 46, 1762–1771. [Google Scholar] [CrossRef]
- Ahmed, S.; Patel, R.; Singhal, R.K.; Kumar, N.; Rana, M.; Chand, S.; Chandra, A.; Rengel, Z. Salinity, alkalinity and their combined stress effects on germination and seedling growth attributes in oats (Avena sativa). Crop Pasture Sci. 2023, 74, 1094–1102. [Google Scholar] [CrossRef]
- Güngör, H.; Çıkılı, Y.; Dumlupınar, Z. Screening of oat varieties and landraces at early vegetative stage under salt stress conditions: Morpho-physiological and PCA biplot analysis. Cereal Res. Commun. 2021, 49, 587–597. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Yan, L.; Zhang, Z.; Zhou, H.; Wu, B. Identification and evaluation of salt-alkali tolerance and screening of salt-alkali tolerant germplasm of oat (Avena sativa L.). Acta Agron. Sin. 2023, 49, 1551–1561. [Google Scholar] [CrossRef]
- Premkumar, R.; Nirmalakumari, A.; Anandakumar, C.R. Correlation studies for yield components in oats germplasm. Electron. J. Plant Breed. 2017, 8, 980–985. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, Y.; Guo, W.; Xue, Y.; Yu, L. Metabolic and physiological changes in the roots of two oat cultivars in response to complex saline-alkali stress. Front. Plant Sci. 2022, 13, 835414. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, Z.; Xu, Y.; Lu, H.; Ji, Z.; Zhou, G. Different types of fertilizers enhanced salt resistance of oat and associated physiological mechanisms in saline soils. Agronomy 2022, 12, 317. [Google Scholar] [CrossRef]
- Quamruzzaman, M.; Manik, S.M.N.; Livermore, M.; Johnson, P.; Zhou, M.; Shabala, S. Multidimensional screening and evaluation of morphophysiological indices for salinity stress tolerance in wheat. J. Agron. Crop Sci. 2022, 208, 454–471. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, X.; Liang, Y.; Dong, L.; Han, C.; Cao, Y.; Ma, W. Comprehensive evaluation of salt tolerance in asparagus germplasm accessions (Asparagus officinalis L.) at different growth stages. J. Food Qual. 2021, 2021, 6626112. [Google Scholar] [CrossRef]
- Wu, L.; Du, J.; Zhang, Y.; Xue, Y.; Jiang, C.; Lu, W.; Zheng, Y.; Zhou, C.; Xiong, A.; Li, M. Identification and evaluation of celery germplasm resources for salt tolerance. Agronomy 2024, 14, 1048. [Google Scholar] [CrossRef]
- Jamshidi, A.; Javanmard, H.R. Evaluation of barley (Hordeum vulgare L.) genotypes for salinity tolerance under field conditions using the stress indices. Ain Shams Eng. J. 2018, 9, 2093–2099. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Wu, B. Comprehensive evaluation of salt tolerance and screening for salt tolerant accessions of naked oat (Avena nuda L.) at germination stage. Sci. Agric. Sin. 2014, 47, 2038–2046. [Google Scholar] [CrossRef]
- Han, F.; Zhuge, Y.; Lou, Y.; Wang, H.; Zhang, N.; He, W.; Zhao, Y. Evaluation of salt tolerance and screening for salt tolerant accessions of 63 foxtail millet germplasm. J. Plant Genet. Resour. 2018, 19, 685–693. [Google Scholar] [CrossRef]
- Ahmed, S.; Patel, R.; Rana, M.; Kumar, N.; Choudhary, M.; Chand, S.; Singh, A.K.; Ghosh, A.; Singhal, R.K. Effect of salt, alkali and combined stresses on root system architecture and ion profiling in a diverse panel of oat (Avena spp.). Funct. Plant Biol. 2023, 51, FP23031. [Google Scholar] [CrossRef]
- Langridge, P.; Waugh, R. Harnessing the potential of germplasm collections. Nat. Genet. 2019, 51, 200–201. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Zhang, L.; Zhou, W. Drip irrigation in agricultural saline-alkali land controls soil salinity and improves crop yield: Evidence from a global meta-analysis. Sci. Total Environ. 2023, 880, 163226. [Google Scholar] [CrossRef]
- Abbasipour, B.H.; Ghazvini, H.; Amiri, B.; Bazrafshan, F.; Nikkhah, H. Responses of barley (Hordeum vulgare L.) genotypes to salinity stress under controlled and field conditions. Gesunde Pflanz. 2022, 75, 499–513. [Google Scholar] [CrossRef]
- Igartua, E. Choice of selection environment for improving crop yields in saline areas. Theor. Appl. Genet. 1995, 91, 1016–1021. [Google Scholar] [CrossRef]
- Song, Y.; Gao, M.; Li, Z. Impacts of straw return methods on crop yield, soil organic matter, and salinity in saline-alkali land in North China. Field Crops Res. 2025, 322, 109752. [Google Scholar] [CrossRef]
- Moustafa, E.S.A.; Ali, M.M.A.; Kamara, M.M.; Awad, M.F.; Hassanin, A.A.; Mansour, E. Field screening of wheat advanced lines for salinity tolerance. Agronomy 2021, 11, 281. [Google Scholar] [CrossRef]
- Ebrahim, F.; Arzani, A.; Rahimmalek, M.; Rezaei, S.; Cheghamirza, K. Yield related traits and tolerance indices to screen salinity tolerant genotypes in cultivated and wild barley. Gesunde Pflanz. 2022, 74, 1099–1108. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Yu, C.; Lin, J.; Liu, J.; Liu, B.; Wang, J.; Huang, A.; Li, H.; Zhao, T. Osmph1 regulates plant height and improves grain yield in rice. PLoS ONE 2017, 12, e0180825. [Google Scholar] [CrossRef] [PubMed]
- Sobczyński, G.; Studnicki, M.; Mądry, W.; Wijata, M.; Gozdowski, D.; Noras, K.; Samborski, S.; Rozbicki, J. Impact of cultivar and environment soil suitability on the contribution of yield components to grain yield variation in spring wheat. Crop Sci. 2020, 60, 428–440. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Mao, L.; Li, Y.; Shen, Y. Divergent response of hay and grain yield of oat: Effects of environmental factors and sowing rate. J. Sci. Food Agric. 2022, 103, 233–242. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Ma, B.L.; Ren, C.Z. Salinity effects on yield and yield components of contrasting naked oat genotypes. J. Plant Nutr. 2009, 32, 1619–1632. [Google Scholar] [CrossRef]
- Muhammad, A.U.; Imdad, A.M.; Muhammad, S.; Muhammad, R. Screening of oat (Avena sativa) varieties in saline-sodic soil. Am. J. Bio. Sci. Res. 2019, 4, 104–106. [Google Scholar] [CrossRef]
- Yu, R.; Wang, G.; Yu, X.; Li, L.; Li, C.; Song, Y.; Xu, Z.; Zhang, J.; Guan, C.; Luo, Z.B. Assessing alfalfa (Medicago sativa L.) tolerance to salinity at seedling stage and screening of the salinity tolerance traits. Plant Biol. 2021, 23, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yi, Z.; Xiao, L.; Sun, G.; Li, M.; Xue, S.; Peng, X.; Duan, M.; Chen, Z. The performance of miscanthus hybrids in saline-alkaline soil. Front. Plant Sci. 2022, 13, 921824. [Google Scholar] [CrossRef]
- Guo, Y.; Kuang, L.; Xu, Y.; Yan, T.; Jiang, L.; Dong, J.; Wu, D. Construction of a worldwide core collection of rapeseed and association analysis for waterlogging tolerance. Plant Growth Regul. 2022, 98, 321–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Gao, J.; Wang, Y.; Zhu, D. Comparative study on the nutritional quality of peanut in saline and non-saline land. Foods 2024, 13, 3751. [Google Scholar] [CrossRef]
- Zeki, M.; Hasan, A.; Özge, D.E.K. Grain yield, quality traits and grain yield stability of local oat cultivars. J. Soil Sci. Plant Nut. 2018, 18, 269–281. [Google Scholar] [CrossRef]
- Farooq, S.; Sahil; Mahajan, G.; Loura, D.; Raymont, K.; Chauhan, B.S. Influence of soil moisture levels on the growth and reproductive behaviour of avena fatua and avena ludoviciana. PLoS ONE 2020, 15, e0234648. [Google Scholar] [CrossRef]






| Experimental Field | pH | Salinity g/kg | OM Content g/kg | AN Content mg/kg | AP Content mg/kg | AK Content mg/kg | TA Content g/kg | TP Content g/kg | TK Content g/kg |
|---|---|---|---|---|---|---|---|---|---|
| Tumt Left Banner | 8.68 | 6.07 | 9.32 | 63.11 | 2.71 | 171.33 | 0.53 | 1.66 | 0.53 |
| Dalat Banner | 8.95 | 9.20 | 7.54 | 59.61 | 3.83 | 98.3 | 0.70 | 0.54 | 10.18 |
| Index | PC1 | PC2 |
|---|---|---|
| Relative germination potential | 0.424 | 0.822 |
| Relative germination rate | 0.516 | 0.126 |
| Relative bud length | 0.527 | −0.394 |
| Relative root length | 0.526 | −0.391 |
| Eigenvalue | 3.105 | 0.605 |
| Contributive ratio (%) | 77.63 | 15.12 |
| Cumulative contributive ratio (%) | 77.63 | 92.74 |
| Trait | Tumt Left Banner | Dalat Banner | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | CV (%) | Min | Max | Mean | SD | CV (%) | |
| Seeding ratio | 38.40 | 92.20 | 62.81 | 11.25 | 17.91 | 43.00 | 88.27 | 65.83 | 11.26 | 17.11 |
| Plant height | 32.16 | 78.67 | 54.91 | 8.96 | 16.32 | 45.56 | 98.85 | 65.67 | 10.70 | 16.30 |
| Ear length | 9.40 | 25.87 | 15.37 | 2.87 | 18.67 | 8.30 | 22.27 | 14.07 | 2.51 | 17.83 |
| Select number | 10.80 | 36.40 | 17.99 | 4.73 | 26.31 | 6.90 | 25.00 | 14.28 | 3.04 | 21.28 |
| Grain number of single plant | 10.30 | 51.40 | 28.26 | 8.20 | 29.02 | 13.00 | 52.10 | 26.42 | 8.83 | 33.40 |
| Grain weight per plant | 0.25 | 1.34 | 0.68 | 0.21 | 30.78 | 0.23 | 1.14 | 0.60 | 0.20 | 33.96 |
| Biological yield | 1667.50 | 11,405.70 | 5338.68 | 2080.53 | 38.97 | 1167.25 | 6303.15 | 3199.71 | 1231.94 | 38.50 |
| Grain yield | 286.74 | 2880.37 | 1318.64 | 713.71 | 54.12 | 347.84 | 1181.59 | 685.80 | 176.02 | 25.67 |
| Crude protein | 12.23 | 15.83 | 14.27 | 0.58 | 4.03 | 9.50 | 15.88 | 12.65 | 0.88 | 6.97 |
| Crude fat | 2.72 | 5.30 | 4.21 | 0.45 | 10.67 | 3.23 | 6.98 | 4.96 | 0.79 | 15.84 |
| β-glucan | 2.56 | 5.87 | 4.05 | 0.77 | 18.96 | 2.30 | 6.76 | 4.15 | 0.86 | 20.76 |
| Index | Tumt Left Banner | Dalat Banner | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
| X1 | 0.415 | −0.312 | −0.004 | −0.172 | 0.015 | −0.130 | 0.099 | 0.571 | −0.097 | 0.028 | −0.014 | −0.059 |
| X2 | 0.205 | 0.029 | 0.575 | 0.325 | −0.152 | 0.116 | 0.353 | −0.082 | 0.017 | 0.277 | −0.459 | 0.439 |
| X3 | 0.220 | 0.155 | 0.563 | −0.069 | 0.284 | 0.210 | 0.369 | 0.017 | 0.225 | 0.497 | −0.176 | 0.058 |
| X4 | 0.188 | 0.460 | 0.206 | −0.052 | −0.028 | 0.187 | 0.456 | −0.014 | 0.028 | −0.047 | −0.026 | 0.039 |
| X5 | 0.336 | 0.490 | −0.278 | −0.022 | −0.017 | −0.106 | 0.491 | −0.095 | −0.171 | −0.208 | 0.309 | −0.212 |
| X6 | 0.346 | 0.456 | −0.277 | 0.001 | −0.030 | −0.147 | 0.511 | −0.107 | −0.139 | −0.231 | 0.211 | −0.126 |
| X7 | 0.451 | −0.335 | 0.068 | −0.141 | −0.028 | −0.146 | 0.105 | 0.591 | −0.088 | −0.030 | −0.007 | −0.071 |
| X8 | 0.447 | −0.266 | −0.054 | −0.113 | −0.089 | −0.137 | −0.001 | 0.523 | −0.105 | 0.086 | 0.052 | 0.095 |
| X9 | 0.104 | −0.074 | −0.133 | 0.499 | 0.817 | −0.137 | −0.071 | −0.083 | −0.542 | 0.323 | 0.517 | 0.539 |
| X10 | 0.192 | −0.152 | −0.342 | −0.039 | 0.080 | 0.893 | 0.016 | 0.035 | 0.562 | 0.460 | 0.555 | −0.191 |
| X11 | 0.141 | −0.085 | −0.116 | 0.757 | −0.461 | 0.037 | 0.046 | 0.126 | 0.512 | −0.502 | 0.200 | 0.630 |
| Eigenvalue | 3.244 | 1.897 | 1.204 | 1.018 | 0.968 | 0.849 | 2.816 | 2.451 | 1.208 | 1.077 | 0.961 | 0.793 |
| Contributive ratio (%) | 29.49 | 17.24 | 10.94 | 9.26 | 8.80 | 7.71 | 25.60 | 22.28 | 10.99 | 9.80 | 8.73 | 7.21 |
| Cumulative contributive ratio (%) | 29.49 | 46.73 | 57.67 | 66.93 | 75.73 | 83.45 | 25.60 | 47.88 | 58.87 | 68.66 | 77.40 | 84.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Mi, J.; Zhao, B.; Xu, Z.; Li, X.; Liu, J.; Yuan, Z. Testing of Saline–Alkali Tolerance in Oat Germplasm Through Integrated Germination and Field Performance Assessments. Agronomy 2025, 15, 1267. https://doi.org/10.3390/agronomy15061267
Zhang B, Mi J, Zhao B, Xu Z, Li X, Liu J, Yuan Z. Testing of Saline–Alkali Tolerance in Oat Germplasm Through Integrated Germination and Field Performance Assessments. Agronomy. 2025; 15(6):1267. https://doi.org/10.3390/agronomy15061267
Chicago/Turabian StyleZhang, Biru, Junzhen Mi, Baoping Zhao, Zhongshan Xu, Xingyan Li, Jinghui Liu, and Zhiqiang Yuan. 2025. "Testing of Saline–Alkali Tolerance in Oat Germplasm Through Integrated Germination and Field Performance Assessments" Agronomy 15, no. 6: 1267. https://doi.org/10.3390/agronomy15061267
APA StyleZhang, B., Mi, J., Zhao, B., Xu, Z., Li, X., Liu, J., & Yuan, Z. (2025). Testing of Saline–Alkali Tolerance in Oat Germplasm Through Integrated Germination and Field Performance Assessments. Agronomy, 15(6), 1267. https://doi.org/10.3390/agronomy15061267






