Metagenomics Reveals the Effects of Organic Material Co-Application on Phosphorus Cycling Functional Genes and Bioavailable Phosphorus
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Determination of Soil Physicochemical Properties and Enzyme Activity
2.4. Soil Bioavailable Phosphorus Grading
2.5. Soil DNA Extraction and Sequencing
2.6. Statistical Analysis
3. Results
3.1. Effects on Soil Physicochemical Properties, Enzyme Activities, and Bioavailable Phosphorus
3.1.1. Effects on Soil Physicochemical Properties and Enzyme Activities
3.1.2. Effects on Bioavailable Phosphorus Components
3.1.3. Correlation Between Soil Physicochemical Properties and Bioavailable Phosphorus Components
3.2. Variations in the Composition and Abundance of Phosphorus Cycling Genes and Their Relationship with Bioavailable Phosphorus
3.2.1. Composition and Abundance of Phosphorus Cycling Genes
3.2.2. Correlation Between Environmental Factors and the Composition of Functional Genes
3.2.3. Linear Regression Analysis of the Correlation Between Bioavailable Phosphorus and Functional Genes
3.3. Effects on the Structure and Composition of Microbial Communities
3.3.1. Structure and Composition of Microbial Communities Involved in Phosphorus Cycling
3.3.2. Analysis of Shared and Endemic Microbial Species
3.3.3. Correlation Analysis Between Microorganisms and Environmental Factors
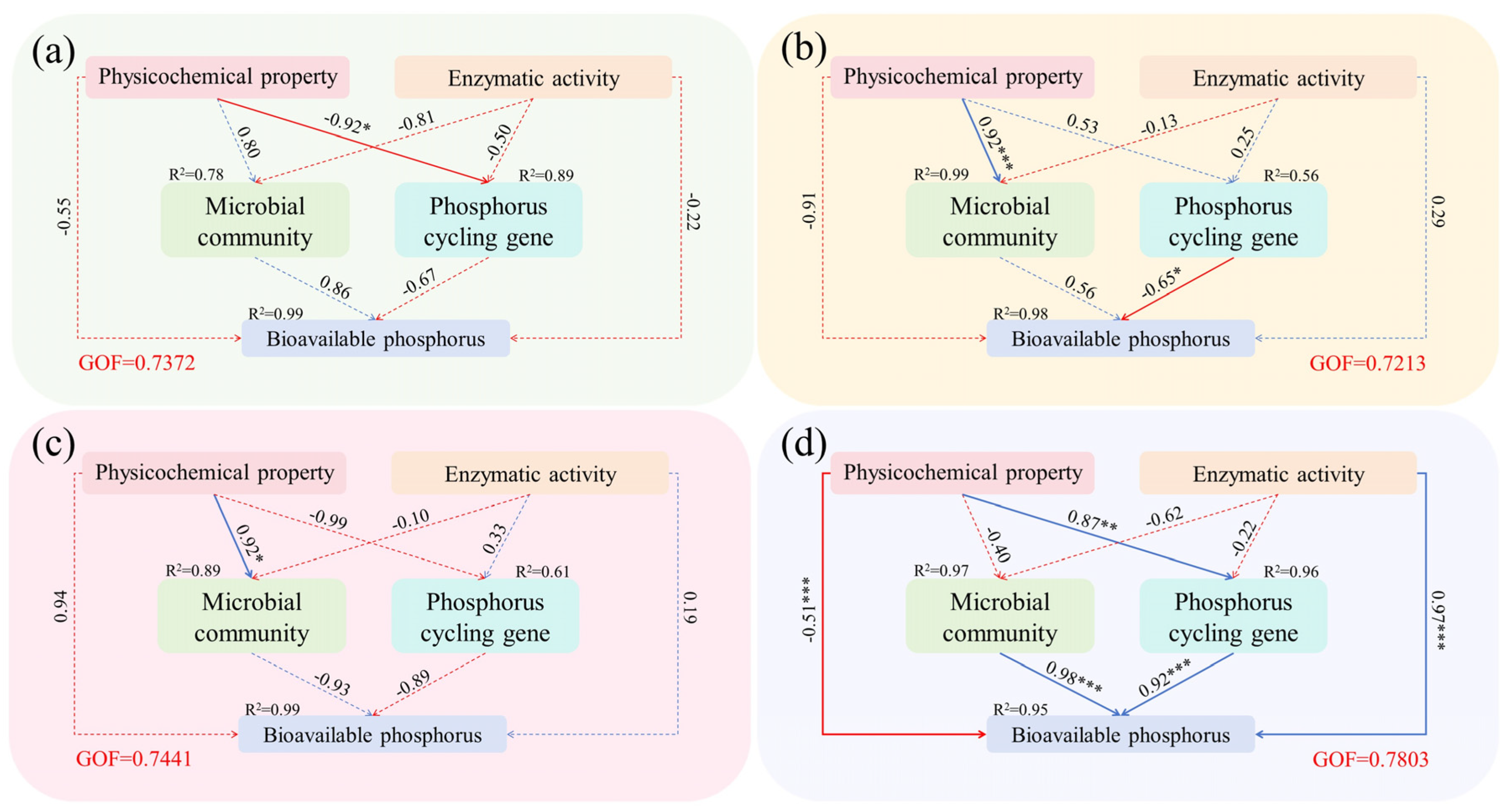
3.4. Relationship Between Microbial Community Composition, Functional Genes, and Environmental Factors
4. Discussion
4.1. Effect of Organic Material Application on Soil Physicochemical Properties and Bioavailable Phosphorus
4.2. Effect of Organic Material Application on Soil Phosphorus Cycling Functional Genes
4.3. Effect of Organic Material Application on Soil Phosphorus Cycling Microorganisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Childers, D.L.; Corman, J.; Edwards, M.; Elser, J.J. Sustainability Challenges of Phosphorus and Food: Solutions from Closing the Human Phosphorus Cycle. BioScience 2011, 61, 117–124. [Google Scholar] [CrossRef]
- Robertson, F.A.; Nash, D.M. Phosphorus and nitrogen in soil, plants, and overland flow from sheep-grazed pastures fertilized with different rates of Superphosphate. Agric. Ecosyst. Environ. 2008, 126, 195–208. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, Q.; Guan, C.-Y.; Yang, X.; Jiang, X.; Wei, M.; Tan, J.; Li, X. Metal oxide modified biochars for fertile soil management: Effects on soil phosphorus transformation, enzyme activity, microbe community, and plant growth. Environ. Res. 2023, 231, 116258. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Margenot, A.J. Navigating limitations and opportunities of soil phosphorus fractionation. Plant Soil 2021, 459, 13–17. [Google Scholar] [CrossRef]
- Velasco-Sánchez, Á.; Bennegadi-Laurent, N.; Trinsoutrot-Gattin, I.; van Groenigen, J.W.; Moinet, G.Y.K. Soil microorganisms increase Olsen phosphorus from poorly soluble organic phosphate: A soil incubation study. Soil Use Manag. 2023, 40, e12960. [Google Scholar] [CrossRef]
- Ai, J.; Banfield, C.C.; Shao, G.; Zamanian, K.; Stürzebecher, T.; Shi, L.; Fan, L.; Liu, X.; Spielvogel, S.; Dippold, M.A. What controls the availability of organic and inorganic P sources in top- and subsoils? A 33P isotopic labeling study with root exudate addition. Soil Biol. Biochem. 2023, 185, 109129. [Google Scholar] [CrossRef]
- Jin, J.; Fang, Y.; He, S.; Liu, Y.; Liu, C.; Li, F.; Khan, S.; Eltohamy, K.M.; Liu, B.; Liang, X. Improved phosphorus availability and reduced degree of phosphorus saturation by biochar-blended organic fertilizer addition to agricultural field soils. Chemosphere 2023, 317, 137809. [Google Scholar] [CrossRef]
- Jia, A.; Song, X.; Li, S.; Liu, Z.; Liu, X.; Han, Z.; Gao, H.; Gao, Q.; Zha, Y.; Liu, Y.; et al. Biochar enhances soil hydrological function by improving the pore structure of saline soil. Agric. Water Manag. 2024, 306, 109170. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, Y.; Sun, Q.; Liu, K.; Chen, X.; Ge, T.; Zhu, B.; Zhu, Z.; Zhang, Z.; Su, Y. Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci. Total Environ. 2018, 628–629, 53–63. [Google Scholar] [CrossRef]
- Chen, X.; Condron, L.M.; Dunfield, K.E.; Wakelin, S.A.; Chen, L. Impact of grassland afforestation with contrasting tree species on soil phosphorus fractions and alkaline phosphatase gene communities. Soil Biol. Biochem. 2021, 159, 108274. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.S.; Ali, O.A.M.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Lin, X.; Ge, Y.; Fahmy, A.E.; et al. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qin, Z.-H.; Zhang, W.-W.; Chen, Y.-H.; Zhu, P.; Peng, C.; Wang, L.; Zhang, S.-X.; Colinet, G. Effect of long-term fertilization on phosphorus fractions in different soil layers and their quantitative relationships with soil properties. J. Integr. Agric. 2022, 21, 2720–2733. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, H.; Xie, W.; Yang, Z.; Zhang, P. Effects of Returning Different Organic Materials in Combination with Inorganic Fertilizers on the Diversity of Eukaryotic Microorganisms in Semi-Arid Northern China. Agronomy 2022, 12, 3116. [Google Scholar] [CrossRef]
- Fernandez, A.L.; Sheaffer, C.C.; Wyse, D.L.; Staley, C.; Gould, T.J.; Sadowsky, M.J. Associations between soil bacterial community structure and nutrient cycling functions in long-term organic farm soils following cover crop and organic fertilizer amendment. Sci. Total Environ. 2016, 566–567, 949–959. [Google Scholar] [CrossRef]
- Borase, D.; Nath, C.; Hazra, K.; Senthilkumar, M.; Singh, S.; Praharaj, C.; Singh, U.; Kumar, N. Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzymes activity. Ecol. Indic. 2020, 114, 106322. [Google Scholar] [CrossRef]
- Peng, Y.; Duan, Y.; Huo, W.; Xu, M.; Yang, X.; Wang, X.; Wang, B.; Blackwell, M.S.A.; Feng, G. Soil microbial biomass phosphorus can serve as an index to reflect soil phosphorus fertility. Biol. Fertil. Soils 2021, 57, 657–669. [Google Scholar] [CrossRef]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; et al. Metagenomic strategies uncover the soil bioavailable phosphorus improved by organic fertilization in Mollisols. Agric. Ecosyst. Environ. 2023, 349, 108462. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, G.; Li, T.; He, B. Fertilization and cultivation management promotes soil phosphorus availability by enhancing soil P-cycling enzymes and the phosphatase encoding genes in bulk and rhizosphere soil of a maize crop in sloping cropland. Ecotoxicol. Environ. Saf. 2023, 264, 115441. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2019, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Heal, K.; Tigabu, M.; Xia, L.; Hu, H.; Yin, D.; Ma, X. Biochar addition to forest plantation soil enhances phosphorus availability and soil bacterial community diversity. For. Ecol. Manag. 2020, 455, 117635. [Google Scholar] [CrossRef]
- Khan, B.A.; Hussain, A.; Elahi, A.; Adnan, M.; Amin, M.; Toor, M.; Aziz, A.; Kashif, M.; Wahab, A.; Ahmad, R. Effect of phosphorus on growth, yield and quality of soybean (Glycine max L.): A Review. Int. J. Agric. Res. 2020, 6, 1–6. [Google Scholar]
- Gong, X.; Feng, Y.; Dang, K.; Jiang, Y.; Qi, H.; Feng, B. Linkages of microbial community structure and root exudates: Evidence from microbial nitrogen limitation in soils of crop families. Sci. Total. Environ. 2023, 881, 163536. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Meng, L.; Liu, X.; Zhang, Y.; Zhao, S.; Zhao, H. Root exudation under maize/soybean intercropping system mediates the arbuscular mycorrhizal fungi diversity and improves the plant growth. Front. Plant Sci. 2024, 15, 1375194. [Google Scholar] [CrossRef]
- Lizcano-Toledo, R.; Reyes-Martín, M.P.; Celi, L.; Fernández-Ondoño, E. Phosphorus dynamics in the soil–plant–environment relationship in cropping systems: A review. Appl. Sci. 2021, 11, 11133. [Google Scholar] [CrossRef]
- Zhang, Z.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, B. Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiol. Biochem. 2020, 155, 828–841. [Google Scholar] [CrossRef]
- Alhassan, A.M.; Ma, W.; Li, G.; Jiang, Z.; Wu, J.; Chen, G. Response of soil organic carbon to vegetation degradation along a moisture gradient in a wet meadow on the Qinghai–Tibet Plateau. Ecol. Evol. 2018, 8, 11999–12010. [Google Scholar] [CrossRef]
- Vekhande, S.; Rao, B. Developing causal loop diagrams for the perusal of soil health in agricultural practices—A case study of rice nursery cultivation practices in India. Agric. Syst. 2024, 217, 103933. [Google Scholar] [CrossRef]
- Zhi, R.; Deng, J.; Xu, Y.; Xu, M.; Zhang, S.; Han, X.; Yang, G.; Ren, C. Altered microbial P cycling genes drive P availability in soil after afforestation. J. Environ. Manag. 2022, 328, 116998. [Google Scholar] [CrossRef]
- Campdelacreu Rocabruna, P.; Domene, X.; Matteazzi, A.; Figl, U.; Fundneider, A.; Fernández-Martínez, M.; Venir, E.; Robatscher, P.; Preece, C.; Peñuelas, J.; et al. Effect of organic fertilisation on soil phosphatase activity, phosphorus availability and forage yield in mountain permanent Meadows. Agric. Ecosyst. Environ. 2024, 368, 109006. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Glanville, H.C.; Harris, M.; Emmett, B.A.; Pingree, M.R.; de Sosa, L.L.; Cerdá-Moreno, C.; Jones, D.L. A novel biologically-based approach to evaluating soil phosphorus availability across complex landscapes. Soil Biol. Biochem. 2015, 88, 110–119. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, N.; Chen, Z.; Tian, J.; Sun, N.; Xu, M.; Chen, L. Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials. Appl. Soil Ecol. 2017, 119, 197–204. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Neal, A.L.; Rossmann, M.; Brearley, C.; Akkari, E.; Guyomar, C.; Clark, I.M.; Allen, E.; Hirsch, P.R. Land-use influences phosphatase gene microdiversity in Soils. Environ. Microbiol. 2017, 19, 2740–2753. [Google Scholar] [CrossRef]
- Thomas, E.; Borchard, N.; Sarmiento, C.; Atkinson, R.; Ladd, B. Key factors determining biochar sorption capacity for metal contaminants: A literature Synthesis. Biochar 2020, 2, 151–163. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Pang, J.; Postma, J.A.; Schrey, S.D.; Lambers, H.; Jablonowski, N.D. The effect of pH on morphological and physiological root traits of Lupinus angustifolius treated with struvite as a recycled phosphorus Source. Plant Soil 2019, 434, 65–78. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Papiernik, S.K.; Malo, D.D.; Clay, D.E.; Kumar, S.; Gulbrandson, D.W. Nitrate sorption and desorption in biochars from fast Pyrolysis. Microporous Mesoporous Mater. 2013, 179, 250–257. [Google Scholar] [CrossRef]
- Gao, S.; Hoffman-Krull, K.; Bidwell, A.L.; DeLuca, T.H. Locally produced wood biochar increases nutrient retention and availability in agricultural soils of the San Juan Islands, USA. Agric. Ecosyst. Environ. 2016, 233, 43–54. [Google Scholar] [CrossRef]
- Pingree, M.R.A.; Makoto, K.; DeLuca, T.H. Interactive effects of charcoal and earthworm activity increase bioavailable phosphorus in sub-boreal forest Soils. Biol. Fertil. Soils 2017, 53, 873–884. [Google Scholar] [CrossRef]
- Yang, F.; Sui, L.; Tang, C.; Li, J.; Cheng, K.; Xue, Q. Sustainable advances on phosphorus utilization in soil via addition of biochar and humic substances. Sci. Total. Environ. 2021, 768, 145106. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tang, C.; Jin, Y.; Cheng, K.; Yang, F. Contribution of exogenous humic substances to phosphorus availability in soil-plant ecosystem: A review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1085–1102. [Google Scholar] [CrossRef]
- Tian, J.; Kuang, X.; Tang, M.; Chen, X.; Huang, F.; Cai, Y.; Cai, K. Biochar application under low phosphorus input promotes soil organic phosphorus mineralization by shifting bacterial phoD gene community Composition. Sci. Total. Environ. 2021, 779, 146556. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Chen, M.; Fei, C.; Zhang, W.; Li, Y.; Ding, X. Fe-modified biochar combined with mineral fertilization promotes soil organic phosphorus mineralization by shifting the diversity of phoD-harboring bacteria within soil aggregates in saline-alkaline paddy Soil. J. Soils Sediments 2023, 23, 619–633. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Y.; Tian, X.; Xu, Q.; Chen, Z.; Lin, W. Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria Abundance. Appl. Soil Ecol. 2017, 112, 90–96. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, J.; Du, X.; Li, F.; Wang, W.; Wang, R. Distribution and sources of Petroleum-hydrocarbon in soil profiles of the Hunpu wastewater-irrigated area, China’s Northeast. Geoderma 2012, 173–174, 215–223. [Google Scholar] [CrossRef]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H. Restoration of carbon and microbial activity in Salt-induced soil by application of peanut shell biochar during short-term incubation Study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef]
- Li, M.; Kang, E.; Wang, J.; Yan, Z.; Zhang, K.; Hu, Z.; Kang, X. Phosphorus accumulation poses less influence than soil physicochemical properties on organic phosphorus adsorption on Ferrasol. Geoderma 2021, 402, 115324. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Improving Phosphorus Availability in an Acid Soil Using Organic Amendments Produced from Agroindustrial Wastes. Sci. World J. 2014, 2014, 506356. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhao, J.; Yi, Q.; Li, J.; Li, Z.; Wu, S.; Zhang, W.; Wang, K. Metagenomic insights into the effects of organic and inorganic agricultural managements on soil phosphorus cycling. Agric. Ecosyst. Environ. 2023, 343, 108281. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Xiangmin, R.; Fei, J.; Peng, J.; Luo, G. Coupling amendment of biochar and organic fertilizers increases maize yield and phosphorus uptake by regulating soil phosphatase activity and phosphorus-acquiring microbiota. Agric. Ecosyst. Environ. 2023, 355, 108582. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Ruan, Y.; Sun, W.; Wang, S.; Wang, H.; Zhang, Y.; Guo, J.; Wang, Y.; Guo, H.; et al. Metagenomes reveal the effect of crop rotation systems on phosphorus cycling functional genes and soil phosphorus avail–Ability. Agric. Ecosyst. Environ. 2024, 364, 108886. [Google Scholar] [CrossRef]
- Long, X.-E.; Yao, H.; Huang, Y.; Wei, W.; Zhu, Y.-G. Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy Soil. Soil Biol. Biochem. 2018, 118, 103–114. [Google Scholar] [CrossRef]
- Ludueña, L.M.; Anzuay, M.S.; Magallanes-Noguera, C.; Tonelli, M.L.; Ibañez, F.J.; Angelini, J.G.; Fabra, A.; McIntosh, M.; Taurian, T. Effects of P limitation and molecules from peanut root exudates on pqqE gene expression and pqq promoter activity in the phosphate-solubilizing strain Serratia sp. S119. Res. Microbiol. 2017, 168, 710–721. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, F.; Wang, K.; Yue, H.; Lan, X. Responses of bacterial phoD gene abundance and diversity to crop rotation and feedbacks to phosphorus uptake in Wheat. Appl. Soil Ecol. 2020, 154, 103604. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Chukwuneme, C.F.; Ayilara, M.S.; Kutu, F.R.; Khantsi, M.; Adeleke, B.S.; Glick, B.R.; Babalola, O.O. Harnessing the rhizosphere soil microbiome of organically amended soil for plant productivity. Agronomy 2022, 12, 3179. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Liu, Q.; Fu, Q.; Hu, H.; Huang, Q. Role of genes encoding microbial Carbohydrate-active enzymes in the accumulation and dynamics of organic carbon in subtropical forest Soils. Sci. Total. Environ. 2024, 918, 170295. [Google Scholar] [CrossRef]
- Shome, S.; Barman, A.; Solaiman, Z.M. Rhizobium and phosphate solubilizing bacteria influence the soil nutrient availability, growth, yield, and quality of soybean. Agriculture 2022, 12, 1136. [Google Scholar] [CrossRef]
- Pastore, G.; Kernchen, S.; Spohn, M. Microbial solubilization of silicon and phosphorus from bedrock in relation to abundance of phosphorus-solubilizing bacteria in temperate forest Soils. Soil Biol. Biochem. 2020, 151, 108050. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: A Review. Pedosphere 2021, 31, 43–75. [Google Scholar] [CrossRef]
- Jarosch, K.; Kandeler, E.; Frossard, E.; Bünemann, E. Is the enzymatic hydrolysis of soil organic phosphorus compounds limited by enzyme or substrate Availability? Soil Biol. Biochem. 2019, 139, 107628. [Google Scholar] [CrossRef]
- Burke, D.J.; Weintraub, M.N.; Hewins, C.R.; Kalisz, S. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood Forest. Soil Biol. Biochem. 2011, 43, 795–803. [Google Scholar] [CrossRef]
- Tang, Q.; Xia, Y.; Ti, C.; Shan, J.; Zhou, W.; Li, C.; Yan, X.; Yan, X. Partial organic fertilizer substitution promotes soil multifunctionality by increasing microbial community diversity and Complexity. Pedosphere 2022, 33, 407–420. [Google Scholar] [CrossRef]
- Siles, J.A.; Starke, R.; Martinovic, T.; Fernandes, M.L.P.; Orgiazzi, A.; Bastida, F. Distribution of phosphorus cycling genes across land uses and microbial taxonomic groups based on metagenome and genome Mining. Soil Biol. Biochem. 2022, 174, 108826. [Google Scholar] [CrossRef]
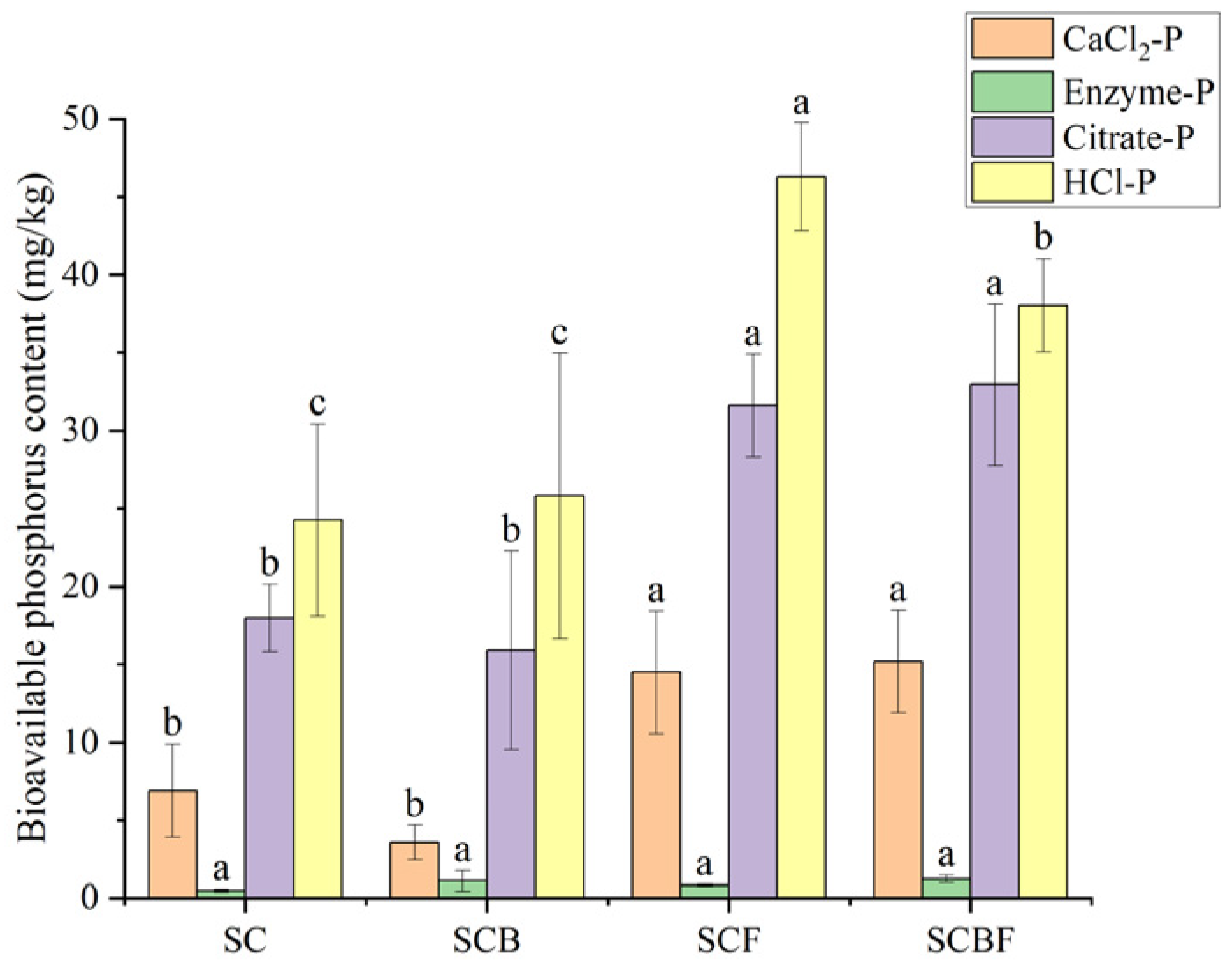
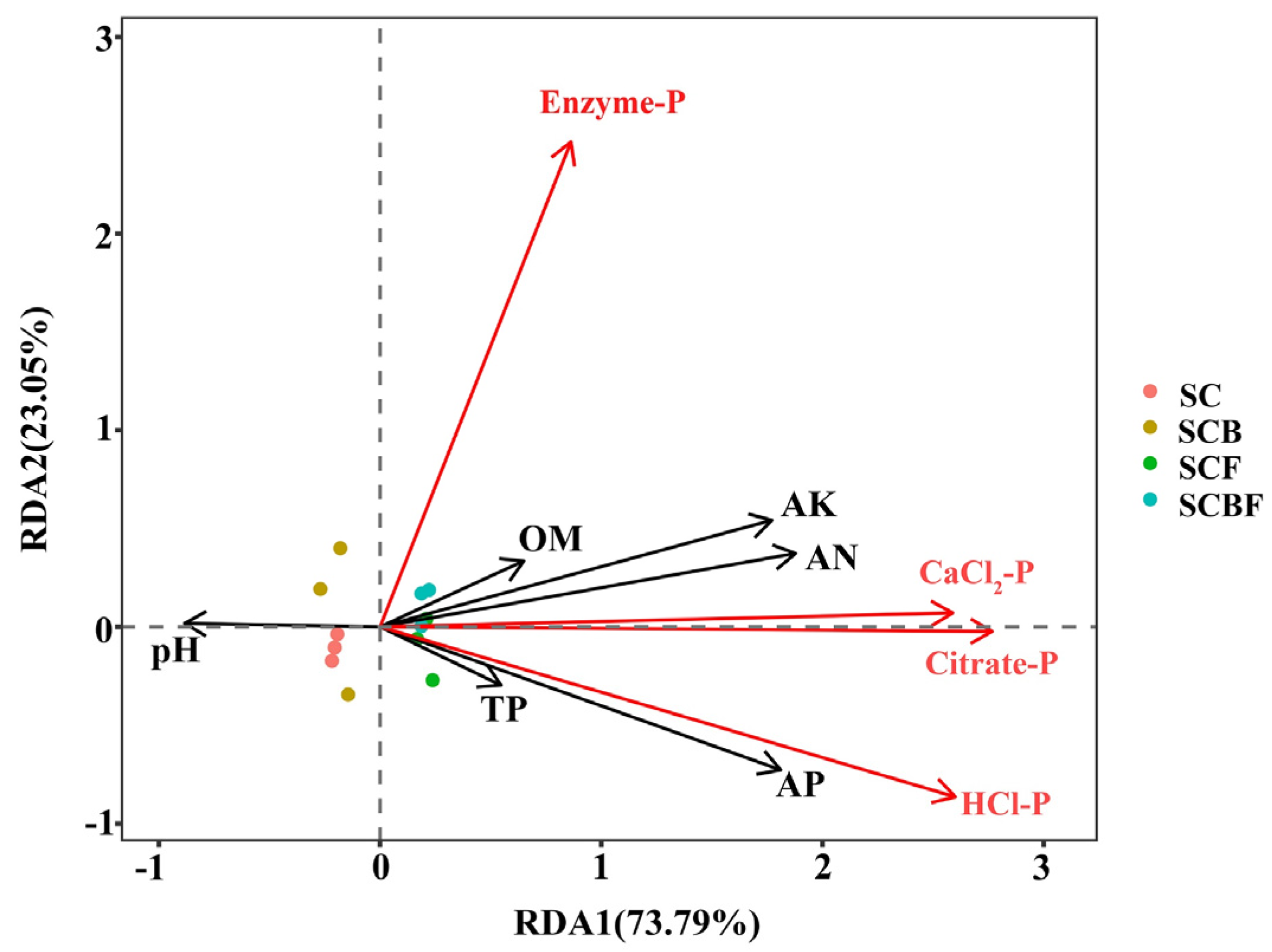

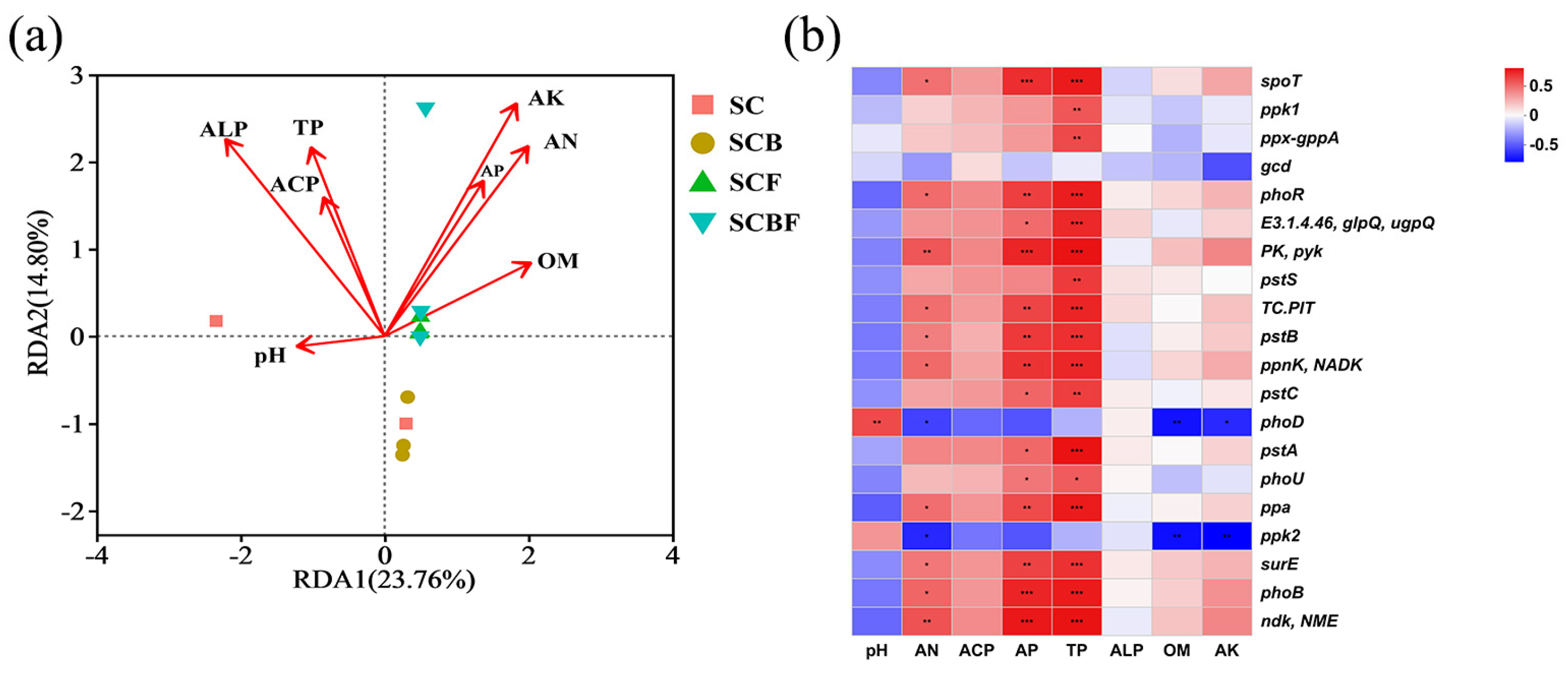

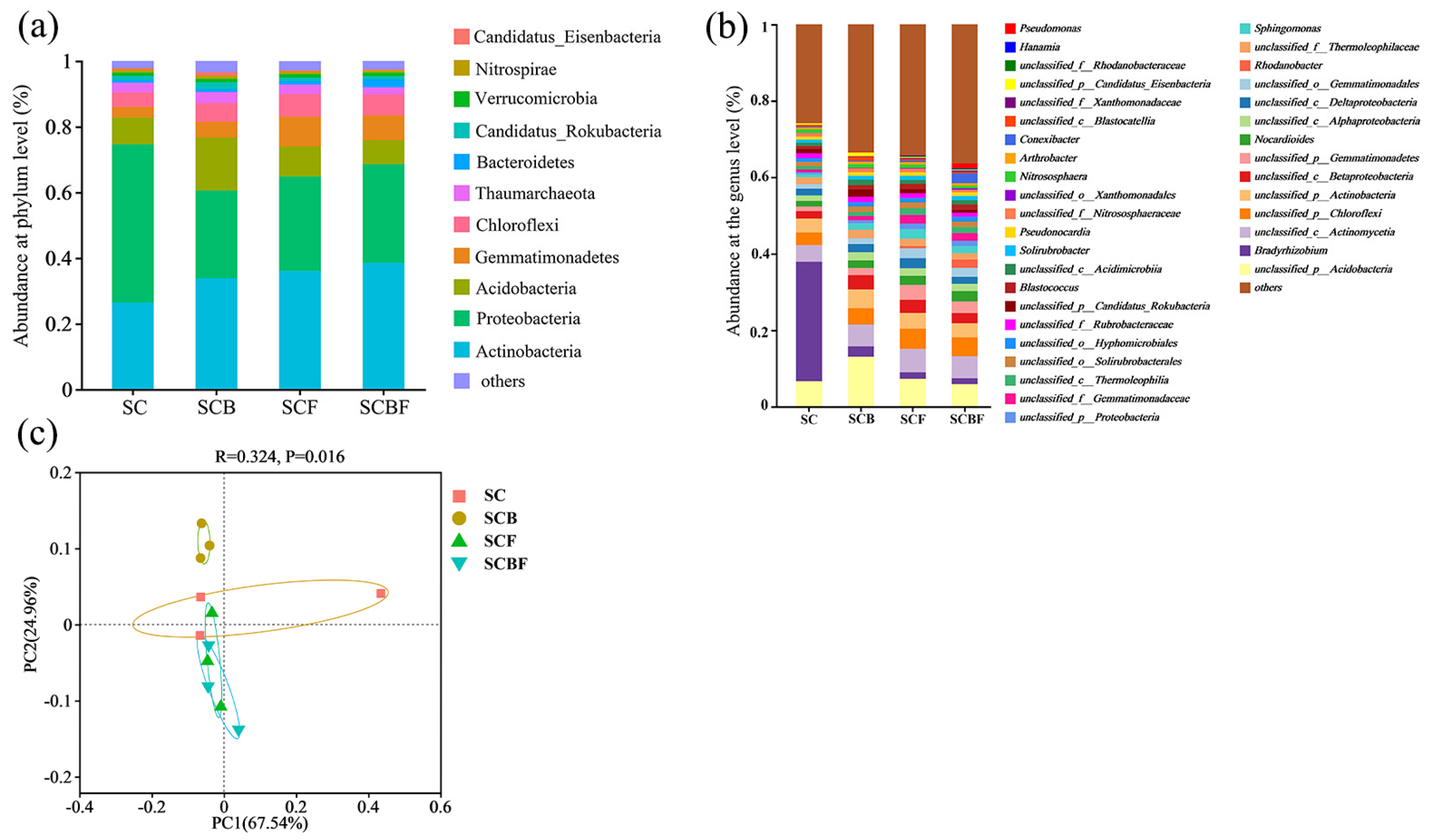
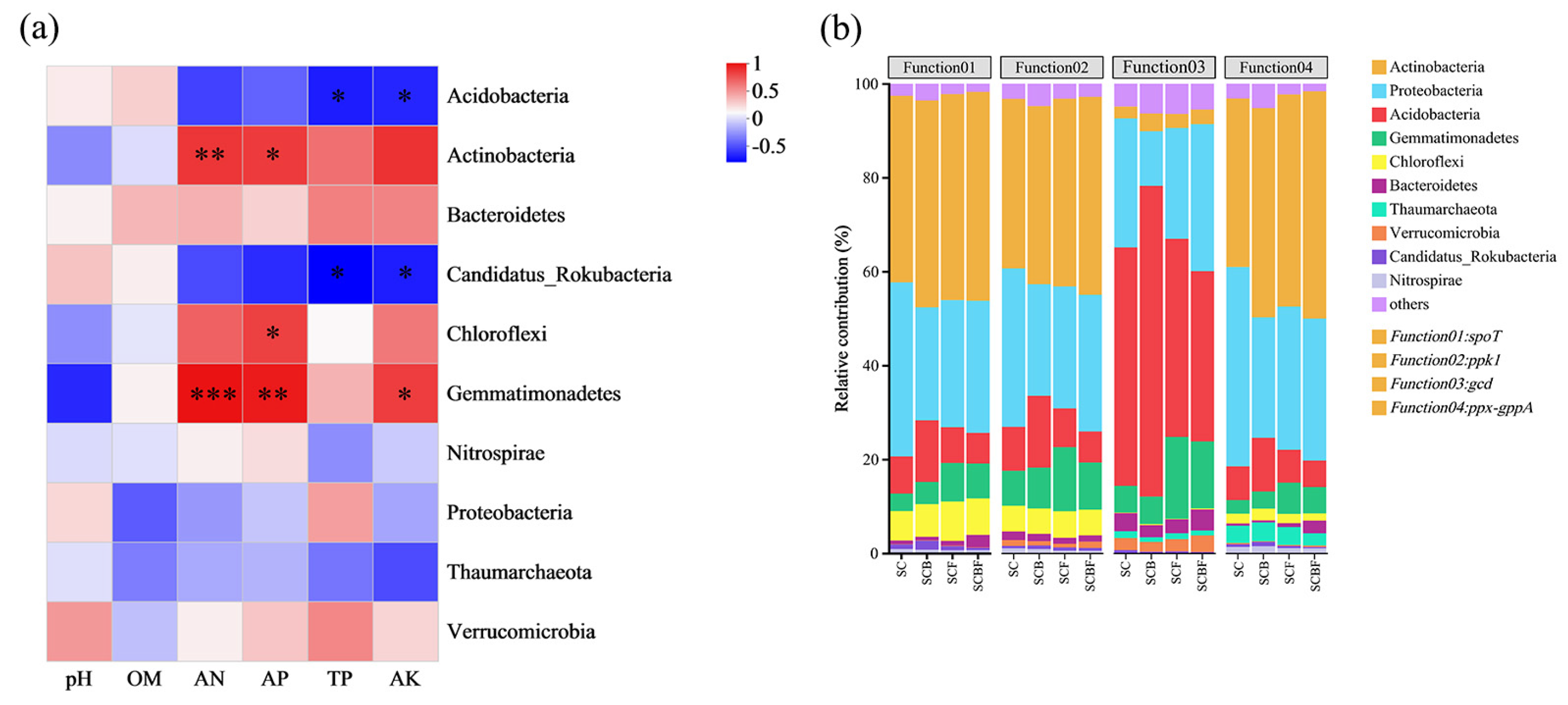
| pH | OM (g/kg) | AP (mg/kg) | TP (%) | AN (mg/kg) | AK (mg/kg) | |
|---|---|---|---|---|---|---|
| SC | 6.71 ± 0.06 a | 2.76 ± 0.06 a | 135.05 ± 19.77 b | 0.67 ± 0.14 a | 96.90 ± 1.27 b | 206.70 ± 35.33 c |
| SCB | 6.57 ± 0.06 a | 3.90 ± 0.26 a | 117.83 ± 11.79 b | 0.57 ± 0.06 a | 98.97 ± 15.88 b | 254.05 ± 19.11 c |
| SCF | 6.31 ± 0.16 b | 3.53 ± 0.56 a | 262.89 ± 14.06 a | 0.66 ± 0.22 a | 149.80 ± 7.97 a | 586.01 ± 50.88 b |
| SCBF | 6.65 ± 0.08 a | 3.87 ± 0.82 a | 241.41 ± 12.34 a | 0.74 ± 0.05 a | 142.90 ± 7.13 a | 902.48 ± 77.33 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Jiang, Y.; Cai, S.; Li, Y.; Sun, L.; Qu, J. Metagenomics Reveals the Effects of Organic Material Co-Application on Phosphorus Cycling Functional Genes and Bioavailable Phosphorus. Agronomy 2025, 15, 1187. https://doi.org/10.3390/agronomy15051187
Wang W, Jiang Y, Cai S, Li Y, Sun L, Qu J. Metagenomics Reveals the Effects of Organic Material Co-Application on Phosphorus Cycling Functional Genes and Bioavailable Phosphorus. Agronomy. 2025; 15(5):1187. https://doi.org/10.3390/agronomy15051187
Chicago/Turabian StyleWang, Wei, Yue Jiang, Shanshan Cai, Yumei Li, Lei Sun, and Juanjuan Qu. 2025. "Metagenomics Reveals the Effects of Organic Material Co-Application on Phosphorus Cycling Functional Genes and Bioavailable Phosphorus" Agronomy 15, no. 5: 1187. https://doi.org/10.3390/agronomy15051187
APA StyleWang, W., Jiang, Y., Cai, S., Li, Y., Sun, L., & Qu, J. (2025). Metagenomics Reveals the Effects of Organic Material Co-Application on Phosphorus Cycling Functional Genes and Bioavailable Phosphorus. Agronomy, 15(5), 1187. https://doi.org/10.3390/agronomy15051187





