The Projected Effects of Climate Change on the Potential Distribution of Planococcus minor Based on Ensemble Species Distribution Models
Abstract
1. Introduction
2. Materials and Methods
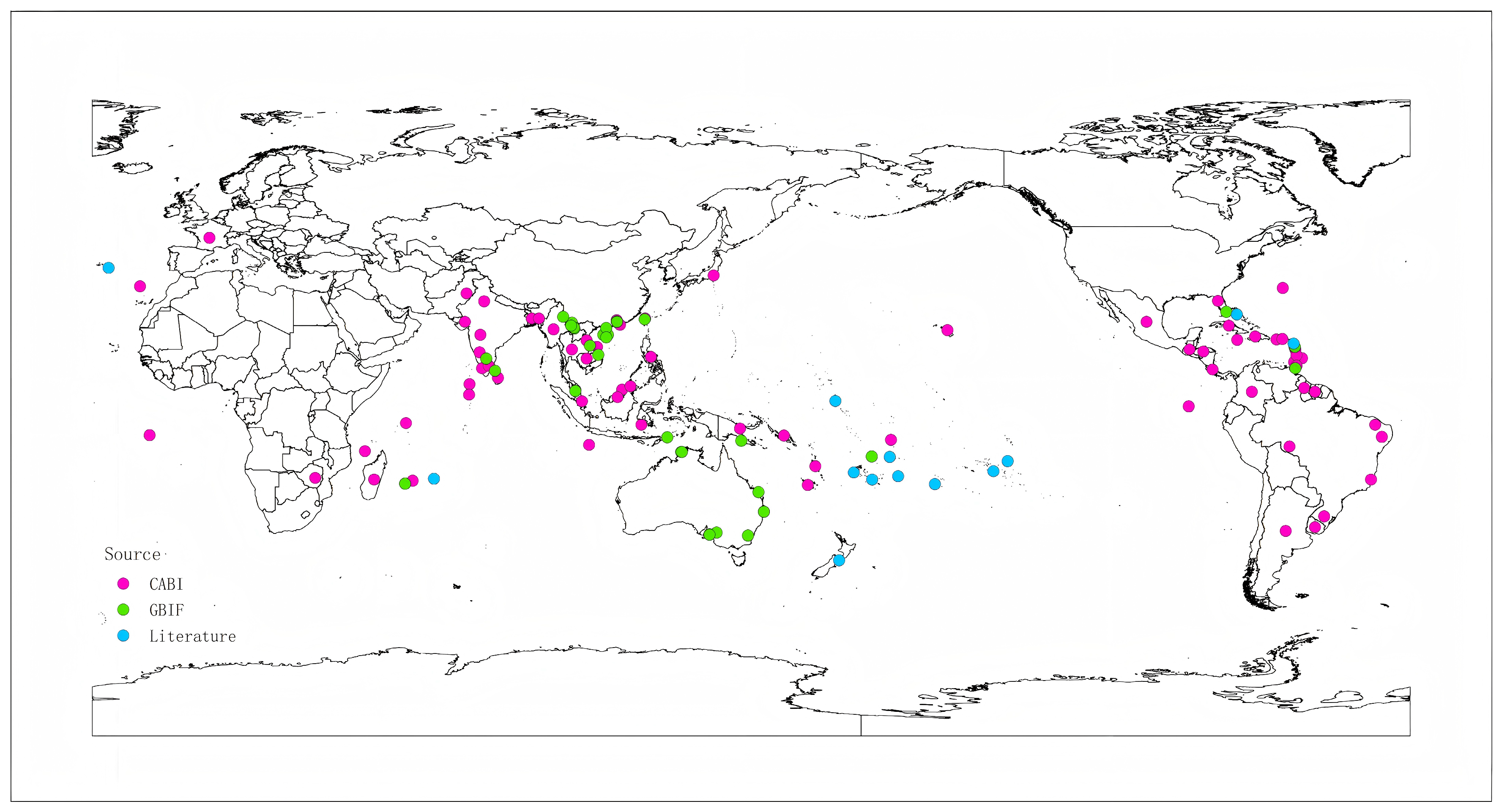
2.1. Collecting and Processing Occurrence Records
2.2. Acquiring and Screening Environmental Variables
2.3. Constructing and Evaluating Ensemble Models
2.4. Dividing and Calculating Suitable Areas
3. Results
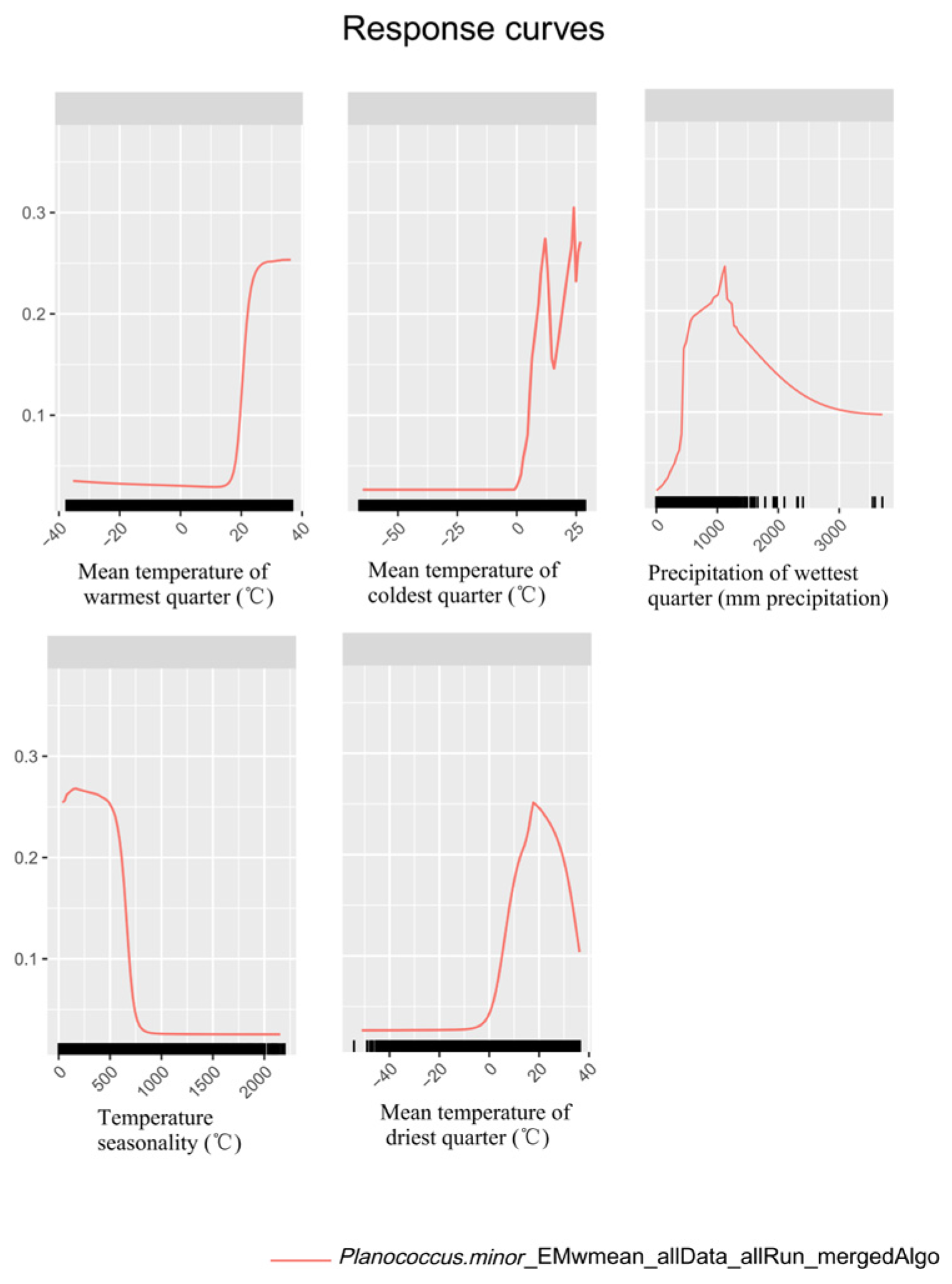
3.1. Contribution and Response of Environmental Variables
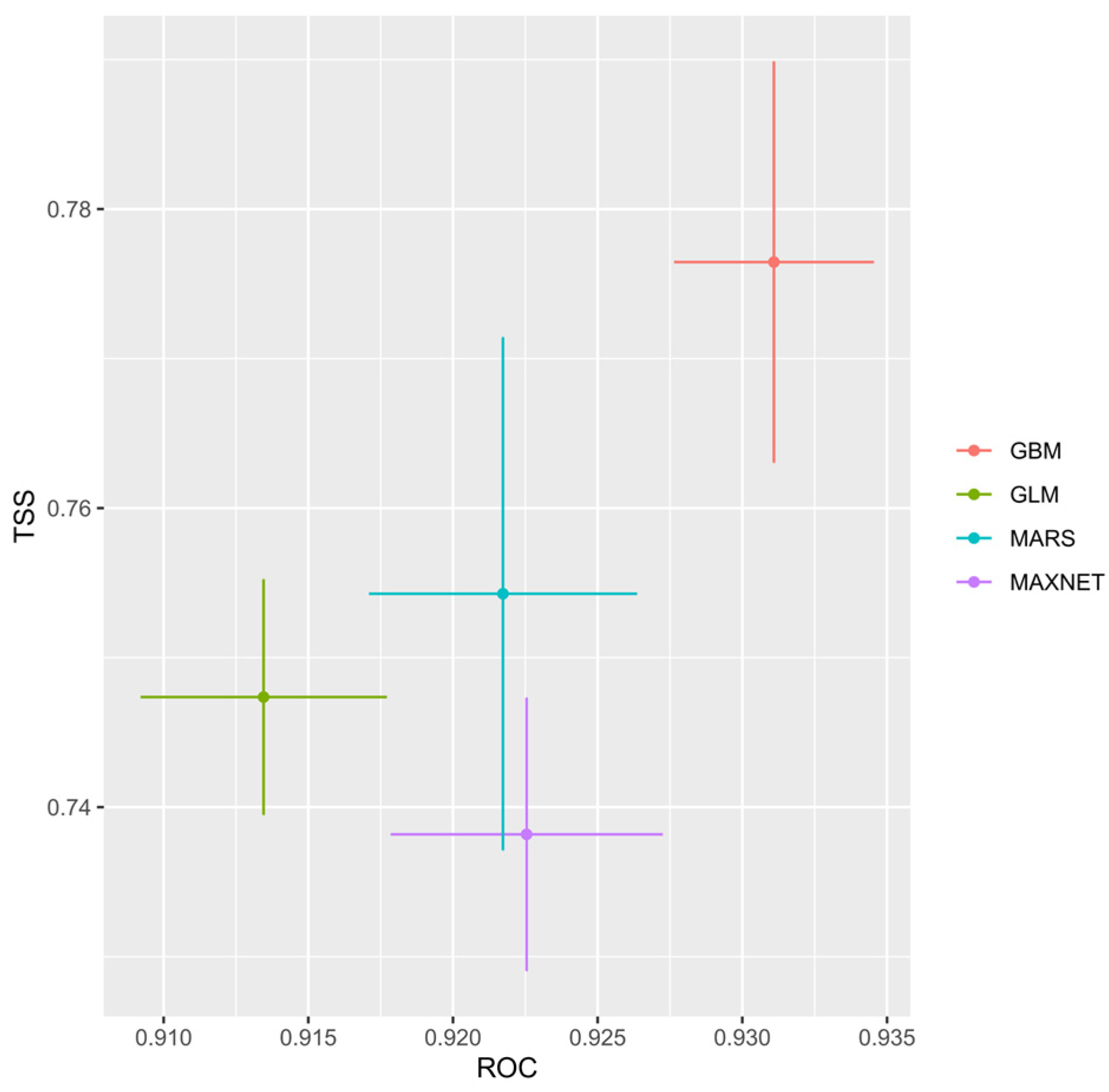
3.2. Evaluation of Ensemble Model
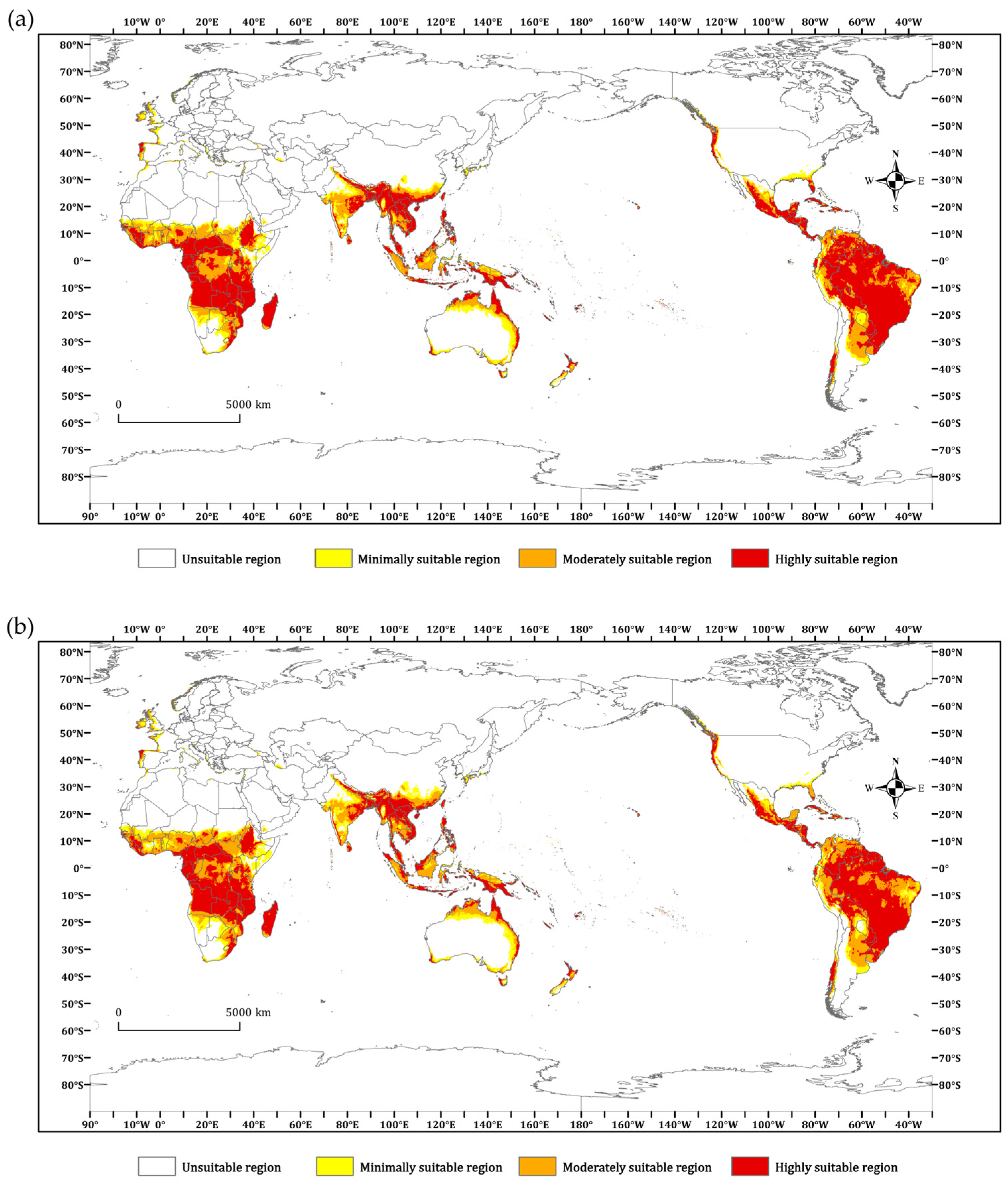
3.3. Current Potential Geographic Distribution of P. minor
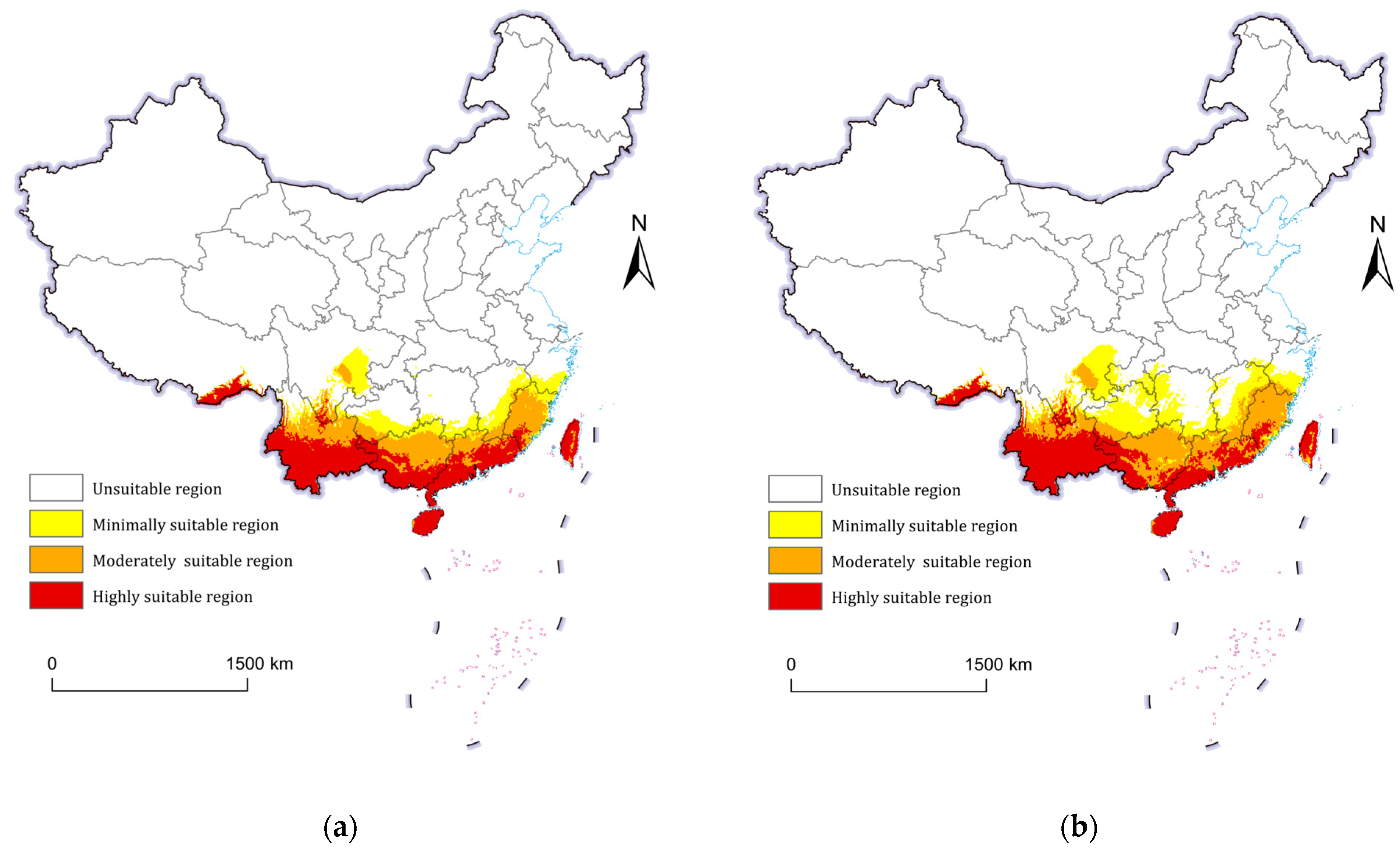
3.4. Future Potential Geographic Distribution of P. minor
4. Discussion
4.1. Accuracy of Model Predictions
4.2. Key Environmental Variables Influencing the Distribution of P. minor
4.3. Geographical Distribution Patterns of P. minor Under Climate Change
4.4. Management and Monitoring Recommendations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, J.; Peng, L.; He, Z.; Lu, Y.; Wang, F. Potential Distribution of Two Invasive Pineapple Pests under Climate Change. Pest Manag. Sci. 2020, 76, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, C.; Zhao, Z.; Pan, X.; Li, Z. Climate Change Impacts on the Global Potential Geographical Distribution of the Agricultural Invasive Pest, Bactrocera Dorsalis (Hendel) (Diptera: Tephritidae). Clim. Change 2019, 155, 145–156. [Google Scholar] [CrossRef]
- Skendzic, S.; Zovko, M.; Zivkovic, I.P.; Lesic, V.; Lemic, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Shrestha, S. Effects of Climate Change in Agricultural Insect Pest. Acta Sci. Agric. 2019, 3, 74–80. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Sixth Assessment Report. Available online: https://www.ipcc.ch/assessment-report/ar6/ (accessed on 20 September 2024).
- Yan, Y.; Wang, Y.; Feng, C.; Wan, P.M.; Chang, K.T.T. Potential Distributional Changes of Invasive Crop Pest Species Associated with Global Climate Change. Appl. Geogr. 2017, 82, 83–92. [Google Scholar] [CrossRef]
- Porter, J.H.; Parry, M.L.; Carter, T.R. The Potential Effects of Climatic Change on Agricultural Insect Pests. Agric. For. Meteorol. 1991, 57, 221–240. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ Warning on Invasive Alien Species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Zhao, W. Meeting the Challenges of Invasive Alien Species. Natl. Sci. Rev. 2024, 11, nwae017. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and Their Control; Roy, H.E., Pauchard, A., Stoett, P., Renard Truong, T., Bacher, S., Galil, B.S., Hulme, P.E., Ikeda, T., Sankaran, K.V., McGeoch, M.A., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2023. [Google Scholar] [CrossRef]
- Westphal, M.I.; Browne, M.; MacKinnon, K.; Noble, I. The Link between International Trade and the Global Distribution of Invasive Alien Species. Biol. Invasions 2008, 10, 391–398. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, Transport and Trouble: Managing Invasive Species Pathways in an Era of Globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate Change and Biological Invasions: Evidence, Expectations, and Response Options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.A.; Beaury, E.M.; Fusco, E.J.; Lopez, B.E. Invasive Species Policy Must Embrace a Changing Climate. Bioscience 2023, 73, 124–133. [Google Scholar] [CrossRef]
- Roda, A.; Francis, A.; Kairo, M.T.K.; Culik, M. Planococcus minor (Hemiptera: Pseudococcidae): Bioecology, Survey and Mitigation Strategies. In CABI Invasives Series; Pena, J.E., Ed.; CABI Publishing: Wallingford, UK, 2013; pp. 288–300. [Google Scholar]
- Bastos, C.S.; De Almeida, R.P.; Vidal Neto, F.D.C.; De Araújo, G.P. Occurrence of Planococcus minor Maskell (Hemiptera: Pseudococcidae) in Cotton Fields of Northeast Region of Brazil. Neotrop. Entomol. 2007, 36, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J.; Ghosh, A.B. Biology of the Mealybug, Planococcus minor (Maskell) on Various Host Plants. Environ. Ecol. 2000, 18, 929–932. [Google Scholar]
- de Sousa, A.L.V.; Souza, B.; Santa-Cecília, L.V.C.; Lima, R.R.; Costa, M.B. Host preference and biology of two cryptic species, Planococcus citri (Risso) and Planococcus minor (Maskell) (Hemiptera: Pseudococcidae). Sci. Agrar. 2018, 19, 71–77. [Google Scholar] [CrossRef]
- Lu, N.; Cai, B.; Ma, X.; Xu, M.; Long, Y.; Meng, R.; Wu, F.; Lin, W.; Xu, W.; Yuan, J.; et al. Warning on the Spread of the Quarantine Pest Planococcus minor in Mainland China. Plant Quar. 2021, 35, 65–69. [Google Scholar] [CrossRef]
- Puspitasari, M.; Susilawati, S.; Hapsari, A.D.; Harni, R. Mealybug (Planococcus spp. Hemiptera: Pseudococcidae) as a Pest on Plantation Crops and Its Control Techniques: A Review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Conference on Modern and Sustainable Agriculture, Online, 8 March 2023; Volume 1133, p. 12032. [Google Scholar] [CrossRef]
- Cox, J.M. The Mealybug Genus Planococcus (Homoptera: Pseudococcidae). Bull. Br. Mus. Nat. Hist. 1989, 58, 1–78. [Google Scholar]
- Shao, W. Study on the Biological Characteristics of Planococcus minor. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2016. [Google Scholar]
- Francis, A.W.; Kairo, M.T.K.; Roda, A.L.; Liburd, O.E.; Polar, P. The passionvine mealybug, Planococcus minor (Maskell) (Hemiptera: Pseudococcidae), and its natural enemies in the cocoa agroecosystem in Trinidad. Biol. Control 2012, 60, 290–296. [Google Scholar] [CrossRef]
- Fu, H.; Wang, F.; Lin, Y.; Zhao, X. First Interception of the Quarantine Pest Planococcus minor in China. Plant Quar. 2008, 22, 382–384. [Google Scholar]
- Gu, Y.; Liu, H.; He, R.; Wu, M.; Chen, K.; Hu, X. Analysis of Quarantine Scale Insects on ASEAN Fruits Exported to China. Plant Quar. 2013, 27, 95–99. [Google Scholar]
- Gu, Y.; Lu, X.; Ding, G.; Huang, F. Analysis of Interceptions on ASEAN Fruits Exported to China. Plant Quar. 2021, 35, 81–85. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Miller, J. Species Distribution Modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Busby, J.R. BIOCLIM: A Bioclimate Analysis and Prediction System. Plant Prot. Q. 1991, 6, 64–68. [Google Scholar]
- Araújo, M.B.; Anderson, R.P.; Márcia Barbosa, A.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for Distribution Models in Biodiversity Assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Eyre, A.C.; Briscoe, N.J.; Harley, D.K.P.; Lumsden, L.F.; McComb, L.B.; Lentini, P.E. Using Species Distribution Models and Decision Tools to Direct Surveys and Identify Potential Translocation Sites for a Critically Endangered Species. Divers. Distrib. 2022, 28, 700–711. [Google Scholar] [CrossRef]
- Peterson, A.T. Predicting the Geography of Species’ Invasions via Ecological Niche Modeling. Q. Rev. Biol. 2003, 78, 419–433. [Google Scholar] [CrossRef]
- Srivastava, V.; Lafond, V.; Griess, V.C. Species Distribution Models (SDM): Applications, Benefits and Challenges in Invasive Species Management. CABI Rev. 2019, 1–13. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can Species Distribution Models Really Predict the Expansion of Invasive Species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.-A.; Guisan, A.; et al. The Role of Biotic Interactions in Shaping Distributions and Realised Assemblages of Species: Implications for Species Distribution Modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Araújo, M.B.; Townsend Peterson, A. Uses and Misuses of Bioclimatic Envelope Modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Thuiller, W.; Araújo, M.B.; Martinez-Meyer, E.; Brotons, L.; McClean, C.; Miles, L.; Segurado, P.; Dawson, T.P.; Lees, D.C. Model-Based Uncertainty in Species Range Prediction. J. Biogeogr. 2006, 33, 1704–1711. [Google Scholar] [CrossRef]
- Bates, J.M.; Granger, C.W.J. The Combination of Forecasts. J. Oper. Res. Soc. 1969, 20, 451–468. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate Warming and the Decline of Amphibians and Reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Thuiller, W. Patterns and Uncertainties of Species’ Range Shifts under Climate Change. Glob. Change Biol. 2004, 10, 2020–2027. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A Review of Evidence about Use and Performance of Species Distribution Modelling Ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Fletcher, R.J.; Hefley, T.J.; Robertson, E.P.; Zuckerberg, B.; McCleery, R.A.; Dorazio, R.M. A Practical Guide for Combining Data to Model Species Distributions. Ecology 2019, 100, e02710. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Zhang, Y.; Fu, W.; Zhang, G.; Li, Z. Predicting the Potential Distribution of Invasive Plant Solanum rostratum Using Ensemble Models. J. Plant Prot. 2022, 49, 1434–1439. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, H.; Tao, S.; Yin, C.; Yu, X. Predicting the Potential Distribution of Pomacea canaliculata in China Using Biomod2 Ensemble Models. Chin. J. Appl. Ecol. 2024, 35, 2237–2246. [Google Scholar] [CrossRef]
- Qi, G.; Chen, T.; Gao, Y.; Lei, Y.; Lü, L. Potential Distribution of Planococcus minor and Planococcus lilacinus in China Based on Maxent. J. Environ. Entomol. 2015, 37, 219–223. [Google Scholar]
- CABI Database. Available online: https://www.cabidigitallibrary.org/doi/abs/10.1079/cabicompendium.41892 (accessed on 26 July 2024).
- GBIF Database. Available online: https://www.gbif.org/species/8319203 (accessed on 26 July 2024).
- Shao, W.; Xu, Z. Research Progress on Planococcus minor. J. Zhejiang For. Sci. Technol. 2014, 34, 70–74. [Google Scholar]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Veloz, S.D. Spatially Autocorrelated Sampling Falsely Inflates Measures of Accuracy for Presence-only Niche Models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Huang, Y.; Li, T.; Chen, W.; Zhang, Y.; Xu, Y.; Guo, T.; Wang, S.; Liu, J.; Qin, Y. Analysis of the Distribution Pattern of Phenacoccus manihoti in China under Climate Change Based on the Biomod2 Model. Biology 2024, 13, 538. [Google Scholar] [CrossRef]
- Xin, X.; Wu, T.; Zhang, J.; Zhang, F.; Li, W.; Zhang, Y.; Lu, Q.; Fang, Y.; Jie, W.; Zhang, L.; et al. Introduction of BCC models and its participation in CMIP6. Adv. Clim. Change Res. 2019, 15, 533–539. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, X.; Sun, J.; Li, T.; Wang, Q.; Ye, X.; Fan, B. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Fawcett, T. An Introduction to ROC Analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Gan, T.; Liu, Q.; Xu, D.; He, Z.; Zhuo, Z. Assessing the Impact of Climate Change on Hippophae neurocarpa in China Using Biomod2 Modeling. Agriculture 2025, 15, 722. [Google Scholar] [CrossRef]
- Chen, B.; Zou, H.; Meng, X.; Wang, L.; Hao, X.; Kang, X.; Wang, C.; Zhang, X. Effects of Climate Change on the Potential Distribution of Bupleurum chinense and Bupleurum scorzonerifolium. Acta Ecol. Sin. 2022, 42, 8471–8482. [Google Scholar]
- Araújo, M.B.; New, M. Ensemble Forecasting of Species Distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Grenouillet, G.; Buisson, L.; Casajus, N.; Lek, S. Ensemble Modelling of Species Distribution: The Effects of Geographical and Environmental Ranges. Ecography 2011, 34, 9–17. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guisan, A. Five (or so) Challenges for Species Distribution Modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Bazzichetto, M.; Lenoir, J.; Da Re, D.; Tordoni, E.; Rocchini, D.; Malavasi, M.; Barták, V.; Sperandii, M.G. Sampling Strategy Matters to Accurately Estimate Response Curves’ Parameters in Species Distribution Models. Glob. Ecol. Biogeogr. 2023, 32, 1717–1729. [Google Scholar] [CrossRef]
- Francis, A.W.; Kairo, M.T.K.; Roda, A.L. Developmental and Reproductive Biology of Planococcus minor (Hemiptera: Pseudococcidae) under Constant Temperatures. Fla. Entomol. 2012, 95, 297–303. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Matthews, R.W. Mealybugs of Central and South America. Ann. Entomol. Soc. Am. 1994, 87, 487–488. [Google Scholar] [CrossRef]
- Roda, A.; Millar, J.G.; Rascoe, J.; Weihman, S.; Stocks, I. Developing Detection and Monitoring Strategies for Planococcus minor (Hemiptera: Pseudococcidae). J. Econ. Entomol. 2012, 105, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.W.; Kairo, M.T.K.; Roda, A. Passionvine Mealybug, Planococcus minor (Maskell) (Hemiptera: Pseudococcidae). EDIS 2012, 2012. [Google Scholar] [CrossRef]
- Buss, E.A.; Dale, A. Managing Scale Insects on Ornamental Plants: ENY-323/MG005, Rev. 7/2016. EDIS 2016, 2016, 6. [Google Scholar] [CrossRef]


| Number | Climate Variable Code | Meaning |
|---|---|---|
| 1 | Bio1 | Annual mean temperature |
| 2 | Bio2 | Mean diurnal range |
| 3 | Bio3 | Isothermality |
| 4 | Bio4 | Temperature seasonality |
| 5 | Bio5 | Max temperature of warmest month |
| 6 | Bio6 | Min temperature of coldest month |
| 7 | Bio7 | Temperature annual range |
| 8 | Bio8 | Mean temperature of wettest quarter |
| 9 | Bio9 | Mean temperature of driest quarter |
| 10 | Bio10 | Mean temperature of warmest quarter |
| 11 | Bio11 | Mean temperature of coldest quarter |
| 12 | Bio12 | Annual precipitation |
| 13 | Bio13 | Precipitation of wettest month |
| 14 | Bio14 | Precipitation of driest month |
| 15 | Bio15 | Precipitation seasonality |
| 16 | Bio16 | Precipitation of wettest quarter |
| 17 | Bio17 | Precipitation of driest quarter |
| 18 | Bio18 | Precipitation of warmest quarter |
| 19 | Bio19 | Precipitation of coldest quarter |
| Climate Scenario | Minimally Suitable Region | Moderately Suitable Region | Highly Suitable Region | |||
|---|---|---|---|---|---|---|
| Area (×104 km2) Percent (%) | Area (×104 km2) Percent (%) | Area (×104 km2) Percent (%) | ||||
| Current conditions | 24.6875 | 2.57 | 35.1806 | 3.66 | 51.3819 | 5.35 |
| SSP1-2.6(2050s) | 28.7847 | 3.00 | 37.4931 | 3.91 | 51.3958 | 5.35 |
| SSP5-8.5(2050s) | 44.2639 | 4.61 | 44.8889 | 4.68 | 52.7639 | 5.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, T.; Wang, S.; Kang, F.; Liu, J.; Qin, Y. The Projected Effects of Climate Change on the Potential Distribution of Planococcus minor Based on Ensemble Species Distribution Models. Agronomy 2025, 15, 1165. https://doi.org/10.3390/agronomy15051165
Xiong T, Wang S, Kang F, Liu J, Qin Y. The Projected Effects of Climate Change on the Potential Distribution of Planococcus minor Based on Ensemble Species Distribution Models. Agronomy. 2025; 15(5):1165. https://doi.org/10.3390/agronomy15051165
Chicago/Turabian StyleXiong, Taohua, Shuping Wang, Fenfen Kang, Jingyuan Liu, and Yujia Qin. 2025. "The Projected Effects of Climate Change on the Potential Distribution of Planococcus minor Based on Ensemble Species Distribution Models" Agronomy 15, no. 5: 1165. https://doi.org/10.3390/agronomy15051165
APA StyleXiong, T., Wang, S., Kang, F., Liu, J., & Qin, Y. (2025). The Projected Effects of Climate Change on the Potential Distribution of Planococcus minor Based on Ensemble Species Distribution Models. Agronomy, 15(5), 1165. https://doi.org/10.3390/agronomy15051165






