Optimal Nitrogen Accumulation and Remobilization Can Synergistically Improve Maize Yield and Nitrogen-Use Efficiency Under Low-Nitrogen Conditions
Abstract
1. Introduction
2. Materials and Methods
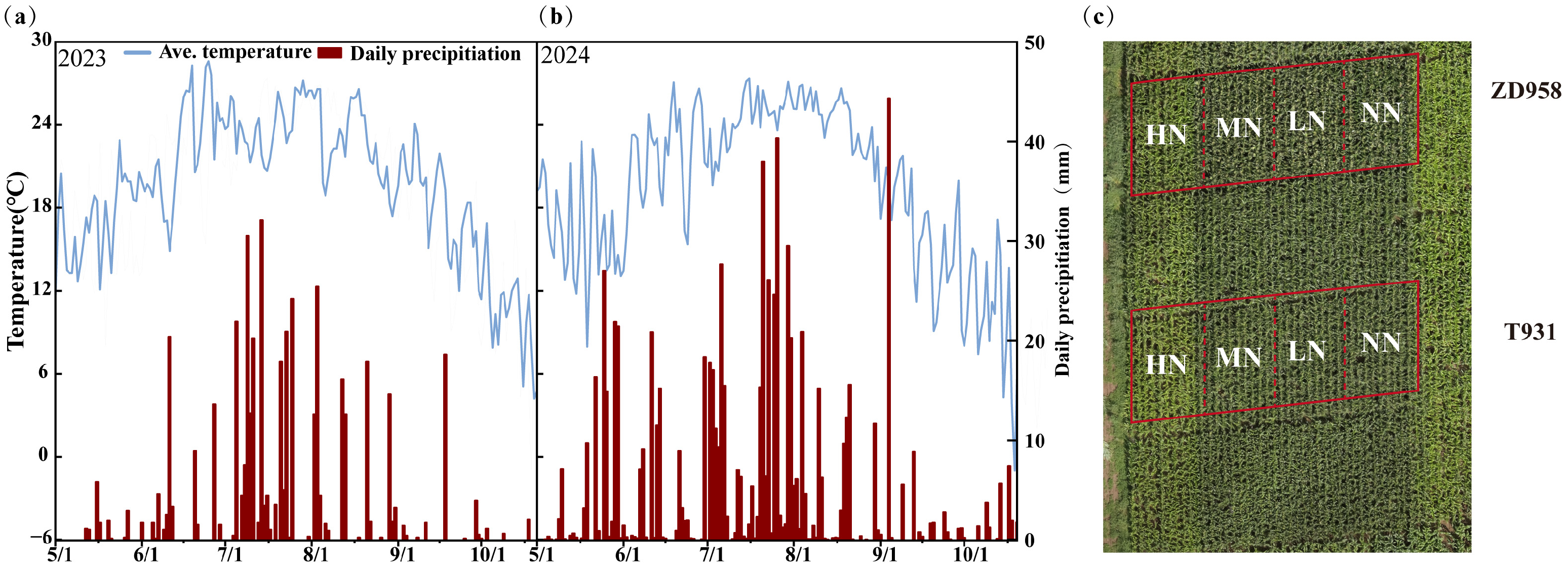
2.1. Experimental Site
2.2. Experiment Design
2.3. Data Collection
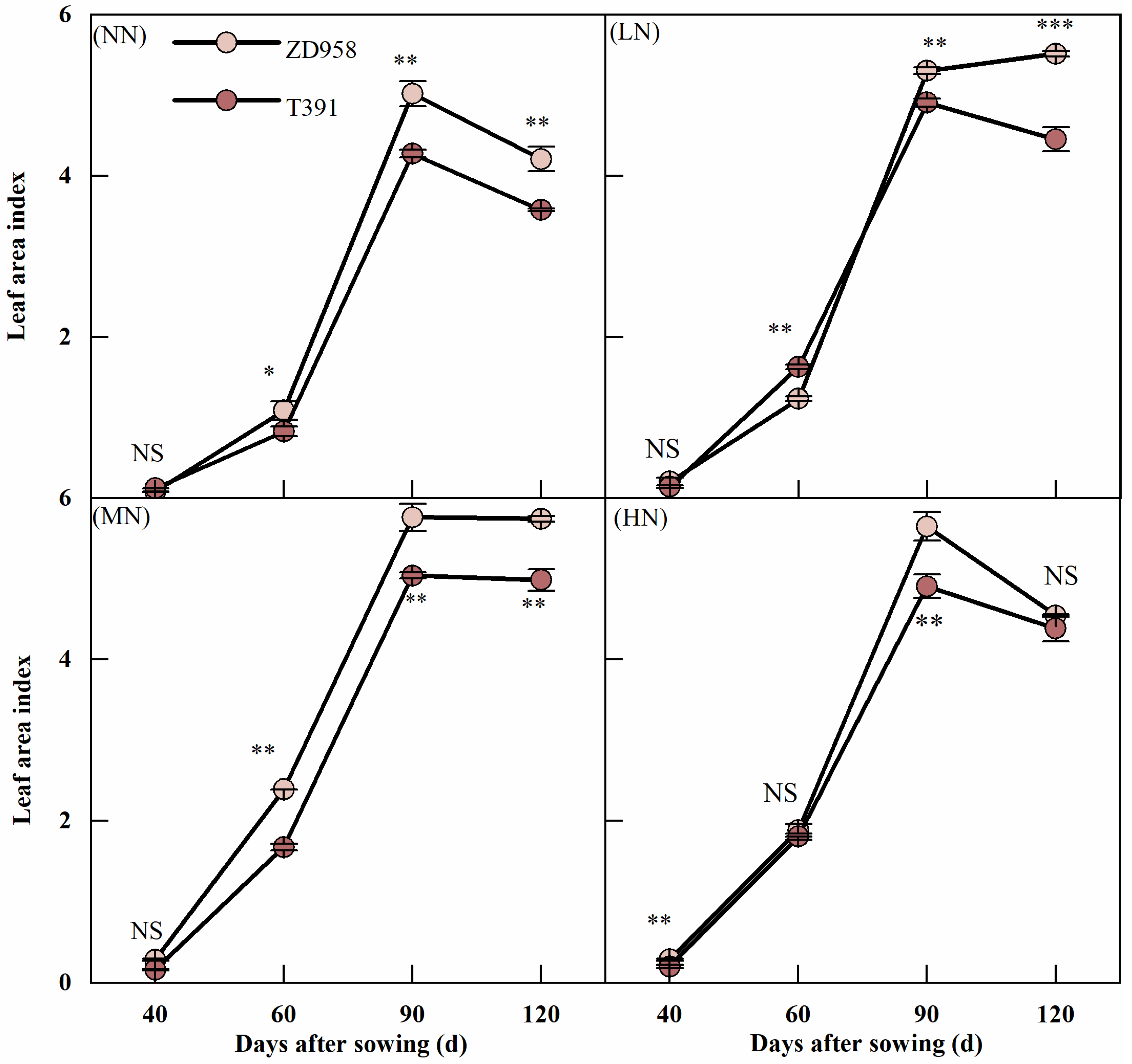
2.3.1. Leaf Area Index (LAI)
2.3.2. Dry Matter and N Accumulation and Reallocation
2.3.3. Grain Yield Measurement
2.4. Statistical Analysis
3. Results
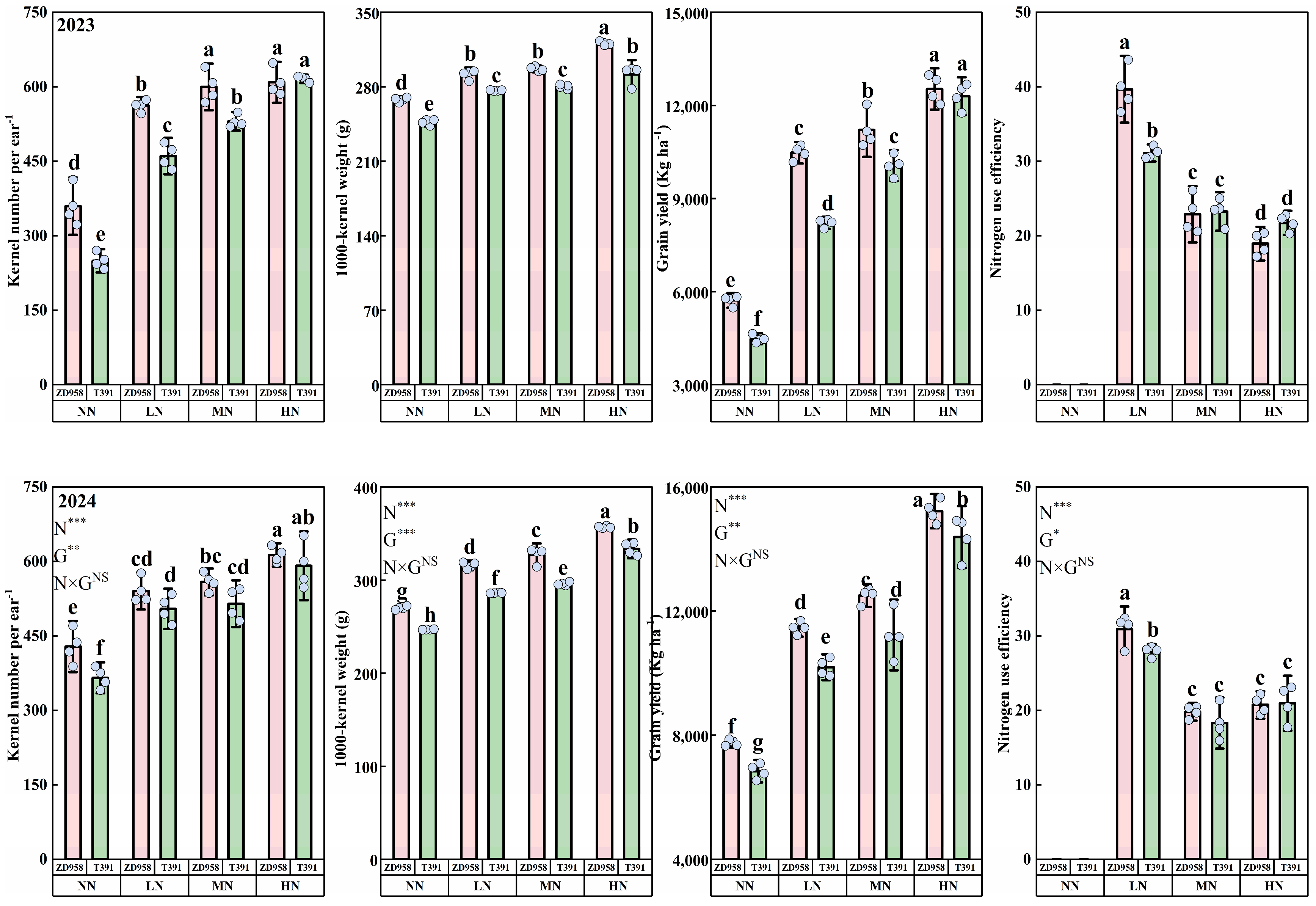
3.1. Grain Yield
3.2. Leaf Area Index
3.3. DM and N Accumulation
3.4. DM and N Reallocation
3.5. Fraction of DM and N Content in Different Organs
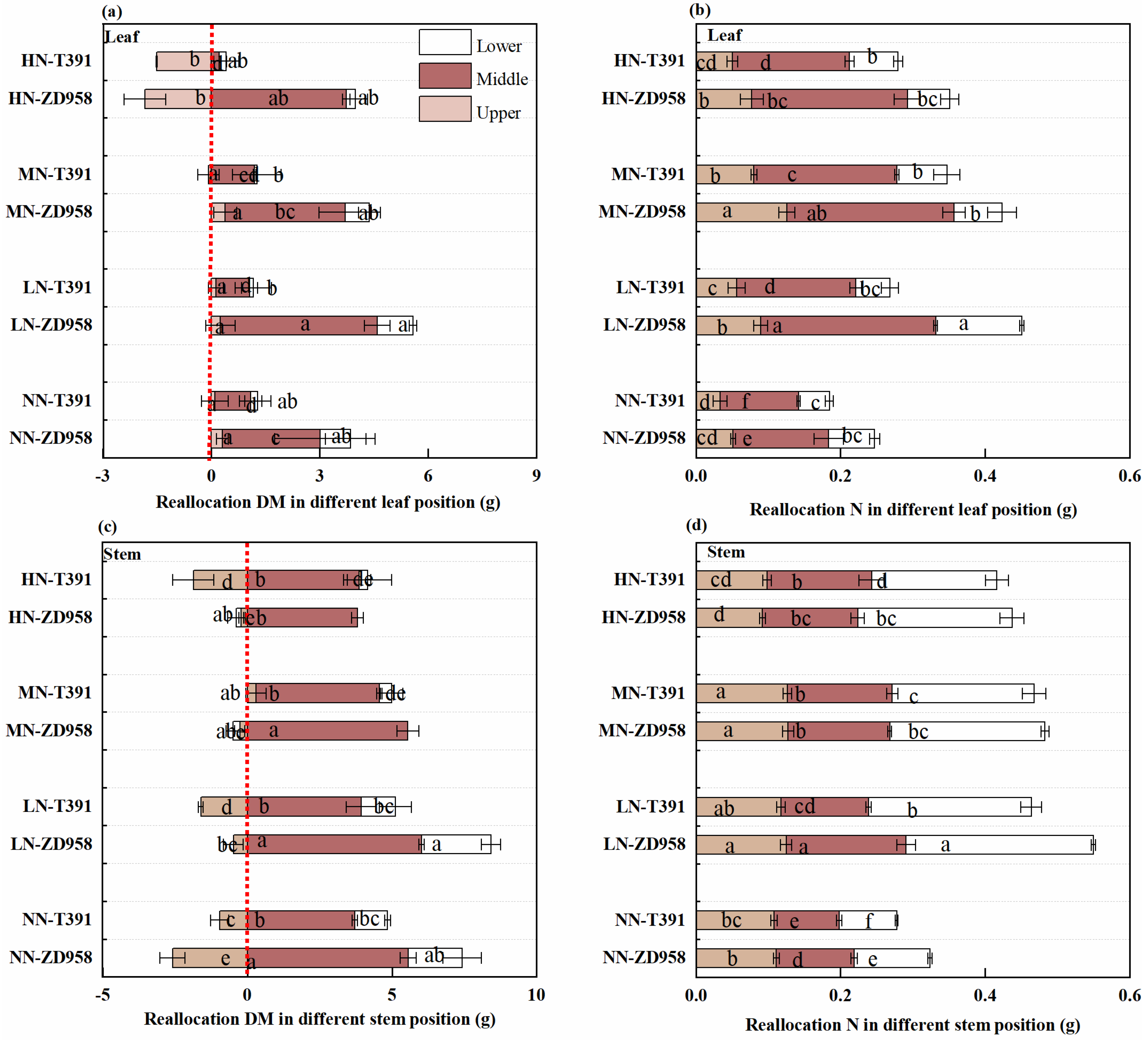
3.6. Reallocation of Leaf and Stem DM and N in Different Canopy Positions
3.7. Correlation Analysis
4. Discussion
4.1. Nitrogen × Hybrids: Grain Yield and Nitrogen-Use Efficiency
4.2. Nitrogen × Hybrids: DM Accumulation and Reallocation
4.3. The Different Patterns of DM and N Reallocation in Various Positions and Organs Within the Maize Canopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Zhu, Y.M.; Wang, G.F.; Liu, R.R.; Huang, D.; Song, M.M.; Zhang, Y.H.; Wang, H.; Wang, Y.C.; Shao, R.X.; et al. Maize yield increased by matching canopy light and nitrogen distribution via controlled-release urea/urea adjustment. Field Crops Res. 2024, 308, 109284. [Google Scholar] [CrossRef]
- Bk, A.; Shrestha, J.; Subedi, R. Grain yield and yield attributing traits of maize genotypes under different planting dates. Malays. J. Sustain. Agric. 2018, 2, 06–08. [Google Scholar] [CrossRef]
- Ali, A.; Jabeen, N.; Farruhbek, R.; Chachar, Z.; Laghari, A.A.; Chachar, S.; Ahmed, N.; Ahmed, S.; Yang, Z. Enhancing nitrogen use efficiency in agriculture by integrating agronomic practices and genetic advances. Front. Plant Sci. 2025, 16, 1543714. [Google Scholar] [CrossRef] [PubMed]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of organic farming for achieving sustainability in agriculture. Farming Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Li, R.F.; Hu, D.D.; Ren, H.; Yang, Q.L.; Dong, S.T.; Zhang, J.W.; Zhao, B.; Liu, P. How delaying post-silking senescence in lower leaves of maize plants increases carbon and nitrogen accumulation and grain yield. Crop J. 2022, 10, 853–863. [Google Scholar] [CrossRef]
- Abdul Aziz, M.; Brini, F.; Rouached, H.; Masmoudi, K. Genetically engineered crops for sustainably enhanced food production systems. Front. Plant Sci. 2022, 13, 1027828. [Google Scholar] [CrossRef]
- Zhang, W.N.; Li, H.G.; Zhang, J.L.; Shen, J.B.; Brown, H.; Wang, E.L. Contrasting patterns of accumulation, partitioning, and remobilization of biomass and phosphorus in a maize cultivar. Crop J. 2022, 10, 254–261. [Google Scholar] [CrossRef]
- Li, Y.Y.; Ming, B.; Fan, P.P.; Liu, Y.; Wang, K.R.; Hou, P.; Xue, J.; Li, S.K.; Xie, R.Z. Quantifying contributions of leaf area and longevity to leaf area duration under increased planting density and nitrogen input regimens during maize yield improvement. Field Crops Res. 2022, 283, 108551. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, M.; Zhou, B.Y.; Zhou, W.B.; Li, K.M.; Qi, H.; Jiang, Y.; Li, C.F. Understanding physiological mechanisms of variation in grain filling of maize under high planting density and varying nitrogen applicate rate. Front. Nutr. 2022, 9, 998946. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xiao, C.X.; Chen, X.C.; Li, Q.; Zhang, J.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Characterization of the plant traits contributed to high grain yield and high grain nitrogen concentration in maize. Field Crops Res. 2014, 159, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, C.H.; Wang, Y.N.; Sha, Y.; Hao, Z.H.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Nitrogen allocation and remobilization contributing to low-nitrogen tolerance in stay-green maize. Field Crops Res. 2021, 263, 108078. [Google Scholar] [CrossRef]
- Liu, Z.; Sha, Y.; Huang, Y.W.; Hao, Z.H.; Guo, W.Q.; Ke, L.H.; Chen, F.J.; Yuan, L.Y.; Mi, G.H. Efficient nitrogen allocation and reallocation into the ear in relation to the superior vascular system in low-nitrogen tolerant maize hybrid. Field Crops Res. 2022, 284, 108580. [Google Scholar] [CrossRef]
- Fan, P.P.; Ming, B.; Evers, J.B.; Li, Y.Y.; Li, S.K.; Xie, R.Z.; Anten, N.P.R. Nitrogen availability determines the vertical patterns of accumulation, partitioning, and reallocation of dry matter and nitrogen in maize. Field Crops Res. 2023, 297, 108927. [Google Scholar] [CrossRef]
- Guo, X.X.; Liu, W.M.; Yang, Y.S.; Liu, G.Z.; Ming, B.; Xie, R.Z.; Wang, K.R.; Li, S.K.; Hou, P. Optimal nitrogen distribution in maize canopy can synergistically improve maize yield and nitrogen utilization efficiency while reduce environmental risks. Agric. Ecosyst. Environ. 2025, 383, 109540. [Google Scholar] [CrossRef]
- Pommel, B.; Gallais, A.; Coque, M.; Quilleré, I.; Hirel, B.; Prioul, J.L.; Andrieu, B.; Floriot, M. Carbon and nitrogen allocation and grain filling in three maize hybrids differing in leaf senescence. Eur. J. Agron. 2006, 24, 203–211. [Google Scholar] [CrossRef]
- Chen, F.J.; Fang, Z.G.; Gao, Q.; Ye, Y.; Jia, L.; Yuan, L.; Mi, G.; Zhang, F. Evaluation of the yield and nitrogen use efficiency of the dominant maize hybrids grown in North and Northeast China. Sci. China Life Sci. 2013, 56, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Ciganda, V.; Gitelson, A.; Schepers, J. Vertical profile and temporal variation of chlorophyll in maize canopy: Quantitative “crop vigor” indicator by means of reflectance-based techniques. Agron. J. 2008, 100, 1409–1417. [Google Scholar] [CrossRef]
- Kumar, R.; Bishop, E.; Bridges, W.C.; Tharayil, N.; Sekhon, R.S. Sugar partitioning and source–sink interaction are key determinants of leaf senescence in maize. Plant Cell Environ. 2019, 42, 2597–2611. [Google Scholar] [CrossRef] [PubMed]
- Gallais, A.; Coque, M.; Quilléré, I. Modelling postsilking nitrogen fluxes in maize (Zea mays L.) using 15N-labelling field experiments. New Phytol. 2006, 172, 696–707. [Google Scholar] [CrossRef]
- Mi, G.H.; Chen, F.J.; Chun, L.; Guo, Y.F.; Tian, Q.Y.; Zhang, F.S. Biological characteristics of nitrogen efficient maize genotypes. J. Plant Nutr. Fertil. 2007, 13, 155–159. [Google Scholar] [CrossRef]
- Winterhalter, L.; Mistele, B.; Schmidhalter, U. Assessing the vertical footprint of reflectance measurements to characterize nitrogen uptake and biomass distribution in maize canopies. Field Crops Res. 2022, 129, 14–20. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Campos, H. Estatística Experimental Não-Paramétrica, 4th ed.; Departamento de Matemáticae Estatística-ESALQ: Piracicaba, Brazil, 1983. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book: New York, NY, USA, 1997. [Google Scholar]
- Ning, P.; Li, S.; Yu, P.; Zhang, Y.; Li, C.J. Post-silking accumulation and partitioning of dry matter, nitrogen, phosphorus and potassium in maize varieties differing in leaf longevity. Field Crops Res. 2013, 144, 19–27. [Google Scholar] [CrossRef]
- Parco, M.; D’Andrea, K.E.; Maddonni, G.Á. Maize prolificacy under contrasting plant densities and N supplies: I. Plant growth, biomass allocation and development of apical and sub-apical ears from floral induction to silking. Field Crops Res. 2022, 284, 108553. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Huang, Y.F.; Li, S.; Chu, X.; Ye, Y.L. Improving the growth, lodging and yield of different density-resistance maize by optimizing planting density and nitrogen fertilization. Plant Soil Environ. 2020, 66, 453–460. [Google Scholar] [CrossRef]
- Lee, E.A.; Tollenaar, M. Physiological basis of successful breeding strategies for maize grain yield. Crop Sci. 2007, 47, S202–S215. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. Physiological perspectives of changes over time in maize yield dependency on nitrogen uptake and associated nitrogen efficiencies: A review. Field Crops Res. 2012, 133, 48–67. [Google Scholar] [CrossRef]
- Tang, Q.; Ren, J.H.; Zhang, X.R.; Wu, C.; Zhang, Y.R.; Bian, D.H.; Liu, G.Z.; Cui, Y.H.; Du, X.; Wang, C.; et al. Plant growth retardant increases nitrogen utilization efficiency and harvest index in maize by optimizing Plant Horizontal-Vertical Ratio and vascular bundles morphology. J. Integr. Agric. 2025, in press. [Google Scholar] [CrossRef]
- Bänziger, M.; Edmeades, G.O.; Beck, D.; Bellon, M. Breeding for Drought and Nitrogen Stress Tolerance in Maize: From Theory to Practice; CIMMYT: El Batán, Mexico, 2000; ISBN 970-648-46-3. [Google Scholar]
- Liu, G.Z.; Yang, Y.S.; Guo, X.X.; Liu, W.M.; Xie, R.Z.; Ming, B.; Xue, J.; Wang, K.R.; Li, S.K.; Hou, P. A global analysis of dry matter accumulation and allocation for maize yield breakthrough from 1.0 to 25.0 Mg ha−1. Resour. Consery. Recy. 2023, 188, 106656. [Google Scholar] [CrossRef]
- Wu, J.; Song, Y.; Wan, G.Y.; Sun, L.Q.; Wang, J.X.; Zhang, Z.S.; Xiang, C.B. Boosting crop yield and nitrogen use efficiency: The hidden power of nitrogen-iron balance. New Crops 2025, 2, 100047. [Google Scholar] [CrossRef]
- Li, R.F.; Zhang, G.Q.; Xie, R.Z.; Hou, P.; Ming, B.; Xue, J.; Wang, K.R.; Li, S.K. Dynamics of high-yielding maize genotypes under intensive management across multiple environments. Eur. J. Agron. 2025, 162, 127368. [Google Scholar] [CrossRef]
- Ning, P.; Fritschi, F.B.; Li, C.J. Temporal dynamics of post-silking nitrogen fluxes and their effects on grain yield in maize under low to high nitrogen inputs. Field Crops Res. 2017, 204, 249–259. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhao, Y.; Guo, S.; Cheng, S.A.; Guan, Y.J.; Cai, H.G.; Mi, G.H.; Yuan, L.X.; Chen, F.J. Enhanced crown root number and length confers potential for yield improvement and fertilizer reduction in nitrogen-efficient maize cultivars. Field Crops Res. 2019, 241, 107562. [Google Scholar] [CrossRef]
- Wei, S.S.; Wang, X.Y.; Li, G.H.; Jiang, D.; Dong, S.T. Maize Canopy Apparent Photosynthesis and 13C-Photosynthate Reallocation in Response to Different Density and N Rate Combinations. Front. Plant Sci. 2019, 10, 1113. [Google Scholar] [CrossRef]
- Li, G.H.; Wang, L.F.; Li, L.; Lu, D.L.; Lu, W.P. Effects of fertilizer management strategies on maize yield and nitrogen use efficiencies under different densities. Agron. J. 2020, 112, 368–381. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Anten, N.P.R.; Schieving, F.; Werger, M.J.A. Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono and dicotyledonous species. Oecologia 1995, 101, 504–513. [Google Scholar] [CrossRef]
- Archontoulis, S.V.; Vos, J.; Yin, X.; Bastiaans, L.; Danalatos, N.G.; Struik, P.C. Temporal dynamics of light and nitrogen vertical distributions in canopies of sunflower, kenaf and cynara. Field Crops Res. 2011, 122, 186–198. [Google Scholar] [CrossRef]
- Shao, H.; Xia, T.T.; Wu, D.L.; Chen, F.J.; Mi, G.H. Root growth and root system architecture of field-grown maize in response to high planting density. Plant Soil 2018, 430, 395–411. [Google Scholar] [CrossRef]
- Qi, D.H.; Pan, C. Responses of shoot biomass accumulation, distribution, and nitrogen use efficiency of maize to nitrogen application rates under waterlogging. Agric. Water Manag. 2022, 261, 107352. [Google Scholar] [CrossRef]
- Han, C.; Wang, H.L.; Shi, W.; Bai, M.Y. The molecular associations between the SnRK1 complex and carbon/nitrogen metabolism in plants. New Crops 2024, 1, 100008. [Google Scholar] [CrossRef]



| N Level | Genotype | Dry Matter per Plant (DM) | Post DM/Grain DM (%) | HI | N Content per Plant (N) | NHI | Post N/Grain N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMR1 | DMR6 | Post DM (g) | Grain | NR1 | NR6 | Post N | Grain N (g) | ||||||
| (g) | (g) | DM (g) | (g) | (g) | |||||||||
| N0 | ZD958 | 115.2 g | 274.9 f | 159.7 e | 141.0 f | 113.3 ab | 0.51 d | 1.16 f | 1.92 g | 0.76 f | 1.22 e | 63.67 a | 61.93 d |
| T391 | 112.1 h | 254.8 g | 142.7 f | 125.1 g | 114.1 a | 0.49 e | 1.09 g | 1.64 h | 0.55 g | 0.95 f | 58.26 c | 57.40 e | |
| N1 | ZD958 | 132.8 e | 305.9 d | 173.1 d | 163.0 d | 106.2 d | 0.53 c | 2.03 d | 3.25 e | 1.22 e | 1.79 c | 55.21 d | 67.91 c |
| T391 | 123.2 f | 287.5 e | 164.2 e | 145.7 e | 112.7 ab | 0.51 d | 1.68 e | 2.43 f | 0.76 f | 1.42 d | 58.34 c | 53.31 f | |
| N2 | ZD958 | 146.5 b | 352.0 b | 205.5 b | 193.1 b | 106.5 d | 0.55 b | 2.44 b | 4.25 c | 1.81 c | 2.39 b | 56.37 d | 75.37 b |
| T391 | 134.5 d | 331.8 c | 197.3 c | 180.7 c | 109.1 c | 0.54 b | 2.22 c | 3.81 d | 1.59 d | 2.29 b | 60.24 b | 69.42 c | |
| N3 | ZD958 | 148.4 a | 389.8 a | 241.3 a | 219.5 a | 110.0 c | 0.56 a | 2.53 a | 5.18 a | 2.65 a | 3.13 a | 60.46 b | 84.51 a |
| T391 | 140.7 c | 381.4 a | 240.8 a | 215.0 a | 112.0 b | 0.56 a | 2.43 b | 4.96 b | 2.53 b | 3.06 a | 61.56 b | 82.81 a | |
| ANOVA | |||||||||||||
| N level (N) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Genotype (G) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | NS | *** | |
| N × G | *** | ** | ** | ** | *** | *** | *** | *** | *** | *** | *** | *** | |
| N Level | Genotype | Dry Matter Reallocation per Plant (DM) | N Reallocation per Plant (N) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amount (g) | Fraction (%) | Contribution to Grain DM (%) | Amount (g) | Fraction (%) | Contribution to Grain N (%) | ||||||||
| Leaf | Stem | Leaf | Stem | Leaf | Stem | Leaf | Stem | Leaf | Stem | Leaf | Stem | ||
| N0 | ZD958 | 3.23 b | 4.83 b | 9.32 b | 6.88 b | 6.66 a | 4.88 b | 0.25 e | 0.32 e | 45.57 a | 68.93 a | 37.38 b | 56.52 b |
| T391 | 1.29 c | 3.89 c | 3.94 cd | 5.63 bc | 3.17 c | 4.50 b | 0.18 f | 0.28 f | 37.85 b | 61.45 c | 39.70 a | 64.51 a | |
| N1 | ZD958 | 5.58 a | 7.94 a | 13.34 a | 9.98 a | 8.19 a | 6.13 a | 0.45 a | 0.55 a | 43.52 a | 65.26 b | 24.26 c | 36.39 d |
| T391 | 1.16 c | 3.51 c | 3.21 cd | 4.67 cd | 2.20 c | 3.21 c | 0.27 d | 0.46 bc | 32.07 cd | 65.86 b | 22.61 c | 46.44 c | |
| N2 | ZD958 | 4.50 a | 5.05 b | 9.46 b | 5.91 b | 4.90 b | 3.06 c | 0.42 b | 0.48 b | 33.09 c | 48.59 d | 13.84 d | 20.32 e |
| T391 | 1.52 c | 4.99 b | 3.59 cd | 6.21 b | 1.99 c | 3.43 c | 0.35 c | 0.47 b | 30.50 d | 49.00 d | 13.29 d | 21.36 e | |
| N3 | ZD958 | 2.90 b | 3.42 c | 6.00 c | 3.93 de | 2.74 c | 1.79 d | 0.35 c | 0.44 cd | 26.84 e | 40.65 e | 8.57 e | 12.98 f |
| T391 | 1.27 c | 2.44 d | 2.72 d | 3.04 e | 1.27 c | 1.41 d | 0.28 d | 0.42 d | 22.04 f | 41.93 e | 7.21 e | 13.72 f | |
| ANOVA | |||||||||||||
| N level (N) | * | *** | ** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Genotype (G) | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | NS | *** | |
| N × G | ** | *** | * | *** | ** | *** | *** | ** | *** | *** | ** | *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Piao, L.; Duan, W.; Bai, Y.; Zhu, N.; Tang, Q.; He, F.; Ren, H.; Gu, Y. Optimal Nitrogen Accumulation and Remobilization Can Synergistically Improve Maize Yield and Nitrogen-Use Efficiency Under Low-Nitrogen Conditions. Agronomy 2025, 15, 1159. https://doi.org/10.3390/agronomy15051159
Li X, Piao L, Duan W, Bai Y, Zhu N, Tang Q, He F, Ren H, Gu Y. Optimal Nitrogen Accumulation and Remobilization Can Synergistically Improve Maize Yield and Nitrogen-Use Efficiency Under Low-Nitrogen Conditions. Agronomy. 2025; 15(5):1159. https://doi.org/10.3390/agronomy15051159
Chicago/Turabian StyleLi, Xiang, Lin Piao, Wenhao Duan, Yan Bai, Nanheng Zhu, Qingquan Tang, Fangming He, Hong Ren, and Yan Gu. 2025. "Optimal Nitrogen Accumulation and Remobilization Can Synergistically Improve Maize Yield and Nitrogen-Use Efficiency Under Low-Nitrogen Conditions" Agronomy 15, no. 5: 1159. https://doi.org/10.3390/agronomy15051159
APA StyleLi, X., Piao, L., Duan, W., Bai, Y., Zhu, N., Tang, Q., He, F., Ren, H., & Gu, Y. (2025). Optimal Nitrogen Accumulation and Remobilization Can Synergistically Improve Maize Yield and Nitrogen-Use Efficiency Under Low-Nitrogen Conditions. Agronomy, 15(5), 1159. https://doi.org/10.3390/agronomy15051159





