Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks
Abstract
1. Introduction
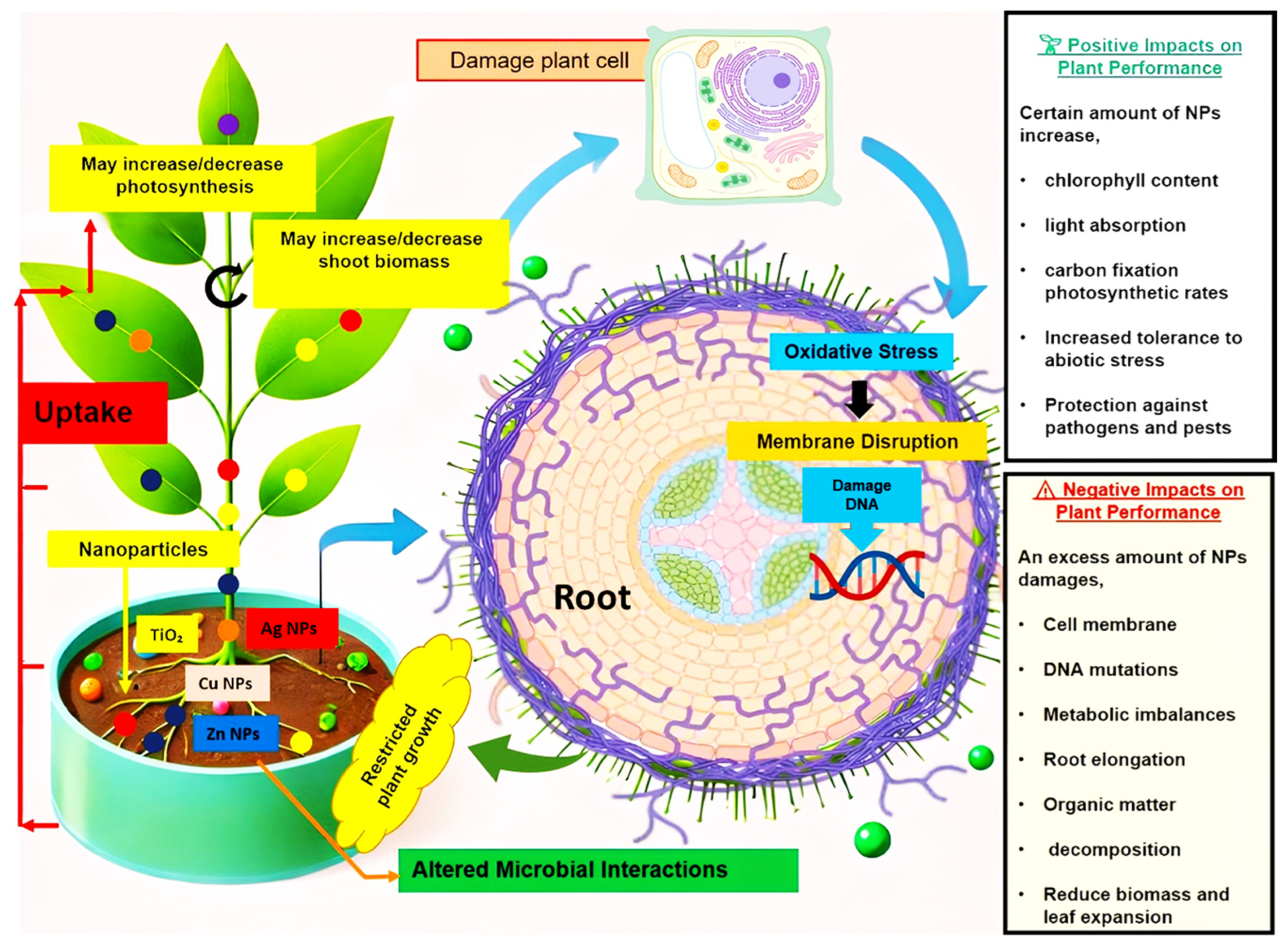
2. Mechanisms of Nanoparticle Action on Plants
2.1. Nanoparticles: Cellular Interactions and Physiological Effects in Plants
2.2. Modulation of Plant Defense Responses by Nanoparticles
| Plant | NPs Nature | Potential Effects | Reference |
|---|---|---|---|
| Saffron (Crocus sativus) | ZnO NPs | Increasing POX (peroxidase) and CAT (catalase) activity | [47] |
| Wheat (Triticum aestivum) | ZnO NPs | lowering oxidative stress (higher activity of SOD (superoxide dismutase) and POD (peroxidase)) | [89] |
| Soybean (Glycine max) | ZnO NPs | Higher activities of SOD, CAT, POD, and APX enzymes. | [90] |
| Rice (Oryza sativa) callus | Ag NPs | Decreasing levels of H2O2 | [46] |
| Spinach (Spinacia oleracea) | TiO2 NPs | Increasing activity of SOD, CAT, APX, and guaiacol peroxidase. Decreased level of superoxide radicals, H2O2 | [91] |
| Maize (Zea mays L.) | TiO2 NPs | Activation of SOD (superoxide dismutase) and glutathione S-transferase (GST) | [92] |
| Rice (Oryza sativa) | Tio2, Si NPs | Lowering oxidative stress (increasing activity of CAT, POD, and APX | [93] |
| (Oryza sativa cv. Gobindabhog L.) | Fe-0 NPs | Increasing the activity of SOD, CAT and glutathione peroxidase, | [60] |
| Evening primrose (Oenthera biennis) | Fe2O3 NPs | Increasing the activity of CAT, SOD, and POD | [94] |
| Brassica napus L. | γ-Fe2O3 NP | Decreasing the level of H2O2 | [95] |
| Sunflower (Helianthus annuus) | Fe-0 NPs | Increasing the activity of antioxidant enzymes superoxide dismutase, peroxidase, catalase and ascorbate peroxidase | [78] |
| Wheat (Triticum aestivum) | [96] | ||
| Maize (Zea mays) | Fe3O4 | [97] | |
| Arabidopsis thaliana L. | CeO2 NPs | Scavenging of hydroxyl radicals, superoxide anions, and H2O2 in chloroplasts | [98] |
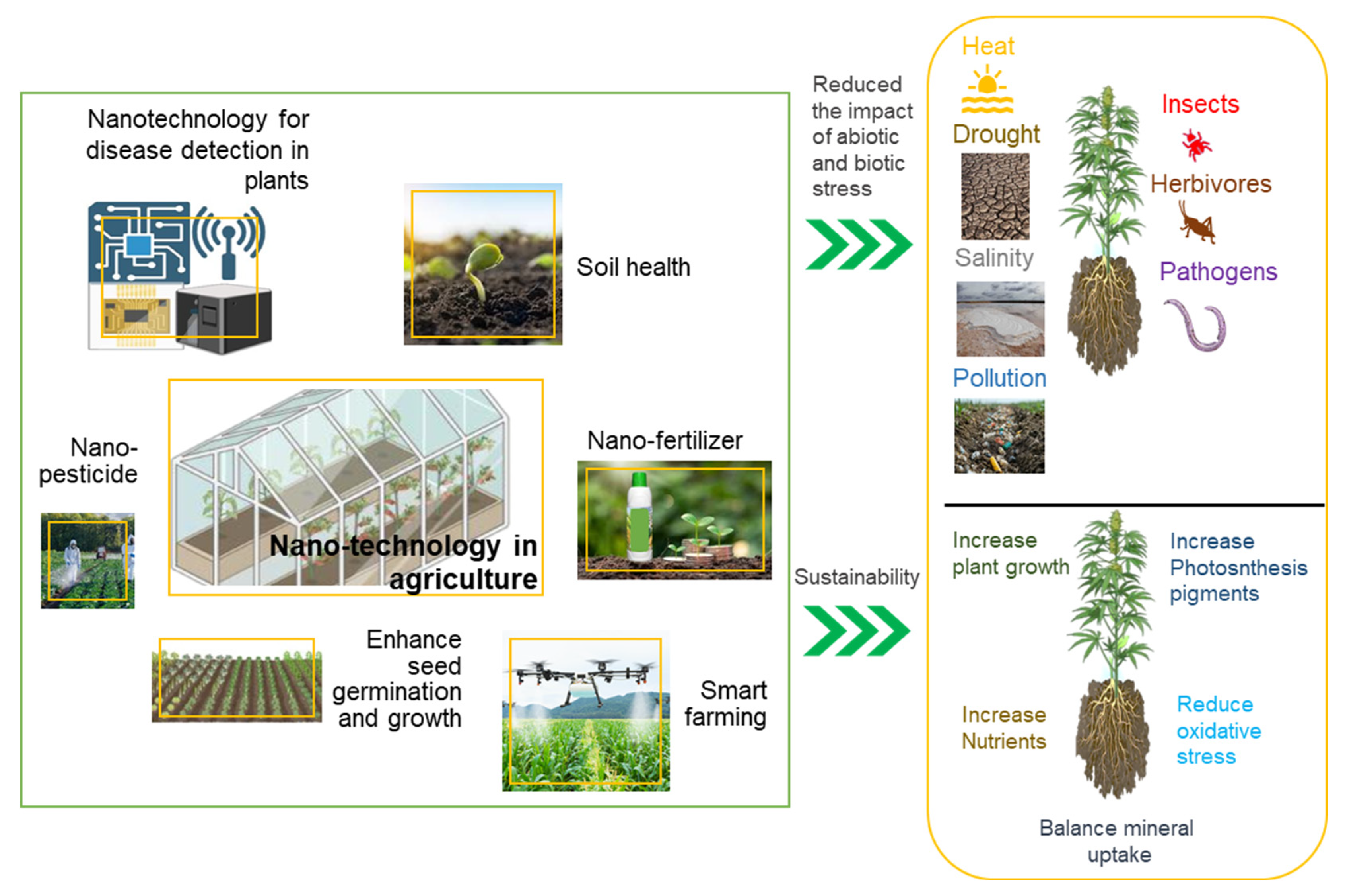
3. Applications of Nanoparticles in Agriculture
3.1. Improving Growth, Development of Plant Nutrition
| Plant | NPs Nature | Potential Effects | Reference |
|---|---|---|---|
| Rice (Oryza sativa) | Ag NPs | Increasing water uptake into the seeds. Up-regulation of aquaporin genes | [45] |
| Arabidopsis thaliana | Increasing the rate of evapotranspiration | [117] | |
| Common bean (Phaseolus vulgaris) | Increasing the concentration of indole-3acetic acid (IAA), gibberellin GA3 and total cytokinins | [118] | |
| Two orchids (Lilium cv. Mona Lisa and cv. Little John) | Increasing the chlorophyll and carotenoid content, potassium, calcium, and sulfur content | [119] | |
| Wheat (Triticum aestivum, variety Galaxy-2013) | TiO2 NPs | Stimulating activity of microorganisms in rhizosphere, increased phosphorus content in shoots | [120] |
| Tobacco (Nicotiana benthamiana) | Significant increases in plant biomass | [121] | |
| Rice (Oryza sativa) | higher levels of chlorophyll and carotenoids, higher transpiration rate | [122] | |
| Maize (Zea mays L.) | Increasing glycine, serine and threonine accumulation and promoted energy metabolism (citrate and galactose cycle) | [123] | |
| Vetiveria zizanioides | Increasing chlorophyll content and photochemical efficiency of photosystem II, increased activity of nitrate reductase and carbonic anhydrase | [48] | |
| Sorghum (Sorghum bicolor var. 251) | ZnO NPs | Increasing uptake of zinc, boron and copper into plants, increased chlorophyll content | [124] |
| Saffron (Crocus sativus) | Increasing content of chlorophyll, relative water content, soluble protein content, | [47] | |
| Lemon balm (Melissa officinalis) seedlings | Increasing accumulation of potassium, iron and zinc, increased activity of nitrate reductase. | [125] | |
| Foxtail millet (Setaria italica) | Increasing oil and nitrogen content in grains | [126] | |
| Wheat (Triticum aestivum) | increasing zinc content in grains, | [127] | |
| Arabidopsis thaliana | Fe NPs | Increasing photosynthesis rate, assimilation rate, intracellular CO2 concentration, transpiration rate, and stomatal conductance) as consequence of increased stomatal opening. Decreased pH in rhizosphere resulting increased P availability | [59] |
| Wheat (Triticum aestivum) | Increasing root length, plant height, biomass growth and chlorophyll content | [128] | |
| Muskmelon (Cucumis melo) | NPs served as a source of Fe supporting chlorophyll synthesis | [129] | |
| Rice (Oryza sativa) | Higher water content, higher activity of hydrolytic enzymes amylase and protease | [60] | |
| Lettuce (Lactuca sativa) | TiO2 and Fe3O4 | Increasing P uptake | [130] |
| Soybean (Glycine max) | CeO2 NPs | Increasing stomatal conductance, enhanced photosynthesis rate, increased Rubisco activity, increased NADPH regeneration rate and synthesis of ribulose-1,5-bisphosphate | [131] |
| Jalapeño pepper (Capsicum annuum) | Mn NPs | Source of manganese as micronutrient | [132] |
3.2. Protecting Plants Against Pathogens
3.3. Increasing Plant Tolerance to Abiotic Stresses
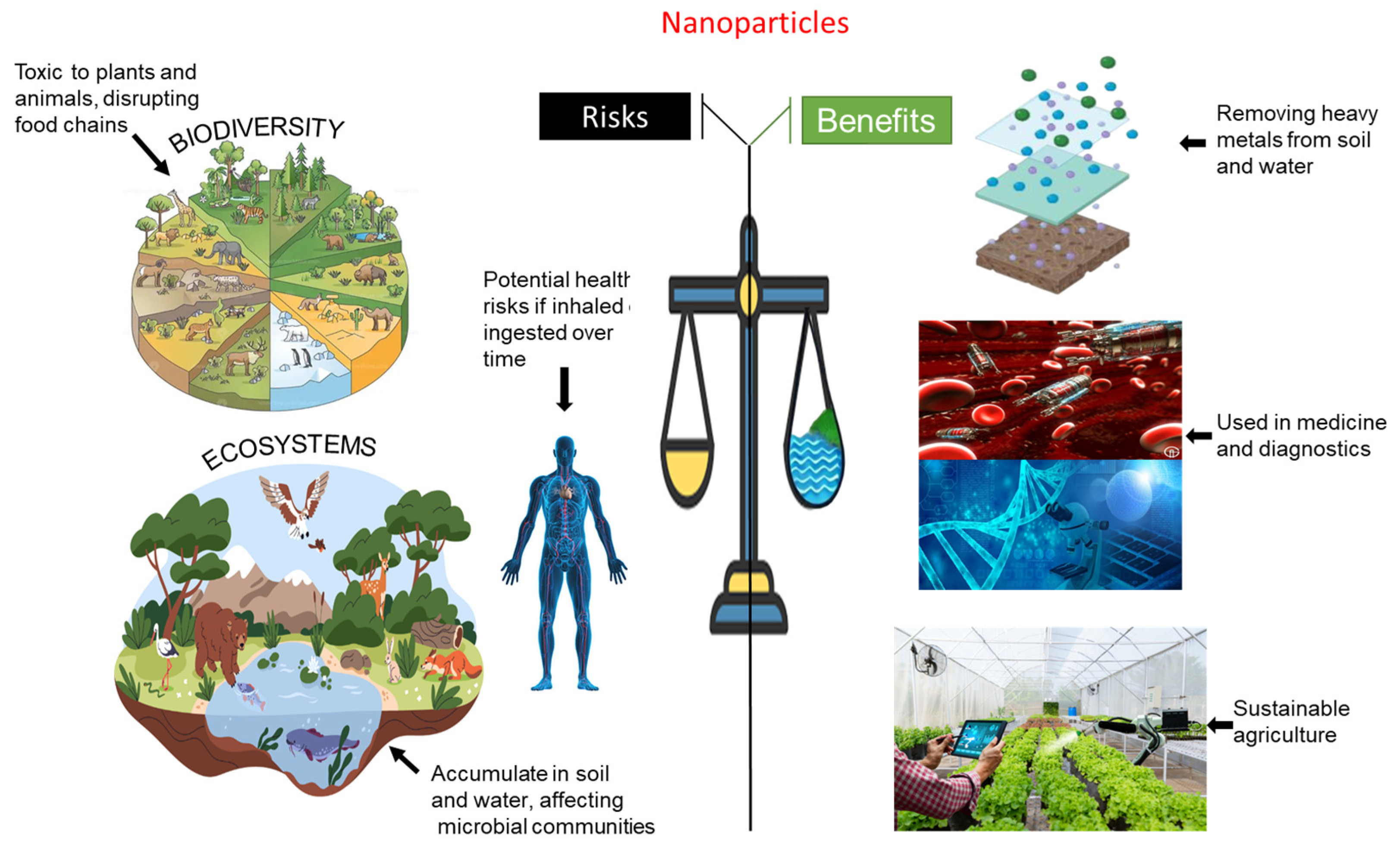
4. Environmental Aspects of Nanoparticles
4.1. Environmental Toxicity of Nanoparticles (NPs)
| Plant | NPs Type | Effects | Reference |
|---|---|---|---|
| Wheat (Triticum aestivum L.) Sorghum (Sorghum bicolor L. Moench) Garden cress (Lepidium sativum L.) Mustard (Sinapis alba L.) | AgNPs | Germination inhibition Inhibition of root and shoot growth Disintegration of cell membranes | [207] |
| Wheat (Triticum aestivum L.) | Ag NPs | Growth suppression and changes in cell division and structure | [208] |
| Camelina sativa L | Ag NPs | Affecting the seedling growth and photosynthetic pigments. | [209] |
| Cucumber (Cucumis sativus L.). | SiO2/ZnO NPs | Upregulated or downregulated the contents of sugars, amino acids, glycosides and organic acids, and secondary metabolites | [210] |
| Glycine max | ZnO/TiO2 NPs | Alteration of primary and secondary metabolites levels regulate the stress level in plant | [211] |
| A. thaliana seedlings | Yttrium oxide (Y2O3) NPs | Reduced the lignin synthesis-related gene expression, and increased abscisic acid and ethylene signaling pathway | [212] |
| Duckweed (Lemna minor L.) | CeO2 NPs | Hindered the root elongation | [213] |
| Bean crop (Phaseolus vulgaris L.) | CeO2 NPs | Chromosome abnormality and malformations in pollen grains and defects in pollen walls | [214] |
| Rice (Oryza sativa L.) | CuO NPs | Damaging the enzymes by the accumulation of excess Cu and ROS induced oxidative damage | [215] |
| Pepper (Capsicum annuum L.) | Cu NPs | Enhanced lipid peroxidation level and H2O2 content, thus implying oxidative stress and potentially causing impairment in plasma membrane integrity | [216] |
| Reduced the expression of the Mevalonate kinase (MVK) gene involved in terpenoid metabolism, which limits photosynthesis and decreases energy production efficiency, thereby leading to reduced transcription |
4.2. Potential Impact of Nanoparticles on Biodiversity
4.3. Economic Costs and Benefits of Nanoparticle Use
5. Future Trends in Nanoparticle Research in Agriculture
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic stress in crop production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 228798. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, B.; Singh, B.N.; Hidangmayum, A.; Jatav, H.S.; Chandra, K.; Singhal, R.K.; Sathyanarayana, E.; Patra, A.; Mohapatra, K.K. Physiological mechanisms and adaptation strategies of plants under nutrient deficiency and toxicity conditions. In Plant Perspectives to Global Climate Changes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 173–194. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Lamsaadi, N.; Farissi, M. Biostimulatory effects of ascorbic acid in improving plant growth, photosynthesis-related parameters and mitigating oxidative damage in alfalfa (Medicago sativa L.) under salt stress condition. Biologia. 2024, 79, 2375–2385. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Wani, M.Y.; Hashim, M.A.; Nabi, F.; Malik, M.A. Nanotoxicity: Dimensional and morphological concerns. Adv. Phys. Chem. 2011, 2011, 450912. [Google Scholar] [CrossRef]
- Mcoyi, M.P.; Mpofu, K.T.; Sekhwama, M.; Mthunzi-Kufa, P. Developments in Localized Surface Plasmon Resonance. In Plasmonics; Springer Nature: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Juma, M.W.; Birech, Z.; Mwenze, N.M.; Ondieki, A.M.; Maaza, M.; Mokhotjwa, S.D. Localized surface plasmon resonance sensing of Trenbolone acetate dopant using silver nanoparticles. Sci. Rep. 2024, 14, 5721. [Google Scholar] [CrossRef]
- Mondal, S.; Truong, T.T.; Doan, V.H.M.; Phan, T.T.V.; Choi, J.; Lee, B.; Oh, J. Nano-biosensors for Detecting Microbial Pathogens in Agriculture. In Nano-Microbiology for Sustainable Development; Springer Nature: Cham, Switzerland, 2025; pp. 193–212. [Google Scholar] [CrossRef]
- Muthukrishnan, L. An overview on the nanotechnological expansion, toxicity assessment and remediating approaches in Agriculture and Food industry. Environ. Technol. Innov. 2022, 25, 102136. [Google Scholar] [CrossRef]
- Semaltianos, N.G. Nanoparticles by Laser Ablation. Crit. Rev. Solid State Mater. Sci. 2010, 35, 105–124. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Pantidos, N.; Horsfall, L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Pérez-Hernández, H.; Fernández-Luqueño, F.; Huerta-Lwanga, E.; Mendoza-Vega, J.; Álvarez-Solís José, D. Effect of engineered nanoparticles on soil biota: Do they improve the soil quality and crop production or jeopardize them? Land Degrad. Dev. 2020, 31, 2213–2230. [Google Scholar] [CrossRef]
- Achari, G.A.; Kowshik, M. Recent developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. J. Agric. Food Chem. 2018, 66, 8647–8661. [Google Scholar] [CrossRef]
- Phogat, N.; Khan, S.A.; Shankar, S.; Ansary, A.A.; Uddin, I. Fate of inorganic nanoparticles in agriculture. Adv. Mater. Lett 2016, 7, 3–12. [Google Scholar] [CrossRef]
- Saritha, G.N.G.; Anju, T.; Kumar, A. Nanotechnology—Big impact: How nanotechnology is changing the future of agriculture? J. Agric. Food Res. 2022, 10, 100457. [Google Scholar] [CrossRef]
- Jafir, M.; Irfan, M.; Zia-ur-Rehman, M.; Hafeez, F.; Ahmad, J.N.; Sabir, M.A.; Zulfiqar, U.; Iqbal, R.; Zulfiqar, F.; Moosa, A. The global trend of nanomaterial usage to control the important agricultural arthropod pests: A comprehensive review. Plant Stress 2023, 10, 100208. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Mathur, T.; Maheshwari, J.; Nagella, P. Interaction of Nanomaterials with Plant Macromolecules: Nucleic Acid, Proteins and Hormones. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications; Springer International Publishing: Cham, Switzerland, 2023; pp. 231–271. [Google Scholar] [CrossRef]
- Francis, D.V.; Abdalla, A.K.; Mahakham, W.; Sarmah, A.K.; Ahmed, Z.F. Interaction of plants and metal nanoparticles: Exploring its molecular mechanisms for sustainable agriculture and crop improvement. Environ. Int. 2024, 190, 108859. [Google Scholar] [CrossRef]
- Zou, M.; Yu, K.; Huang, Y.; Sheng, Q.; Chen, Y.; Liu, H.; Zhu, Z.; Feang, N.; Ma, D.; Dou, D. Activation of ROS signaling molecule by AgNPs: Molecular mechanisms in roses under abiotic stress, exploration of stress memory, and impact on root microbiota. In Plant Soil; Springer Nature: Cham, Switzerland, 2025; pp. 1–22. [Google Scholar] [CrossRef]
- Omar, S.A.; Elsheery, N.I.; Pashkovskiy, P.; Kuznetsov, V.; Allakhverdiev, S.I.; Zedan, A.M. Impact of Titanium Oxide Nanoparticles on Growth, Pigment Content, Membrane Stability, DNA Damage, and Stress-Related Gene Expression in Vicia faba under Saline Conditions. Horticulturae 2023, 9, 1030. [Google Scholar] [CrossRef]
- Mokhtarabadi, E.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Saadatmand, S.; Ebadi, M. Selenium nanoparticles affected growth and secondary metabolism in chicory seedlings epigenetically by modifying DNA methylation and transcriptionally by upregulating DREB1A transcription factor and stimulating genes involved in phenylpropanoid metabolism. Environ. Pollut. 2025, 370, 125907. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Buja, I.; Sabella, E.; Monteduro, A.G.; Chiriacò, M.S.; De Bellis, L.; Luvisi, A.; Maruccio, G. Advances in Plant Disease Detection and Monitoring: From Traditional Assays to In-Field Diagnostics. Sensors 2021, 21, 2129. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.V.; Asif, A.; Ahmed, Z.F.R. Nanoparticle-Enhanced Plant Defense Mechanisms Harnessed by Nanotechnology for Sustainable Crop Protection. In Nanoparticles in Plant Biotic Stress Management; Springer Nature: Cham, Switzerland, 2024; pp. 451–491. [Google Scholar] [CrossRef]
- Sun, X.D.; Ma, J.Y.; Feng, L.J.; Duan, J.L.; Yuan, X.Z. Precise tracking of nanoparticles in plant roots. Nat. Protoc. 2024, 20, 248–271. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E.J. Nanoparticle exposure and hormetic dose–responses: An update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Cameron, S.J.; Sheng, J.; Hosseinian, F.; Willmore, W.G. Nanoparticle effects on stress response pathways and nanoparticle–protein interactions. Int. J. Mol. Sci. 2022, 23, 7962. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; MEbrahim, A.A.; Abd El-Mageed, T.A.; et al. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, R.; Li, Y.S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Landa, P.; Prerostova, S.; Petrova, S.; Knirsch, V.; Vankova, R.; Vanek, T. The transcriptomic response of Arabidopsis thaliana to zinc oxide: A comparison of the impact of nanoparticle, bulk, and ionic zinc. Environ. Sci. Technol. 2015, 49, 14537–14545. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Banerjee, K.; Pramanik, P.; Maity, A.; Joshi, D.; Wani, S.; Krishnan, P. Methods of Using Nanomaterials to Plant Systems and Their Delivery to Plants (Mode of Entry, Uptake, Translocation, Accumulation, Biotransformation and Barriers). In Advances in Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 123–152. [Google Scholar] [CrossRef]
- Newkirk, G.M.; De Allende, P.; Jinkerson, R.E.; Giraldo, J.P. Nanotechnology Approaches for Chloroplast Biotechnology Advancements. Front. Plant Sci. 2021, 12, 691295. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Fatemi, H.; Rizwan, M. Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: A review. Ecotoxicol. Environ. Saf. 2021, 225, 112769. [Google Scholar] [CrossRef]
- Mohammed, J.; Rashmi, R.; Surya Ulhas, R.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.; Shehata, W.F.; Almaghasla, M.I. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Manickavasagam, M.; Pavan, G.; Vasudevan, V. A comprehensive study of the hormetic influence of biosynthesized AgNPs on regenerating rice calli of indica cv. IR64. Sci. Rep. 2019, 9, 8821. [Google Scholar] [CrossRef]
- Rostami, M.; Talarposhti, R.M.; Mohammadi, H.; Demyan, M.S. Morpho-physiological Response of Saffron (Crocus sativus L.) to Particle Size and Rates of Zinc Fertilizer. Commun. Soil Sci. Plant Anal. 2019, 50, 1250–1257. [Google Scholar] [CrossRef]
- Shabbir, A.; Khan, M.M.A.; Ahmad, B.; Sadiq, Y.; Jaleel, H.; Uddin, M. Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash. Photosynth. 2019, 57, 599–606. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Vojodi Mehrabani, L.; Bonabian, Z.; Aazami, M.A.; Rasouli, F.; Feldo, M.; Strzemski, M.; Dresler, S. Foliar Application of Cerium Oxide-Salicylic Acid Nanoparticles (CeO2:SA Nanoparticles) Influences the Growth and Physiological Responses of Portulaca oleracea L. Under Salinity. Int. J. Mol. Sci. 2021, 23, 5093. [Google Scholar] [CrossRef] [PubMed]
- Mawale, K.S.; Nandini, B.; Giridhar, P. Copper and Silver Nanoparticle Seed Priming and Foliar Spray Modulate Plant Growth and Thrips Infestation in Capsicum spp. ACS Omega 2024, 9, 3430–3444. [Google Scholar] [CrossRef] [PubMed]
- Çekiç, F.Ö.; Ekinci, S.; İnal, M.S.; Özakça, D. Silver nanoparticles induced genotoxicity and oxidative stress in tomato plants. Turk. J. Biol. 2017, 41, 700–707. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, J.; Zhang, Y.; Han, S.; Zhang, J.; Jia, G. DNA oxidative damage as a sensitive genetic endpoint to detect the genotoxicity induced by titanium dioxide nanoparticles. Nanomaterials 2022, 12, 2616. [Google Scholar] [CrossRef]
- Panda, K.K.; Golari, D.; Venugopal, A.; Achary, V.M.; Phaomei, G.; Parinandi, N.L.; Sahu, H.K.; Panda, B.B. Green synthesized zinc oxide (ZnO) nanoparticles induce oxidative stress and DNA damage in Lathyrus sativus L. root bioassay system. Antioxidants 2017, 6, 35. [Google Scholar] [CrossRef]
- Atha, D.H.; Wang, H.; Petersen, E.J.; Cleveland, D.; Holbrook, R.D.; Jaruga, P.; Dizdaroglu, M.; Xing, B.S.; Nelson, B.C. Copper Oxide Nanoparticle Mediated DNA Damage in Terrestrial Plant Models. Environ. Sci. Technol. 2012, 46, 1819–1827. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Abd El-Aziz, M.; Abd Elwahed, M.S.; Shaaban, E. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kang, Y.; Chang, Y.; Kim, J. Effects of Zerovalent Iron Nanoparticles on Photosynthesis and Biochemical Adaptation of Soil-Grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef]
- Guha, T.; Ravikumar, K.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the mobilization of native phosphorus in the mung bean rhizosphere using ZnO nanoparticles synthesized by soil fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman, M.; Mfarrej, M.F.B.; Usman, M.; Anayatullah, S.; Rizwan, M.; Alharby, H.F.; Abu Zeid, I.M.; Alabdallah, N.M.; Ali, S. Effect of iron nanoparticles and conventional sources of Fe on growth, physiology and nutrient accumulation in wheat plants grown on normal and salt-affected soils. J. Hazard. Mater. 2023, 458, 131861. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Hassan, Z.U.; Akram, M.A.; Tariq, R.; Brestic, M.; Xie, W. Nanoparticle’s uptake and translocation mechanisms in plants via seed priming, foliar treatment, and root exposure: A review. Environ. Sci. Pollut. Res. 2022, 29, 89823–89833. [Google Scholar] [CrossRef]
- Feigl, G. The impact of copper oxide nanoparticles on plant growth: A comprehensive review. J. Plant Interact. 2023, 18, 2243098. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Bouhadi, M.; El Kouali, M.H.; Samir, K.; Elbouhmadi, K.; Talbi, M.; Fougrach, H. Exogenous Application of Thiamine and Nicotinic Acid Improves Tolerance and Morpho-physiological Parameters of Lens culinaris Under Lead (Pb) Exposure. J. Plant Growth Regul. 2024, 43, 4185–4198. [Google Scholar] [CrossRef]
- Soujoud, R.; Lamsaadi, N.; Bouhadi, M.; El Moukhtari, A.; Ourribane, M. Physiological and Biochemical Responses of Maize (Zea mays L.) to the Application of Re-Treated Urban Wastewater Using Wood Waste Biochar. J. Ecol. Eng. 2024, 25, 180–193. [Google Scholar] [CrossRef]
- Bouhadi, M.; Daoui, O.; El Hajjouji, H.; Elkhattabi, S.; Chtita, S.; El Kouali, M.; Talbi, M.; Fougrach, H. Study of the competition between Pi and Cr (VI) for the use of Pi-transporter at Vicia faba L. Using molecular modeling. Plant Physiol. Biochem. 2023, 196, 695–702. [Google Scholar] [CrossRef]
- Bouhadi, M.; Daoui, O.; El Hajjouji, H.; Elkhattabi, S.; Chtita, S.; El Kouali, M.; Talbi, M.; Fougrach, H. Physiological and molecular modeling investigations of the relationship between sulfate and chromium VI uptake in Vicia faba L. Biocatal. Agric. Biotechnol. 2022, 47, 102554. [Google Scholar] [CrossRef]
- Bouhadi, M.; Elkouali, M.H.; Talbi, M.; Amegrissi, F.; Fougrach, H. Phytoextraction of chromium VI by Raphanus sativus L. under exogenous application of citric acid. Bot. Pacifica 2022, 11, 89–93. [Google Scholar] [CrossRef]
- Bouhadi, M.; Abchir, O.; Yamari, I.; El Hamsas El Youbi, A.; Azgaoui, A.; Chtita, S.; El Hajjouji, H.; El Kouali, M.; Talbi, M.; Fougrach, H. Genotoxic effects and mitosis aberrations of chromium (VI) on root cells of Vicia faba and its molecular docking analysis. Plant Physiol. Biochem. 2024, 207, 108361. [Google Scholar] [CrossRef]
- El moukhtari, A.; Lamsaadi, N.; Bouhadi, M.; Abchir, O.; Chtita, S.; Samir, K.; El Rasafi, T.; Ghoulam, C.; Farissi, M. Uptake and competition between cadmium nanoparticles and essential nutrients (Fe, Mg and Mn) in Phaseolus vulgaris L. using a molecular docking approach. Euro-Mediterr. J. Environ. Integr. 2025, 1–11. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Thomas, M.B.; Thomas, R.; Raichur, A.M.; Chakravortty, D. Interaction of Silver Nanoparticles with Serum Proteins Affects Their Antimicrobial Activity In Vivo. Antimicrob. Agents Chemother. 2013, 57, 4945. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef]
- Madany, M.M.; Saleh, A.M.; Habeeb, T.H.; Hozzein, W.N.; Abd Elgawad, H. Silicon dioxide nanoparticles alleviate the threats of broomrape infection in tomato by inducing cell wall fortification and modulating ROS homeostasis. Environ. Sci. Nano 2020, 7, 1415–1430. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Explicating the cross-talks between nanoparticles, signaling pathways and nutrient homeostasis during environmental stresses and xenobiotic toxicity for sustainable cultivation of cereals. Chemosphere 2022, 286, 131827. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Seed priming with zinc oxide nanoparticles to enhance crop tolerance to environmental stresses. Int. J. Mol. Sci. 2023, 24, 17612. [Google Scholar] [CrossRef]
- Mohammadi, H.; Amani-Ghadim, A.R.; Matin, A.A.; Ghorbanpour, M. Fe0 nanoparticles improve physiological and antioxidative attributes of sunflower (Helianthus annuus) plants grown in soil spiked with hexavalent chromium. 3 Biotech 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.W.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Bhatti, K.H.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.M.A.; Elansary, H.O. Seed Priming with the Selenium Nanoparticles Maintains the Redox Status in the Water Stressed Tomato Plants by Modulating the Antioxidant Defense Enzymes. Plants 2022, 12, 1556. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. S. Afr. J. Bot. 2022, 151, 889–899. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Humbal, A.; Pathak, B. Application of Nanotechnology in Plant Growth and Diseases Management: Tool for Sustainable Agriculture. In Agricultural and Environmental Nanotechnology; Interdisciplinary Biotechnological, Advances; Fernandez-Luqueno, F., Patra, J.K., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Şahin, E.Ç.; Aydın, Y.; Utkan, G.; Uncuoğlu, A.A. Nanotechnology in agriculture for plant control and as biofertilizer. In Synthesis of Bionanomaterials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 469–492. [Google Scholar] [CrossRef]
- Li, W.; Keller, A.A. Assessing the Impacts of Cu and Mo Engineered Nanomaterials on Crop Plant Growth Using a Targeted Proteomics Approach. ACS Agric. Sci. Technol. 2023, 4, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, H.; Esmaiel Pour, B.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Chattha, M.U.; Khan, I.; Khan, T.A.; Nawaz, M.; Tang, H.; Noor, M.A.; Asseri, T.A.; Hashem, M.; Guoqin, H. Zinc Seed Priming Alleviates Salinity Stress and Enhances Sorghum Growth by Regulating Antioxidant Activities, Nutrient Homeostasis, and Osmolyte Synthesis. Agronomy 2024, 14, 1815. [Google Scholar] [CrossRef]
- Fouda, H.M.; Saied, E.; Abdelmouty, E.S.; Osman, M.S. Ameliorative role of copper nanoparticle in alleviating salt-induced oxidative stress in fenugreek (Trigonella foenum-graecum L.) plants. Biocatal. Agric. Biotechnol. 2024, 57, 103095. [Google Scholar] [CrossRef]
- Taran, N.; Storozhenko, V.; Svietlova, N.; Batsmanova, L.; Shvartau, V.; Kovalenko, M. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 2017, 12, 60. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc Oxide Nanoparticles Application Alleviates Arsenic (As) Toxicity in Soybean Plants by Restricting the Uptake of as and Modulating Key Biochemical Attributes, Antioxidant Enzymes, Ascorbate-Glutathione Cycle and Glyoxalase System. Plants 2020, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Mingyu, S.; Xiao, W.; Chao, L.; Chunxiang, Q.; Liang, C.; Huang Hao, H.; Xiaoqing, L.; Fashui, H. Antioxidant Stress is Promoted by Nano-anatase in Spinach Chloroplasts Under UV-B Radiation. Biol. Trace Elem. Res. 2018, 121, 69–79. [Google Scholar] [CrossRef]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2019, 239, 124794. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Malik, S.; Qayyum, M.F.; Alamri, S.; Alyemeni, M.; Ahmad, P. Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 72. [Google Scholar] [CrossRef]
- Asadi-Kavan, Z.; Khavari-Nejad, R.A.; Iranbakhsh, A.; Najafi, F. Cooperative effects of iron oxide nanoparticle (α-Fe2O3) and citrate on germination and oxidative system of evening primrose (Oenthera biennis L.). J. Plant Interact. 2020, 15, 166–179. [Google Scholar] [CrossRef]
- Palmqvist, N.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedlings. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef]
- Jalali, M.; Ghanati, F.; Modarres-Sanavi, A.M. Effect of Fe3O4 nanoparticles and iron chelate on the antioxidant capacity and nutritional value of soil cultivated maize (Zea mays) plants. Crop Pasture Sci. 2016, 67, 621–628. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef]
- Garg, S.; Rumjit, N.P.; Roy, S. Smart agriculture and nanotechnology: Technology, challenges, and new perspective. Adv. Agrochem 2024, 3, 115–125. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Nelwamondo, A.A.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal nanoparticles in agriculture: A review of possible use. Coatings 2020, 12, 1586. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, H.; Joshi, P. Nanofertilisers, nanopesticides and nanosensors in agriculture. Nanosci. Food Agric. 2016, 1, 247–282. [Google Scholar] [CrossRef]
- Beegum, S.; Das, S. Nanosensors in agriculture. In Agricultural Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 465–478. [Google Scholar] [CrossRef]
- Chhipa, H. Applications of nanotechnology in agriculture. Methods Microbiol. 2018, 46, 115–142. [Google Scholar] [CrossRef]
- Mai, K.; Williams, R.A. Response of Oak and Maple Seed Germination and Seedling Growth to Different Manganese Fertilizers in a Cultured Substratum. Forests 2019, 10, 547. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Exploring the potential of nanofertilizers for a sustainable agriculture. Plant Nano Biol. 2023, 5, 100044. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Brestic, M.; Raza, M.A.; Pang, T.; Langham, D.R.; Safdar, M.E.; Ahmed, S.; Wen, B.; Gao, Y.; et al. Changes in morphology, chlorophyll fluorescence performance and Rubisco activity of soybean in response to foliar application of ionic titanium under normal light and shade environment. Sci. Total Environ. 2019, 658, 626–637. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 237149. [Google Scholar] [CrossRef]
- Dinler, B.S.; Cetinkaya, H.; Koc, F.N.; Gül, V.; Sefaoğlu, F. Effects of titanium dioxide nanoparticles against salt and heat stress in safflower cultivars. Acta Bot. Bras. 2024, 38, e20230136. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Zivcak, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Jiang, M.; Song, Y.; Kanwar, M.K.; Ahammed, G.J.; Shao, S.; Zhou, J. Phytonanotechnology applications in modern agriculture. J. Nanobiotechnol. 2021, 19, 430. [Google Scholar] [CrossRef] [PubMed]
- Rehmanullah, R.Z.; Inayat, N.; Majeed, A. Application of nanoparticles in agriculture as fertilizers and pesticides: Challenges and opportunities. In New Frontiers in Stress Management for Durable Agriculture; Springer: Singapore, 2020; pp. 281–293. [Google Scholar]
- Kumar, Y.; Tiwari, K.N.; Singh, T.; Raliya, R. Nanofertilizers and their role in sustainable agriculture. Ann. Plant Soil Res. 2021, 23, 238–255. [Google Scholar] [CrossRef]
- Santas-Miguel, V.; Arias-Estevez, M.; Rodriguez-Seijo, A.; Arenas—Lago, D. Use of metal nanoparticles in agriculture. A review on the effects on plant germination. Environ. Pollut. 2023, 334, 122222. [Google Scholar] [CrossRef] [PubMed]
- Kusumavathi, K.; Rautaray, S.K.; Sarkar, S.; Dash, S.; Sahoo, T.R.; Swain, S.K.; Sethi, D. Nano-herbicides a sustainable strategy for weed control. Plant Nano Biol. 2025, 11, 100132. [Google Scholar] [CrossRef]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Gharib, F.A.; Ghazi, S.M.; Hegazi, A.Z.; Hafz, A.G. Physiological responses of two varieties of common bean (Phaseolus vulgaris L.) to foliar application of silver nanoparticles. Nanomater. Nanotechnol. 2016, 6, 13. [Google Scholar] [CrossRef]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory effect of silver nanoparticles on the growth and flowering of potted oriental lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef]
- Zahra, Z.; Maqbool, T.; Arshad, M.; Badshah, M.A.; Choi, H.K.; Hur, J. Changes in fluorescent dissolved organic matter and their association with phytoavailable phosphorus in soil amended with TiO2 nanoparticles. Chemosphere 2019, 227, 17–25. [Google Scholar] [CrossRef]
- Hao, Y.; Yuan, W.; Ma, C.; White, J.C.; Zhang, Z.; Adeel, M.; Zhou, T.; Rui, Y.; Xing, B. Engineered nanomaterials suppress Turnip mosaic virus infection in tobacco (Nicotiana benthamiana). Environ. Sci. Nano 2018, 5, 1685–1693. [Google Scholar] [CrossRef]
- Kiany, T.; Pishkar, L.; Sartipnia, N.; Iranbakhsh, A.; Barzin, G. Effects of silicon and titanium dioxide nanoparticles on arsenic accumulation, phytochelatin metabolism, and antioxidant system by rice under arsenic toxicity. Environ. Sci. Pollut. Res. 2022, 29, 34725–34737. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Shahid, M.; Ahmed, T.; Tahir, M.; Naqqash, T.; Muhammad, S.; Song, F.; Abid, H.M.A.; Aslam, Z. Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol. Environ. Saf. 2020, 192, 110303. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem 2017, 65, 8552–8559. [Google Scholar] [CrossRef] [PubMed]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. Int 2019, 26, 24430–24444. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions. Nanomaterials 2019, 9, 1559. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Sanabria, J.; Bindraban, P.S.; Singh, U.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci. Total Environ. 2020, 722, 137808. [Google Scholar] [CrossRef]
- Al-Amri, N.; Tombuloglu, H.; Slimani, Y.; Akhtar, S.; Barghouthi, M.; Almessiere, M.; Alshammari, T.; Baykal, A.; Sabit, H.; Ercan, I.; et al. Size effect of iron (III) oxide nanomaterials on the growth, and their uptake and translocation in common wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2020, 194, 110377. [Google Scholar] [CrossRef]
- Shah, A.A.; Yasin, N.A.; Mudassir, M.; Ramzan, M.; Hussain, I.; Siddiqui, M.H.; Ali, H.M.; Shabbir, Z.; Ali, A.; Ahmed, S.; et al. Iron oxide nanoparticles and selenium supplementation improve growth and photosynthesis by modulating antioxidant system and gene expression of chlorophyll synthase (CHLG) and protochlorophyllide oxidoreductase (POR) in arsenic-stressed Cucumis melo. Environ. Pollut. 2022, 307, 119413. [Google Scholar] [CrossRef]
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic Nanoparticle (TiO2 and Fe3O4) Application Modifies Rhizosphere Phosphorus Availability and Uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882. [Google Scholar] [CrossRef]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of Soybean (Glycine max L.). Environ. Sci Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hernandez-Viezcas, J.A.; Valdes, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: A sustainable approach for agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Moradi, A.; Maceroni, M.; Reinhardt, D.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Elmer, W.; Ma, C.; White, J. Nanoparticles for plant disease management. Curr. Opin. Environ. Sci. Health 2018, 6, 66–70. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Kalia, A.; Bhardwaj, K.; Bhardwaj, P.A.K.; Valis, M.; Kuca, K. Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives. J. Fungi 2020, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Choudhary, P.; Kumar, S.; Daima, H.K. Mechanistic approaches for crosstalk between nanomaterials and plants: Plant immunomodulation, defense mechanisms, stress resilience, toxicity, and perspectives. Environ. Sci. Nano 2024, 11, 2324–2351. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, A.; Silletti, M.F.; Faraone, I.; Milella, L. Nanoparticulate Antibiotic Systems as Antibacterial Agents and Antibiotic Delivery Platforms to Fight Infections. J. Nanomater. 2020, 2020, 6905631. [Google Scholar] [CrossRef]
- Hojjat, S.S. Effect of interaction between Ag nanoparticles and salinity on germination stages of Lathyrus sativus L. J. Environ. Soil Sci 2019, 2, 186–191. [Google Scholar]
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticle on fenugreek seed germination under salinity levels. Russ. Agric. Sci 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Nejatzadeh, F. Effect of silver nanoparticles on salt tolerance of Satureja hortensis L. during in vitro and in vivo germination tests. Heliyon 2021, 7, e05981. [Google Scholar] [PubMed]
- Ekhtiyari, R.; Moraghebi, F. Effect of nanosilver particles on salinity tolerance of cumin (Cuminum cyminum L.). J. Plant Biotechnol 2012, 25, 99–107. [Google Scholar]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant Nutr. 2016, 39, 172–180. [Google Scholar] [CrossRef]
- Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Pollut. 2016, 219, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Alam, M.A.; Syed, M.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Sedghi, M.; Mitra, H.; Sahar, T. Effect of nano zinc oxide on the germination of soybean seeds under drought stress. Ann. West Univ. Timiş. Ser. Biol 2013, 16, 73–78. [Google Scholar]
- Basit, F.; Shahid, M.; Abbas, S.; Naqqash, T.; Akram, M.S.; Tahir, M.; Azeem, M.; Cai, Y.; Jia, S.; Hu, J.; et al. Protective role of ZnO nanoparticles in soybean seedlings growth and stress management under Cr-enriched conditions. Plant Growth Regul. 2023, 100, 703–716. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Turgeon, R. The puzzle of phloem pressure. Plant Physiol. 2010, 154, 578–581. [Google Scholar] [CrossRef]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomášková, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. For. Res. Pap 2015, 76, 350–359. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata Grand Nain’Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Afshari, M.; Pazoki, A.; Sadeghipour, O. Foliar-applied silicon and its nanoparticles stimulates physio-chemical changes to improve growth, yield and active constituents of coriander (Coriandrum sativum L.) essential oil under different irrigation regimes. Silicon 2021, 13, 4177–4188. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Bagci, E.G.; Coban, S. Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J. Plant Interact 2007, 2, 105–113. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019, 26, 19859–19870. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa L.) under salinity conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Mohasseli, V.; Farbood, F.; Moradi, A. Antioxidant defense and metabolic responses of lemon balm (Melissa officinalis L.) to Fe-nano-particles under reduced irrigation regimes. Ind. Crops Prod. 2020, 149, 112338. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front. Environ. Sci 2016, 4, 46. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Zhang, S. Mechanism of Migration and Transformation of Nano Selenium and Mitigates Cadmium Stress in Plants. Master’s Thesis, Shandong University, Jinan, China, 2019. [Google Scholar]
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2015, 22, 2837–2845. [Google Scholar] [CrossRef]
- Kumar, J.A.; Krithiga, T.; Manigandan, S.; Sathish, S.; Renita, A.A.; Prakash, P.; Prasad, B.N.; Kumar, T.P.; Rajasimman, M.; Hosseini-Bandegharaei, A. A focus to green synthesis of metal/metal based oxide nanoparticles: Various mechanisms and applications towards ecological approach. J. Clean. Prod. 2021, 324, 129198. [Google Scholar] [CrossRef]
- Ullah, S.; Ikram, M.; Xiao, J.; Khan, A.; Din, I.; Huang, J. Influence of Foliar Application of Nanoparticles on Low Temperature Resistance of Rice Seedlings. Plants 2023, 13, 2949. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc Oxide Nanoparticles Alleviate Chilling Stress in Rice (Oryza Sativa L.) by Regulating Antioxidative System and Chilling Response Transcription Factors. Molecules 2020, 26, 2196. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Prasad, P.V. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef]
- Rahmatizadeh, R.; Javad Arvin, S.M.; Jamei, R.; Mozaffari, H.; Nejhad, F.R. Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant Interact 2019, 14, 474–481. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, W.; Li, Q.; Wang, Z.; Liu, S. The effects and the potential mechanism of environmental transformation of metal nanoparticles on their toxicity in organisms. Environ. Sci. Nano 2018, 5, 2482–2499. [Google Scholar] [CrossRef]
- Ghazaryan, K.; Agrawal, S.; Margaryan, G.; Harutyunyan, A.; Rajput, P.; Movsesyan, H.; Rajput, V.D.; Singh, R.K.; Minkina, T.; Elshikh, M.S. Soil pollution: An agricultural and environmental problem with nanotechnological remediation opportunities and challenges. Discov. Sustain. 2024, 5, 453. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Tolra, R.; Hajiboland, R.; Arroyave, C.; Barceló, J. Mechanisms of hyper-resistance and hyper-tolerance to aluminum in plants. Alum. Stress Adapt. Plants 2015, 24, 81–98. [Google Scholar] [CrossRef]
- Panda, S.K.; Baluška, F.; Matsumoto, H. Aluminum stress signaling in plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 517877. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Rubilar, O.; Fernández-Baldo, M.A.; Bertolino, F.A.; Durán, N.; Seabra, A.; Tortella, G. The nanotechnology among US: Are metal and metal oxides nanoparticles a nano or mega risk for soil microbial communities? Crit. Rev. Biotechnol. 2019, 39, 157–172. [Google Scholar] [CrossRef]
- Ahmed, A.; He, P.; He, P.; Wu, Y.; He, Y.; Munir, S. Environmental effect of agriculture-related manufactured nano-objects on soil microbial communities. Environ. Int. 2023, 173, 107819. [Google Scholar] [CrossRef]
- Maurice, N. Impact of nanoparticles on soil microbes for enhancing soil fertility and productivity. In Nanotechnology for Sustainable Agriculture; Apple Academic Press: Cambridge, MA, USA, 2023; pp. 75–127. [Google Scholar]
- Huang, W. Boosting Soil Health: The Role of Rhizobium in Legume Nitrogen Fixation. Mol. Soil Biol. 2024, 15, 129–139. [Google Scholar] [CrossRef]
- Raza, T.; Qadir, M.F.; Khan, K.S.; Eash, N.S.; Yousuf, M.; Chatterjee, S.; Manzoor, R.; Ur Rehman, S.; Oetting, J.N. Unrevealing the potential of microbes in decomposition of organic matter and release of carbon in the ecosystem. J. Environ. Manag. 2023, 344, 118529. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Mcgee, C.F. The effects of silver nanoparticles on the microbial nitrogen cycle: A review of the known risks. Environ. Sci. Pollut. Res. 2020, 27, 31061–31073. [Google Scholar] [CrossRef]
- Iqbal, S.; Xu, J.; Allen, S.D.; Khan, S.; Nadir, S.; Arif, M.S.; Yasmeen, T. Unraveling consequences of soil micro-and nano-plastic pollution on soil-plant system: Implications for nitrogen (N) cycling and soil microbial activity. Chemosphere 2020, 260, 127578. [Google Scholar] [CrossRef] [PubMed]
- Abdullatif, Y.; Soliman, E.; Hammad, S.; El-Ghamry, A.; Mansour, M. Impact of Carbon Nanoparticles on Aggregation and Carbon Sequestration under Soil Degradation–a Review. J. Soil Sci. Agric. Eng. 2024, 15, 85–92. [Google Scholar] [CrossRef]
- Sun, W.; Dou, F.; Li, C.; Ma, X.; Ma, L.Q. Impacts of metallic nanoparticles and transformed products on soil health. Crit. Rev. Environ. Sci. Technol. 2021, 51, 973–1002. [Google Scholar] [CrossRef]
- Faisal, M.; Saquib, Q.; Alatar, A.A.; Al-Khedhairy, A.A. Phytotoxicity of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A prospective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Dashora, K.; Mishra, M.; Fasake, V.D.; Srivastva, A. Effect of accumulation of nanoparticles in soil health-a concern on future. Front. Nanosci. Nanotechnol 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Ping, Y.; Cao, D.; Hu, J.; Lin, Y.; Dang, C.; Xue, D. The application, safety, and challenge of nanomaterials on plant growth and stress tolerance. Ind. Crops Prod. 2024, 222, 119691. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, T.; Wang, Y.; Li, T.; Chi, Q. Multifaceted impacts of nanoparticles on plant nutrient absorption and soil microbial communities. Front. Plant Sci. 2024, 15, 1497006. [Google Scholar] [CrossRef]
- Bhardwaj, L.K.; Rath, P.; Choudhury, M. A comprehensive review on the classification, uses, sources of nanoparticles (NPs) and their toxicity on health. Aerosol Sci. Eng. 2023, 7, 69–86. [Google Scholar] [CrossRef]
- Das, P.; Penton, C.R.; Westerhoff, P.; Perreault, F. Prospects of 2D graphene nanomaterials in plant-based agriculture and their fate in terrestrial soil: A critical review. Environ. Sci. Nano 2023, 10, 2936–2956. [Google Scholar] [CrossRef]
- Zhang, K.; Rengel, Z.; Zhang, F.; White, P.J.; Shen, J. Rhizosphere engineering for sustainable crop production: Entropy-based insights. Trends Plant Sci. 2023, 28, 390–398. [Google Scholar] [CrossRef]
- Bhavya, G.; Belorkar, S.A.; Mythili, R.; Geetha, N.; Shetty, H.S.; Udikeri, S.S.; Jogaiah, S. Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 2021, 275, 129975. [Google Scholar] [CrossRef]
- Aravinthan, A.; Govarthanan, M.; Selvam, K.; Praburaman, L.; Selvankumar, T.; Balamurugan, R.; Kamala-Kannan, S.; Kim, J.H. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int. J. Nanomed. 2015, 10, 1977–1983. [Google Scholar] [CrossRef]
- Hayes, K.L.; Mui, J.; Song, B.; Sani, E.S.; Eisenman, S.W.; Sheffield, J.B.; Kim, B. Effects, uptake, and translocation of aluminum oxide nanoparticles in lettuce: A comparison study to phytotoxic aluminum ions. Sci. Total Environ. 2020, 719, 137393. [Google Scholar] [CrossRef]
- Chauhan, P.; Kumar, D.; Sharma, R. Biogenic magnetic iron oxide nanoparticles for multifunctional applications in environmental remediation and biomedical field. J. Environ. Chem. Eng. 2025, 13, 115646. [Google Scholar] [CrossRef]
- Chinnappan, S.; Kandasamy, S.; Arumugam, S.; Seralathan, K.K.; Thangaswamy, S.; Muthusamy, G. Biomimetic synthesis of silver nanoparticles using flower extract of Bauhinia purpurea and its antibacterial activity against clinical pathogens. Environ. Sci. Pollut. Res. 2018, 25, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; AlYahya, S.; Govarthanan, M.; ALjahdali, N.; Al-Enazi, N.; Alsamhary, K.; Alshehri, W.; Alwakeel, S.; Alharbi, S. Soil bacteria Cupriavidus sp. Mediates the extracellular synthesis of antibacterial silver nanoparticles. J. Mol. Struct. 2020, 1202, 127233. [Google Scholar] [CrossRef]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S. Mycogenic Selenium Nanoparticles as Potential New Generation Broad Spectrum Antifungal Molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef]
- Jogaiah, S.; Kurjogi, M.; Abdelrahman, M.; Hanumanthappa, N.; Tran, L.P. Ganoderma applanatum-mediated green synthesis of silver nanoparticles: Structural characterization, and in vitro and in vivo biomedical and agrochemical properties. Arab. J. Chem. 2019, 12, 1108–1120. [Google Scholar] [CrossRef]
- Moll, J.; Klingenfuss, F.; Widmer, F.; Gogos, A.; Bucheli, T.D.; Hartmann, M.; Van Der Heijden, M.G. Effects of titanium dioxide nanoparticles on soil microbial communities and wheat biomass. Soil Biol. Biochem. 2017, 111, 85–93. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.S.A.; Borah, K.K.; Goswami, Y.; Hakeem, K.R.; Chakrabartty, I. The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A review. Environ. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S. Low concentrations of copper oxide nanoparticles alter microbial community structure and function of sediment biofilms. Sci. Total Environ. 2019, 653, 705–713. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xu, M.; Xiao, L.; Dai, Z.; Li, J. The impacts of γ-Fe2O3 and Fe3O4 nanoparticles on the physiology and fruit quality of muskmelon (Cucumis melo) plants. Environ. Pollut. 2019, 249, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic nanoparticles: Synthesis, characterisation and applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Pociecha, E.; Przemieniecki, S.W.; Oćwieja, M. Phytotoxicity of Silver Nanoparticles with Different Surface Properties on Monocots and Dicots Model Plants. J. Soil Sci. Plant Nutr. 2022, 22, 1647–1664. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Mirmoeini, T.; Pishkar, L.; Kahrizi, D.; Barzin, G.; Karimi, N. Phytotoxicity of green synthesized silver nanoparticles on Camelina sativa L. Physiol. Mol. Biol. Plants 2021, 27, 417–427. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wang, Y.; Gao, Y.; Zhang, Z.; Xu, J.; Xing, G. Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur. 2021, 10, e269. [Google Scholar] [CrossRef]
- Leopold, L.F.; Coman, C.; Clapa, D.; Oprea, I.; Toma, A.; Iancu, Ș.D.; Barbu-Tudoran, L.; Suciu, M.; Ciorîță, A.; Cadiș, A.I. The effect of 100–200 nm ZnO and TiO2 nanoparticles on the in vitro-grown soybean plants. Colloids Surf. B Biointerfaces 2022, 216, 112536. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Li, Z.; Chai, J.; Feng, J.; Han, R. Phytotoxicity and the molecular response in yttrium oxide nanoparticle–treated Arabidopsis thaliana seedlings. Protoplasma 2023, 260, 955–966. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Ma, Y.; Dai, W.; Song, Z.; Wang, Y.; Shen, J.; He, X.; Yang, F.; Zhang, Z. Interaction of cerium oxide nanoparticles and ionic cerium with duckweed (Lemna minor L.): Uptake, distribution, and phytotoxicity. Nanomaterials 2023, 13, 2523. [Google Scholar] [CrossRef]
- Salehi, H.; Chehregani Rad, A.; Raza, A.; Chen, J.T. Foliar application of CeO2 nanoparticles alters generative components fitness and seed productivity in bean crop (Phaseolus vulgaris L.). Nanomaterials 2021, 11, 862. [Google Scholar] [CrossRef]
- Desmau, M.; Carboni, A.; Le Bars, M.; Doelsch, E.; Benedetti, M.F.; Auffan, M.; Levard, C.; Gelabert, A. How microbial biofilms control the environmental fate of engineered nanoparticles? Front. Environ. Sci. 2020, 8, 82. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V. Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef]
- Schulz, R.; Bundschuh, M.; Entling, M.H.; Jungkunst, H.F.; Lorke, A.; Schwenk, K.; Schäfer, R.B. A synthesis of anthropogenic stress effects on emergence-mediated aquatic-terrestrial linkages and riparian food webs. Sci. Total Environ. 2024, 908, 168186. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, H.; Teng, Y. Impact of Nanoparticles on Soil Ecosystems. In The Role of Nanoparticles in Plant Nutrition under Soil Pollution: Nanoscience in Nutrient Use Efficiency; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef]
- Wang, W.; Do, A.T.N.; Kwon, J.-H. Ecotoxicological effects of micro-and nanoplastics on terrestrial food web from plants to human beings. Sci. Total Environ. 2022, 834, 155333. [Google Scholar] [CrossRef]
- Mccracken, C.; Dutta, P.K.; Waldman, W.J. Critical assessment of toxicological effects of ingested nanoparticles. Environ. Sci. Nano 2016, 3, 256–282. [Google Scholar] [CrossRef]
- Arif, Y.; Mir, A.R.; Zieliński, P.; Hayat, S.; Bajguz, A. Microplastics and nanoplastics: Source, behavior, remediation, and multi-level environmental impact. J. Environ. Manag. 2024, 356, 120618. [Google Scholar] [CrossRef] [PubMed]
- Dagar, G.; Bagchi, G. Nanoparticles as potential endocrine disruptive chemicals. In NanoBioMedicine; Saxena, S.K., Khurana, S.M.P., Eds.; Springer: Singapore, 2020; pp. 411–429. [Google Scholar]
- Milivojević, T.; Glavan, G.; Božič, J.; Sepčić, K.; Mesarič, T.; Drobne, D. Neurotoxic potential of ingested ZnO nanomaterials on bees. Chemosphere 2015, 120, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Kumar, M.; Bolan, N.S.; Singh, L.; Kumar, S.; Kumar, R.; You, S. Are microplastics destabilizing the global network of terrestrial and aquatic ecosystem services? Environ. Res. 2021, 198, 111243. [Google Scholar] [CrossRef]
- Araújo, C.V.; Laissaoui, A.; Silva, D.C.; Ramos-Rodríguez, E.; González-Ortegón, E.; Espíndola, E.L.; Baldó, F.; Mena, F.; Parra, G.; Blasco, J. Not only toxic but repellent: What can organisms’ organisms’ responses tell us about contamination and what are the ecological consequences when they flee from an environment? Toxics 2020, 8, 118. [Google Scholar] [CrossRef]
- Brar, K.K.; Magdouli, S.; Othmani, A.; Ghanei, J.; Narisetty, V.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Awasthi, M.K.; Pandey, A. Green route for recycling of low-cost waste resources for the biosynthesis of nanoparticles (NPs) and nanomaterials (NMs)-A review. Environ. Res. 2022, 207, 112202. [Google Scholar] [CrossRef]
- Khan, M.R.; Rizvi, T.F. Application of nanofertilizer and nanopesticides for improvements in crop production and protection. In Nanoscience and Plant–Soil Systems; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 405–427. [Google Scholar]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Shegokar, R.; Nakach, M. Large-scale manufacturing of nanoparticles—An industrial outlook. In Drug Delivery Aspects; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Tassey, G. Policy issues for R&D investment in a knowledge-based economy. J. Technol. Transf. 2004, 29, 153–185. [Google Scholar]
- Hobson, D.W. Commercialization of nanotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 189–202. [Google Scholar] [CrossRef]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Grillo, R.; Fraceto, L.F.; Amorim, M.J.; Scott-Fordsmand, J.J.; Schoonjans, R.; Chaudhry, Q. Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. J. Hazard. Mater. 2021, 404, 124148. [Google Scholar] [CrossRef]
- Corsi, I.; Venditti, I.; Trotta, F.; Punta, C. Environmental safety of nanotechnologies: The eco-design of manufactured nanomaterials for environmental remediation. Sci. Total Environ. 2023, 864, 161181. [Google Scholar] [CrossRef] [PubMed]
- Opacic, T.; Paefgen, V.; Lammers, T.; Kiessling, F. Status and trends in the development of clinical diagnostic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1441. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N. Nanoparticles: Environmental problems or problem solvers? Bioscience 2018, 68, 241–246. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Sintes, J.R.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Isibor, P.O. Regulations and policy considerations for nanoparticle safety. In Environmental Nanotoxicology: Combatting the Minute Contaminants; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Ramachandran, G.; Wolf, S.M.; Paradise, J.; Kuzma, J.; Hall, R.; Kokkoli, E.; Fatehi, L. Recommendations for oversight of nanobiotechnology: Dynamic oversight for complex and convergent technology. J Nanopart Res. 2011, 13, 1345–1371. [Google Scholar] [CrossRef]
- Mcalea, E.M.; Mullins, M.; Murphy, F.; Tofail, S.A.; Carroll, A.G. Engineered nanomaterials: Risk perception, regulation and insurance. J. Risk Res. 2016, 19, 444–460. [Google Scholar] [CrossRef]
- Śliwiński, A.; Janowicz-Lomott, M. The risk of nanotechnology: A challenge to the insurance industry. J. Rev. Glob. Econ. 2020, 6, 404–419. [Google Scholar]
- Nielsen, M.B.; Skjolding, L.; Baun, A.; Hansen, S.F. European nanomaterial legislation in the past 20 years—Closing the final gaps. NanoImpact 2023, 32, 100487. [Google Scholar] [CrossRef]
- Bharti, A.; Jain, U.; Chauhan, N. From lab to field: Nano-biosensors for real-time plant nutrient tracking. Plant Nano Biol. 2024, 9, 100079. [Google Scholar] [CrossRef]
- Mgadi, K.; Ndaba, B.; Roopnarain, A.; Rama, H.; Adeleke, R. Nanoparticle applications in agriculture: Overview and response of plant-associated microorganisms. Front. Microbiol. 2024, 15, 1354440. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouhadi, M.; Javed, Q.; Jakubus, M.; Elkouali, M.; Fougrach, H.; Ansar, A.; Ban, S.G.; Ban, D.; Heath, D.; Černe, M. Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks. Agronomy 2025, 15, 1131. https://doi.org/10.3390/agronomy15051131
Bouhadi M, Javed Q, Jakubus M, Elkouali M, Fougrach H, Ansar A, Ban SG, Ban D, Heath D, Černe M. Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks. Agronomy. 2025; 15(5):1131. https://doi.org/10.3390/agronomy15051131
Chicago/Turabian StyleBouhadi, Mohammed, Qaiser Javed, Monika Jakubus, M’hammed Elkouali, Hassan Fougrach, Ayesha Ansar, Smiljana Goreta Ban, Dean Ban, David Heath, and Marko Černe. 2025. "Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks" Agronomy 15, no. 5: 1131. https://doi.org/10.3390/agronomy15051131
APA StyleBouhadi, M., Javed, Q., Jakubus, M., Elkouali, M., Fougrach, H., Ansar, A., Ban, S. G., Ban, D., Heath, D., & Černe, M. (2025). Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks. Agronomy, 15(5), 1131. https://doi.org/10.3390/agronomy15051131










