Abstract
Nanotechnology is rapidly emerging as a transformative force in agriculture, offering innovative solutions to support sustainable crop production. This review examines the interactions between nanoparticles (NPs) and plants, elucidating the underlying mechanisms that govern NP uptake, translocation, and interactions at the cellular level. We explore how NPs influence key physiological processes and modulate plant defense responses to both biotic and abiotic stresses, highlighting their potential for enhancing stress resistance. The diverse applications of NPs in agriculture are also comprehensively surveyed, encompassing targeted delivery of nutrients, enhanced biocontrol of phytopathogens, and engineering improved tolerance to environmental extremes. We also address the broader environmental and socioeconomic implications of the widespread use of NPs in agriculture, critically evaluating their ecotoxicity, impacts on biodiversity, and the associated economic costs and benefits. Finally, we offer a perspective on future directions for research, including emerging trends in NPs synthesis and characterization, challenges to sustainable implementation, and the prospects for large-scale deployment of nanotechnology-enabled agricultural solutions. This review provides a rigorous and balanced assessment of the potential of nanotechnology to revolutionize agricultural practices while acknowledging the need for responsible innovation and risk mitigation.
1. Introduction
Abiotic stresses, including drought, low temperatures, salinity and excess water (Excess runoff), alongside biotic stresses such as bacteria, viruses, fungi, parasites, insect pests and weeds, represent major constraints on agricultural productivity worldwide. These environmental stresses induce physiological responses in plants that can alter their growth and development. Abiotic stresses are responsible for an estimated 20–50% loss in agricultural yield each year worldwide [1]. Drought alone can reduce cereal yields by 50% or more [2]. When stressed, plants implement elaborate defense mechanisms that often involve the production of signaling molecules, such as abscisic acid (ABA) [3]. Plants can also modulate their metabolic pathways and gene expression, synthesizing protective compounds that enhance stress tolerance. These modifications can have long-term consequences for both the plant and the ecosystem. In the case of abiotic stresses, this can result in reduced growth, altered photosynthesis, hormonal disruption, increased susceptibility to pathogens, and loss of product quality [4]. Several strategies have been explored to address these challenges, including varietal selection, biotechnology (genetic engineering, genome editing), biostimulants, nanotechnology, and precision agronomy [5].
Among these emerging approaches, nanotechnology, especially nanoparticles, holds significant promise due to its versatility, scalability, and potential to enhance plant performance under stress conditions.
As the global population rises, demand for food increases, and with it, there is an urgency to ensure food safety and sustainable production. In this context, the use of nanoparticles in agricultural systems seems increasingly justified. As one of the most important sectors of a country’s economy, agriculture faces many challenges, including the need to produce biomass for food, feed and fiber while maintaining soil health and following sustainable agriculture principles. Attaining this balance is especially challenging with climate change, intensive land use, and continual degradation of the environment, all of which lead to reductions in soil productivity and long-term ecosystem stability.
Nanotechnology offers many solutions for enhancing crop resilience to abiotic and biotic stresses and ensuring long-term food security [5,6]. Nanotechnology involves manipulating, studying, and applying matter on an atomic, molecular, and supramolecular scale, typically at dimensions between 1 and 100 nanometers [7]. At this scale, materials like nanoparticles have unique physicochemical properties that distinguish them from macroscopic materials. This nanometric dimension increases their specific surface area, increasing their reactivity and environmental interactions [8]. In addition, quantum size effects, such as electronic energy quantization and surface plasmon resonance, give nanoparticles unique optical, electrical, and magnetic properties, opening up unprecedented prospects in agriculture [9]. Usually, metallic nanoparticles exhibit vibrant optical properties primarily due to surface plasmon resonance (SPR), a phenomenon where incident light causes collective oscillations of their surface electrons [10]. This resonance leads to strong absorption and scattering of specific wavelengths of light, highly dependent on the nanoparticle’s size, shape, material, and surrounding environment [11]. This extreme sensitivity to their surroundings makes SPR invaluable for developing nanosensors that can detect minute changes in agricultural environments, such as the presence of pathogens or changes in nutrient levels [12]. Furthermore, the enhanced electromagnetic fields associated with SPR can be utilized for targeted delivery of agrochemicals and for advanced imaging techniques to monitor plant health at a cellular level [13].
Nanoparticle synthesis is the subject of intensive research. Synthesis methods vary, and the choice of method depends on the type of nanoparticle and its final application. They can be produced from solid materials using physical techniques, including mechanical grinding and laser ablation [14], although chemical methods, such as precipitation, chemical reduction or sol–gel, offer greater control over their composition and morphology [15]. In addition, biological methods involving bacteria or fungi can be used to produce nanoparticles more ecologically and with specific properties such as controlled size, specific surface functionalization, enhanced biocompatibility, and targeted catalytic or biological activities [16].
The interest in the agricultural application of nanoparticles is driven by their potential to improve crop productivity and quality by interacting with plants and soil at a molecular level.
Nanoparticles can address many of the current agricultural challenges, such as improving plant growth, yield, and resilience to pathogens by acting as fertilizers or targeted nutrient delivery systems [17,18]. Their unique physicochemical properties, such as high surface area and reactivity, facilitate enhanced biomass production, improved nutritional quality, and increased disease resistance. Recent studies have highlighted their ability to optimize nutrient use efficiency, enable the controlled release of agrochemicals, and support the precise application of bioactive compounds, thereby minimizing over-fertilization and pesticide use through their high surface area, small size, and tunable properties that enable targeted delivery, controlled release, and enhanced interaction with plants and their environment [19,20]. They can also contribute to soil restoration and sustainability by decontaminating polluted soils, refining key physicochemical properties, enhancing soil structure, improving water retention and nutrient availability, and supporting beneficial microbial communities [21,22].
Within plants, the introduction of nanoparticles can lead to interactions with fundamental biomolecules, including proteins, nucleic acids (DNA and RNA), and lipids, which may result in altered biological activities [23]. Notably, these interactions can extend to the regulation of gene expression. By engaging with DNA or RNA, nanoparticles can influence genes associated with critical pathways governing growth, development, and responses to environmental stresses [24]. Furthermore, Zou et al.’s [25] transcriptomic analysis demonstrated that low silver nanoparticle (AgNP) concentrations likely enhance stress resistance and induce stress memory in rose seedlings by activating ROS signaling pathways, which in turn leads to the upregulation of genes within crucial metabolic pathways (riboflavin, peroxisome, glutathione, cysteine/methionine metabolism) and key transcription factor families (WRKY, TIFY, bHLH). In addition, Omar et al. [26] showed that titanium dioxide nanoparticles (nTiO2) can mitigate the negative impacts of salinity stress in broad bean plants by enhancing growth, reducing oxidative damage and genetic instability, and upregulating protective antioxidant and heat shock protein genes. On the other hand, Mokhtarabadi et al. [27] revealed that, while low concentrations of nanoscale red elemental selenium (nSe) enhanced the fresh weight of chicory seedlings, high concentrations proved toxic. Their biosafety assessment further indicated distinct epigenetic responses to nSe compared to selenate, identifying DNA hypomethylation as a key mechanism, with the DREB1A gene’s activity increasing alongside nSe levels. Additionally, the researchers found that nSe upregulated genes involved in phenylpropanoid synthesis (PAL, HQT, HCT), increased proline content, and stimulated the accumulation of beneficial phenylpropanoids such as caffeic, chlorogenic, and cichoric acids, as well as antioxidants like ascorbate and glutathione. Their large surface area also boosts nutrient uptake by adsorbing and delivering nutrients to plant roots, improving assimilation [28]. Moreover, nanoparticles can also be used to create diagnostic tools for early disease detection and plant growth monitoring [29] and to strengthen plant defenses by activating resistance mechanisms against pathogens, abiotic stresses, and disease [5,30].
While the potential of nanoparticles to revolutionize agriculture is evident, their widespread adoption necessitates a thorough understanding of the intricate interplay between their mechanisms of action in plants, diverse agricultural applications, and the potential environmental and socioeconomic ramifications, particularly concerning toxicity, regulation, and cost. Moving beyond a descriptive overview of these areas, this comprehensive review, synthesizing findings from over 200 scientific publications, critically examines the current evidence to identify key knowledge gaps and conflicting findings that hinder the development of sustainable and responsible nano-agricultural practices. By highlighting these critical junctures, we aim to propose a future research agenda that prioritizes interdisciplinary approaches and addresses the most pressing uncertainties, ultimately paving the way for the translation of promising nanotechnologies into viable and safe agricultural solutions.
2. Mechanisms of Nanoparticle Action on Plants
2.1. Nanoparticles: Cellular Interactions and Physiological Effects in Plants
Due to their small size, nanoparticles can be taken up by plants through roots, leaves, or other pathways and subsequently translocated via the vascular systems [31,32]. Their effects vary depending on many factors, such as the nature of the nanoparticle, dose, duration of exposure and environmental conditions [33]. For example, they can stimulate growth by increasing nutrient availability or inhibit growth by causing cell damage [34]. In addition, they can cause oxidative stress, interact with biomolecules, and disrupt cellular signals [35,36]. However, a key challenge in nanoparticle research is separating the effects attributed to their nanoscale size from those caused by the material. For instance, metal and metal oxide NP dissolution, influenced by the specific metal’s properties, significantly impacts their biological activity, both positive and negative [37,38,39,40] (Figure 1).

Figure 1.
Nanoparticle-plant interactions: uptake, translocation, oxidative stress, cellular and physiological responses of plants to nanoparticles exposure: positive and negative impacts.
Nanoparticles enter plant cells through various mechanisms, including endocytosis and passive diffusion [41]. For instance, highly charged nanoparticles can passively penetrate plant cell membranes and chloroplast envelopes, potentially via disruption of the lipid bilayer, though the cell wall and nanoparticle coatings add complexity to this process [42]. Once inside, they can interact with cellular components such as membranes, organelles (mitochondria, chloroplasts), and DNA [43]. Extensively studied nanoparticles, including Ag NPs, Au NPs, TiO2 NPs, ZnO NPs, SiO2 NPs, CuO NPs, and CaCO3 NPs, can trigger rapid molecular interactions in plants, leading to a dual response involving both ROS protection and induction of oxidative stress that subsequently activates the plant’s antioxidant defense system. This ultimately enables plants to more effectively adjust to stressful environmental conditions and enhance overall productivity by mitigating abiotic stress and influencing a range of their morphological, anatomical, physiological, biochemical, and molecular characteristics [44]. Mahakham et al. [45] demonstrated how soaking rice seeds in 10 or 20 mg/L of silver nanoparticles (Ag NPs) for 24 h significantly enhanced germination and seedling growth. This effect was attributed to increased water uptake, the upregulation of aquaporin genes (PIP1;1 and PIP2;1), and elevated amylase, dehydrogenase, and catalase activity.
In addition, Manickavasagam et al. [46] observed that treating rice (Oryza sativa) callus grown with 1-Ag NPs at 5 and 10 mg/L significantly enhanced callus regeneration. This positive effect was correlated with a reduction in oxidative stress markers, including hydrogen peroxide (H2O2), malondialdehyde (MDA), and proline levels. Furthermore, the researchers reported a down-regulation of genes involved in the plant’s hormonal responses, specifically those related to ethylene, abscisic acid, auxins, cytokinins, and gibberellic acid [39].
Likewise, Rostami et al. [47] reported that foliar application of zinc oxide nanoparticles (ZnO NP) at concentrations of 2–9 g/L increased saffron flower yield, chlorophyll content, water retention, protein levels, and antioxidant enzyme activity (POX and CAT). Similarly, Shabbir et al. [48] demonstrated that foliar application of TiO2 nanoparticles on Vetiveria zizanioides significantly enhanced biomass growth, essential oil and khusimol production, chlorophyll content, photosystem II efficiency, and the activity of nitrate reductase and carbonic anhydrase. In addition, Hassanpouraghdam et al. [49] revealed that the foliar treatment with CeO2:SA-nanoparticles alleviated the side effects of salinity by improving the physiological responses and growth-related traits of purslane plants (Portulaca oleracea L.). Furthermore, foliar treatment with silver, copper, and copper–silver nanoparticles at various concentrations enhanced the growth and productivity of chili plant, leading to increased plant height and number of fruits compared to the untreated control groups [50].
In contrast, high concentrations of TiO2, ZnO, and Ag NPs can generate oxidative stress, disrupt cellular metabolism, and even damage DNA [51,52,53]. Studies have shown that copper oxide NPs (CuO-NPs) cause oxidative DNA lesions and growth suppression in various agricultural and grassland plants (radish, perennial ryegrass, and annual ryegrass) [54]. Similarly, high concentrations of ZnO-NPs also act as genotoxic agents, leading to DNA damage. Such damage occurs as membrane integrity loss, increased chromosome aberrations, and DNA strand breaks in onion root cells (Allium cepa) [55]. Furthermore, ZnO-NPs have been shown to induce reactive oxygen species (ROS) production in broad bean (Vicia faba) and tobacco (Nicotiana tabacum), which in turn also contribute to DNA damage and strand breaks [56,57,58].
Nanoparticle interactions can trigger a range of physiological effects. For instance, Yoon et al. [59] demonstrated that 500 mg/kg of 54 nm Fe-NPs in Arabidopsis thaliana soil increased plant biomass, carbohydrate, and phosphorus levels by enhancing photosynthesis due to increased stomatal opening and improved phosphorus availability via reduced rhizosphere pH. In addition, ref. [60] demonstrated that soaking rice seeds (cv. Gobindabhog) in 20 mg/L of Fe-NPs for three days significantly enhanced seedling growth. According to the same study, this result was achieved by improving water content, increasing the activity of hydrolytic and antioxidant enzymes, improving cell membrane integrity and viability, and raising chlorophyll and iron levels. Moreover, Raliya et al. [61] demonstrated that foliar application of 10 mg/L of zinc oxide nanoparticles (ZnO NPs) on mung bean plants significantly enhanced growth (longer stems, larger root volume), nutrient uptake (increased phosphorus accumulation via stimulated phosphatase and phytase activity), photosynthetic capacity (higher chlorophyll and protein levels), and beneficial rhizospheric microbial populations. In addition, applying iron nanoparticles (Fe NPs) at 25 mg/kg significantly enhanced wheat growth in both normal and salt-affected soils, increasing the dry weights of roots, shoots, and grains more substantially in the salt-affected soil than in the normal soil, and outperformed other iron sources like FeSO4 and Fe-EDTA [62]. Moreover, Fe nanoparticles can boost the response to oxidative stress through biochemical pathways and improve plant growth by promoting seed germination and root growth [63]. Feigl [64] show that CuO NPs can positively influence plant growth and development by enhancing photosynthesis, nutrient uptake, and root growth at appropriate levels; however, high concentrations can have detrimental effects, causing oxidative stress and cell damage that lead to reduced growth and yield.
In contrast, high concentrations of NPs can be harmful to plants. While both silver (Ag) and titanium dioxide nanoparticles (TiO2 NPs) induce increased reactive oxygen species (ROS), a common marker of toxicity, AgNPs have paradoxically been observed to stimulate growth in certain plant species [65]. TiO2 NPs caused oxidative stress and DNA oxidative damage in BEAS-2B cells, as demonstrated by [51]. Oxidative stress, mediated by reactive oxygen species (ROS) production, is a key factor in cellular damage [66,67,68]. These highly reactive molecules target and damage vital cellular constituents such as lipids, proteins, and DNA, impairing essential cellular functions [69,70,71,72]. Moreover, silver nanoparticles (AgNPs) can bind to proteins and enzymes essential for cellular respiration, reducing metabolic efficiency [73].
2.2. Modulation of Plant Defense Responses by Nanoparticles
Nanoparticle interactions with plants can significantly enhance their defense mechanisms by stimulating the production of defense-related molecules (Table 1), such as phenolic compounds and defense proteins, thereby increasing tolerance and resistance to pathogens and insect herbivores [27,74]. Specifically, Silica nanoparticles (SiNPs) enhance plant disease resistance through multiple mechanisms. Firstly, they strengthen cell walls, providing a physical barrier against pathogen invasion [75]. Secondly, SiNPs activate plant signaling pathways, initiating a cascade of defense responses [76]. Furthermore, nanoparticle-mediated priming is observed. For instance, ZnONPs can induce a state of heightened preparedness in plants, pre-activating defense mechanisms against future pathogen attacks [77]. In addition, the study by Mohammadi et al. [78] revealed that Fe-0 nanoparticles (35–45 nm, 8–14 m²/g) alleviate hexavalent chromium (Cr (VI)) stress in sunflower plants through a dual mechanism: immobilization of Cr within the soil and upregulation of antioxidant enzyme activity (superoxide dismutase, peroxidase, catalase, and ascorbate peroxidase). Furthermore, seed priming with 25 ppm of zinc oxide nanoparticles ZnO NPs effectively alleviates drought stress in rice plants by reducing oxidative damage, enhancing antioxidant enzyme activity and proline levels [79]. Additionally, priming tomato seeds with 75 ppm selenium nanoparticles (SeNPs) enhances drought tolerance by lowering oxidative stress, boosting antioxidant defense mechanisms and the ascorbate–glutathione cycle, and increasing the accumulation of beneficial bioactive compounds [80]. Similarly, under drought stress, canola seed nano-priming with 75 ppm CaONPs significantly enhanced plant performance by improving germination (30%), seedling fresh weight (34%), leaf number (16%), total chlorophyll (28.9%), pod and seed production (up to 73%), 100 seed weight (35.13%), and overall yield (35.18%), likely due to improved antioxidant enzyme activity and reduced stress indicators [81].
However, under different conditions, such as high concentrations or prolonged exposure, NPs may impair these defenses, and excessive ROS production induced by NPs can disrupt signaling pathways or damage cellular components, potentially leading to cell death and growth inhibition [82].
Furthermore, engineered nanomaterials (ENMs) have attracted considerable attention in the field of agriculture, especially nanopesticides and nanofertilizers aimed at boosting agricultural sustainability and productivity [83,84]. Similarly, Li et al. [85] demonstrated that exposing wheat plants to molybdenum (Mo) engineered nanomaterials (ENMs) leads to significant Mo uptake by the roots and its subsequent translocation to the leaves. While copper-based ENMs offer protection against fungi and other pests, they can elicit stress responses and metabolic adaptations, particularly in the early stages of exposure. In addition, Fatemi et al. [86] demonstrated that foliar application of 1.5 mM ENM silicon nanoparticles (SiNPs) to Coriandrum sativum under salt stress mitigated the negative effects by enhancing the antioxidant defense system (increased SOD, CAT, POD activity), and reducing oxidative stress markers like MDA and H2O2. Similarly, Umair Hassan et al. [87] noted that priming Sorghum bicolor seeds with ZnNPs under salt stress improved shoot and root length, significantly decreased the Na+/K+ ratio (indicating better ion homeostasis), and mitigated oxidative stress, leading to enhanced growth and stress tolerance. Fouda et al. [88] showed that foliar application of CuNPs (20 mg/L) on Trigonella foenum-graecum L. under salt stress improved plant growth and biomass, promoted the production of plant pigments, osmolytes, anthocyanin, shikimic acid, and phenols, and upregulated antioxidant enzyme activity, suggesting a positive role in mitigating salt stress and enhancing bioactive compound production.

Table 1.
Potential effects of NPs on the modulation of plant defense responses.
Table 1.
Potential effects of NPs on the modulation of plant defense responses.
| Plant | NPs Nature | Potential Effects | Reference |
|---|---|---|---|
| Saffron (Crocus sativus) | ZnO NPs | Increasing POX (peroxidase) and CAT (catalase) activity | [47] |
| Wheat (Triticum aestivum) | ZnO NPs | lowering oxidative stress (higher activity of SOD (superoxide dismutase) and POD (peroxidase)) | [89] |
| Soybean (Glycine max) | ZnO NPs | Higher activities of SOD, CAT, POD, and APX enzymes. | [90] |
| Rice (Oryza sativa) callus | Ag NPs | Decreasing levels of H2O2 | [46] |
| Spinach (Spinacia oleracea) | TiO2 NPs | Increasing activity of SOD, CAT, APX, and guaiacol peroxidase. Decreased level of superoxide radicals, H2O2 | [91] |
| Maize (Zea mays L.) | TiO2 NPs | Activation of SOD (superoxide dismutase) and glutathione S-transferase (GST) | [92] |
| Rice (Oryza sativa) | Tio2, Si NPs | Lowering oxidative stress (increasing activity of CAT, POD, and APX | [93] |
| (Oryza sativa cv. Gobindabhog L.) | Fe-0 NPs | Increasing the activity of SOD, CAT and glutathione peroxidase, | [60] |
| Evening primrose (Oenthera biennis) | Fe2O3 NPs | Increasing the activity of CAT, SOD, and POD | [94] |
| Brassica napus L. | γ-Fe2O3 NP | Decreasing the level of H2O2 | [95] |
| Sunflower (Helianthus annuus) | Fe-0 NPs | Increasing the activity of antioxidant enzymes superoxide dismutase, peroxidase, catalase and ascorbate peroxidase | [78] |
| Wheat (Triticum aestivum) | [96] | ||
| Maize (Zea mays) | Fe3O4 | [97] | |
| Arabidopsis thaliana L. | CeO2 NPs | Scavenging of hydroxyl radicals, superoxide anions, and H2O2 in chloroplasts | [98] |
3. Applications of Nanoparticles in Agriculture
Nanoparticles offer many potential agricultural uses, including crop growth, production, processing, storage, packaging, and transportation [99]. A key challenge in plant production is maintaining abundant available nutrients and effective plant protection against pests and diseases, typically achieved using fertilizers and pesticides, such as herbicides, insecticides, and fungicides. This approach, although widely used by farmers, unfortunately, when used intensively, often leads to environmental degradation, soil and water pollution and diminishing soil quality due to inefficient nutrient uptake by plants, leading to nutrient loss [100]. Nanofertilizers and nanopesticides can enhance plant nutrient and pesticide delivery [101,102]. Moreover, nanosensors facilitate the early detection of plant diseases and pests, enabling timely intervention and yield protection [103]. Furthermore, nanoparticles can improve soil health by enhancing water retention and nutrient availability [104].
3.1. Improving Growth, Development of Plant Nutrition
The unique role of NPs in agriculture is emphasized in crop protection and fertilization, improving precision farming and stress tolerance. Mai et al. [105] found that irrigating red oak, chestnut oak, and red maple seedlings with Mn (OH)2 NPs increased seedling size and nitrogen/phosphorus uptake over a period of 12 weeks.
In addition, NPs are considered a good transport tool that can ultimately be used for fertilizers, pesticides, synthetic hormones or genetic material. [21]. They can also promote plant productivity by increasing tolerance to adverse conditions such as abiotic stress and unfavorable environmental conditions (e.g., salinity, heavy metals, Figure 2, Table 2).
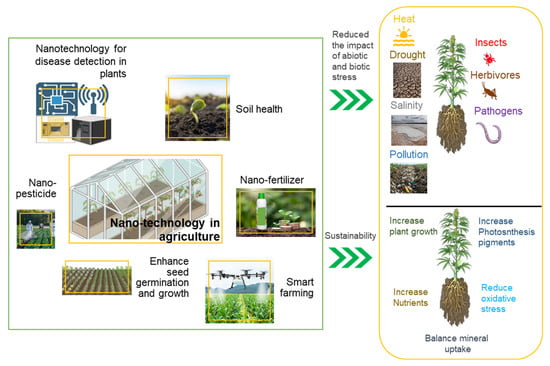
Figure 2.
Schematic representation of nanotechnology in agriculture: enhancing plant growth, mitigating abiotic (drought) and biotic (pests, pathogens) stressors, and promoting sustainability through nano-enabled solutions (nano-fertilizers, nano-pesticides, nanosensors).
Nanofertilizers, as described by Yadav et al. [106], include metal or metal oxide nanoparticles (e.g., FeO, ZnO) and organic compounds such as chitosan and humic acid. The authors outline the various production methods and delivery systems for commercial nanofertilizers, including encapsulation, coating, and blending with organic, inorganic, or polymeric materials. Extending this concept of targeted nutrient delivery, the application of titanium, potentially in nano-formulations, has also demonstrated significant improvements in plant physiological parameters [46]. Studies have shown that titanium fertilization enhances nitrate reductase enzymatic activity, chlorophyll and carotenoid concentrations, photosynthetic rate, symbiotic nitrogen fixation, and the uptake of essential elements like iron and magnesium [107,108]. Incorporating titanium into nanofertilizer delivery systems could offer a promising avenue for optimizing plant nutrition and enhancing productivity. In addition, Dinler et al. [109] study reveals that priming Carthamus tinctorius L. (safflower) seeds with 200 ppm ENM TiO2 nanoparticles positively impacts radicle length and plumula fresh weight by boosting the conversion of inorganic nitrogen into protein and chlorophyll structures, likely through the regulation of nitrate reductase, glutamine synthase, glutamate dehydrogenase, and glutamic-pyruvic transaminase enzymatic activity. Under salinity stress, Gohari et al. [110] research indicates that irrigating Dracocephalum moldavica with 100 mg/L titanium nanoparticles (Ti NPs) increased plant height, enhanced nutrient uptake, and boosted antioxidant enzyme activities.
Silica nanoparticles have been shown to exhibit pesticidal properties, while mesoporous Si-NPs can serve as nanocarriers for the controlled release of commercial pesticides and fertilizers [111]. Additionally, Si-NPs and nano-zeolites can enhance the soil’s water-holding capacity by improving porosity and moisture retention.
In Garg et al.’s [99] opinion, smart nano-sensors can enhance productivity by improving fertilization management, reducing input costs, and protecting the environment when combined with precision farming. Thanks to nanosensors, the detection of plant pathogens and nutrient deficiency in soil will be possible. Moreover, precision farming based on nanosensors allows the sustainable use of natural resources without damaging them, while meeting the principles of safe food production.
Based on their functionality, nanofertilizers can be classified into three types: nanocomposite fertilizers, controlled-release fertilizers, and hybrid nano-devices designed to deliver both macro- and micronutrients [112]. Rehmanullah et al. [113] also categorize nanoparticles (NPs) used in fertilizers as derived from macronutrients, micronutrients, and NPs that enhance fertilizer performance. Irrespective of their classification, nanofertilizers outperform conventional fertilizers due to their reduced toxicity and enhanced efficiency in nutrient delivery [114].
Jiang et al. [112] also list the many benefits of using nanofertilizers, such as a slow release mechanism, reduction in transportation and application cost, low salt accumulation compared to conventional fertilizers, and improved nutrient bioavailability to meet crop demands efficiently. They also offer advantages over conventional fertilizers, including enhanced nutrient uptake, nutrient use efficiency, improved crop nutritional quality, root biomass development, microbial regulation of the rhizosphere, and higher yields [115]. Their properties allow for more precise delivery and controlled release, reducing the overall application rate and increasing cost and time savings [22]. They also help reduce eutrophication by minimizing nutrient runoff [21]. Additionally, nanoparticles are beneficial in promoting seed germination and early plant development [115].
When addressing nutrient uptake, another factor to consider is mitigating nutrient competition, specifically through weed control. Here, nanoparticles can contribute by enhancing herbicide efficacy via NP-mediated transport and delivery. Kusumavathi et al. [116] show how nano-herbicides can control weeds using inorganic, organic, and hybrid compounds. According to the authors cited, nanoherbicides offer more precise and less environmentally harmful solutions for agricultural weed control. The affinity for the target can be increased by providing a larger specific surface area, potentially lowering the required dosages and minimizing adverse effects on non-target organisms and soil health. Encapsulation of nano-herbicides also increases the uniformity of application, avoiding adverse effects on crop plants such as phytotoxicity, yield reduction, environmental contamination, and harm to beneficial organisms [112].

Table 2.
Potential beneficial effects of NPs on plant growth, physiology, and nutrition.
Table 2.
Potential beneficial effects of NPs on plant growth, physiology, and nutrition.
| Plant | NPs Nature | Potential Effects | Reference |
|---|---|---|---|
| Rice (Oryza sativa) | Ag NPs | Increasing water uptake into the seeds. Up-regulation of aquaporin genes | [45] |
| Arabidopsis thaliana | Increasing the rate of evapotranspiration | [117] | |
| Common bean (Phaseolus vulgaris) | Increasing the concentration of indole-3acetic acid (IAA), gibberellin GA3 and total cytokinins | [118] | |
| Two orchids (Lilium cv. Mona Lisa and cv. Little John) | Increasing the chlorophyll and carotenoid content, potassium, calcium, and sulfur content | [119] | |
| Wheat (Triticum aestivum, variety Galaxy-2013) | TiO2 NPs | Stimulating activity of microorganisms in rhizosphere, increased phosphorus content in shoots | [120] |
| Tobacco (Nicotiana benthamiana) | Significant increases in plant biomass | [121] | |
| Rice (Oryza sativa) | higher levels of chlorophyll and carotenoids, higher transpiration rate | [122] | |
| Maize (Zea mays L.) | Increasing glycine, serine and threonine accumulation and promoted energy metabolism (citrate and galactose cycle) | [123] | |
| Vetiveria zizanioides | Increasing chlorophyll content and photochemical efficiency of photosystem II, increased activity of nitrate reductase and carbonic anhydrase | [48] | |
| Sorghum (Sorghum bicolor var. 251) | ZnO NPs | Increasing uptake of zinc, boron and copper into plants, increased chlorophyll content | [124] |
| Saffron (Crocus sativus) | Increasing content of chlorophyll, relative water content, soluble protein content, | [47] | |
| Lemon balm (Melissa officinalis) seedlings | Increasing accumulation of potassium, iron and zinc, increased activity of nitrate reductase. | [125] | |
| Foxtail millet (Setaria italica) | Increasing oil and nitrogen content in grains | [126] | |
| Wheat (Triticum aestivum) | increasing zinc content in grains, | [127] | |
| Arabidopsis thaliana | Fe NPs | Increasing photosynthesis rate, assimilation rate, intracellular CO2 concentration, transpiration rate, and stomatal conductance) as consequence of increased stomatal opening. Decreased pH in rhizosphere resulting increased P availability | [59] |
| Wheat (Triticum aestivum) | Increasing root length, plant height, biomass growth and chlorophyll content | [128] | |
| Muskmelon (Cucumis melo) | NPs served as a source of Fe supporting chlorophyll synthesis | [129] | |
| Rice (Oryza sativa) | Higher water content, higher activity of hydrolytic enzymes amylase and protease | [60] | |
| Lettuce (Lactuca sativa) | TiO2 and Fe3O4 | Increasing P uptake | [130] |
| Soybean (Glycine max) | CeO2 NPs | Increasing stomatal conductance, enhanced photosynthesis rate, increased Rubisco activity, increased NADPH regeneration rate and synthesis of ribulose-1,5-bisphosphate | [131] |
| Jalapeño pepper (Capsicum annuum) | Mn NPs | Source of manganese as micronutrient | [132] |
3.2. Protecting Plants Against Pathogens
The use of silica, zinc oxide, iron oxide, titanium dioxide, silver, and copper nanoparticles presents a promising strategy for enhancing crop yield and addressing global food security. SiO2 NPs have been investigated for their potential to provide controlled nano-delivery of Si and other beneficial compounds to plants. Moradi et al. [133] demonstrated that low concentrations of SiO2 NPs effectively protect the model plant Arabidopsis against Pseudomonas infection. This protective effect is achieved by activating salicylic acid (SA)-dependent plant immunity. The mechanism involves a dual approach: the gradual release of Si (OH)4 from the NPs within the plant tissue, following stomatal uptake and distribution through the spongy mesophyll, and potentially, direct NP-induced SA responses. Furthermore, nanoparticle carriers can minimize environmental contamination by delivering smaller amounts of active molecules. Moreover, AgNPs and CuNPs exhibit inherent antimicrobial properties, disrupting pathogen cell walls or membranes and producing ROS with toxic effects. In addition, ref. [74] found that Cu-NPs, with EC50 values ranging from 162 to 310 μg/mL, were the most effective in inhibiting fungal growth compared to ZnO-NPs (EC50 235–848 μg/mL), silver, and CuO-NPs, demonstrating higher potency than bulk metal counterparts and a standard copper hydroxide fungicide, especially against spores, and silver nanoparticles completely inhibited gray mold symptoms on plum fruit, suggesting their potential as protective antifungal agents. A wide range of NPs, including metal oxides (e.g., ZnO, TiO2, NiO, MgO, Fe3O₄, CuO, CeO2, Ag2O, Au2O3, Al2O3), metalloids (e.g., B, Si), non-metals (e.g., fullerenes, graphene oxide), and other nanomaterials (e.g., quantum dots, liposomes), have been explored for their potential in plant pathology [134,135]. As previously mentioned, TiO2 NPs can trigger a plant immune system, producing defense-related enzymes and proteins [136]. Nanoparticles can also act as carriers for fungicides, bactericides, and other antimicrobial agents, delivering them directly to the site of infection [137,138].
3.3. Increasing Plant Tolerance to Abiotic Stresses
Nanoparticles (NPs) offer a promising approach to enhancing plant tolerance against various abiotic stresses, including salinity, drought, and heavy metals toxicity [34]. Under saline conditions, NPs like Ag, Cu, ZnO, CeO, and Fe3O4 have positively affected various plant species. Furthermore, Ag NPs improved germination and seedling growth in Lathyrus sativus and basil [139,140], enhanced salt tolerance in wheat (through altered antioxidant enzyme activity) [141], S. hortensis [142], and cumin, and reduced salt-stress effects [143]. In addition, Cu NPs reduced oxidative stress and increased yield in wheat [144]. Likewise, ZnO NPs increased sunflower dry weight [145], while CeO NPs enhanced physiological responses in B. napus and boosted biomass in canola [146]. Moreover, ref. [147] demonstrated that soaking lupine (Lupinus termis) seeds in ZnO NPsat 20, 40, and 60 mg/L for 12 h enhanced seedling growth under normal conditions and effectively mitigated the negative impacts of 150 mM NaCl salt stress.
Specifically, ZnO NP treatment, compared to salt-stressed controls, resulted in increased levels of photosynthetic pigments, organic solutes, total phenols, ascorbic acid, and zinc, along with elevated activities of antioxidant enzymes (SOD, CAT, POD, APX), and decreased levels of malondialdehyde and sodium For drought stress, NPs like analcite, ZnO, Cu, Zn, SiO2, TiO2, and Si have demonstrated beneficial effects. Analcite NPs promoted germination and growth in wheat, while ZnO NPs increased soybean germination [148,149]. Additionally, Cu and Zn NPs enhanced wheat’s antioxidant enzyme activity and relative moisture content [150]. Moreover, SiO2 NPs increased shoot length and relative water content in barley, while TiO2 NPs mitigated yield reduction in wheat [150,151].
Silicon NPs, although reported to reduce photosynthesis and stomatal conductivity in hawthorn [152], have also been shown to ameliorate drought stress effects in bananas [153], coriander [154], chickpeas [155], and rice (especially when combined with Se-NPs) [156]. Furthermore, they can reduce the negative impact of drought-enhanced Cd stress in wheat when used with ZnO NPs [157]. In addition, Mohasseli et al. [158] found that irrigating lemon balm (Melissa officinalis) with 40 nm iron oxide nanoparticles (Fe2O3 NPs) at concentrations of 5, 10, 20, 30, and 40 μM alleviated oxidative stress induced by drought by increased essential oil content, restored chlorophyll levels, and decreased proline, malondialdehyde (MDA), and hydrogen peroxide (H2O2) levels in drought-stressed plants. Similarly, ref. [95] demonstrated that irrigating Brassica napus with γ-Fe2O3 NP at concentrations of 0.5, 0.8, 1, or 2 mg/mL effectively alleviated drought stress, leading to increased chlorophyll content, enhanced plant growth, and a reduction in H2O2 levels.
Regarding heavy metal stress, NPs can reduce heavy metal concentration in soil, regulate the transfer of genes, increase antioxidant activity, and stimulate the production of protective substances. Si NPs reduced As stress effects in maize [159], while TiO2 NPs mitigated Cd toxicity in Zea mays [92]. Similarly, SiO2 NPs increased antioxidant enzyme activity in pea seedlings under Cr stress, reduced Cr toxicity by reducing Cr accumulation and oxidative stress, and upregulated the antioxidant defense system and nutrient elements [160]. Konate et al. [96] showed that Fe3O4 NPs significantly improved wheat (Triticum aestivum) seedling growth under heavy metal stress. A 2000 mg/L aqueous suspension of the NPs reduced heavy metal uptake (cadmium, zinc, lead, copper), enhanced antioxidant enzyme activity (peroxidase, SOD), and limited oxidative damage (decreased MDA). In addition, Se NPs alleviated Cd stress in Chinese cabbage [161], and Si NPs reduced Cd stress in rice [162]. Previous studies showed that combined applications of ZnO NPs with biochar and Fe NPs with biochar proved effective against Cd stress. It has also been shown that iron oxide NPs reduce Cd content and improve antioxidant enzyme activity in wheat [34]. Similarly, the foliar application of Fe NPs, particularly Fe3O4, reduced Cd accumulation and toxicity in tomato plants [163]. In addition, data from Ullah et al. [164] showed that the application of ZnO, Fe2O3, TiO2, and CeO2 NPs to rice (Oryza sativa L.) effectively reduced cold stress damage by improving plant defense mechanisms, in particular by regulating antioxidant enzyme activity, as well as significantly enhancing plant development and chlorophyll production. Similarly, Song et al. [165] found that spraying ZnO nanoparticles on the leaves of rice seedlings significantly reduced the negative effects of cold stress. This was evident in taller plants, longer roots, and increased overall dry weight. Additionally, the ZnO nanoparticles helped the plants maintain their chlorophyll levels and lessened the oxidative damage caused by the cold, as shown by lower amounts of harmful substances (H2O2, MDA, proline) and increased activity of protective enzymes (SOD, CAT, POD) [165]. The study of Djanaguiraman et al. [166] demonstrated that foliar application of Se-NPs enhanced the antioxidant defense system of sorghum [Sorghum bicolor (L.) Moench], improved pollen germination, and significantly increased seed yield under high-temperature conditions.
4. Environmental Aspects of Nanoparticles
4.1. Environmental Toxicity of Nanoparticles (NPs)
The environmental toxicity of NPs is a concern, primarily due to their persistence, bioaccumulation potential, and interaction with biological systems at the cellular level [167,168,169]. Nanoparticles in pesticides, fertilizers, and soil amendments are directly applied to ecosystems (Figure 3), leading to contamination [170,171]. Soil parameters are also important factors. For example, soil pH plays a critical role in the availability and toxicity of Al and Mn. In acidic soils (pH < 5), Al (Al3⁺) becomes soluble and poses a significant threat to plant health [172,173]. Al toxicity primarily inhibits root cell division, leading to stunted, swollen, and damaged roots with impaired water uptake [174]. While Al toxicity is well documented, studies indicate the potential stimulatory effects of Al2O3 NPs on plant growth. Similarly, Mn availability is also highly dependent on soil conditions. Manganese deficiency is prevalent in dry, well-aerated, and calcareous soils and those rich in organic matter. Conversely, Mn toxicity is more common in poorly drained and acidic soils [175].

Figure 3.
A comparative overview of nanoparticle risks (ecosystem disruption, human health) and benefits (heavy metal removal, medicine, sustainable agriculture).
Metal oxide nanoparticles (e.g., TiO2, ZnO, AgNPs) have been shown to disrupt essential soil microbial communities [176,177,178]. Soil microorganisms play a crucial role in vital processes such as nitrogen fixation [179], organic matter decomposition [180], and carbon cycling [181], all of which are fundamental for maintaining soil fertility and healthy ecosystems. NPs accumulation in soil can disrupt critical ecological functions [182], impaired nutrient cycling [183], and consequently, diminished soil fertility and carbon sequestration capacity [184,185]. In turn, this may contribute indirectly to climate change by altering carbon storage processes in the soil [186]. Additionally, the high reactivity of nanoparticles can lead to phytotoxicity [187].
Excessive NPs accumulation in the soil can also cause adverse effects on plant growth (Table 3), including stunted root and shoot development, reduced seed germination rates, and chlorosis (yellowing of leaves) [188]. These symptoms indicate the stress plants experience due to nanoparticle exposure, which can interfere with essential metabolic processes such as photosynthesis [189,190]. The disruption of plant growth not only impairs agricultural productivity but also poses significant challenges to the sustainability of agricultural systems, especially in regions where soil health is already compromised by factors like erosion, salinization, or nutrient depletion [191].
Airborne nanoparticles, including carbon-based materials and metal oxides, reduce air quality and settle onto vegetation and water bodies, introducing contaminants into terrestrial and aquatic systems [192]. This deposition exacerbates environmental pollution and ecological imbalances. By altering beneficial plant microbial communities’ structure, composition, and diversity, nanoparticles can negatively impact the plant–soil-microbe system, contrasting with the enhanced microbial activity and nutrient cycling fostered by a healthy soil structure [193,194].
However, the widespread use of nanoparticles necessitates implementing strategies to mitigate their adverse environmental impacts [195,196]. Also, biological and eco-friendly nanoparticle synthesis methods can significantly reduce their environmental footprint [197], while developing biodegradable nanoparticles that break down into non-toxic components offers a promising solution to prevent their long-term persistence in the environment [198]. Within the past decade, substantial research efforts have focused on the synthesis of nanoparticles utilizing a spectrum of biological sources, including plant extracts [199,200], actinomycetes [201], bacteria [201], fungi [202], and basidiomycetes [202]. These biogenically synthesized nanoparticles exhibit distinctive characteristics, rendering them suitable for a broad array of applications encompassing catalysis, biosensing, bio-labeling, tissue engineering, electronics, agriculture [203], optical devices, and environmental remediation [204,205,206].

Table 3.
Certain potential adverse effects of NPs on plant growth and physiology.
Table 3.
Certain potential adverse effects of NPs on plant growth and physiology.
| Plant | NPs Type | Effects | Reference |
|---|---|---|---|
| Wheat (Triticum aestivum L.) Sorghum (Sorghum bicolor L. Moench) Garden cress (Lepidium sativum L.) Mustard (Sinapis alba L.) | AgNPs | Germination inhibition Inhibition of root and shoot growth Disintegration of cell membranes | [207] |
| Wheat (Triticum aestivum L.) | Ag NPs | Growth suppression and changes in cell division and structure | [208] |
| Camelina sativa L | Ag NPs | Affecting the seedling growth and photosynthetic pigments. | [209] |
| Cucumber (Cucumis sativus L.). | SiO2/ZnO NPs | Upregulated or downregulated the contents of sugars, amino acids, glycosides and organic acids, and secondary metabolites | [210] |
| Glycine max | ZnO/TiO2 NPs | Alteration of primary and secondary metabolites levels regulate the stress level in plant | [211] |
| A. thaliana seedlings | Yttrium oxide (Y2O3) NPs | Reduced the lignin synthesis-related gene expression, and increased abscisic acid and ethylene signaling pathway | [212] |
| Duckweed (Lemna minor L.) | CeO2 NPs | Hindered the root elongation | [213] |
| Bean crop (Phaseolus vulgaris L.) | CeO2 NPs | Chromosome abnormality and malformations in pollen grains and defects in pollen walls | [214] |
| Rice (Oryza sativa L.) | CuO NPs | Damaging the enzymes by the accumulation of excess Cu and ROS induced oxidative damage | [215] |
| Pepper (Capsicum annuum L.) | Cu NPs | Enhanced lipid peroxidation level and H2O2 content, thus implying oxidative stress and potentially causing impairment in plasma membrane integrity | [216] |
| Reduced the expression of the Mevalonate kinase (MVK) gene involved in terpenoid metabolism, which limits photosynthesis and decreases energy production efficiency, thereby leading to reduced transcription |
4.2. Potential Impact of Nanoparticles on Biodiversity
Nanoparticles can positively or negatively impact plant biodiversity and are directly linked to agricultural practices. Their potential to improve stress tolerance, nutrient supply and pest control could increase crop yields and reduce chemical inputs, indirectly promoting biodiversity by reducing the need for extensive land use. However, the risks of nanoparticle toxicity, soil disturbance, and bioaccumulation pose a direct threat to plant biodiversity, which could compromise the long-term sustainability of agriculture. NPs can harm ecosystems by impacting microorganisms and higher-order species [217]. NPs such as Ag, TiO2, and ZnO possess antimicrobial properties, which can disrupt the balance of microbial communities in both soil and aquatic environments [218].
Furthermore, NPs can alter the structure and function of microbial biofilms, which are essential for maintaining ecological balance in aquatic systems [219]. High concentrations of NPs, such as CeO2 and CuO, can impair plant growth by inhibiting seed germination, reducing root elongation, and decreasing photosynthetic efficiency [219]. These effects reverberate throughout the food web, influencing herbivores and other species dependent on plants [220]. Soil-dwelling organisms, including earthworms and nematodes, are also sensitive to NPs [221]. Exposure to these particles can alter their population dynamics and behavior, affecting processes like soil aeration, organic matter turnover, and overall plant productivity [222].
Previous studies indicate that nanoparticle exposure can significantly impact plant development. Vannini et al. [223] demonstrated that silver nanoparticles reduce shoot and root length, decrease biomass, and alter metabolic and defense protein expression in wheat. Similarly, [224] observed that Ag NPs lead to reduced root elongation, shoot and root fresh weights, decreased chlorophyll and carotenoid content, and increased rice’s ROS production. Mccracken et al. [225] reported that zinc (Zn) and copper nanoparticles negatively affect wheat, resulting in reduced root and shoot length, decreased biomass and chlorophyll, and increased antioxidant enzyme activity. Furthermore, Rif et al. [226] found that TiO2 nanoparticles in maize cause delayed seed germination, reduced root length, decreased mitotic index, and increased chromosomal aberrations. Considering the fundamental role of plant health in maintaining biodiversity and ensuring sustainable agricultural practices, these nanoparticle-induced disruptions pose a significant concern. Reduced plant vigor and reproductive success, as evidenced by decreased biomass, chlorophyll content, and increased cellular stress, can negatively impact crop yields and the overall resilience of agricultural ecosystems.
Terrestrial animals may also be exposed to NPs through contaminated water, soil, or food [227]. Once ingested, these particles can cross biological barriers, accumulate in tissues, and lead to oxidative stress, inflammation, and toxicity, affecting animal health [228]. Thus, NPs present a multi-level environmental threat, impacting aquatic and terrestrial ecosystems through bioaccumulation and disruption of biological functions [229].
Nanoparticles have been shown to disrupt endocrine systems, leading to reproductive challenges and developmental anomalies [230]. In particular, pollinators, such as bees, exposed to ZnO NPs through contaminated pollen or water have exhibited altered foraging behavior, reduced navigation abilities, and impaired learning [231]. The application of NPs also affects ecosystem engineers, such as corals and keystone species, leading to habitat degradation and the loss of biodiversity [232]. The persistent exposure to NPs can push sensitive species toward extinction, reducing biodiversity and ecological resilience [233]. This decline in biodiversity alters critical ecosystem functions, such as nutrient cycling, water filtration, and primary production, ultimately reducing the ecosystem services supporting wildlife and human populations (Figure 3).
In summary, nanoparticles present both opportunities and challenges for biodiversity. While they have revolutionary applications, their release into the environment poses significant risks to various life and ecological systems. A balanced approach integrating innovation with environmental stewardship is critical to minimizing their adverse impacts and preserving global biodiversity.
4.3. Economic Costs and Benefits of Nanoparticle Use
The economic implications of nanoparticles (NPs) in agriculture are multifaceted, with significant costs and substantial benefits [234]. Understanding the balance between these factors is crucial for assessing the long-term viability and sustainability of NPs. As reported above, in agriculture, nanoparticles such as nano-fertilizers and nano-pesticides improve nutrient delivery and pest control, which can reduce the overall quantity of inputs required and increase crop yields [235]. This leads to cost savings for farmers and boosts productivity. For instance, applying nanoparticles enhances nutrient absorption in plants, thereby offering a strategy to reduce the over-application of chemical fertilizers and mitigate associated environmental pollution [236].
The development and large-scale manufacturing of nanoparticles (NPs) involves significant costs, particularly in research and development (R&D) [237]. The R&D phase requires substantial financial investment, specialized equipment, skilled personnel, and lengthy testing procedures, all contributing to the high upfront costs [238]. These investments are necessary to produce nanoparticles with the required precision, quality, and safety standards [239]. Manufacturing NPs on a large scale presents its own set of challenges. The need for sophisticated technology, strict quality control measures, and consistent production processes means that scaling up nanoparticle production is costly [237]. Additionally, ensuring the safety and efficacy of nanoparticles at a large scale requires constant testing, which adds to the operational costs [237]. These high production costs, combined with scalability limitations, can hinder the adoption of nanoparticles in industries where cost sensitivity is crucial [238].
The potential environmental toxicity and health risks NPs pose further increase the manufacturers’ economic burden. As the release of NPs into the environment could have harmful effects, industries must invest in rigorous testing procedures and comply with stringent safety regulations [239,240]. This regulatory compliance adds to production costs and increases the time required for approval and commercialization of NP-based products [241]. Without adequate oversight, the release of nanoparticles into ecosystems could result in pollution clean-up costs [238], biodiversity loss [242], public health concerns [243], and additional financial liabilities for companies [244].
While the economic benefits of nanoparticles (NPs) are substantial, particularly in enhancing efficiency, productivity, and sustainability, the associated costs pose significant challenges that must be addressed. Insurance and liability costs for businesses working with nanoparticles can be substantial [245]. Given the potential risks of nanoparticle exposure and environmental contamination, companies must often carry specialized insurance to cover potential damages [246]. This is especially true in industries where nanoparticles’ long-term environmental and health effects are not fully understood, leading to more significant uncertainty for investors. This uncertainty can make it difficult for companies to secure funding, potentially slowing market growth.
Additionally, adopting nanoparticles across various industries often requires a considerable investment in new infrastructure, including specialized production facilities, storage systems, and waste disposal measures, particularly relevant in industries like agriculture, where production and disposal must adhere to strict regulatory standards [247]. Developing streamlined regulatory frameworks that ensure safety without imposing excessive financial burdens is crucial, thus encouraging broader adoption. Furthermore, increasing public understanding of the benefits and risks of nanoparticles can help mitigate resistance to their use, fostering a supportive market environment.
5. Future Trends in Nanoparticle Research in Agriculture
Biosynthesis of nanoparticles offers enormous potential to alleviate biotic and abiotic stress, boost plant growth and improve agricultural production. Most studies indicate numerous benefits resulting from the use of nanoparticles. However, it should be emphasized that the studies are not yet advanced and present only preliminary results derived under controlled conditions, often confirmed for selected plants and chosen soil and climatic conditions [21,22]. Moreover, there remain limitations and gaps in knowledge concerning using NPs in agriculture and their long-term impact on the environment (soil, plant, water, air, humans, and animals).
Specifically, we lack comprehensive knowledge regarding the long-term fate of NPs in soil, water, and air, including their transformation, degradation, and accumulation. More research is also needed on their ecotoxicological effects on non-target organisms (beneficial insects and soil microbes), their potential bioaccumulation and biomagnification, the mechanisms of plant uptake and translocation and subsequent effects on plant growth, metabolism, and nutritional value, as well as the potential for transfer to humans through the food chain. Notably, the potential human health risks associated with exposure to NPs, including ingestion, inhalation, dermal contact, and interactions with human biological systems, also require further study.
The best application method of NPs, foliar or soil, should also be studied. In this respect, there is little data on the long-term impact of the NPs on plants in crop rotation and soil properties. Studies are also lacking regarding the interaction of NPs with basic soil properties, such as soil reaction, clay minerals or organic matter, which can effectively change the effectiveness of the introduced substances. Similarly, more information related to environmental and public health issues from the agricultural use of NPs is needed. For instance, the dispersion of NPs into the atmosphere during application may lead to respiratory disorders, allergic reactions, and accumulation in bodily organs.
Regarding policy, gaps remain concerning appropriate guidelines for the production and law regarding NPs usage and the public’s perception of these substances. A separate issue is the introduction of uniform safety rules and standards for the production and prospective agricultural use of NPs. Currently, no such regulations indicate the need for process control or further monitoring of the fate and transformation of NPs in the environment. Moreover, the general public is unaware of the presence of NPs and the possibilities associated with their use. In this context, public participation cannot be ignored, and the public should be informed about the presence of NPs in the environment and the pros and cons associated with NPs in the context of food and feed production.
Nanotechnology is a promising interdisciplinary research area that various fields, including agriculture, have adopted. The development of agrochemicals in the nanoscale will continue to develop and be observed, but the direction must be carefully indicated so that the use of NPs is smart and safe without any adverse effects on the environment, plants, animals, and humans. This approach sets future trends in research related to the appropriate application and dosage of NPs. It is necessary to track the transport paths of NPs to plants and in plants and the transformation of NPs in the soil, depending on their properties. This effort requires the development of suitable standards that should be developed in the scope of the use and production of NPs without detriment. Many experts believe that NPs in the form of nanofertilizers and nanopesticides can increase the nutrient efficiency of plants and lead to reduced losses resulting from leaching, volatilization, precipitation or immobilization. While nanoparticles offer an excellent opportunity for advancing precision farming and their development should be encouraged, their potential for rapid entry into the food chain necessitates careful monitoring of environmental safety to protect animal and human health.
Future trends in nanoparticle research in agriculture include precision agriculture with nanosensors for real-time monitoring [248] and targeted delivery of agrochemicals, enhanced crop production via nanofertilizers and nanopesticides, improved plant health through nanomaterials for disease management, environmental sustainability with nanoremediation and efficient water management, advancements in food safety and quality using nanotechnology in packaging and for nutritional enhancement, exploration of novel bio-based and multifunctional nanoparticles, and a growing focus on risk assessment and regulatory development to ensure safe and responsible application [249].
6. Conclusions and Perspectives
Despite the recognized challenges, the trajectory of nanotechnology in agriculture points towards a fundamental shift in how we approach crop production, moving beyond incremental improvements to potentially transformative changes in sustainability and efficiency. Years of research now allow us to envision a future where nano-enabled agriculture facilitates truly precision-based farming, optimizing resource allocation at the individual plant level and fostering resilient agroecosystems. This necessitates a move beyond simply applying nanoscale versions of existing inputs. Instead, the focus should shift towards designing smart nanomaterials that dynamically interact with plants and their environment, responding in real-time to changing conditions and minimizing unintended consequences.
Furthermore, the convergence of nanotechnology with other advanced fields like biotechnology and artificial intelligence holds immense promise. Imagine nanosensors providing real-time data that inform AI-driven robotic systems for targeted interventions, creating a closed-loop system for optimized crop management. This integrated approach could unlock unprecedented levels of resource efficiency and significantly reduce agriculture’s environmental footprint. However, realizing this vision demands a parallel evolution in our understanding of the long-term ecological and health implications of nanomaterials. Future research must prioritize the development of inherently safer and more sustainable nanomaterials, drawing inspiration from biological systems and focusing on biodegradability and minimal off-target effects. Moreover, proactive and globally harmonized regulatory frameworks are essential, not as a barrier to innovation, but as a crucial element in building public trust and ensuring the responsible adoption of these powerful technologies.
Ultimately, the future of nano-enabled agriculture lies not just in the application of tiny particles, but in harnessing their unique properties to create intelligent, responsive, and sustainable agricultural systems that can meet the growing global demand for food while safeguarding our planet. This requires a bold, interdisciplinary approach that fosters innovation while prioritizing safety and long-term sustainability.
Author Contributions
Conceptualization, M.B., Q.J. and A.A.; validation, D.B., M.E. and H.F.; data curation, S.G.B. and M.E.; writing—original draft preparation, M.B., M.J. and Q.J.; writing—review and editing, D.H., S.G.B. and M.B.; Literature review, M.J. and D.B.; Investigation, D.H., M.E. and A.A.; visualization, H.F.; supervision, M.Č.; project administration, M.Č.; funding acquisition, M.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation under the project numbers HRZZ-IPS-2022-02-2099 and HRZZ-MOBDOL-2023-08-5800.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Croatian Science Foundation under the project numbers HRZZ- IPS-2022-02-2099 and HRZZ-MOBDOL-2023-08-5800.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic stress in crop production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 228798. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, B.; Singh, B.N.; Hidangmayum, A.; Jatav, H.S.; Chandra, K.; Singhal, R.K.; Sathyanarayana, E.; Patra, A.; Mohapatra, K.K. Physiological mechanisms and adaptation strategies of plants under nutrient deficiency and toxicity conditions. In Plant Perspectives to Global Climate Changes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 173–194. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Lamsaadi, N.; Farissi, M. Biostimulatory effects of ascorbic acid in improving plant growth, photosynthesis-related parameters and mitigating oxidative damage in alfalfa (Medicago sativa L.) under salt stress condition. Biologia. 2024, 79, 2375–2385. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Wani, M.Y.; Hashim, M.A.; Nabi, F.; Malik, M.A. Nanotoxicity: Dimensional and morphological concerns. Adv. Phys. Chem. 2011, 2011, 450912. [Google Scholar] [CrossRef]
- Mcoyi, M.P.; Mpofu, K.T.; Sekhwama, M.; Mthunzi-Kufa, P. Developments in Localized Surface Plasmon Resonance. In Plasmonics; Springer Nature: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Juma, M.W.; Birech, Z.; Mwenze, N.M.; Ondieki, A.M.; Maaza, M.; Mokhotjwa, S.D. Localized surface plasmon resonance sensing of Trenbolone acetate dopant using silver nanoparticles. Sci. Rep. 2024, 14, 5721. [Google Scholar] [CrossRef]
- Mondal, S.; Truong, T.T.; Doan, V.H.M.; Phan, T.T.V.; Choi, J.; Lee, B.; Oh, J. Nano-biosensors for Detecting Microbial Pathogens in Agriculture. In Nano-Microbiology for Sustainable Development; Springer Nature: Cham, Switzerland, 2025; pp. 193–212. [Google Scholar] [CrossRef]
- Muthukrishnan, L. An overview on the nanotechnological expansion, toxicity assessment and remediating approaches in Agriculture and Food industry. Environ. Technol. Innov. 2022, 25, 102136. [Google Scholar] [CrossRef]
- Semaltianos, N.G. Nanoparticles by Laser Ablation. Crit. Rev. Solid State Mater. Sci. 2010, 35, 105–124. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Pantidos, N.; Horsfall, L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Pérez-Hernández, H.; Fernández-Luqueño, F.; Huerta-Lwanga, E.; Mendoza-Vega, J.; Álvarez-Solís José, D. Effect of engineered nanoparticles on soil biota: Do they improve the soil quality and crop production or jeopardize them? Land Degrad. Dev. 2020, 31, 2213–2230. [Google Scholar] [CrossRef]
- Achari, G.A.; Kowshik, M. Recent developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. J. Agric. Food Chem. 2018, 66, 8647–8661. [Google Scholar] [CrossRef]
- Phogat, N.; Khan, S.A.; Shankar, S.; Ansary, A.A.; Uddin, I. Fate of inorganic nanoparticles in agriculture. Adv. Mater. Lett 2016, 7, 3–12. [Google Scholar] [CrossRef]
- Saritha, G.N.G.; Anju, T.; Kumar, A. Nanotechnology—Big impact: How nanotechnology is changing the future of agriculture? J. Agric. Food Res. 2022, 10, 100457. [Google Scholar] [CrossRef]
- Jafir, M.; Irfan, M.; Zia-ur-Rehman, M.; Hafeez, F.; Ahmad, J.N.; Sabir, M.A.; Zulfiqar, U.; Iqbal, R.; Zulfiqar, F.; Moosa, A. The global trend of nanomaterial usage to control the important agricultural arthropod pests: A comprehensive review. Plant Stress 2023, 10, 100208. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Mathur, T.; Maheshwari, J.; Nagella, P. Interaction of Nanomaterials with Plant Macromolecules: Nucleic Acid, Proteins and Hormones. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications; Springer International Publishing: Cham, Switzerland, 2023; pp. 231–271. [Google Scholar] [CrossRef]
- Francis, D.V.; Abdalla, A.K.; Mahakham, W.; Sarmah, A.K.; Ahmed, Z.F. Interaction of plants and metal nanoparticles: Exploring its molecular mechanisms for sustainable agriculture and crop improvement. Environ. Int. 2024, 190, 108859. [Google Scholar] [CrossRef]
- Zou, M.; Yu, K.; Huang, Y.; Sheng, Q.; Chen, Y.; Liu, H.; Zhu, Z.; Feang, N.; Ma, D.; Dou, D. Activation of ROS signaling molecule by AgNPs: Molecular mechanisms in roses under abiotic stress, exploration of stress memory, and impact on root microbiota. In Plant Soil; Springer Nature: Cham, Switzerland, 2025; pp. 1–22. [Google Scholar] [CrossRef]
- Omar, S.A.; Elsheery, N.I.; Pashkovskiy, P.; Kuznetsov, V.; Allakhverdiev, S.I.; Zedan, A.M. Impact of Titanium Oxide Nanoparticles on Growth, Pigment Content, Membrane Stability, DNA Damage, and Stress-Related Gene Expression in Vicia faba under Saline Conditions. Horticulturae 2023, 9, 1030. [Google Scholar] [CrossRef]
- Mokhtarabadi, E.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Saadatmand, S.; Ebadi, M. Selenium nanoparticles affected growth and secondary metabolism in chicory seedlings epigenetically by modifying DNA methylation and transcriptionally by upregulating DREB1A transcription factor and stimulating genes involved in phenylpropanoid metabolism. Environ. Pollut. 2025, 370, 125907. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Buja, I.; Sabella, E.; Monteduro, A.G.; Chiriacò, M.S.; De Bellis, L.; Luvisi, A.; Maruccio, G. Advances in Plant Disease Detection and Monitoring: From Traditional Assays to In-Field Diagnostics. Sensors 2021, 21, 2129. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.V.; Asif, A.; Ahmed, Z.F.R. Nanoparticle-Enhanced Plant Defense Mechanisms Harnessed by Nanotechnology for Sustainable Crop Protection. In Nanoparticles in Plant Biotic Stress Management; Springer Nature: Cham, Switzerland, 2024; pp. 451–491. [Google Scholar] [CrossRef]
- Sun, X.D.; Ma, J.Y.; Feng, L.J.; Duan, J.L.; Yuan, X.Z. Precise tracking of nanoparticles in plant roots. Nat. Protoc. 2024, 20, 248–271. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E.J. Nanoparticle exposure and hormetic dose–responses: An update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Cameron, S.J.; Sheng, J.; Hosseinian, F.; Willmore, W.G. Nanoparticle effects on stress response pathways and nanoparticle–protein interactions. Int. J. Mol. Sci. 2022, 23, 7962. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; MEbrahim, A.A.; Abd El-Mageed, T.A.; et al. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, R.; Li, Y.S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Landa, P.; Prerostova, S.; Petrova, S.; Knirsch, V.; Vankova, R.; Vanek, T. The transcriptomic response of Arabidopsis thaliana to zinc oxide: A comparison of the impact of nanoparticle, bulk, and ionic zinc. Environ. Sci. Technol. 2015, 49, 14537–14545. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Banerjee, K.; Pramanik, P.; Maity, A.; Joshi, D.; Wani, S.; Krishnan, P. Methods of Using Nanomaterials to Plant Systems and Their Delivery to Plants (Mode of Entry, Uptake, Translocation, Accumulation, Biotransformation and Barriers). In Advances in Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 123–152. [Google Scholar] [CrossRef]
- Newkirk, G.M.; De Allende, P.; Jinkerson, R.E.; Giraldo, J.P. Nanotechnology Approaches for Chloroplast Biotechnology Advancements. Front. Plant Sci. 2021, 12, 691295. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Fatemi, H.; Rizwan, M. Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: A review. Ecotoxicol. Environ. Saf. 2021, 225, 112769. [Google Scholar] [CrossRef]
- Mohammed, J.; Rashmi, R.; Surya Ulhas, R.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.; Shehata, W.F.; Almaghasla, M.I. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Manickavasagam, M.; Pavan, G.; Vasudevan, V. A comprehensive study of the hormetic influence of biosynthesized AgNPs on regenerating rice calli of indica cv. IR64. Sci. Rep. 2019, 9, 8821. [Google Scholar] [CrossRef]
- Rostami, M.; Talarposhti, R.M.; Mohammadi, H.; Demyan, M.S. Morpho-physiological Response of Saffron (Crocus sativus L.) to Particle Size and Rates of Zinc Fertilizer. Commun. Soil Sci. Plant Anal. 2019, 50, 1250–1257. [Google Scholar] [CrossRef]
- Shabbir, A.; Khan, M.M.A.; Ahmad, B.; Sadiq, Y.; Jaleel, H.; Uddin, M. Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash. Photosynth. 2019, 57, 599–606. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Vojodi Mehrabani, L.; Bonabian, Z.; Aazami, M.A.; Rasouli, F.; Feldo, M.; Strzemski, M.; Dresler, S. Foliar Application of Cerium Oxide-Salicylic Acid Nanoparticles (CeO2:SA Nanoparticles) Influences the Growth and Physiological Responses of Portulaca oleracea L. Under Salinity. Int. J. Mol. Sci. 2021, 23, 5093. [Google Scholar] [CrossRef] [PubMed]
- Mawale, K.S.; Nandini, B.; Giridhar, P. Copper and Silver Nanoparticle Seed Priming and Foliar Spray Modulate Plant Growth and Thrips Infestation in Capsicum spp. ACS Omega 2024, 9, 3430–3444. [Google Scholar] [CrossRef] [PubMed]
- Çekiç, F.Ö.; Ekinci, S.; İnal, M.S.; Özakça, D. Silver nanoparticles induced genotoxicity and oxidative stress in tomato plants. Turk. J. Biol. 2017, 41, 700–707. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, J.; Zhang, Y.; Han, S.; Zhang, J.; Jia, G. DNA oxidative damage as a sensitive genetic endpoint to detect the genotoxicity induced by titanium dioxide nanoparticles. Nanomaterials 2022, 12, 2616. [Google Scholar] [CrossRef]
- Panda, K.K.; Golari, D.; Venugopal, A.; Achary, V.M.; Phaomei, G.; Parinandi, N.L.; Sahu, H.K.; Panda, B.B. Green synthesized zinc oxide (ZnO) nanoparticles induce oxidative stress and DNA damage in Lathyrus sativus L. root bioassay system. Antioxidants 2017, 6, 35. [Google Scholar] [CrossRef]
- Atha, D.H.; Wang, H.; Petersen, E.J.; Cleveland, D.; Holbrook, R.D.; Jaruga, P.; Dizdaroglu, M.; Xing, B.S.; Nelson, B.C. Copper Oxide Nanoparticle Mediated DNA Damage in Terrestrial Plant Models. Environ. Sci. Technol. 2012, 46, 1819–1827. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Abd El-Aziz, M.; Abd Elwahed, M.S.; Shaaban, E. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kang, Y.; Chang, Y.; Kim, J. Effects of Zerovalent Iron Nanoparticles on Photosynthesis and Biochemical Adaptation of Soil-Grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef]
- Guha, T.; Ravikumar, K.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the mobilization of native phosphorus in the mung bean rhizosphere using ZnO nanoparticles synthesized by soil fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Zia-ur-Rehman, M.; Mfarrej, M.F.B.; Usman, M.; Anayatullah, S.; Rizwan, M.; Alharby, H.F.; Abu Zeid, I.M.; Alabdallah, N.M.; Ali, S. Effect of iron nanoparticles and conventional sources of Fe on growth, physiology and nutrient accumulation in wheat plants grown on normal and salt-affected soils. J. Hazard. Mater. 2023, 458, 131861. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Hassan, Z.U.; Akram, M.A.; Tariq, R.; Brestic, M.; Xie, W. Nanoparticle’s uptake and translocation mechanisms in plants via seed priming, foliar treatment, and root exposure: A review. Environ. Sci. Pollut. Res. 2022, 29, 89823–89833. [Google Scholar] [CrossRef]
- Feigl, G. The impact of copper oxide nanoparticles on plant growth: A comprehensive review. J. Plant Interact. 2023, 18, 2243098. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Bouhadi, M.; El Kouali, M.H.; Samir, K.; Elbouhmadi, K.; Talbi, M.; Fougrach, H. Exogenous Application of Thiamine and Nicotinic Acid Improves Tolerance and Morpho-physiological Parameters of Lens culinaris Under Lead (Pb) Exposure. J. Plant Growth Regul. 2024, 43, 4185–4198. [Google Scholar] [CrossRef]
- Soujoud, R.; Lamsaadi, N.; Bouhadi, M.; El Moukhtari, A.; Ourribane, M. Physiological and Biochemical Responses of Maize (Zea mays L.) to the Application of Re-Treated Urban Wastewater Using Wood Waste Biochar. J. Ecol. Eng. 2024, 25, 180–193. [Google Scholar] [CrossRef]
- Bouhadi, M.; Daoui, O.; El Hajjouji, H.; Elkhattabi, S.; Chtita, S.; El Kouali, M.; Talbi, M.; Fougrach, H. Study of the competition between Pi and Cr (VI) for the use of Pi-transporter at Vicia faba L. Using molecular modeling. Plant Physiol. Biochem. 2023, 196, 695–702. [Google Scholar] [CrossRef]
- Bouhadi, M.; Daoui, O.; El Hajjouji, H.; Elkhattabi, S.; Chtita, S.; El Kouali, M.; Talbi, M.; Fougrach, H. Physiological and molecular modeling investigations of the relationship between sulfate and chromium VI uptake in Vicia faba L. Biocatal. Agric. Biotechnol. 2022, 47, 102554. [Google Scholar] [CrossRef]
- Bouhadi, M.; Elkouali, M.H.; Talbi, M.; Amegrissi, F.; Fougrach, H. Phytoextraction of chromium VI by Raphanus sativus L. under exogenous application of citric acid. Bot. Pacifica 2022, 11, 89–93. [Google Scholar] [CrossRef]
- Bouhadi, M.; Abchir, O.; Yamari, I.; El Hamsas El Youbi, A.; Azgaoui, A.; Chtita, S.; El Hajjouji, H.; El Kouali, M.; Talbi, M.; Fougrach, H. Genotoxic effects and mitosis aberrations of chromium (VI) on root cells of Vicia faba and its molecular docking analysis. Plant Physiol. Biochem. 2024, 207, 108361. [Google Scholar] [CrossRef]
- El moukhtari, A.; Lamsaadi, N.; Bouhadi, M.; Abchir, O.; Chtita, S.; Samir, K.; El Rasafi, T.; Ghoulam, C.; Farissi, M. Uptake and competition between cadmium nanoparticles and essential nutrients (Fe, Mg and Mn) in Phaseolus vulgaris L. using a molecular docking approach. Euro-Mediterr. J. Environ. Integr. 2025, 1–11. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Thomas, M.B.; Thomas, R.; Raichur, A.M.; Chakravortty, D. Interaction of Silver Nanoparticles with Serum Proteins Affects Their Antimicrobial Activity In Vivo. Antimicrob. Agents Chemother. 2013, 57, 4945. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef]
- Madany, M.M.; Saleh, A.M.; Habeeb, T.H.; Hozzein, W.N.; Abd Elgawad, H. Silicon dioxide nanoparticles alleviate the threats of broomrape infection in tomato by inducing cell wall fortification and modulating ROS homeostasis. Environ. Sci. Nano 2020, 7, 1415–1430. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Explicating the cross-talks between nanoparticles, signaling pathways and nutrient homeostasis during environmental stresses and xenobiotic toxicity for sustainable cultivation of cereals. Chemosphere 2022, 286, 131827. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Seed priming with zinc oxide nanoparticles to enhance crop tolerance to environmental stresses. Int. J. Mol. Sci. 2023, 24, 17612. [Google Scholar] [CrossRef]
- Mohammadi, H.; Amani-Ghadim, A.R.; Matin, A.A.; Ghorbanpour, M. Fe0 nanoparticles improve physiological and antioxidative attributes of sunflower (Helianthus annuus) plants grown in soil spiked with hexavalent chromium. 3 Biotech 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.W.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Bhatti, K.H.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.M.A.; Elansary, H.O. Seed Priming with the Selenium Nanoparticles Maintains the Redox Status in the Water Stressed Tomato Plants by Modulating the Antioxidant Defense Enzymes. Plants 2022, 12, 1556. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. S. Afr. J. Bot. 2022, 151, 889–899. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Humbal, A.; Pathak, B. Application of Nanotechnology in Plant Growth and Diseases Management: Tool for Sustainable Agriculture. In Agricultural and Environmental Nanotechnology; Interdisciplinary Biotechnological, Advances; Fernandez-Luqueno, F., Patra, J.K., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Şahin, E.Ç.; Aydın, Y.; Utkan, G.; Uncuoğlu, A.A. Nanotechnology in agriculture for plant control and as biofertilizer. In Synthesis of Bionanomaterials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 469–492. [Google Scholar] [CrossRef]
- Li, W.; Keller, A.A. Assessing the Impacts of Cu and Mo Engineered Nanomaterials on Crop Plant Growth Using a Targeted Proteomics Approach. ACS Agric. Sci. Technol. 2023, 4, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, H.; Esmaiel Pour, B.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Chattha, M.U.; Khan, I.; Khan, T.A.; Nawaz, M.; Tang, H.; Noor, M.A.; Asseri, T.A.; Hashem, M.; Guoqin, H. Zinc Seed Priming Alleviates Salinity Stress and Enhances Sorghum Growth by Regulating Antioxidant Activities, Nutrient Homeostasis, and Osmolyte Synthesis. Agronomy 2024, 14, 1815. [Google Scholar] [CrossRef]
- Fouda, H.M.; Saied, E.; Abdelmouty, E.S.; Osman, M.S. Ameliorative role of copper nanoparticle in alleviating salt-induced oxidative stress in fenugreek (Trigonella foenum-graecum L.) plants. Biocatal. Agric. Biotechnol. 2024, 57, 103095. [Google Scholar] [CrossRef]
- Taran, N.; Storozhenko, V.; Svietlova, N.; Batsmanova, L.; Shvartau, V.; Kovalenko, M. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res. Lett. 2017, 12, 60. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc Oxide Nanoparticles Application Alleviates Arsenic (As) Toxicity in Soybean Plants by Restricting the Uptake of as and Modulating Key Biochemical Attributes, Antioxidant Enzymes, Ascorbate-Glutathione Cycle and Glyoxalase System. Plants 2020, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Mingyu, S.; Xiao, W.; Chao, L.; Chunxiang, Q.; Liang, C.; Huang Hao, H.; Xiaoqing, L.; Fashui, H. Antioxidant Stress is Promoted by Nano-anatase in Spinach Chloroplasts Under UV-B Radiation. Biol. Trace Elem. Res. 2018, 121, 69–79. [Google Scholar] [CrossRef]
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2019, 239, 124794. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Malik, S.; Qayyum, M.F.; Alamri, S.; Alyemeni, M.; Ahmad, P. Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 72. [Google Scholar] [CrossRef]
- Asadi-Kavan, Z.; Khavari-Nejad, R.A.; Iranbakhsh, A.; Najafi, F. Cooperative effects of iron oxide nanoparticle (α-Fe2O3) and citrate on germination and oxidative system of evening primrose (Oenthera biennis L.). J. Plant Interact. 2020, 15, 166–179. [Google Scholar] [CrossRef]
- Palmqvist, N.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedlings. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef]
- Jalali, M.; Ghanati, F.; Modarres-Sanavi, A.M. Effect of Fe3O4 nanoparticles and iron chelate on the antioxidant capacity and nutritional value of soil cultivated maize (Zea mays) plants. Crop Pasture Sci. 2016, 67, 621–628. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef]
- Garg, S.; Rumjit, N.P.; Roy, S. Smart agriculture and nanotechnology: Technology, challenges, and new perspective. Adv. Agrochem 2024, 3, 115–125. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Nelwamondo, A.A.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal nanoparticles in agriculture: A review of possible use. Coatings 2020, 12, 1586. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, H.; Joshi, P. Nanofertilisers, nanopesticides and nanosensors in agriculture. Nanosci. Food Agric. 2016, 1, 247–282. [Google Scholar] [CrossRef]
- Beegum, S.; Das, S. Nanosensors in agriculture. In Agricultural Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 465–478. [Google Scholar] [CrossRef]
- Chhipa, H. Applications of nanotechnology in agriculture. Methods Microbiol. 2018, 46, 115–142. [Google Scholar] [CrossRef]
- Mai, K.; Williams, R.A. Response of Oak and Maple Seed Germination and Seedling Growth to Different Manganese Fertilizers in a Cultured Substratum. Forests 2019, 10, 547. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Exploring the potential of nanofertilizers for a sustainable agriculture. Plant Nano Biol. 2023, 5, 100044. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Brestic, M.; Raza, M.A.; Pang, T.; Langham, D.R.; Safdar, M.E.; Ahmed, S.; Wen, B.; Gao, Y.; et al. Changes in morphology, chlorophyll fluorescence performance and Rubisco activity of soybean in response to foliar application of ionic titanium under normal light and shade environment. Sci. Total Environ. 2019, 658, 626–637. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 237149. [Google Scholar] [CrossRef]
- Dinler, B.S.; Cetinkaya, H.; Koc, F.N.; Gül, V.; Sefaoğlu, F. Effects of titanium dioxide nanoparticles against salt and heat stress in safflower cultivars. Acta Bot. Bras. 2024, 38, e20230136. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Zivcak, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Jiang, M.; Song, Y.; Kanwar, M.K.; Ahammed, G.J.; Shao, S.; Zhou, J. Phytonanotechnology applications in modern agriculture. J. Nanobiotechnol. 2021, 19, 430. [Google Scholar] [CrossRef] [PubMed]
- Rehmanullah, R.Z.; Inayat, N.; Majeed, A. Application of nanoparticles in agriculture as fertilizers and pesticides: Challenges and opportunities. In New Frontiers in Stress Management for Durable Agriculture; Springer: Singapore, 2020; pp. 281–293. [Google Scholar]
- Kumar, Y.; Tiwari, K.N.; Singh, T.; Raliya, R. Nanofertilizers and their role in sustainable agriculture. Ann. Plant Soil Res. 2021, 23, 238–255. [Google Scholar] [CrossRef]
- Santas-Miguel, V.; Arias-Estevez, M.; Rodriguez-Seijo, A.; Arenas—Lago, D. Use of metal nanoparticles in agriculture. A review on the effects on plant germination. Environ. Pollut. 2023, 334, 122222. [Google Scholar] [CrossRef] [PubMed]
- Kusumavathi, K.; Rautaray, S.K.; Sarkar, S.; Dash, S.; Sahoo, T.R.; Swain, S.K.; Sethi, D. Nano-herbicides a sustainable strategy for weed control. Plant Nano Biol. 2025, 11, 100132. [Google Scholar] [CrossRef]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Gharib, F.A.; Ghazi, S.M.; Hegazi, A.Z.; Hafz, A.G. Physiological responses of two varieties of common bean (Phaseolus vulgaris L.) to foliar application of silver nanoparticles. Nanomater. Nanotechnol. 2016, 6, 13. [Google Scholar] [CrossRef]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory effect of silver nanoparticles on the growth and flowering of potted oriental lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef]
- Zahra, Z.; Maqbool, T.; Arshad, M.; Badshah, M.A.; Choi, H.K.; Hur, J. Changes in fluorescent dissolved organic matter and their association with phytoavailable phosphorus in soil amended with TiO2 nanoparticles. Chemosphere 2019, 227, 17–25. [Google Scholar] [CrossRef]
- Hao, Y.; Yuan, W.; Ma, C.; White, J.C.; Zhang, Z.; Adeel, M.; Zhou, T.; Rui, Y.; Xing, B. Engineered nanomaterials suppress Turnip mosaic virus infection in tobacco (Nicotiana benthamiana). Environ. Sci. Nano 2018, 5, 1685–1693. [Google Scholar] [CrossRef]
- Kiany, T.; Pishkar, L.; Sartipnia, N.; Iranbakhsh, A.; Barzin, G. Effects of silicon and titanium dioxide nanoparticles on arsenic accumulation, phytochelatin metabolism, and antioxidant system by rice under arsenic toxicity. Environ. Sci. Pollut. Res. 2022, 29, 34725–34737. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Shahid, M.; Ahmed, T.; Tahir, M.; Naqqash, T.; Muhammad, S.; Song, F.; Abid, H.M.A.; Aslam, Z. Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol. Environ. Saf. 2020, 192, 110303. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem 2017, 65, 8552–8559. [Google Scholar] [CrossRef] [PubMed]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. Int 2019, 26, 24430–24444. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions. Nanomaterials 2019, 9, 1559. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Sanabria, J.; Bindraban, P.S.; Singh, U.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci. Total Environ. 2020, 722, 137808. [Google Scholar] [CrossRef]
- Al-Amri, N.; Tombuloglu, H.; Slimani, Y.; Akhtar, S.; Barghouthi, M.; Almessiere, M.; Alshammari, T.; Baykal, A.; Sabit, H.; Ercan, I.; et al. Size effect of iron (III) oxide nanomaterials on the growth, and their uptake and translocation in common wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2020, 194, 110377. [Google Scholar] [CrossRef]
- Shah, A.A.; Yasin, N.A.; Mudassir, M.; Ramzan, M.; Hussain, I.; Siddiqui, M.H.; Ali, H.M.; Shabbir, Z.; Ali, A.; Ahmed, S.; et al. Iron oxide nanoparticles and selenium supplementation improve growth and photosynthesis by modulating antioxidant system and gene expression of chlorophyll synthase (CHLG) and protochlorophyllide oxidoreductase (POR) in arsenic-stressed Cucumis melo. Environ. Pollut. 2022, 307, 119413. [Google Scholar] [CrossRef]
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic Nanoparticle (TiO2 and Fe3O4) Application Modifies Rhizosphere Phosphorus Availability and Uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882. [Google Scholar] [CrossRef]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of Soybean (Glycine max L.). Environ. Sci Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hernandez-Viezcas, J.A.; Valdes, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: A sustainable approach for agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Moradi, A.; Maceroni, M.; Reinhardt, D.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Elmer, W.; Ma, C.; White, J. Nanoparticles for plant disease management. Curr. Opin. Environ. Sci. Health 2018, 6, 66–70. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Kalia, A.; Bhardwaj, K.; Bhardwaj, P.A.K.; Valis, M.; Kuca, K. Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives. J. Fungi 2020, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Choudhary, P.; Kumar, S.; Daima, H.K. Mechanistic approaches for crosstalk between nanomaterials and plants: Plant immunomodulation, defense mechanisms, stress resilience, toxicity, and perspectives. Environ. Sci. Nano 2024, 11, 2324–2351. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, A.; Silletti, M.F.; Faraone, I.; Milella, L. Nanoparticulate Antibiotic Systems as Antibacterial Agents and Antibiotic Delivery Platforms to Fight Infections. J. Nanomater. 2020, 2020, 6905631. [Google Scholar] [CrossRef]
- Hojjat, S.S. Effect of interaction between Ag nanoparticles and salinity on germination stages of Lathyrus sativus L. J. Environ. Soil Sci 2019, 2, 186–191. [Google Scholar]
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticle on fenugreek seed germination under salinity levels. Russ. Agric. Sci 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Nejatzadeh, F. Effect of silver nanoparticles on salt tolerance of Satureja hortensis L. during in vitro and in vivo germination tests. Heliyon 2021, 7, e05981. [Google Scholar] [PubMed]
- Ekhtiyari, R.; Moraghebi, F. Effect of nanosilver particles on salinity tolerance of cumin (Cuminum cyminum L.). J. Plant Biotechnol 2012, 25, 99–107. [Google Scholar]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant Nutr. 2016, 39, 172–180. [Google Scholar] [CrossRef]
- Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Pollut. 2016, 219, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Alam, M.A.; Syed, M.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Sedghi, M.; Mitra, H.; Sahar, T. Effect of nano zinc oxide on the germination of soybean seeds under drought stress. Ann. West Univ. Timiş. Ser. Biol 2013, 16, 73–78. [Google Scholar]
- Basit, F.; Shahid, M.; Abbas, S.; Naqqash, T.; Akram, M.S.; Tahir, M.; Azeem, M.; Cai, Y.; Jia, S.; Hu, J.; et al. Protective role of ZnO nanoparticles in soybean seedlings growth and stress management under Cr-enriched conditions. Plant Growth Regul. 2023, 100, 703–716. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Turgeon, R. The puzzle of phloem pressure. Plant Physiol. 2010, 154, 578–581. [Google Scholar] [CrossRef]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomášková, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. For. Res. Pap 2015, 76, 350–359. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata Grand Nain’Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Afshari, M.; Pazoki, A.; Sadeghipour, O. Foliar-applied silicon and its nanoparticles stimulates physio-chemical changes to improve growth, yield and active constituents of coriander (Coriandrum sativum L.) essential oil under different irrigation regimes. Silicon 2021, 13, 4177–4188. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Bagci, E.G.; Coban, S. Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J. Plant Interact 2007, 2, 105–113. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019, 26, 19859–19870. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa L.) under salinity conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Mohasseli, V.; Farbood, F.; Moradi, A. Antioxidant defense and metabolic responses of lemon balm (Melissa officinalis L.) to Fe-nano-particles under reduced irrigation regimes. Ind. Crops Prod. 2020, 149, 112338. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front. Environ. Sci 2016, 4, 46. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Zhang, S. Mechanism of Migration and Transformation of Nano Selenium and Mitigates Cadmium Stress in Plants. Master’s Thesis, Shandong University, Jinan, China, 2019. [Google Scholar]
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2015, 22, 2837–2845. [Google Scholar] [CrossRef]
- Kumar, J.A.; Krithiga, T.; Manigandan, S.; Sathish, S.; Renita, A.A.; Prakash, P.; Prasad, B.N.; Kumar, T.P.; Rajasimman, M.; Hosseini-Bandegharaei, A. A focus to green synthesis of metal/metal based oxide nanoparticles: Various mechanisms and applications towards ecological approach. J. Clean. Prod. 2021, 324, 129198. [Google Scholar] [CrossRef]
- Ullah, S.; Ikram, M.; Xiao, J.; Khan, A.; Din, I.; Huang, J. Influence of Foliar Application of Nanoparticles on Low Temperature Resistance of Rice Seedlings. Plants 2023, 13, 2949. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc Oxide Nanoparticles Alleviate Chilling Stress in Rice (Oryza Sativa L.) by Regulating Antioxidative System and Chilling Response Transcription Factors. Molecules 2020, 26, 2196. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Prasad, P.V. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef]
- Rahmatizadeh, R.; Javad Arvin, S.M.; Jamei, R.; Mozaffari, H.; Nejhad, F.R. Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant Interact 2019, 14, 474–481. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. 2015, 22, 4122–4143. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, W.; Li, Q.; Wang, Z.; Liu, S. The effects and the potential mechanism of environmental transformation of metal nanoparticles on their toxicity in organisms. Environ. Sci. Nano 2018, 5, 2482–2499. [Google Scholar] [CrossRef]
- Ghazaryan, K.; Agrawal, S.; Margaryan, G.; Harutyunyan, A.; Rajput, P.; Movsesyan, H.; Rajput, V.D.; Singh, R.K.; Minkina, T.; Elshikh, M.S. Soil pollution: An agricultural and environmental problem with nanotechnological remediation opportunities and challenges. Discov. Sustain. 2024, 5, 453. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Tolra, R.; Hajiboland, R.; Arroyave, C.; Barceló, J. Mechanisms of hyper-resistance and hyper-tolerance to aluminum in plants. Alum. Stress Adapt. Plants 2015, 24, 81–98. [Google Scholar] [CrossRef]
- Panda, S.K.; Baluška, F.; Matsumoto, H. Aluminum stress signaling in plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 517877. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Rubilar, O.; Fernández-Baldo, M.A.; Bertolino, F.A.; Durán, N.; Seabra, A.; Tortella, G. The nanotechnology among US: Are metal and metal oxides nanoparticles a nano or mega risk for soil microbial communities? Crit. Rev. Biotechnol. 2019, 39, 157–172. [Google Scholar] [CrossRef]
- Ahmed, A.; He, P.; He, P.; Wu, Y.; He, Y.; Munir, S. Environmental effect of agriculture-related manufactured nano-objects on soil microbial communities. Environ. Int. 2023, 173, 107819. [Google Scholar] [CrossRef]
- Maurice, N. Impact of nanoparticles on soil microbes for enhancing soil fertility and productivity. In Nanotechnology for Sustainable Agriculture; Apple Academic Press: Cambridge, MA, USA, 2023; pp. 75–127. [Google Scholar]
- Huang, W. Boosting Soil Health: The Role of Rhizobium in Legume Nitrogen Fixation. Mol. Soil Biol. 2024, 15, 129–139. [Google Scholar] [CrossRef]
- Raza, T.; Qadir, M.F.; Khan, K.S.; Eash, N.S.; Yousuf, M.; Chatterjee, S.; Manzoor, R.; Ur Rehman, S.; Oetting, J.N. Unrevealing the potential of microbes in decomposition of organic matter and release of carbon in the ecosystem. J. Environ. Manag. 2023, 344, 118529. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Mcgee, C.F. The effects of silver nanoparticles on the microbial nitrogen cycle: A review of the known risks. Environ. Sci. Pollut. Res. 2020, 27, 31061–31073. [Google Scholar] [CrossRef]
- Iqbal, S.; Xu, J.; Allen, S.D.; Khan, S.; Nadir, S.; Arif, M.S.; Yasmeen, T. Unraveling consequences of soil micro-and nano-plastic pollution on soil-plant system: Implications for nitrogen (N) cycling and soil microbial activity. Chemosphere 2020, 260, 127578. [Google Scholar] [CrossRef] [PubMed]
- Abdullatif, Y.; Soliman, E.; Hammad, S.; El-Ghamry, A.; Mansour, M. Impact of Carbon Nanoparticles on Aggregation and Carbon Sequestration under Soil Degradation–a Review. J. Soil Sci. Agric. Eng. 2024, 15, 85–92. [Google Scholar] [CrossRef]
- Sun, W.; Dou, F.; Li, C.; Ma, X.; Ma, L.Q. Impacts of metallic nanoparticles and transformed products on soil health. Crit. Rev. Environ. Sci. Technol. 2021, 51, 973–1002. [Google Scholar] [CrossRef]
- Faisal, M.; Saquib, Q.; Alatar, A.A.; Al-Khedhairy, A.A. Phytotoxicity of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A prospective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Dashora, K.; Mishra, M.; Fasake, V.D.; Srivastva, A. Effect of accumulation of nanoparticles in soil health-a concern on future. Front. Nanosci. Nanotechnol 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Ping, Y.; Cao, D.; Hu, J.; Lin, Y.; Dang, C.; Xue, D. The application, safety, and challenge of nanomaterials on plant growth and stress tolerance. Ind. Crops Prod. 2024, 222, 119691. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, T.; Wang, Y.; Li, T.; Chi, Q. Multifaceted impacts of nanoparticles on plant nutrient absorption and soil microbial communities. Front. Plant Sci. 2024, 15, 1497006. [Google Scholar] [CrossRef]
- Bhardwaj, L.K.; Rath, P.; Choudhury, M. A comprehensive review on the classification, uses, sources of nanoparticles (NPs) and their toxicity on health. Aerosol Sci. Eng. 2023, 7, 69–86. [Google Scholar] [CrossRef]
- Das, P.; Penton, C.R.; Westerhoff, P.; Perreault, F. Prospects of 2D graphene nanomaterials in plant-based agriculture and their fate in terrestrial soil: A critical review. Environ. Sci. Nano 2023, 10, 2936–2956. [Google Scholar] [CrossRef]
- Zhang, K.; Rengel, Z.; Zhang, F.; White, P.J.; Shen, J. Rhizosphere engineering for sustainable crop production: Entropy-based insights. Trends Plant Sci. 2023, 28, 390–398. [Google Scholar] [CrossRef]
- Bhavya, G.; Belorkar, S.A.; Mythili, R.; Geetha, N.; Shetty, H.S.; Udikeri, S.S.; Jogaiah, S. Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 2021, 275, 129975. [Google Scholar] [CrossRef]
- Aravinthan, A.; Govarthanan, M.; Selvam, K.; Praburaman, L.; Selvankumar, T.; Balamurugan, R.; Kamala-Kannan, S.; Kim, J.H. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int. J. Nanomed. 2015, 10, 1977–1983. [Google Scholar] [CrossRef]
- Hayes, K.L.; Mui, J.; Song, B.; Sani, E.S.; Eisenman, S.W.; Sheffield, J.B.; Kim, B. Effects, uptake, and translocation of aluminum oxide nanoparticles in lettuce: A comparison study to phytotoxic aluminum ions. Sci. Total Environ. 2020, 719, 137393. [Google Scholar] [CrossRef]
- Chauhan, P.; Kumar, D.; Sharma, R. Biogenic magnetic iron oxide nanoparticles for multifunctional applications in environmental remediation and biomedical field. J. Environ. Chem. Eng. 2025, 13, 115646. [Google Scholar] [CrossRef]
- Chinnappan, S.; Kandasamy, S.; Arumugam, S.; Seralathan, K.K.; Thangaswamy, S.; Muthusamy, G. Biomimetic synthesis of silver nanoparticles using flower extract of Bauhinia purpurea and its antibacterial activity against clinical pathogens. Environ. Sci. Pollut. Res. 2018, 25, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; AlYahya, S.; Govarthanan, M.; ALjahdali, N.; Al-Enazi, N.; Alsamhary, K.; Alshehri, W.; Alwakeel, S.; Alharbi, S. Soil bacteria Cupriavidus sp. Mediates the extracellular synthesis of antibacterial silver nanoparticles. J. Mol. Struct. 2020, 1202, 127233. [Google Scholar] [CrossRef]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S. Mycogenic Selenium Nanoparticles as Potential New Generation Broad Spectrum Antifungal Molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef]
- Jogaiah, S.; Kurjogi, M.; Abdelrahman, M.; Hanumanthappa, N.; Tran, L.P. Ganoderma applanatum-mediated green synthesis of silver nanoparticles: Structural characterization, and in vitro and in vivo biomedical and agrochemical properties. Arab. J. Chem. 2019, 12, 1108–1120. [Google Scholar] [CrossRef]
- Moll, J.; Klingenfuss, F.; Widmer, F.; Gogos, A.; Bucheli, T.D.; Hartmann, M.; Van Der Heijden, M.G. Effects of titanium dioxide nanoparticles on soil microbial communities and wheat biomass. Soil Biol. Biochem. 2017, 111, 85–93. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.S.A.; Borah, K.K.; Goswami, Y.; Hakeem, K.R.; Chakrabartty, I. The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A review. Environ. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S. Low concentrations of copper oxide nanoparticles alter microbial community structure and function of sediment biofilms. Sci. Total Environ. 2019, 653, 705–713. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xu, M.; Xiao, L.; Dai, Z.; Li, J. The impacts of γ-Fe2O3 and Fe3O4 nanoparticles on the physiology and fruit quality of muskmelon (Cucumis melo) plants. Environ. Pollut. 2019, 249, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic nanoparticles: Synthesis, characterisation and applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Pociecha, E.; Przemieniecki, S.W.; Oćwieja, M. Phytotoxicity of Silver Nanoparticles with Different Surface Properties on Monocots and Dicots Model Plants. J. Soil Sci. Plant Nutr. 2022, 22, 1647–1664. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Mirmoeini, T.; Pishkar, L.; Kahrizi, D.; Barzin, G.; Karimi, N. Phytotoxicity of green synthesized silver nanoparticles on Camelina sativa L. Physiol. Mol. Biol. Plants 2021, 27, 417–427. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wang, Y.; Gao, Y.; Zhang, Z.; Xu, J.; Xing, G. Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur. 2021, 10, e269. [Google Scholar] [CrossRef]
- Leopold, L.F.; Coman, C.; Clapa, D.; Oprea, I.; Toma, A.; Iancu, Ș.D.; Barbu-Tudoran, L.; Suciu, M.; Ciorîță, A.; Cadiș, A.I. The effect of 100–200 nm ZnO and TiO2 nanoparticles on the in vitro-grown soybean plants. Colloids Surf. B Biointerfaces 2022, 216, 112536. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Li, Z.; Chai, J.; Feng, J.; Han, R. Phytotoxicity and the molecular response in yttrium oxide nanoparticle–treated Arabidopsis thaliana seedlings. Protoplasma 2023, 260, 955–966. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Ma, Y.; Dai, W.; Song, Z.; Wang, Y.; Shen, J.; He, X.; Yang, F.; Zhang, Z. Interaction of cerium oxide nanoparticles and ionic cerium with duckweed (Lemna minor L.): Uptake, distribution, and phytotoxicity. Nanomaterials 2023, 13, 2523. [Google Scholar] [CrossRef]
- Salehi, H.; Chehregani Rad, A.; Raza, A.; Chen, J.T. Foliar application of CeO2 nanoparticles alters generative components fitness and seed productivity in bean crop (Phaseolus vulgaris L.). Nanomaterials 2021, 11, 862. [Google Scholar] [CrossRef]
- Desmau, M.; Carboni, A.; Le Bars, M.; Doelsch, E.; Benedetti, M.F.; Auffan, M.; Levard, C.; Gelabert, A. How microbial biofilms control the environmental fate of engineered nanoparticles? Front. Environ. Sci. 2020, 8, 82. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V. Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef]
- Schulz, R.; Bundschuh, M.; Entling, M.H.; Jungkunst, H.F.; Lorke, A.; Schwenk, K.; Schäfer, R.B. A synthesis of anthropogenic stress effects on emergence-mediated aquatic-terrestrial linkages and riparian food webs. Sci. Total Environ. 2024, 908, 168186. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, H.; Teng, Y. Impact of Nanoparticles on Soil Ecosystems. In The Role of Nanoparticles in Plant Nutrition under Soil Pollution: Nanoscience in Nutrient Use Efficiency; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef]
- Wang, W.; Do, A.T.N.; Kwon, J.-H. Ecotoxicological effects of micro-and nanoplastics on terrestrial food web from plants to human beings. Sci. Total Environ. 2022, 834, 155333. [Google Scholar] [CrossRef]
- Mccracken, C.; Dutta, P.K.; Waldman, W.J. Critical assessment of toxicological effects of ingested nanoparticles. Environ. Sci. Nano 2016, 3, 256–282. [Google Scholar] [CrossRef]
- Arif, Y.; Mir, A.R.; Zieliński, P.; Hayat, S.; Bajguz, A. Microplastics and nanoplastics: Source, behavior, remediation, and multi-level environmental impact. J. Environ. Manag. 2024, 356, 120618. [Google Scholar] [CrossRef] [PubMed]
- Dagar, G.; Bagchi, G. Nanoparticles as potential endocrine disruptive chemicals. In NanoBioMedicine; Saxena, S.K., Khurana, S.M.P., Eds.; Springer: Singapore, 2020; pp. 411–429. [Google Scholar]
- Milivojević, T.; Glavan, G.; Božič, J.; Sepčić, K.; Mesarič, T.; Drobne, D. Neurotoxic potential of ingested ZnO nanomaterials on bees. Chemosphere 2015, 120, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Kumar, M.; Bolan, N.S.; Singh, L.; Kumar, S.; Kumar, R.; You, S. Are microplastics destabilizing the global network of terrestrial and aquatic ecosystem services? Environ. Res. 2021, 198, 111243. [Google Scholar] [CrossRef]
- Araújo, C.V.; Laissaoui, A.; Silva, D.C.; Ramos-Rodríguez, E.; González-Ortegón, E.; Espíndola, E.L.; Baldó, F.; Mena, F.; Parra, G.; Blasco, J. Not only toxic but repellent: What can organisms’ organisms’ responses tell us about contamination and what are the ecological consequences when they flee from an environment? Toxics 2020, 8, 118. [Google Scholar] [CrossRef]
- Brar, K.K.; Magdouli, S.; Othmani, A.; Ghanei, J.; Narisetty, V.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Awasthi, M.K.; Pandey, A. Green route for recycling of low-cost waste resources for the biosynthesis of nanoparticles (NPs) and nanomaterials (NMs)-A review. Environ. Res. 2022, 207, 112202. [Google Scholar] [CrossRef]
- Khan, M.R.; Rizvi, T.F. Application of nanofertilizer and nanopesticides for improvements in crop production and protection. In Nanoscience and Plant–Soil Systems; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 405–427. [Google Scholar]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Shegokar, R.; Nakach, M. Large-scale manufacturing of nanoparticles—An industrial outlook. In Drug Delivery Aspects; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Tassey, G. Policy issues for R&D investment in a knowledge-based economy. J. Technol. Transf. 2004, 29, 153–185. [Google Scholar]
- Hobson, D.W. Commercialization of nanotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 189–202. [Google Scholar] [CrossRef]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Grillo, R.; Fraceto, L.F.; Amorim, M.J.; Scott-Fordsmand, J.J.; Schoonjans, R.; Chaudhry, Q. Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. J. Hazard. Mater. 2021, 404, 124148. [Google Scholar] [CrossRef]
- Corsi, I.; Venditti, I.; Trotta, F.; Punta, C. Environmental safety of nanotechnologies: The eco-design of manufactured nanomaterials for environmental remediation. Sci. Total Environ. 2023, 864, 161181. [Google Scholar] [CrossRef] [PubMed]
- Opacic, T.; Paefgen, V.; Lammers, T.; Kiessling, F. Status and trends in the development of clinical diagnostic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1441. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N. Nanoparticles: Environmental problems or problem solvers? Bioscience 2018, 68, 241–246. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Sintes, J.R.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Isibor, P.O. Regulations and policy considerations for nanoparticle safety. In Environmental Nanotoxicology: Combatting the Minute Contaminants; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Ramachandran, G.; Wolf, S.M.; Paradise, J.; Kuzma, J.; Hall, R.; Kokkoli, E.; Fatehi, L. Recommendations for oversight of nanobiotechnology: Dynamic oversight for complex and convergent technology. J Nanopart Res. 2011, 13, 1345–1371. [Google Scholar] [CrossRef]
- Mcalea, E.M.; Mullins, M.; Murphy, F.; Tofail, S.A.; Carroll, A.G. Engineered nanomaterials: Risk perception, regulation and insurance. J. Risk Res. 2016, 19, 444–460. [Google Scholar] [CrossRef]
- Śliwiński, A.; Janowicz-Lomott, M. The risk of nanotechnology: A challenge to the insurance industry. J. Rev. Glob. Econ. 2020, 6, 404–419. [Google Scholar]
- Nielsen, M.B.; Skjolding, L.; Baun, A.; Hansen, S.F. European nanomaterial legislation in the past 20 years—Closing the final gaps. NanoImpact 2023, 32, 100487. [Google Scholar] [CrossRef]
- Bharti, A.; Jain, U.; Chauhan, N. From lab to field: Nano-biosensors for real-time plant nutrient tracking. Plant Nano Biol. 2024, 9, 100079. [Google Scholar] [CrossRef]
- Mgadi, K.; Ndaba, B.; Roopnarain, A.; Rama, H.; Adeleke, R. Nanoparticle applications in agriculture: Overview and response of plant-associated microorganisms. Front. Microbiol. 2024, 15, 1354440. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).