Organic Amendments Enhance Agroecosystem Multifunctionality via Divergent Regulation of Energy Flow Uniformity in Soil Nematode Food Webs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Plant and Soil Sampling
2.4. Physicochemical and Biological Analyses
2.5. Nematode Extraction and Identification
2.6. Statistical Analyses
2.6.1. Assessment of Ecosystem Multifunctionality
2.6.2. Analysis of Nematode Community
2.6.3. Data Analysis
3. Results
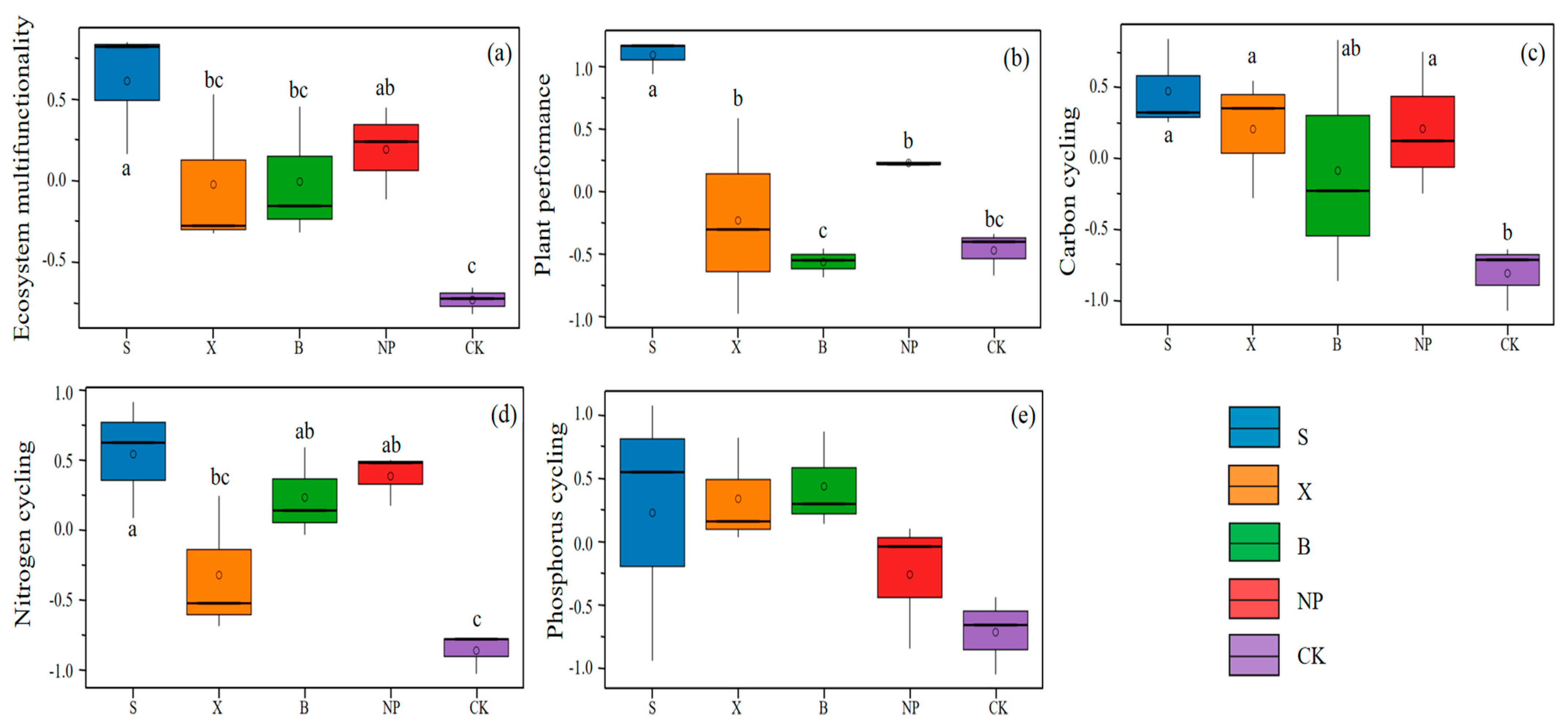
3.1. Ecosystem Multifunctionality
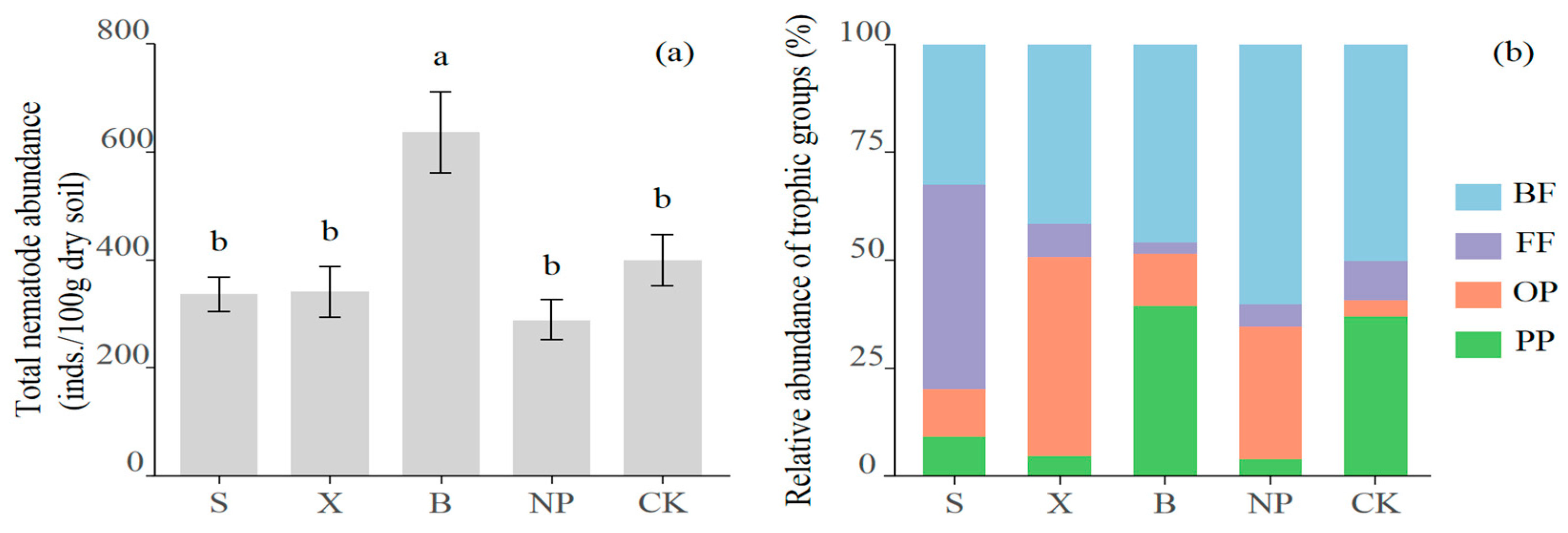
3.2. The Composition and Diversity of Soil Nematode Communities
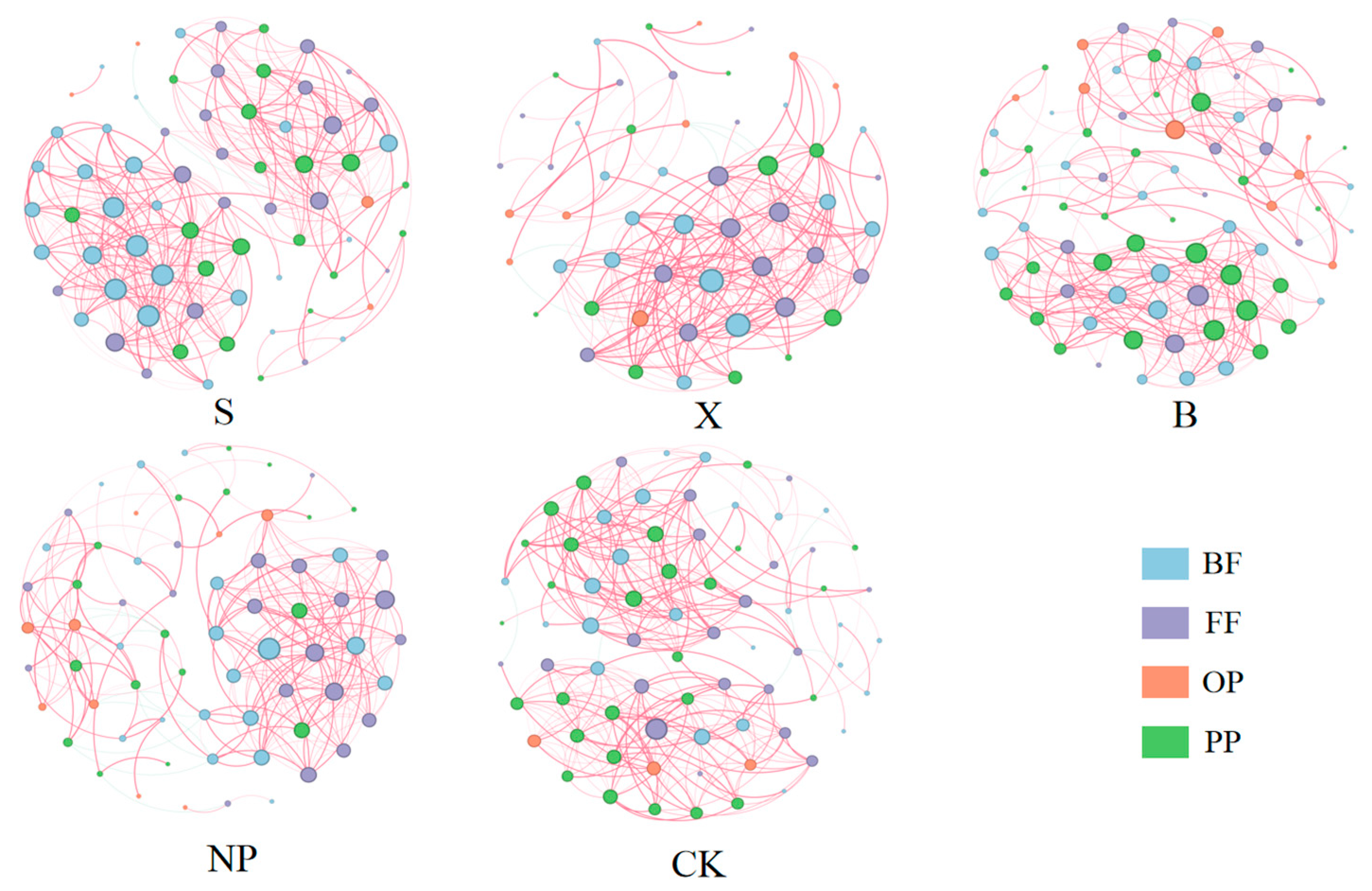
3.3. The Characteristics of Soil Nematode Co-Occurrence Networks
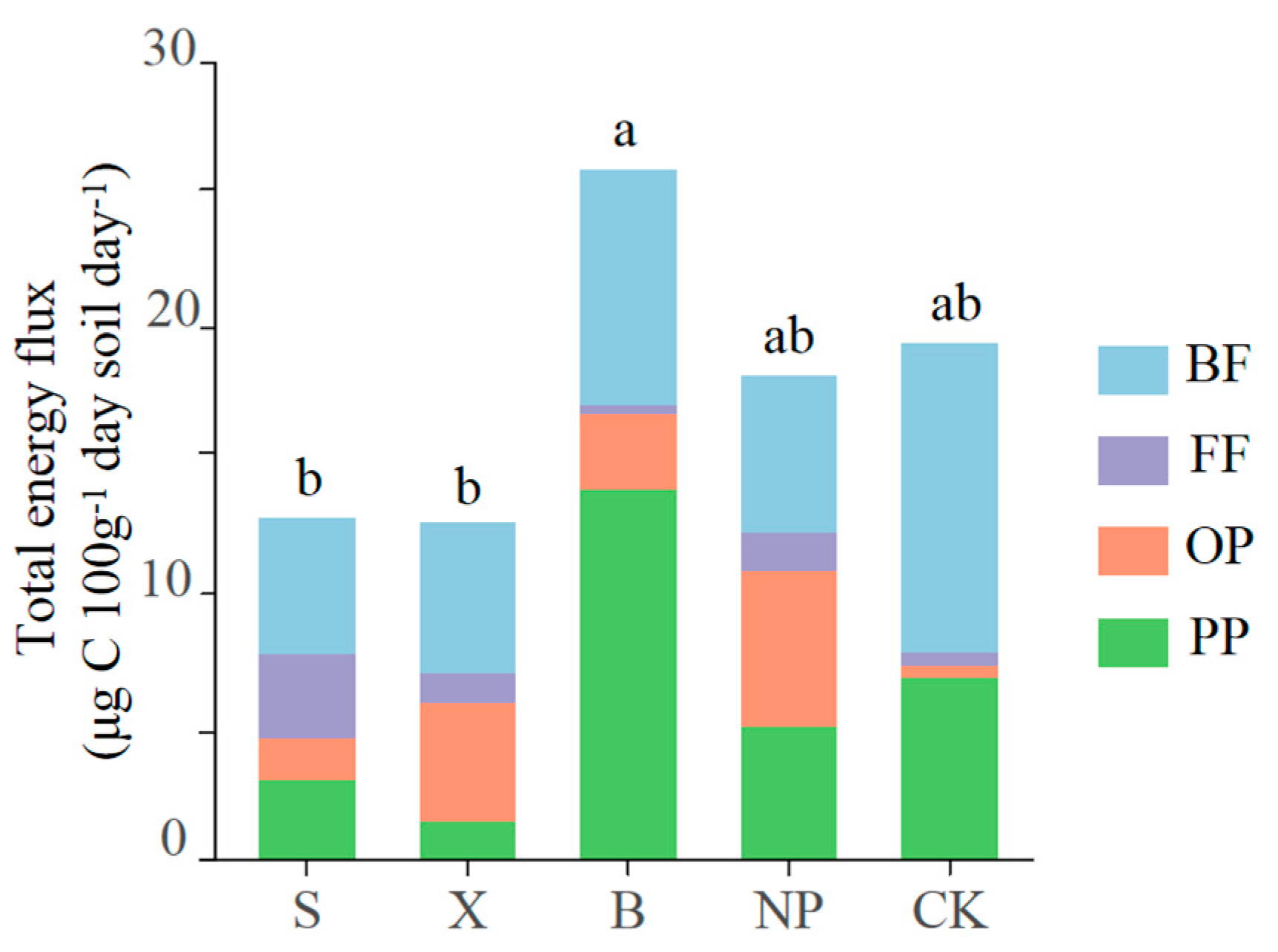
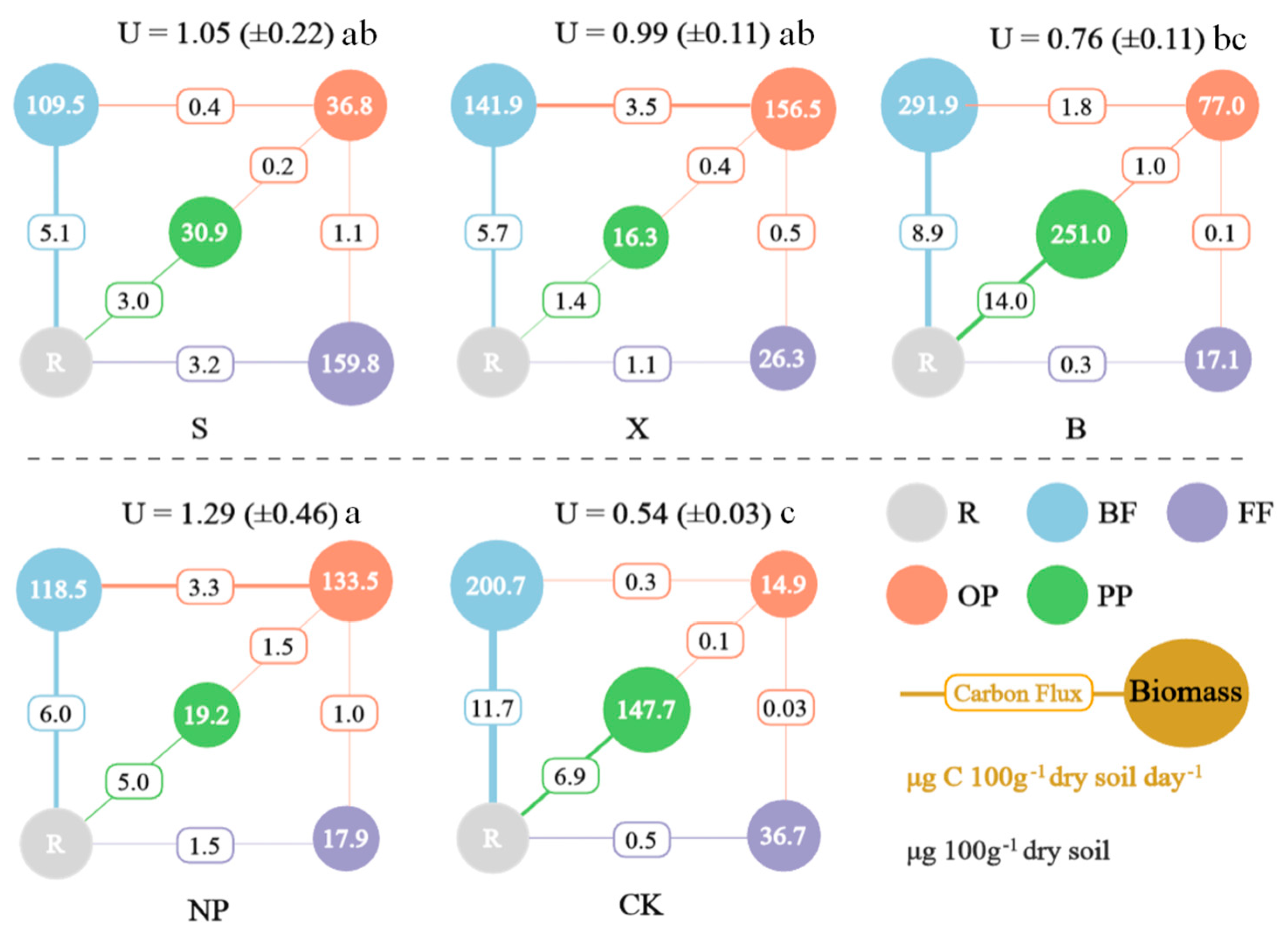
3.4. The Energetic Structure of Soil Nematode Food Webs
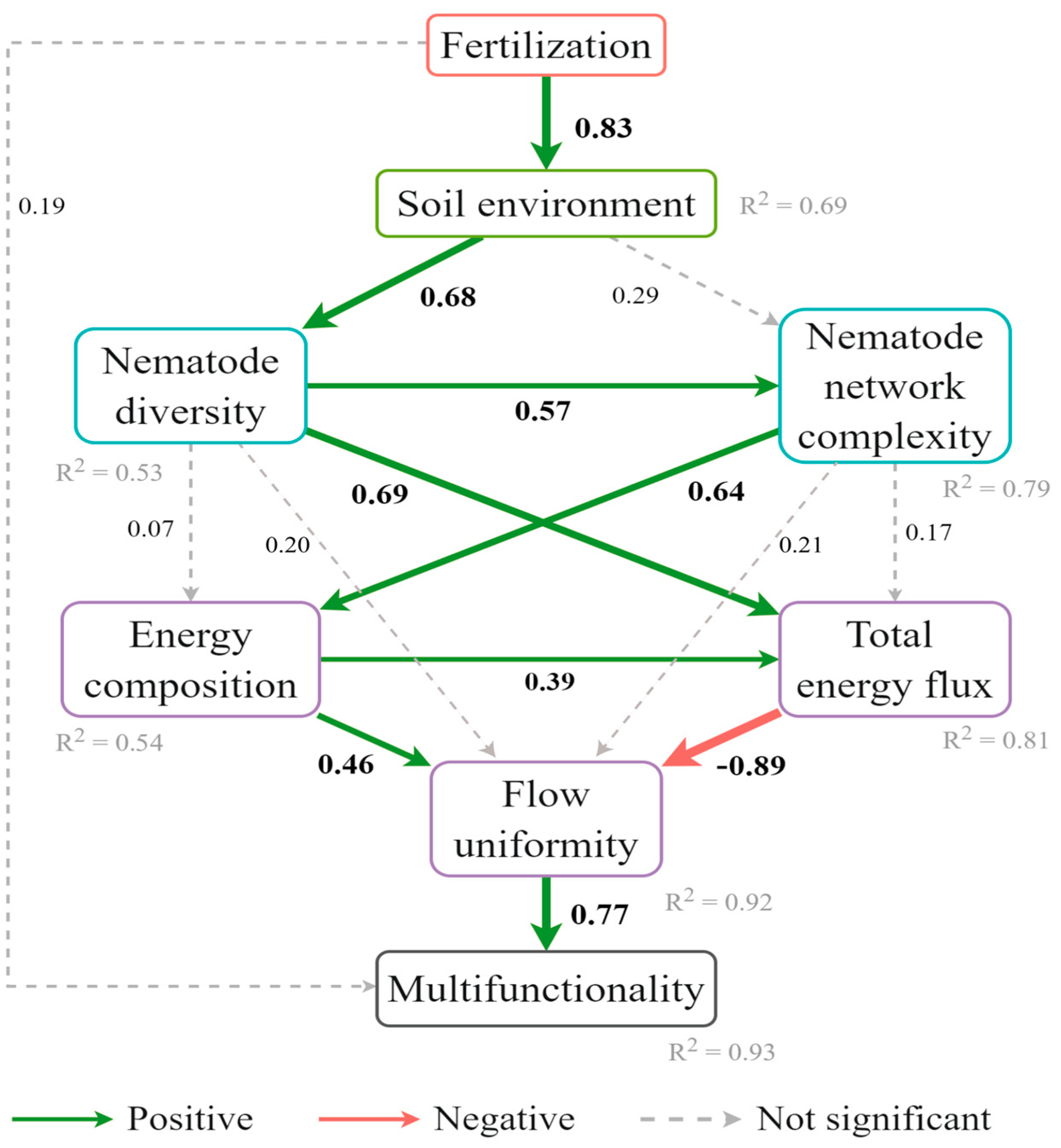
3.5. Relationships Between Soil Nematode Communities and Ecosystem Multifunctionality
4. Discussion
4.1. Effects of Organic Amendments on Soil Nematode Diversity and Network Complexity
4.2. Effects of Organic Amendments on the Energetic Structure of Soil Nematode Food Webs
4.3. Soil Nematode Communities as Drivers of Agroecosystem Multifunctionality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A. Biodiversity–ecosystem functioning research: Brief history, major trends and perspectives. Biol. Conserv. 2023, 285, 110210. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.K.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.S.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Ekschmitt, K.; Bakonyi, G.; Bongers, M.; Bongers, T.; Boström, S.; Dogan, H.; Harrison, A.; Nagy, P.; O’Donnell, A.G.; Papatheodorou, E.M.; et al. Nematode community structure as indicator of soil functioning in European grassland soils. Eur. J. Soil Biol. 2001, 37, 263–268. [Google Scholar] [CrossRef]
- Tamburini, G.; Bommarco, R.; Wanger, T.C.; Kremen, C.; van der Heijden, M.G.A.; Liebman, M.; Hallin, S. Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 2020, 6, eaba1715. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Huang, T.; Li, G.; Wang, Z.; Zhao, S. Soil nematode biodiversity mediates the impact of altered precipitation on dryland agroecosystem multifunctionality in the loess tableland area of China. Agric. Ecosyst. Environ. 2024, 376, 109221. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y.; Wei, G. Soil multitrophic network complexity enhances the link between biodiversity and multifunctionality in agricultural systems. Glob. Change Biol. 2022, 28, 140–153. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Zhu, B.; Wan, B.; Liu, T.; Zhang, C.; Cheng, L.; Cheng, Y.; Tian, S.; Chen, X.; Hu, F.; Whalen, J.K.; et al. Biochar enhances multifunctionality by increasing the uniformity of energy flow through a soil nematode food web. Soil Biol. Biochem. 2023, 183, 109056. [Google Scholar] [CrossRef]
- Gan, H.; Wickings, K. Root herbivory and soil carbon cycling: Shedding “green” light onto a “brown” world. Soil Biol. Biochem. 2020, 150, 107972. [Google Scholar] [CrossRef]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Pollierer, M.M.; Dyckmans, J.; Scheu, S.; Haubert, D. Carbon flux through fungi and bacteria into the forest soil animal food web as indicated by compound-specific fatty acid analysis. Funct. Ecol. 2012, 26, 978–990. [Google Scholar] [CrossRef]
- Richter, A.; Kern, T.; Wolf, S.; Struck, U.; Ruess, L. Trophic and non-trophic interactions in binary links affect carbon flow in the soil micro-food web. Soil Biol. Biochem. 2019, 135, 239–247. [Google Scholar] [CrossRef]
- Barnes, A.D.; Jochum, M.; Lefcheck, J.S.; Eisenhauer, N.; Scherber, C.; O’Connor, M.I.; de Ruiter, P.; Brose, U. Energy Flux: The Link between Multitrophic Biodiversity and Ecosystem Functioning. Trends Ecol. Evol. 2018, 33, 186–197. [Google Scholar] [CrossRef]
- Moore, J.C.; de Ruiter, P.C. Energetic Food Webs: An Analysis of Real and Model Ecosystems; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef]
- Holtkamp, R.; Kardol, P.; van der Wal, A.; Dekker, S.C.; van der Putten, W.H.; de Ruiter, P.C. Soil food web structure during ecosystem development after land abandonment. Appl. Soil Ecol. 2008, 39, 23–34. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Neher, D.A. Ecology of Plant and Free-Living Nematodes in Natural and Agricultural Soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef]
- Ritz, K.; Trudgill, D.L. Utility of nematode community analysis as an integrated measure of the functional state of soils: Perspectives and challenges. Plant Soil 1999, 212, 1–11. [Google Scholar] [CrossRef]
- Huang, J.; Huang, T.; Chen, J.; Li, G.; Wang, Z.; Huo, N. Nematode Community Characteristics Indicate Soil Restoration under Different Revegetation Approaches in the Semiarid Area of the Chinese Loess Plateau. Forests 2023, 14, 1886. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Ewald, M.; Glavatska, O.; Ruess, L. Effects of resource manipulation on nematode community structure and metabolic footprints in an arable soil across time and depth. Nematology 2020, 22, 1025–1043. [Google Scholar] [CrossRef]
- Zhang, X.; Ferris, H.; Mitchell, J.; Liang, W. Ecosystem services of the soil food web after long-term application of agricultural management practices. Soil Biol. Biochem. 2017, 111, 36–43. [Google Scholar] [CrossRef]
- Jochum, M.; Eisenhauer, N. Out of the dark: Using energy flux to connect above- and belowground communities and ecosystem functioning. Eur. J. Soil Sci. 2022, 73, e13154. [Google Scholar] [CrossRef]
- Barnes, A.D.; Weigelt, P.; Jochum, M.; Ott, D.; Hodapp, D.; Haneda, N.F.; Brose, U. Species richness and biomass explain spatial turnover in ecosystem functioning across tropical and temperate ecosystems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150279. [Google Scholar] [CrossRef]
- Thakur, M.P.; Geisen, S. Trophic Regulations of the Soil Microbiome. Trends Microbiol. 2019, 27, 771–780. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S. Nematodes as Drivers of Plant Performance in Natural Systems. Trends Plant Sci. 2021, 26, 237–247. [Google Scholar] [CrossRef]
- Yousaf, M.; Li, J.; Lu, J.; Ren, T.; Cong, R.; Fahad, S.; Li, X. Effects of fertilization on crop production and nutrient-supplying capacity under rice-oilseed rape rotation system. Sci. Rep. 2017, 7, 1270. [Google Scholar] [CrossRef]
- Zhang, Z.; He, P.; Hao, X.; Li, L.-J. Long-term mineral combined with organic fertilizer supports crop production by increasing microbial community complexity. Appl. Soil Ecol. 2023, 188, 104930. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Y.; Liang, W.; Lou, Y.; Zhang, E.; Liang, C. Long-term effect of fertility management on the soil nematode community in vegetable production under greenhouse conditions. Appl. Soil Ecol. 2010, 46, 111–118. [Google Scholar] [CrossRef]
- Villenave, C.; Saj, S.; Pablo, A.-L.; Sall, S.; Djigal, D.; Chotte, J.-L.; Bonzi, M. Influence of long-term organic and mineral fertilization on soil nematofauna when growing Sorghum bicolor in Burkina Faso. Biol. Fertil. Soils 2010, 46, 659–670. [Google Scholar] [CrossRef]
- Herren, G.L.; Habraken, J.; Waeyenberge, L.; Haegeman, A.; Viaene, N.; Cougnon, M.; Reheul, D.; Steel, H.; Bert, W. Effects of synthetic fertilizer and farm compost on soil nematode community in long-term crop rotation plots: A morphological and metabarcoding approach. PLoS ONE 2020, 15, e0230153. [Google Scholar] [CrossRef]
- Hu, C.; Qi, Y. Effect of compost and chemical fertilizer on soil nematode community in a Chinese maize field. Eur. J. Soil Biol. 2010, 46, 230–236. [Google Scholar] [CrossRef]
- Wan, B.; Hu, Z.; Liu, T.; Yang, Q.; Li, D.; Zhang, C.; Chen, X.; Hu, F.; Kardol, P.; Griffiths, B.S.; et al. Organic amendments increase the flow uniformity of energy across nematode food webs. Soil Biol. Biochem. 2022, 170, 108695. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Xu, M.; Zhang, S.; Huang, S.; Liang, W. Responses of soil micro-food web to long-term fertilization in a wheat–maize rotation system. Appl. Soil Ecol. 2016, 98, 56–64. [Google Scholar] [CrossRef]
- Katayama, N.; Osada, Y.; Mashiko, M.; Baba, Y.G.; Tanaka, K.; Kusumoto, Y.; Okubo, S.; Ikeda, H.; Natuhara, Y. Organic farming and associated management practices benefit multiple wildlife taxa: A large-scale field study in rice paddy landscapes. J. Appl. Ecol. 2019, 56, 1970–1981. [Google Scholar] [CrossRef]
- Quist, C.W.; Schrama, M.; de Haan, J.J.; Smant, G.; Bakker, J.; van der Putten, W.H.; Helder, J. Organic farming practices result in compositional shifts in nematode communities that exceed crop-related changes. Appl. Soil Ecol. 2016, 98, 254–260. [Google Scholar] [CrossRef]
- van der Werf, H.M.G.; Knudsen, M.T.; Cederberg, C. Towards better representation of organic agriculture in life cycle assessment. Nat. Sustain. 2020, 3, 419–425. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef]
- Puissant, J.; Villenave, C.; Chauvin, C.; Plassard, C.; Blanchart, E.; Trap, J. Quantification of the global impact of agricultural practices on soil nematodes: A meta-analysis. Soil Biol. Biochem. 2021, 161, 108383. [Google Scholar] [CrossRef]
- Malerba, M.E.; White, C.R.; Marshall, D.J. Eco-energetic consequences of evolutionary shifts in body size. Ecol. Lett. 2018, 21, 54–62. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.A.; Ostle, N.; Bardgett, R.D.; Hopkins, D.W.; Pereira, M.G.; Vanbergen, A.J. Soil biota, carbon cycling and crop plant biomass responses to biochar in a temperate mesocosm experiment. Plant Soil 2019, 440, 341–356. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in agriculture—A systematic review of 26 global meta-analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Wan, B.; Barnes, A.D.; Potapov, A.; Yang, J.; Zhu, M.; Chen, X.; Hu, F.; Liu, M. Altered litter stoichiometry drives energy dynamics of food webs through changing multiple facets of soil biodiversity. Soil Biol. Biochem. 2024, 191, 109331. [Google Scholar] [CrossRef]
- Vestergård, M.; Bang-Andreasen, T.; Buss, S.M.; Cruz-Paredes, C.; Bentzon-Tilia, S.; Ekelund, F.; Kjøller, R.; Hindborg Mortensen, L.; Rønn, R. The relative importance of the bacterial pathway and soil inorganic nitrogen increase across an extreme wood-ash application gradient. GCB Bioenergy 2018, 10, 320–334. [Google Scholar] [CrossRef]
- Fan, T.; Stewart, B.A.; Yong, W.; Junjie, L.; Guangye, Z. Long-term fertilization effects on grain yield, water-use efficiency and soil fertility in the dryland of Loess Plateau in China. Agric. Ecosyst. Environ. 2005, 106, 313–329. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.-Z.; Dang, T.-H.; Sainju, U.M. Nitrogen Fertilization Effect on Soil Water and Wheat Yield in the Chinese Loess Plateau. Agron. J. 2013, 105, 143–149. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, G.; Feng, H.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J.; Dyck, M.; Wang, X.; Zhang, J.; et al. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. 2017, 24, 10108–10120. [Google Scholar] [CrossRef]
- Wu, T.; Schoenau, J.J.; Li, F.; Qian, P.; Malhi, S.S.; Shi, Y. Influence of fertilization and organic amendments on organic-carbon fractions in Heilu soil on the loess plateau of China. J. Plant Nutr. Soil Sci. 2005, 168, 100–107. [Google Scholar] [CrossRef]
- Li, W.; Hou, Y.; Long, M.; Wen, X.; Han, J.; Liao, Y. Long-term effects of biochar application on rhizobacteria community and winter wheat growth on the Loess Plateau in China. Geoderma 2023, 429, 116250. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; ASA and SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; ASA: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis; ASA and SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Wu, J.; O’Donnell, A.G.; He, Z.L.; Syers, J.K. Fumigation-extraction method for the measurement of soil microbial biomass-S. Soil Biol. Biochem. 1994, 26, 117–125. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- DeForest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J. Vis. Exp. 2013, 81, e50961. [Google Scholar] [CrossRef]
- Liu, M.; Chen, X.; Qin, J.; Wang, D.; Griffiths, B.; Hu, F. A sequential extraction procedure reveals that water management affects soil nematode communities in paddy fields. Appl. Soil Ecol. 2008, 40, 250–259. [Google Scholar] [CrossRef]
- Geisen, S.; Snoek, L.B.; ten Hooven, F.C.; Duyts, H.; Kostenko, O.; Bloem, J.; Martens, H.; Quist, C.W.; Helder, J.A.; van der Putten, W.H. Integrating quantitative morphological and qualitative molecular methods to analyse soil nematode community responses to plant range expansion. Methods Ecol. Evol. 2018, 9, 1366–1378. [Google Scholar] [CrossRef]
- Sun, Y.; Du, X.; Li, Y.; Han, X.; Fang, S.; Geisen, S.; Li, Q. Database and primer selections affect nematode community composition under different vegetations of Changbai Mountain. Soil Ecol. Lett. 2023, 5, 142–150. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Chao, A.; Bunge, J. Estimating the Number of Species in a Stochastic Abundance Model. Biometrics 2004, 58, 531–539. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Guseva, K.; Darcy, S.; Simon, E.; Alteio, L.V.; Montesinos-Navarro, A.; Kaiser, C. From diversity to complexity: Microbial networks in soils. Soil Biol. Biochem. 2022, 169, 108604. [Google Scholar] [CrossRef]
- Gauzens, B.; Barnes, A.; Giling, D.P.; Hines, J.; Jochum, M.; Lefcheck, J.S.; Rosenbaum, B.; Wang, S.; Brose, U. fluxweb: A R package to easily estimate energy fluxes in food webs. bioRxiv 2017. [Google Scholar] [CrossRef]
- Mulder, C.; Cohen, J.E.; Setälä, H.; Bloem, J.; Breure, A.M. Bacterial traits, organism mass, and numerical abundance in the detrital soil food web of Dutch agricultural grasslands. Ecol. Lett. 2005, 8, 80–90. [Google Scholar] [CrossRef]
- Klekowski, R.Z.; Wasilewska, L.; Paplinska, E. Oxygen Consumption by Soil-Inhabiting Nematodes. Nematologica 1972, 18, 391–403. [Google Scholar] [CrossRef]
- Atkinson, H.J. Respiration in nematodes. Nematodes Biol. Models 1980, 2, 101–142. [Google Scholar]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef]
- Cui, S.; Liang, S.; Zhang, X.; Li, Y.; Liang, W.; Sun, L.; Wang, J.; Martijn Bezemer, T.; Li, Q. Long-term fertilization management affects the C utilization from crop residues by the soil micro-food web. Plant Soil 2018, 429, 335–348. [Google Scholar] [CrossRef]
- Jochum, M.; Barnes, A.D.; Ott, D.; Lang, B.; Klarner, B.; Farajallah, A.; Scheu, S.; Brose, U. Decreasing Stoichiometric Resource Quality Drives Compensatory Feeding across Trophic Levels in Tropical Litter Invertebrate Communities. Am. Nat. 2017, 190, 131–143. [Google Scholar] [CrossRef]
- Hunt, H.W.; Coleman, D.C.; Ingham, E.R.; Ingham, R.E.; Elliott, E.T.; Moore, J.C.; Rose, S.L.; Reid, C.P.P.; Morley, C.R. The detrital food web in a shortgrass prairie. Biol. Fertil. Soils 1987, 3, 57–68. [Google Scholar] [CrossRef]
- Schwarz, B.; Barnes, A.D.; Thakur, M.P.; Brose, U.; Ciobanu, M.; Reich, P.B.; Rich, R.L.; Rosenbaum, B.; Stefanski, A.; Eisenhauer, N. Warming alters energetic structure and function but not resilience of soil food webs. Nat. Clim. Chang. 2017, 7, 895–900. [Google Scholar] [CrossRef]
- Buzhdygan, O.Y.; Meyer, S.T.; Weisser, W.W.; Eisenhauer, N.; Ebeling, A.; Borrett, S.R.; Buchmann, N.; Cortois, R.; De Deyn, G.B.; de Kroon, H.; et al. Biodiversity increases multitrophic energy use efficiency, flow and storage in grasslands. Nat. Ecol. Evol. 2020, 4, 393–405. [Google Scholar] [CrossRef]
- Sanchez, G.; Trinchera l, R.G. Tools for partial least squares path modeling (PLS-PM). In R Package Version 0.4; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Yang, B.; Banerjee, S.; Herzog, C.; Ramírez, A.C.; Dahlin, P.; van der Heijden, M.G.A. Impact of land use type and organic farming on the abundance, diversity, community composition and functional properties of soil nematode communities in vegetable farming. Agric. Ecosyst. Environ. 2021, 318, 107488. [Google Scholar] [CrossRef]
- Haraguchi, S.; Yoshiga, T. Potential of the fungal feeding nematode Aphelenchus avenae to control fungi and the plant parasitic nematode Ditylenchus destructor associated with garlic. Biol. Control 2020, 143, 104203. [Google Scholar] [CrossRef]
- Huang, W.-k.; Ji, H.-l.; Gheysen, G.; Debode, J.; Kyndt, T. Biochar-amended potting medium reduces the susceptibility of rice to root-knot nematode infections. BMC Plant Biol. 2015, 15, 267. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Sánchez-Moreno, S. Biochar addition rate determines contrasting shifts in soil nematode trophic groups in outdoor mesocosms: An appraisal of underlying mechanisms. Appl. Soil Ecol. 2021, 158, 103788. [Google Scholar] [CrossRef]
- Li, G.; Liu, T.; Whalen, J.K.; Wei, Z. Nematodes: An overlooked tiny engineer of plant health. Trends Plant Sci. 2024, 29, 52–63. [Google Scholar] [CrossRef]

| Genus | Abbr. | Trophic Group | c-p Value | Treatments | ||||
|---|---|---|---|---|---|---|---|---|
| S | X | B | NP | CK | ||||
| Acrobeloides | Acr | BF | 2 | 6.28 | 11.74 | 11.62 | 8.94 | 9.22 |
| Alaimus | Ala | BF | 4 | 3.21 | 0.03 | 0 | 10.55 | 0.31 |
| Cephalobus | Cep1 | BF | 2 | 8.8 | 23.39 | 23.69 | 14.4 | 24.55 |
| Chiloplacus | Chi | BF | 2 | 0.99 | 0.92 | 0.24 | 1.74 | 3.24 |
| Mesorhabditis | Mes2 | BF | 1 | 5.56 | 0 | 0 | 0.83 | 4.78 |
| Odontolaimus | Odo | BF | 3 | 0.92 | 2.65 | 0.17 | 0.1 | 0.2 |
| Panagrolaimus | Pan | BF | 1 | 0 | 0 | 0 | 0 | 1.91 |
| Pelodera | Pel | BF | 1 | 0.2 | 0.03 | 0.2 | 1.09 | 0 |
| Plectus | Ple | BF | 2 | 3.72 | 0 | 0.07 | 1.06 | 3.24 |
| Prismatolaimus | Pri | BF | 3 | 2.08 | 2.75 | 8.76 | 2.06 | 1.33 |
| Pseudacrobeles | Pse | BF | 2 | 0.2 | 0.03 | 0.55 | 0.24 | 0.51 |
| Steinernema | Ste | BF | 2 | 0.27 | 0 | 0.1 | 0 | 0.79 |
| Zeldia | Zel | BF | 2 | 0.03 | 0.07 | 0.41 | 0.07 | 0.1 |
| Aphelenchoides | Aph1 | FF | 2 | 30.16 | 2.68 | 0.95 | 2.71 | 1.54 |
| Aphelenchus | Aph2 | FF | 2 | 4.47 | 0.24 | 0.51 | 0.37 | 4.13 |
| Diphtherophora | Dip | FF | 3 | 0 | 1.63 | 0 | 0 | 0.1 |
| Filenchus | Fil | FF | 2 | 9.86 | 3.09 | 0.55 | 1.91 | 2.8 |
| Miculenchus | Mic | FF | 2 | 3.17 | 0.07 | 0.55 | 1.06 | 0.51 |
| Amplimerlinius | Amp | PP | 3 | 0.1 | 0.17 | 10.16 | 0.28 | 12.19 |
| Basiria | Bas | PP | 2 | 0.2 | 0.27 | 0.48 | 0.28 | 0.41 |
| Boleodorus | Bol | PP | 2 | 1.47 | 0 | 0 | 0 | 0.2 |
| Bursaphelenchus | Bur | PP | 2 | 0 | 0.03 | 0 | 0.2 | 0.07 |
| Cephalenchus | Cep2 | PP | 2 | 0.03 | 0.14 | 0.31 | 0.14 | 0.27 |
| Discotylenchus | Dis | PP | 2 | 0.48 | 0.03 | 0.14 | 0.58 | 0.14 |
| Ditylenchus | Dit | PP | 2 | 1.77 | 0.75 | 15.51 | 2.29 | 4.61 |
| Irantylenchus | Ira | PP | 2 | 0.03 | 0 | 0 | 0.24 | 0.44 |
| Longidorus | Lon1 | PP | 5 | 0.82 | 0.1 | 0.14 | 0.72 | 0.03 |
| Longidorella | Lon2 | PP | 4 | 0.1 | 0.03 | 0.03 | 0.07 | 0.03 |
| Merlinius | Mer | PP | 3 | 1.02 | 2.27 | 10.74 | 0.72 | 15.94 |
| Neopsilenchus | Neo | PP | 2 | 1.16 | 0.03 | 0 | 0.1 | 0 |
| Pratylenchus | Pra | PP | 3 | 0.89 | 0.88 | 0.68 | 0.83 | 1.88 |
| Psilenchus | Psi | PP | 2 | 0.75 | 0 | 0.95 | 0 | 0 |
| Scutylenchus | Scu | PP | 3 | 0.03 | 0.03 | 0.1 | 0.03 | 0.03 |
| Tylenchus | Tyl1 | PP | 3 | 0.17 | 0.03 | 0 | 0.1 | 0.65 |
| Tylenchorhynchus | Tyl2 | PP | 3 | 0.03 | 0 | 0.31 | 0 | 0.07 |
| Campydora | Cam | OP | 4 | 0.07 | 13.95 | 2.22 | 0.1 | 0.75 |
| Ecumenicus | Ecu | OP | 4 | 3.92 | 15.41 | 4.74 | 22.22 | 1.3 |
| Mesodorylaimus | Mes1 | OP | 5 | 6.79 | 16.36 | 5.04 | 23.32 | 1.64 |
| Prodorylaimus | Pro | OP | 4 | 0.1 | 0.14 | 0 | 0.07 | 0.07 |
| Sectonema | Sec | OP | 5 | 0.1 | 0.03 | 0.1 | 0.58 | 0 |
| Diversity Index | S | X | B | NP | CK |
|---|---|---|---|---|---|
| Richness | 56.67 ± 6.01 a | 31.00 ± 4.58 b | 47.67 ± 3.33 ab | 42.00 ± 8.39 ab | 58.00 ± 7.94 a |
| H′ | 3.92 ± 0.52 a | 2.93 ± 0.10 ab | 3.43 ± 0.13 ab | 2.63 ± 0.48 b | 3.47 ± 0.39 ab |
| J′ | 0.67 ± 0.07 a | 0.60 ± 0.04 ab | 0.62 ± 0.02 ab | 0.48 ± 0.06 b | 0.59 ± 0.06 ab |
| λ | 0.88 ± 0.05 a | 0.81 ± 0.02 ab | 0.86 ± 0.01 ab | 0.72 ± 0.06 b | 0.80 ± 0.05 ab |
| Chao1 | 33.55 ± 2.13 a | 23.11 ± 4.35 b | 33.64 ± 2.26 a | 30.19 ± 1.00 ab | 37.90 ± 4.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Huang, J.; Zhang, J.; Li, G.; Zhao, S. Organic Amendments Enhance Agroecosystem Multifunctionality via Divergent Regulation of Energy Flow Uniformity in Soil Nematode Food Webs. Agronomy 2025, 15, 1048. https://doi.org/10.3390/agronomy15051048
Huang T, Huang J, Zhang J, Li G, Zhao S. Organic Amendments Enhance Agroecosystem Multifunctionality via Divergent Regulation of Energy Flow Uniformity in Soil Nematode Food Webs. Agronomy. 2025; 15(5):1048. https://doi.org/10.3390/agronomy15051048
Chicago/Turabian StyleHuang, Tianyuan, Jinghua Huang, Jing Zhang, Guoqing Li, and Shiwei Zhao. 2025. "Organic Amendments Enhance Agroecosystem Multifunctionality via Divergent Regulation of Energy Flow Uniformity in Soil Nematode Food Webs" Agronomy 15, no. 5: 1048. https://doi.org/10.3390/agronomy15051048
APA StyleHuang, T., Huang, J., Zhang, J., Li, G., & Zhao, S. (2025). Organic Amendments Enhance Agroecosystem Multifunctionality via Divergent Regulation of Energy Flow Uniformity in Soil Nematode Food Webs. Agronomy, 15(5), 1048. https://doi.org/10.3390/agronomy15051048






