Soil pH and Nutrient Stoichiometry as Key Drivers of Phosphorus Availability in Crop Rotation Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Soil Sampling
2.3. Analysis of Soil Physicochemical Properties
2.4. Analysis of Soil P Fractions and Soil Phosphatase Activity
2.5. Soil DNA Extraction and Quantitative Polymerase Chain Reaction (PCR) of P Functional Genes
2.6. Data Analysis
3. Results
3.1. Soil Physicochemical Properties
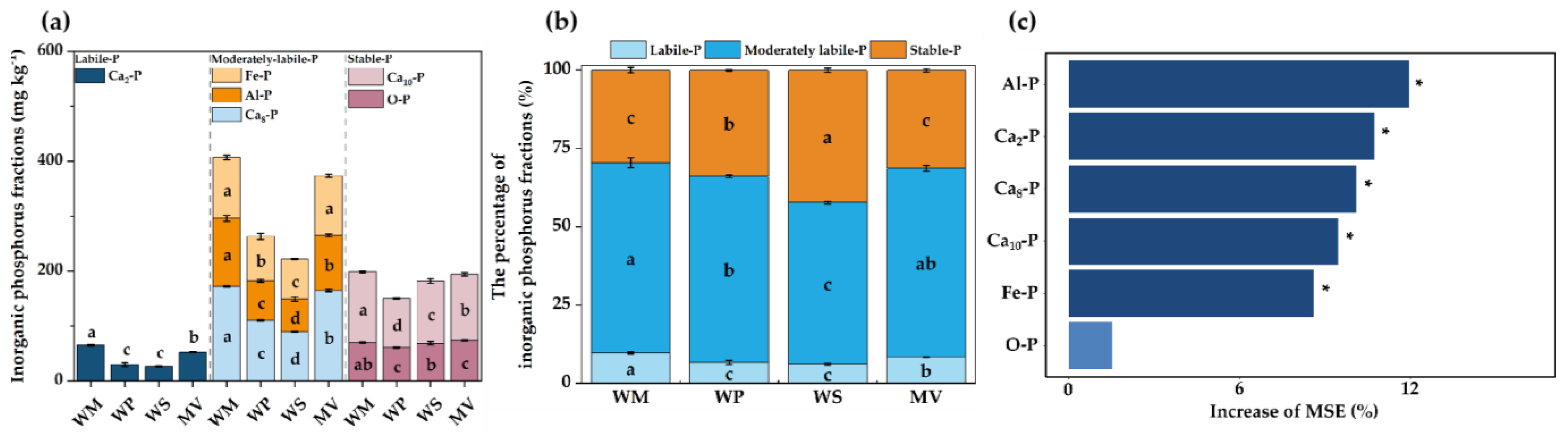
3.2. The Content and Relative Proportions of Inorganic P Fractions
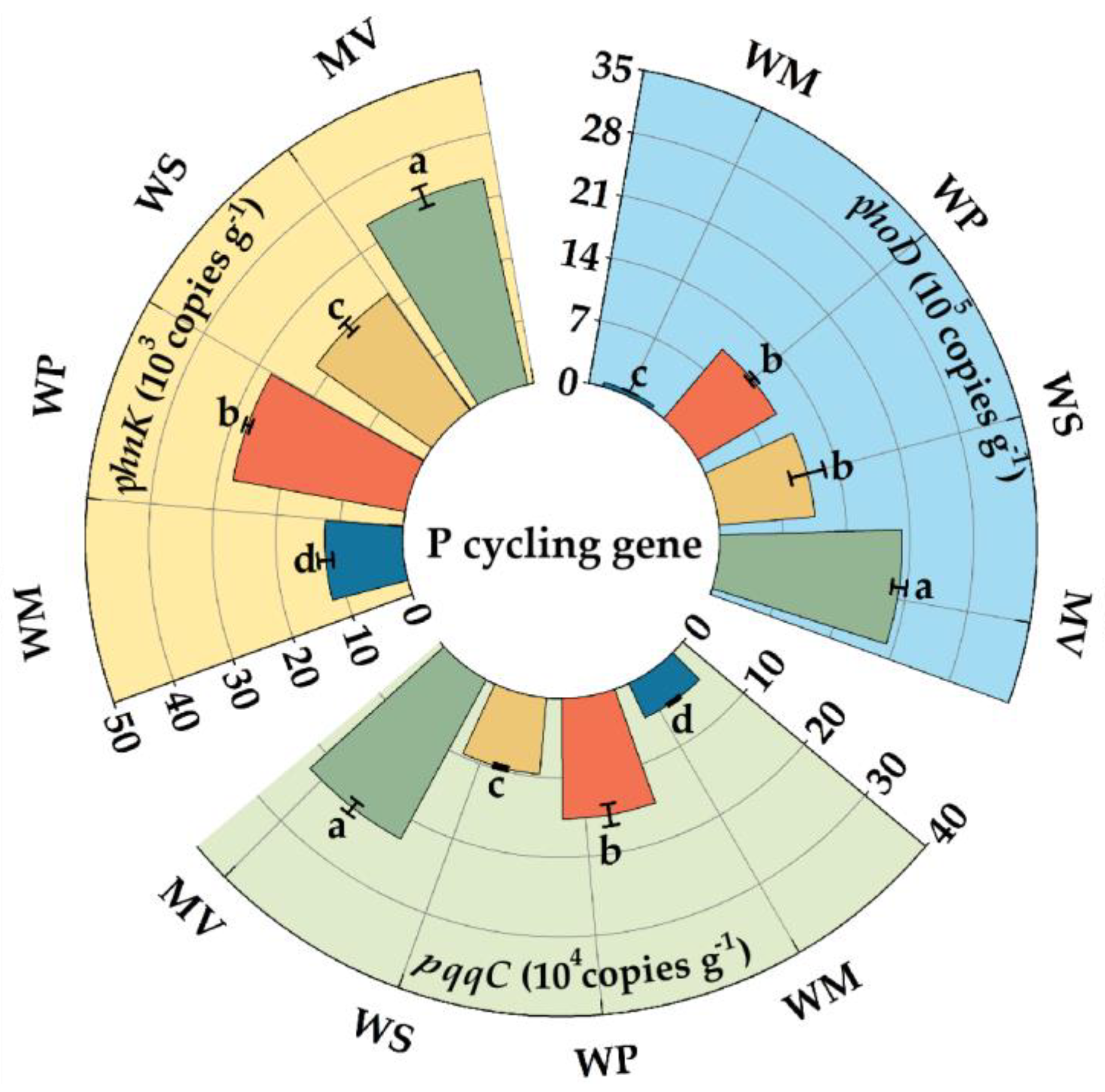
3.3. Soil Enzyme Activities and the Abundance of P-Cycling Genes
3.4. Relationships of P Fractions, Phosphatase Activities, P-Cycling Genes, and Soil Physicochemical Properties
4. Discussion
4.1. Effects of Different Crop Rotation Systems on Soil P Fractions
4.2. Response of P-Cycling Process to Crop Rotation Regimes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amadou, I.; Faucon, M.P.; Houben, D. Role of soil minerals on organic phosphorus availability and phosphorus uptake by plants. Geoderma 2022, 428, 116125. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption in a black soil from Northeast China. Soil Till. Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Fan, B.; Ding, J.; Fenton, O.; Daly, K.; Chen, Q. Understanding phosphate sorption characteristics of mineral amendments in relation to stabilising high legacy P calcareous soil. Environ. Pollut. 2020, 261, 114175. [Google Scholar] [CrossRef] [PubMed]
- Izhar, S.M.; Adnan, M.; Fahad, S.; Wahid, F.; Khan, A.; Yue, Z. Application of single superphosphate with humic acid improves the growth, yield and phosphorus uptake of wheat (Triticum aestivum L.) in calcareous soil. Agronomy 2020, 10, 1224. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Guo, Z.; Xu, L.; Zhao, H.; Zhao, P.; Ma, C.; Yi, K.; Jia, X. Revealing the underlying molecular basis of phosphorus recycling in the green manure crop Astragalus sinicus. J. Clean. Prod. 2022, 341, 130924. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, Z.; Li, T. Distribution of inorganic phosphorus fractions in water-stable aggregates of soil from tea plantations converted from farmland in the hilly region of western Sichuan, China. J. Soil. Sediment. 2018, 18, 906–916. [Google Scholar] [CrossRef]
- Zhai, X.F.; Lu, P.; Zhang, R.F.; Bai, W.M.; Zhang, W.H.; Chen, J.; Tian, Q.Y. Mowing accelerates phosphorus cycling without depleting soil phosphorus pool. Ecol. Appl. 2023, 24, e2861. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dorodnikov, M.; Dijkstra, F.A. Sensitivities to nitrogen and water addition vary among microbial groups within soil aggregates in a semiarid grassland. Biol. Fert. Soils 2017, 53, 129–140. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.Z.; He, X.X.; Ye, S.M.; Wang, S.Q. Dynamics of soil inorganic phosphorus fractions at aggregate scales in a chrono sequence of Chinese fir plantations. J. Mt. Sci. 2022, 19, 136–150. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Lin, H.; Chang, Z.; Li, Z.; Riaz, A.; Hou, E. Magnesium-doped biochar increase soil phosphorus availability by regulating phosphorus retention, microbial solubilization and mineralization. Biochar 2024, 6, 68. [Google Scholar] [CrossRef]
- Li, M.H.; Hu, J.L.; Lin, X.G. Profiles and interrelationships of functional soil microbiomes involved in phosphorus cycling in diversified agricultural land-use systems. Food Energy Secur. 2021, 10, e315. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Ghimire, R.; Zhang, N.; Zhou, S.; Zhao, F.; Wang, J. Linking soil phosphorus fractions to associated microbial functional profiles under crop rotation on the Loess Plateau of China. Soil Till. Res. 2023, 233, 105809. [Google Scholar] [CrossRef]
- Yang, L.; Du, L.L.; Li, W.J.; Wang, R.; Guo, S.L. Divergent responses of phoD- and pqqC-harbouring bacterial communities across soil aggregates to long fertilization practices. Soil Till. Res. 2023, 228, 105634. [Google Scholar] [CrossRef]
- Zhi, R.C.; Deng, J.; Xu, Y.L.; Xu, M.P.; Zhang, S.H.; Han, X.H.; Yang, G.H.; Ren, C.J. Altered microbial P cycling genes drive P availability in soil after afforrstation. J. Environ. Manag. 2023, 328, 116998. [Google Scholar] [CrossRef]
- Bernatchez, L.; Ferchaud, A.L.; Berger, C.S.; Venney, C.J.; Xuereb, A.T. Genomics for monitoring and understanding species responses to global climate change. Nat. Rev. Genet. 2024, 3, 165–183. [Google Scholar] [CrossRef]
- Hodapp, D.; Torrecilla, R.I.; Fiorentino, D.; Garilao, C.; Kaschner, K.; Kesner-Reyes, K.N.; Schneider, B.; Segschneider, J.; Kocsis, Á.T.; Kiessling, W.; et al. Climate change disrupts core habitats of marine species. Glob. Change Biol. 2023, 29, 3304–3317. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.; Punzón, A.; Hidalgo, M.; Pécuchet, L.; Sáinz Bariáin, M.; González-Irusta, J.M.; Esteban, A.; García, E.; Vivas, M.; GildeSolá, L.; et al. Community’s ecological traits reflect spatio-temporal variability of climate change impacts. Enivorn Sustain. Ind. 2024, 23, 100421. [Google Scholar] [CrossRef]
- Yang, L.; Wen, M.; Hu, R.; Zhao, F.; Wang, J. Regulation of wheat yield by soil multifunctionality and metagenomic-based microbial degradation potentials under crop rotations. J. Environ. Manag. 2024, 370, 122897. [Google Scholar]
- Zhang, Y.; Chen, J.; Du, M.; Ruan, Y.; Wang, Y.; Guo, J.; Shao, R.; Wang, H. Metagenomic insights into microbial variation and carbon cycling function in crop rotation systems. Sci. Total Env. 2024, 947, 174529. [Google Scholar] [CrossRef]
- Calonego, J.C.; Rosolem, C.A. Phosphorus and potassium balance in a corn–soybean rotation under no-till and chiseling. Nutr. Cycl. Agroecosys 2013, 96, 123–131. [Google Scholar] [CrossRef]
- Espinosa, D.; Sale, P.; Tang, C. Effect of soil phosphorus availability and residue quality on phosphorus transfer from crop residues to the following wheat. Plant Soil. 2017, 416, 361–375. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, J.; Chen, Y.; Yang, X.; Lu, W.; Ding, S.; Jiang, Y.; Zhou, X.; Mi, G.; Xu, J.; et al. Long-term cropping rotation with soybean enhances soil health as evidenced by improved nutrient cycles through keystone phylotypes interaction. Soil. Ecol. Lett. 2024, 6, 240251. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Li, J.; Mo, Q.; Tang, J.; Liu, W.; Batyrbek, M.; Liu, T.; Zhang, X.; Han, Q. Shaping the succession patterns of different soil nutrients, enzyme stoichiometry, and microbial communities through rotation systems. Catena 2024, 236, 107740. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanbe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA: Washington, DC, USA, 1954.
- David, D.J. The determination of exchangeable sodium, potassium, calcium and magnesium in soils by atomic-absorption spectrophotometry. Analyst 1960, 85, 495–503. [Google Scholar] [CrossRef]
- Jiang, B.; Gu, Y. A suggested fractionation scheme of inorganic phosphorus in calcareous soils. Fert. Res. 1989, 20, 159–165. [Google Scholar] [CrossRef]
- Alvez, C.M.; Varela, C.P.; Barrios, P.G.; Guimaraes, A.B.; Machado, A.P. Lupine Cultivation Affects Soil’s P Availability and Nutrient Uptake in Four Contrasting Soils. Agronomy 2024, 14, 389. [Google Scholar] [CrossRef]
- Wei, K.; Chen, Z.H.; Zhu, A.N.; Zhang, J.B.; Chen, L.J. Application of 31P NMR spectroscopy in determining phosphatase activities and P composition in soil aggregates influenced by tillage and residue management practices. Soil Till. Res. 2014, 138, 35–43. [Google Scholar] [CrossRef]
- Giannini, A.P.; Andriulo, A.E.; Wyngaard, N.; Irizar, A.B. Long-term tillage impact on soil phosphorus under different crop sequences. Soil Use Manag. 2024, 40, e13018. [Google Scholar] [CrossRef]
- Wiche, O.; Edwards, C.; Pourret, O.; Monei, N.; Heim, J.; Lambers, H. Relationships between carboxylate-based nutrient-acquisition strategies, phosphorus-nutritional status and rare earth element accumulation in plants. Plant Soil 2023, 489, 645–666. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Zhang, S.; Li, N.; Wei, X.; Wei, Y.; Wei, L.; Li, J.; Huang, S.; Chen, Q.; et al. Soil organic carbon stability mediate soil phosphorus in greenhouse vegetable soil by shifting phoD-harboring bacterial communities and keystone taxa. Sci. Total Environ. 2023, 873, 162400. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Qin, L.; Kong, L.Y.; Tian, W.; Zhao, C.L. Temporal variation of soil phosphorus fractions and nutrient stoichiometry during wetland restoration: Implications for phosphorus management. Environ. Res. 2025, 266, 120486. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Cao, M.; He, H.; Song, Y.; Jin, C.; Xin, G.; He, C. Application of Italian ryegrass residue changes the soil biochemical properties and bacterial communities in Southern China. Appl. Soil Ecol. 2024, 195, 105329. [Google Scholar] [CrossRef]
- Zhou, G.; Chang, D.; Gao, S.; Liang, T.; Liu, R.; Cao, W. Co-incorporating leguminous green manure and rice straw drives the synergistic release of carbon and nitrogen, increases hydrolase activities, and changes the composition of main microbial groups. Biol. Fert. Soils 2021, 57, 547–561. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J.; Chen, K.; Lan, W.; Ye, Y.; Ma, X.; Lin, K. Responses of Soil Phosphorus Cycling-Related Microbial Genes to Thinning Intensity in Cunninghamia lanceolata Plantations. Forests 2024, 15, 440. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.Y.; Gao, Y.; Mahmood, M.; Jiao, H.J.; Wang, Z.H.; Liu, J.S. Long-term high-P fertilizer input shifts soil P cycle genes and microorganism communities in dryland wheat production systems. Agric. Ecosyst. Environ. 2023, 342, 108226. [Google Scholar] [CrossRef]
- Hu, M.J.; Sardans, J.; Le, Y.X.; Yan, R.B.; Peñuelas, J. Coastal wetland conversion to aquaculture pond reduced soil P availability by altering P fractions, phosphatase activity, and associated microbial properties. Chemosphere 2023, 311, 137083. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Ge, Y. Global distribution pattern of soil phosphorus-cycling microbes under the influence of human activities. Glob. Change Biol. 2024, 30, e17477. [Google Scholar] [CrossRef]
- Smith, T. Phosphatase gene regulation in acidic soils: Impacts on phosphorus cycling. Soil Biol. Biochem. 2021, 150, 110082. [Google Scholar]
- Wan, W.; Hao, X.; Xing, Y.; Liu, S.; Zhang, X.; Li, X.; Chen, W.; Huang, Q. Spatial differences in soil microbial diversity caused by pH-driven organic phosphorus mineralization. Land Degrad. Dev. 2021, 32, 766–776. [Google Scholar] [CrossRef]
- Liu, Y.; Hosseini Bai, S.; Wang, D.; Zhang, L.; Hu, D.N.; Wen, J.; Zhang, W.; Zhang, M. Relationships among phosphatase activities, functional genes and soil properties following amendment with the bacterium Burkholderia sp. ZP-4. Land Degrad. Dev. 2022, 33, 3427–3437. [Google Scholar] [CrossRef]
- Richardson, A.; George, T.S.; Hens, M.; Delhaize, E.; Ryan, P.R.; Simpson, R.J.; Hocking, P.J. Organic anions facilitate the mobilization of soil organic phosphorus and its subsequent lability to phosphatases. Plant Soil 2022, 476, 161–180. [Google Scholar] [CrossRef]
- Eslamian, F.; Qi, Z.; Tate, M.J.; Romaniuk, N. Lime application to reduce phosphorus release in different textured intact and small repacked soil columns. J. Soils Sediment. 2020, 20, 2053–2066. [Google Scholar] [CrossRef]
- Rocabruna, P.; Domene, X.; Preece, C.; Peñuelas, J. Relationship among Soil Biophysicochemical Properties, Agricultural Practices and Climate Factors Influencing Soil Phosphatase Activity in Agricultural Land. Agriculture 2024, 14, 288. [Google Scholar] [CrossRef]
- Adomako, M.O.; Xue, W.; Du, D.; Yu, F.H. Soil Microbe-Mediated N:P Stoichiometric Effects on Solidago canadensis Performance Depend on Nutrient Levels. Microb. Ecol. 2022, 83, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, S.B.; Song, C.H.; Zhao, R.; Jia, L.M.; Wei, Z.M. Response of phosphorus fractions transformation and microbial community to carbon-to-phosphorus ratios during sludge composting. J. Environ. Manag. 2024, 360, 121145. [Google Scholar] [CrossRef]
- DeForest, J.L.; Moorhead, D.L. Effects of elevated pH and phosphorus fertilizer on soil C, N and P enzyme stoichiometry in an acidic mixed mesophytic deciduous forest. Soil Biol. Biochem. 2020, 150, 107996. [Google Scholar] [CrossRef]




| Soil Properties | Crop Rotation Regimes | |||
|---|---|---|---|---|
| WM | WP | WS | MV | |
| pH | 6.29c | 8.13a | 7.19b | 7.14b |
| EC (μS cm−1) | 162.8c | 330.6a | 292.4a | 178.6b |
| SOC (g kg−1) | 16.8a | 10.1d | 11.6c | 14.3b |
| Total N (g kg−1) | 1.64b | 1.35d | 2.08a | 1.52c |
| Total P (g kg−1) | 1.09a | 0.72c | 0.73c | 0.95b |
| Olsen-P (mg kg−1) | 81.6a | 32.9d | 43.5c | 59.7b |
| Pi (g kg−1) | 0.66a | 0.44c | 0.43c | 0.62b |
| Po (g kg−1) | 0.43a | 0.28c | 0.30bc | 0.33b |
| C/N | 10.24a | 7.48c | 5.57d | 9.41b |
| C/P | 39.3ab | 36.0b | 39.0ab | 42.9a |
| N/P | 3.83c | 4.81b | 7.01a | 4.55b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Zhu, Y.; Zhao, Y.; Wang, M.; Qu, Z.; Lv, D.; Liu, Y.; Song, Y.; Wang, T.; Li, C.; et al. Soil pH and Nutrient Stoichiometry as Key Drivers of Phosphorus Availability in Crop Rotation Systems. Agronomy 2025, 15, 1023. https://doi.org/10.3390/agronomy15051023
Yuan Y, Zhu Y, Zhao Y, Wang M, Qu Z, Lv D, Liu Y, Song Y, Wang T, Li C, et al. Soil pH and Nutrient Stoichiometry as Key Drivers of Phosphorus Availability in Crop Rotation Systems. Agronomy. 2025; 15(5):1023. https://doi.org/10.3390/agronomy15051023
Chicago/Turabian StyleYuan, Yi, Yi Zhu, Yichen Zhao, Meng Wang, Zhaoming Qu, Dongqing Lv, Yanli Liu, Yan Song, Tingting Wang, Chengliang Li, and et al. 2025. "Soil pH and Nutrient Stoichiometry as Key Drivers of Phosphorus Availability in Crop Rotation Systems" Agronomy 15, no. 5: 1023. https://doi.org/10.3390/agronomy15051023
APA StyleYuan, Y., Zhu, Y., Zhao, Y., Wang, M., Qu, Z., Lv, D., Liu, Y., Song, Y., Wang, T., Li, C., & Feng, H. (2025). Soil pH and Nutrient Stoichiometry as Key Drivers of Phosphorus Availability in Crop Rotation Systems. Agronomy, 15(5), 1023. https://doi.org/10.3390/agronomy15051023





