Response of Agronomic Traits and Phosphorus Uptake to Soil P Deficiency During Rice Cultivars Improvement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Rice Varieties and Growth Conditions
2.3. Experimental Soil, Fertilization, and Management
2.4. Plant and Soil Harvest and Chemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Changes in Agronomic Traits During Historical Rice Variety Replacement
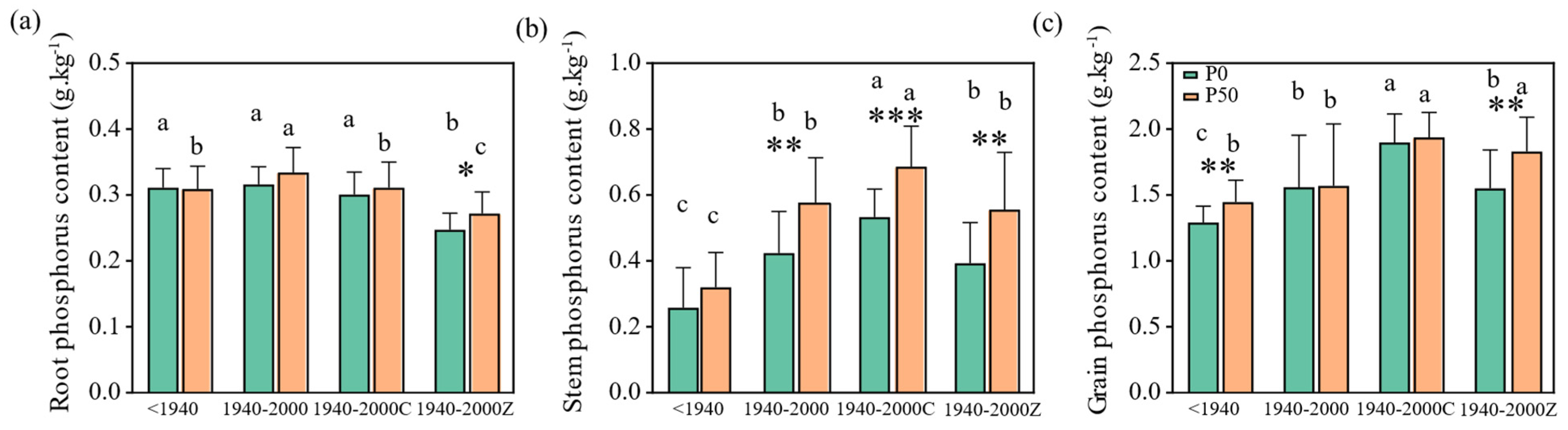
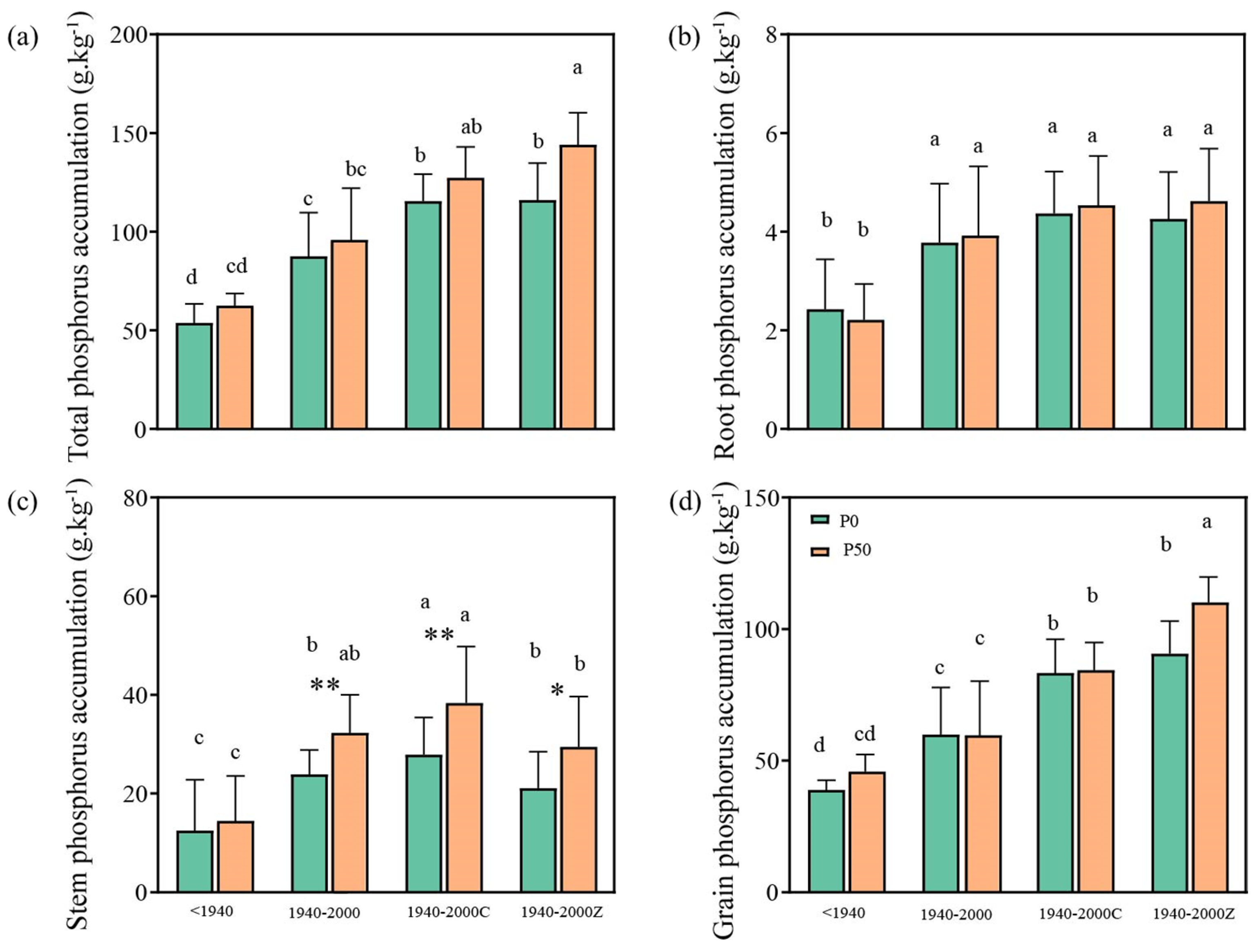
3.2. Variations in Plant P Uptake During the Historical Rice Variety Replacement
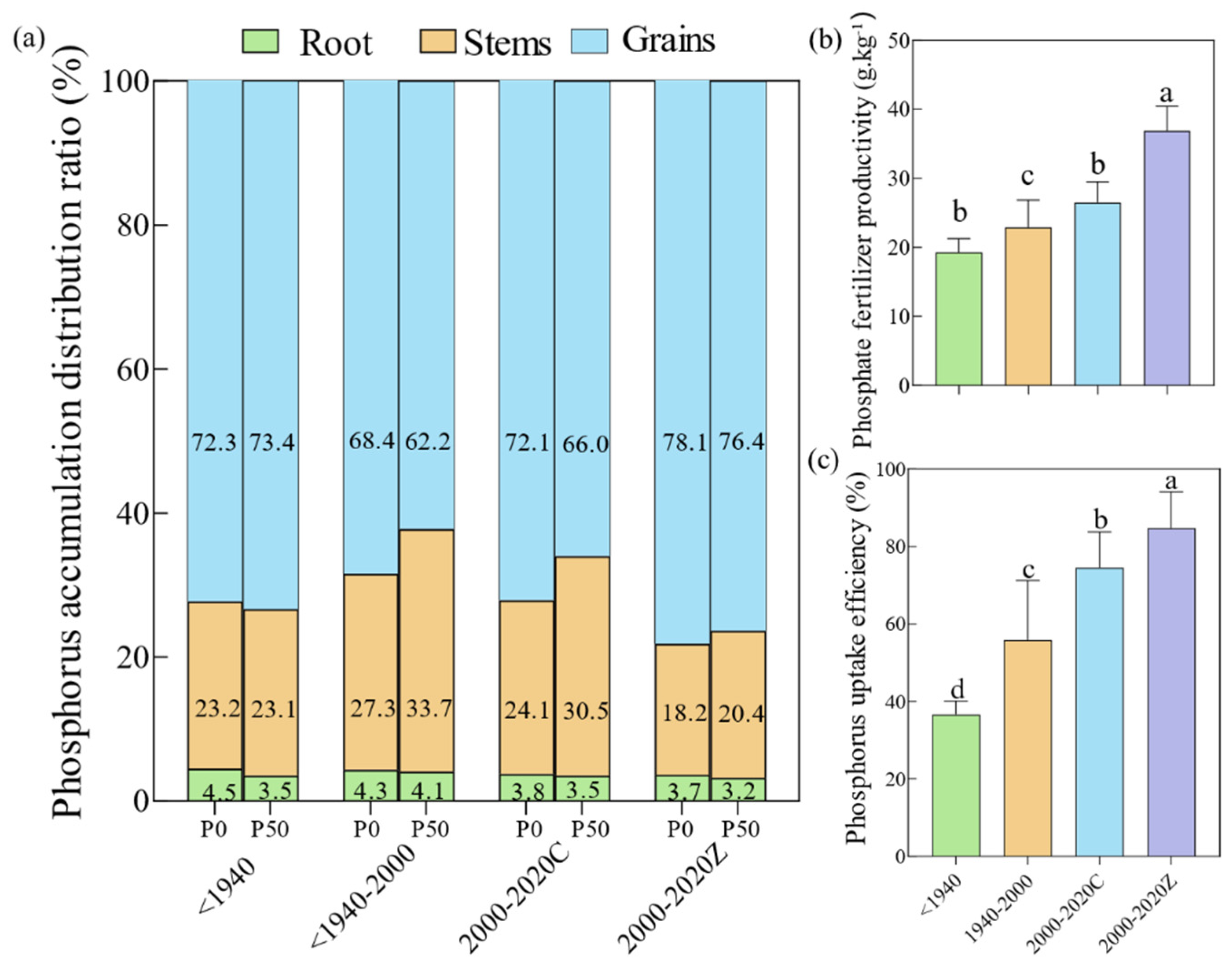
3.3. Characteristics of Rice P Distribution, Accumulation, and Utilization During the Historical Variety Replacement
3.4. Dissimilarity Analysis Across Historical Rice Varieties Under Two P Treatments
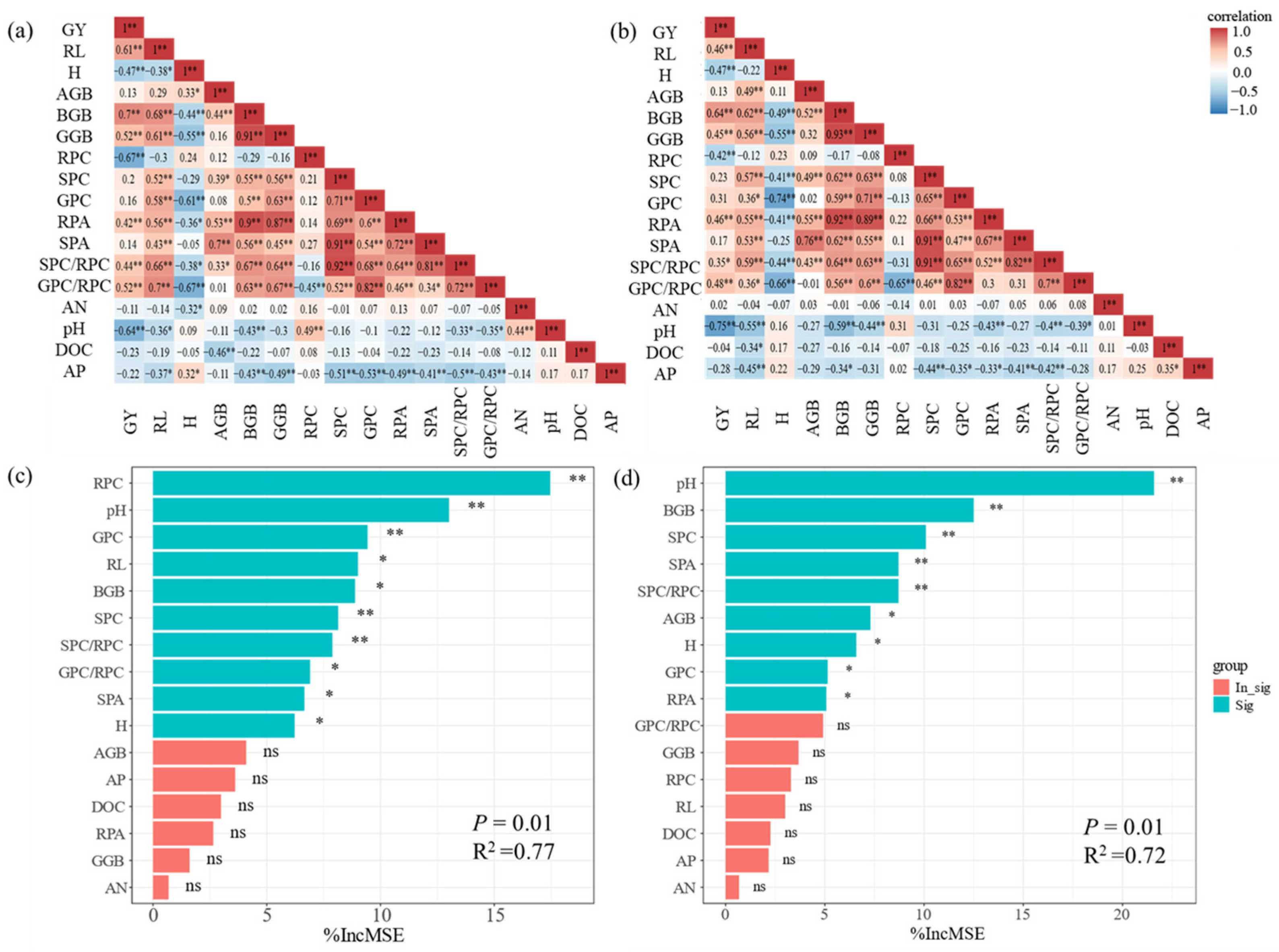
3.5. Correlation of Agronomic Traits, P Utilization, and Yield Drivers Under Two P Treatments
4. Discussion
4.1. Effects of P Limitation on Rice Yield and Agronomic Traits During Historical Varietal Replacement
4.2. Effects of Soil P Limitation on Plant P Dynamics During Historical Varietal Replacement
4.3. Effects of P Application on the Relationship Between Rice Yield, P-Use Efficiency, and Soil Properties During Varietal Replacement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GY | Grain weight |
| H | Plant height |
| RL | Root length |
| BGB | Root biomass |
| AGB | Aboveground biomass |
| GGB | Root-to-shoot ratio |
| RPC | Root P content |
| SPC | Stem P content |
| GPC | Grain P content |
| RPA | Root P accumulation |
| SPA | Stem P accumulation |
| GPA | Grain P accumulation |
| PTPA | Total P accumulation |
| AN | Alkali-hydrolyzable nitrogen |
| DOC | Dissolved organic carbon |
| AP | Soil available P |
References
- Jahan, N.; Mahmud, U.; Khan, M.Z. Sustainable Plant-Soil Phosphorus Management in Agricultural Systems: Challenges, Environmental Impacts and Innovative Solutions. Discov. Soil. 2025, 2, 13. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.-R.; Shane, M.W.; White, P.J.; et al. Opportunities for Improving Phosphorus-Use Efficiency in Crop Plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Kale, R.R.; Anila, M.; Mahadeva Swamy, H.K.; Bhadana, V.P.; Durga Rani, C.V.; Senguttuvel, P.; Subrahmanyam, D.; Hajira, S.K.; Rekha, G.; Ayyappadass, M.; et al. Morphological and Molecular Screening of Rice Germplasm Lines for Low Soil P Tolerance. J. Plant Biochem. Biotechnol. 2021, 30, 275–286. [Google Scholar] [CrossRef]
- Pazhamala, L.T.; Giri, J. Plant Phosphate Status Influences Root Biotic Interactions. J. Exp. Bot. 2023, 74, 2829–2844. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, W.; Liu, Y.; Yun, W.; Luo, B.; Chai, R.; Zhang, C.; Xiang, X.; Su, X. Changes in Phosphorus Mobilization and Community Assembly of Bacterial and Fungal Communities in Rice Rhizosphere under Phosphate Deficiency. Front. Microbiol. 2022, 13, 953340. [Google Scholar] [CrossRef]
- An, R.; Yu, R.-P.; Xing, Y.; Zhang, J.-D.; Bao, X.-G.; Lambers, H.; Li, L. Enhanced Phosphorus-Fertilizer-Use Efficiency and Sustainable Phosphorus Management with Intercropping. Agron. Sustain. Dev. 2023, 43, 57. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, X.; Huang, L.; Liu, X.; Wang, P. Transcriptome Analysis of Pennisetum Americanum × Pennisetum Purpureum and Pennisetum Americanum Leaves in Response to High-Phosphorus Stress. BMC Plant Biol. 2024, 24, 635. [Google Scholar] [CrossRef]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. Key Sustainability Challenges for the Global Phosphorus Resource, Their Implications for Global Food Security, and Options for Mitigation. J. Clean. Prod. 2017, 140, 945–963. [Google Scholar] [CrossRef]
- Gilbert, N. Environment: The Disappearing Nutrient. Nature 2009, 461, 716–718. [Google Scholar] [CrossRef]
- Soumya, P.R.; Vengavasi, K.; Pandey, R. Adaptive Strategies of Plants to Conserve Internal Phosphorus under P Deficient Condition to Improve P Utilization Efficiency. Physiol. Mol. Biol. Plants 2022, 28, 1981–1993. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, A.; Bishi, S.K.; Akhila, P.; Latha, P.C.; Subrahmanyam, D.; Brajendra, P.; Anantha, M.S.; Ch, S.R.; Sakhare, A.S.; et al. Tolerance of Oryza sativa to Low Phosphate Is Associated with Adaptive Changes in Root Architecture and Metabolic Exudates. Plant Sci. 2025, 353, 112415. [Google Scholar] [CrossRef]
- Smith, S.E.; Anderson, I.C.; Smith, F.A. Mycorrhizal Associations and Phosphorus Acquisition: From Cells to Ecosystems. In Annual Plant Reviews Volume 48; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 409–439. ISBN 978-1-118-95884-1. [Google Scholar]
- Dissanayaka, D.M.S.B.; Plaxton, W.C.; Lambers, H.; Siebers, M.; Marambe, B.; Wasaki, J. Molecular Mechanisms Underpinning Phosphorus-Use Efficiency in Rice. Plant Cell Environ. 2018, 41, 1483–1496. [Google Scholar] [CrossRef]
- Lin, H.; Li, T.; Tong, H.; Wang, Z.; Wang, L.; E, Z. Analysis on Evolution of Major Rice Cultivars in China. Chin. J. Rice Sci. 2018, 32, 565–571. [Google Scholar] [CrossRef]
- Wu, B.; Hu, W.; Xing, Y.Z. The History and Prospect of Rice Genetic Breeding in China. Hereditas 2018, 40, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hu, X.; Zhu, Y.; Mao, D. Re-Evaluation of the Rice ‘Green Revolution’ Gene: The Weak Allele SD1-EQ from Japonica Rice May Be Beneficial for Super Indica Rice Breeding in the Post-Green Revolution Era. Mol. Breed. 2020, 40, 84. [Google Scholar] [CrossRef]
- Huang, F. Responses of Grain Yield and Phosphorus Efficiency of Wheat Cultivars Released in Different Decades to Soil Fertility Levels in Guanzhong Plain, Shaanxi. Master’s Thesis, Northwest A&F University, Xianyang, China, 2016. [Google Scholar]
- Zhang, W.; Chen, X.; Ma, L.; Deng, Y.; Cao, N.; Xiao, R.; Zhang, F.; Chen, X. Re-prediction of Phosphate Fertilizer Demand in China Based on Agriculture Green Development. Acta Pedol. Sin. 2023, 60, 1389–1397. [Google Scholar]
- Qian, Q.; Guo, L.; Smith, S.M.; Li, J. Breeding High-Yield Superior Quality Hybrid Super Rice by Rational Design. Natl. Sci. Rev. 2016, 3, 283–294. [Google Scholar] [CrossRef]
- Gómez-Gallego, T.; Sánchez-Castro, I.; Molina, L.; Trasar-Cepeda, C.; García-Izquierdo, C.; Ramos, J.L.; Segura, A. Phosphorus Acquisition by Plants: Challenges and Promising Strategies for Sustainable Agriculture in the 21st Century. Pedosphere 2025, 35, 193–215. [Google Scholar] [CrossRef]
- Raza, S.; Pandey, B.K.; Hawkesford, M.J.; Griffiths, S.; Bennett, M.J.; Mooney, S.J. Future Crop Breeding Needs to Consider Future Soils. Nat. Plants 2025. [Google Scholar] [CrossRef]
- Xu, J.; Lü, T.; Mao, C. Determination of Inorganic Phosphate in Rice by SKALAR San++ Continuous Flowing Analyzer. Anal. Instrum. 2018, 46–50. [Google Scholar]
- Tian, Z.; Li, J.; He, X.; Jia, X.; Yang, F.; Wang, Z. Grain Yield, Dry Weight and Phosphorus Accumulation and Translocation in Two Rice (Oryza Sativa L.) Varieties as Affected by Salt-Alkali and Phosphorus. Sustainability 2017, 9, 1461. [Google Scholar] [CrossRef]
- Du, J.; Han, T.; Qu, X.; Ma, C.; Liu, K.; Huang, J.; Sheng, Z.; Zhang, L.; Liu, L.; Xie, J.; et al. Spatial-Temporal Evolution Characteristics and Driving Factors of Partial Phosphorus Productivity in Major Grain Crops in China. J. Plant Nutr. Fertil. 2022, 28, 191–204. [Google Scholar]
- Yan, J. Effect of Phosphorus Fertilizer Rate on Crop Yield, Phosphorus Utilization and Soil Phosphorus Transformation in a Rice-Oilseed Rape Rotation. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, China, 2022. [Google Scholar]
- Lu, R.K. Analysis Methods of Soil Agricultural Chemistry; China Agricultural Science Press: Beijing, China, 2020. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis. Part 3: Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Shuang, L.; Ni, S.; Du, J.; Teng, Z.; Shi, C. Determination of Organic Carbon in Geochemical Soil Sample by Potassium Dichromate Oxidation-Heating Method. Anhui Chem. Ind. 2016, 42, 110–112. [Google Scholar]
- Aiyelokun, O.O.; Agbede, O.A. Development of Random Forest Model as Decision Support Tool in Water Resources Management of Ogun Headwater Catchments. Appl. Water Sci. 2021, 11, 130. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.; Liu, X. Study on the Renewed Tendency and Key Backbone-Parents of Inbred Rice Varieties (O. sativa L.) in China. Sci. Agric. Sin. 2012, 45, 1455–1464. [Google Scholar]
- Lammerts van Bueren, E.T.; Struik, P.C. Diverse Concepts of Breeding for Nitrogen Use Efficiency. A Review. Agron. Sustain. Dev. 2017, 37, 50. [Google Scholar] [CrossRef]
- Ali, J.; Jewel, Z.A.; Mahender, A.; Anandan, A.; Hernandez, J.; Li, Z. Molecular Genetics and Breeding for Nutrient Use Efficiency in Rice. Int. J. Mol. Sci. 2018, 19, 1762. [Google Scholar] [CrossRef]
- Vinod, K.K.; Heuer, S. Approaches towards Nitrogen- and Phosphorus-Efficient Rice. AoB Plants 2012, 2012, pls028. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Mi, G.; Zhang, H.; Mu, P.; Wang, X. Screening and Identification for Tolerance to Low-phosphorus Stress of Rice Germplasm (Oryza sativa L.). Acta Agron. Sin. 2005, 31, 238–242. [Google Scholar]
- Rajamanickam, V.; Vinod, K.K.; Vengavasi, K.; Kumar, T.; Chinnusamy, V.; Pandey, R. Root Architectural Adaptations to Phosphorus Deficiency: Unraveling Genotypic Variability in Wheat Seedlings. Agriculture 2024, 14, 447. [Google Scholar] [CrossRef]
- Verbeeck, M.; Houben, E.; De Bauw, P.; Rakotoson, T.; Merckx, R.; Smolders, E. Root Biomass Explains Genotypic Differences in Phosphorus Uptake of Rainfed Rice Subjected to Water and Phosphorus Stresses. Plant Soil. 2023, 486, 253–271. [Google Scholar] [CrossRef]
- Yuan, K. Agronomic Performance, Energy Efficiency, and Economic Analysis of Inbred and Hybrid Rice Grown Under Reduced-Input Crop Management Practices. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar] [CrossRef]
- Julia, C.; Wissuwa, M.; Kretzschmar, T.; Jeong, K.; Rose, T. Phosphorus Uptake, Partitioning and Redistribution during Grain Filling in Rice. Ann. Bot. 2016, 118, 1151–1162. [Google Scholar] [CrossRef]
- Baker, A.; Ceasar, S.A.; Palmer, A.J.; Paterson, J.B.; Qi, W.; Muench, S.P.; Baldwin, S.A. Replace, Reuse, Recycle: Improving the Sustainable Use of Phosphorus by Plants. J. Exp. Bot. 2015, 66, 3523–3540. [Google Scholar] [CrossRef]
- El Mazlouzi, M.; Morel, C.; Chesseron, C.; Robert, T.; Mollier, A. Contribution of External and Internal Phosphorus Sources to Grain P Loading in Durum Wheat (Triticum durum L.) Grown Under Contrasting P Levels. Front. Plant Sci. 2020, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Lin, W.-Y.; Hsiao, Y.-M.; Chiou, T.-J. Milestones in Understanding Transport, Sensing, and Signaling of the Plant Nutrient Phosphorus. Plant Cell 2024, 36, 1504–1523. [Google Scholar] [CrossRef]
- Jia, H.; Ren, H.; Gu, M.; Zhao, J.; Sun, S.; Zhang, X.; Chen, J.; Wu, P.; Xu, G. The Phosphate Transporter Gene OsPht1;8 Is Involved in Phosphate Homeostasis in Rice. Plant Physiol. 2011, 156, 1164–1175. [Google Scholar] [CrossRef]
- Yamaji, N.; Takemoto, Y.; Miyaji, T.; Mitani-Ueno, N.; Yoshida, K.T.; Ma, J.F. Reducing Phosphorus Accumulation in Rice Grains with an Impaired Transporter in the Node. Nature 2016, 541, 92–95. [Google Scholar] [CrossRef]
- Wang, D. The Change of Plant Type and Nitrogen Use Efficiency during Rice Cultivar Improvement. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2008. [Google Scholar]
- Fu, Y.; Lu, C.; Zhong, X.; Liang, K.; Pan, J.; Liu, Y.; Hu, X.; Hu, R.; Li, M.-J.; Wang, X.; et al. Post-Heading Dry-Matter Transport and Nutrient Uptake Differentiate Hybrid and Inbred Indica Rice in the Double-Cropping System in South China. Front. Plant Sci. 2024, 15, 1433402. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Manter, D.K.; Fonte, S.J.; Vivanco, J.M. Root Exudate-Derived Compounds Stimulate the Phosphorus Solubilizing Ability of Bacteria. Sci. Rep. 2023, 13, 4050. [Google Scholar] [CrossRef]
- Matsushima, C.; Shenton, M.; Kitahara, A.; Wasaki, J.; Oikawa, A.; Cheng, W.; Ikeo, K.; Tawaraya, K. Multiple Analysis of Root Exudates and Microbiome in Rice (Oryza sativa) under Low P Conditions. Arch. Microbiol. 2021, 203, 5599–5611. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Wang, L.; Zhu, Y.; Shao, G.; Cui, C. Effects of Different Phosphorus Levels on Root Growth of Rice Cultivars and the Absorption of Some Nutrient Elements. J. Southwest Univ. (Nat. Sci. Ed.) 2015, 37, 30–36. [Google Scholar] [CrossRef]
- Liu, X. Screening of Nitrogen- and Phosphorus-Efficient Rice Varieties and Their Morpho-Physiological Characteristics. Master’s Thesis, Yangzhou University, Yangzhou, China, 2024. [Google Scholar]
- Liu, K.; Li, T.; Chen, Y.; Huang, J.; Qiu, Y.; Li, S.; Wang, H.; Zhu, A.; Zhuo, X.; Yu, F.; et al. Effects of Root Morphology and Physiology on the Formation and Regulation of Large Panicles in Rice. Field Crops Res. 2020, 258, 107946. [Google Scholar] [CrossRef]
- Oo, A.Z.; Tsujimoto, Y.; Mukai, M.; Nishigaki, T.; Takai, T.; Uga, Y. Significant Interaction between Root System Architecture and Stratified Phosphorus Availability for the Initial Growth of Rice in a Flooded Soil Culture. Rhizosphere 2024, 31, 100947. [Google Scholar] [CrossRef]
- Gonçalves, B.X.; Lima-Melo, Y.; Maraschin, F.D.S.; Margis-Pinheiro, M. Phosphate Starvation Responses in Crop Roots: From Well-Known Players to Novel Candidates. Environ. Exp. Bot. 2020, 178, 104162. [Google Scholar] [CrossRef]
- Lou, Y.; Yang, W.; Yang, L.; Li, T.; Lu, J.; Lou, Q. Comparison of Roots Plasticity in Two Rice Groups with Adverse Root Architectures. J. Nucl. Agric. Sci. 2018, 32, 1220–1229. [Google Scholar]


| GY | H | GL | BGB | AGB | GGB | RPC | SPC | GPC | PTPA | RPA | SPA | GPA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice type | 178 | 33.79 | 45.16 | 64.05 | 38.11 | 26.06 | 23.92 | 35.52 | 20.77 | 102.1 | 29.62 | 28.75 | 120.80 |
| *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| P treatment | 0.38 | 0.23 | 2.67 | 0.19 | 0.21 | 0.55 | 4.90 | 35.50 | 5.72 | 105.7 | 0.39 | 22.55 | 35.14 |
| ns | ns | ns | ns | ns | ns | * | *** | * | *** | ns | *** | * | |
| Rice type × P treatment | 0.31 | 0.14 | 0.88 | 0.06 | 1.69 | 0.15 | 0.98 | 1.12 | 1.48 | 2.84 | 0.43 | 1.51 | 4.16 |
| ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | ns | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Mo, X.; Li, S.; Liu, Y.; Chen, R.; Yu, S.; Lin, W.; Wang, Y.; Hu, Y. Response of Agronomic Traits and Phosphorus Uptake to Soil P Deficiency During Rice Cultivars Improvement. Agronomy 2025, 15, 983. https://doi.org/10.3390/agronomy15040983
Li C, Mo X, Li S, Liu Y, Chen R, Yu S, Lin W, Wang Y, Hu Y. Response of Agronomic Traits and Phosphorus Uptake to Soil P Deficiency During Rice Cultivars Improvement. Agronomy. 2025; 15(4):983. https://doi.org/10.3390/agronomy15040983
Chicago/Turabian StyleLi, Chunqin, Xu Mo, Shuwei Li, Yuxi Liu, Rongxin Chen, Shuying Yu, Wenqiang Lin, Yifeng Wang, and Yajun Hu. 2025. "Response of Agronomic Traits and Phosphorus Uptake to Soil P Deficiency During Rice Cultivars Improvement" Agronomy 15, no. 4: 983. https://doi.org/10.3390/agronomy15040983
APA StyleLi, C., Mo, X., Li, S., Liu, Y., Chen, R., Yu, S., Lin, W., Wang, Y., & Hu, Y. (2025). Response of Agronomic Traits and Phosphorus Uptake to Soil P Deficiency During Rice Cultivars Improvement. Agronomy, 15(4), 983. https://doi.org/10.3390/agronomy15040983






