Abstract
Elaeis oleifera and Elaeis guineensis, two oil palm species capable of intercrossing to produce interspecific Elaeis oleifera × Elaeis guineensis (O × G) hybrids, exhibit genetic variability in key agronomic traits such as fruit development, oil accumulation, and bunch composition. This variability influences the productivity and oil quality of the resulting hybrids. Harvesting, a critical practice in oil palm production, significantly impacts oil yield and quality. Therefore, this study aimed to ascertain the optimum harvest point (OHP) in widely cultivated O × G hybrids and its correlation with genetic backgrounds. The O × G cultivars, “Coari × La Mé” (C × LM), “Manaos × Compacta” (M × C), and “Brazil × Djongo” (B × DJ), were examined to identify notable changes during various phenological stages of bunch ripening using the O × G BBCH scale, a standardized system for describing plant growth stages based on phenological development. The research was conducted in the Southwest Colombian oil palm zone during dry and rainy seasons. Observations revealed distinctive fruit coloration patterns and increased bunch weights throughout the maturation process. However, final fruit coloration did not consistently align with maximum oil rates, indicating it as an unsuitable descriptor for OHP. The C × LM cultivar exhibited the shortest ripening period (173 days after anthesis, DAA), while M × C showed the longest at 207 DAA, followed by B × DJ at 187 DAA. Pollination efficiency varied among cultivars, with C × LM and M × C displaying higher proportions of parthenocarpic fruits. Findings suggest harvesting can occur for all cultivars between phenological stages 807 and 809—corresponding to late maturity stages in fruit development—regardless of the time of year, when maximum oil per bunch is attained. Fruit opacity, fruit cracking, and fruit detachment at stages 807 and 809 were identified as pivotal descriptors for determining the right OHP, albeit unique to each cultivar. Implementing two of these three descriptors by field workers will likely result in the highest oil yields for O × G cultivars. In conclusion, this research provides valuable insights into optimizing oil palm harvest practices, emphasizing the importance of considering genetic variability and phenological indicators for determining the optimum harvest point in interspecific O × G hybrids.
1. Introduction
Oil palm (Elaeis guineensis) stands as one of the most important crops globally, serving as a cornerstone in producing edible oils, biofuels, and many industrial applications. Its significance stems from its high oil yield per hectare, making it an indispensable component of the global vegetable oil market [1]. With its origins in West Africa, oil palm cultivation has expanded rapidly across tropical regions, particularly Southeast Asia and South America, owing to its adaptability to diverse climatic conditions [2]. Malaysia and Indonesia emerge as dominant players, collectively accounting for over 80% of global palm oil production [3,4]. This expansion underscores the economic importance of oil palm, contributing significantly to these nations’ GDP (Gross Domestic Product) and providing livelihoods for millions of people [5]. In Colombia, the cultivated area reached 580,000 hectares, of which 14% corresponds to interspecific O × G hybrids (Elaeis oleifera × Elaeis guineensis) [3]
Interspecific hybrids, or O × G hybrids, result from the cross between two oil palm species: the American oil palm Elaeis oleifera (HBK) Cortés and the African oil palm Elaeis guineensis (Jacq) [6]. The O × G hybrids show agronomic characteristics such as slow stem growth [7,8], high oil quality [9], and disease resistance [6]. Furthermore, O × G hybrids are the most important and feasible alternative for oil palm growers affected by diseases such as Bud Rot (BR) [10], the most devasting disease in oil palm in Latin America [11].
Due to the rapid expansion of the O × G hybrids in South America to replace areas affected by but rot (BR), the agronomic management of the hybrids was transferred from the African oil palm with minimum or no adaptation of the technologies. For instance, critical activities toward oil yield, such as pollination and harvest criteria, were conducted empirically. Thus, it is essential to determine the optimum harvest point to maximize the oil extraction efficiency of the O × G hybrids.
A standardized approach to monitoring crop development is critical for optimizing agronomic practices and improving plantation efficiency. The BBCH scale (Biologische Bundesanstalt, Bundessortenamt, and CHemical industry) provides a universal framework for describing the phenological growth stages of crops [12]. This decimal-based system categorizes crop development into principal growth phases (0–9), including germination, leaf development, stem elongation, inflorescence emergence, flowering, fruit development, and ripening. Each principal stage is further subdivided into meso-stages, allowing a finer level of detail for describing crop phenology in a standardized and reproducible manner.
In the oil palm industry, phenological scales have been developed to track reproductive development and fruit ripening, essential for efficient plantation management. These scales help establish precise harvesting criteria to optimize oil yield and quality. The BBCH scale for O × G hybrids, as proposed by Hormaza et al. [13], has become a widely adopted standard across Latin American plantations, where it is now routinely applied to guide assisted pollination and harvesting operations. By providing an objective and reproducible way to describe the phenological changes in fruit and bunch development, this scale enhances decision-making in plantation management, ensuring better synchronization of pollination practices and determining the optimal harvest window based on physiological maturity rather than empirical observations.
The initial stage in determining the optimum harvest time for the O × G hybrids involves examining the growth and ripening patterns of its fruits and bunches. In a study conducted by Rincón et al. [14] on eight-year-old Coari × La Mé cultivar palms of the O × G hybrid in Colombia’s Eastern Zone, they investigated the growth dynamics of the fruits. Their findings revealed a sigmoidal growth curve, with fruits reaching their maximum size approximately 80 days after anthesis (DAA). This period aligns with phenological stage 709, as previously described [13]. After this stage, the fruits begin the maturation process. Considering these growth dynamics and phenological stages is crucial when determining the optimal harvest point for O × G hybrids, as they provide valuable insights into fruit development and maturation. Additionally, monitoring other physiological parameters, such as oil content and fatty acid composition, could further refine the harvest timing for maximizing oil yield and quality.
In E. guineensis, fruit maturation is characterized by a progressive softening of the mesocarp, a bright orange coloration of the exocarp, and eventual fruit abscission [15]. Depending on environmental conditions, 1–2 weeks after stage 805, the mesocarp becomes softer and turns bright orange, while the shell darkens to brown [16]. At stage 807, fruit detachment begins at the upper part of the bunch, and harvesting is recommended when three to five loose fruits are observed on the ground, as this is associated with peak oil content and optimal mesocarp-to-kernel ratio [17,18]. If harvesting is delayed beyond this stage, the fruit enters senescence (stage 809), where 10–40% of the exocarp surface may appear gray and cracked, with the rachilla becoming more open and lignified. At this point, the bunch is overripe and unsuitable for processing.
In contrast, E. oleifera exhibits a different ripening dynamic, with some variations depending on the palms’ geographical origin [19]. At stage 807, fruits are at their maximum weight, thickness, and length. The epidermis is bright orange, with a smooth and shiny surface; only a tiny proportion of loose fruits are present, with some ecotypes not shading fruits [20]. These characteristics mark this species’ optimum harvest point (OHP) [21].
For O × G hybrids, the ripening process integrates traits from both parental species, necessitating a multi-parameter approach to determine the optimal harvest point (OHP). The BBCH scale adapted for O × G hybrids [13] provides a standardized framework to classify phenological stages based on fruit and bunch development, facilitating objective maturity assessments.
In the O × G hybrids, ripening begins at phenological stage 800 (PS 800), where the fruits display a homogeneous dark green external coloration, indicating the completion of fruit growth. By stage PS 803, a faint yellowing appears at the apex of some fruits, marking the onset of chlorophyll degradation. At stage PS 805, this yellowing extends across the entire fruit surface, signifying early ripening. At stage PS 806, fruits transition from yellow to orange hues, accompanied by a slight softening of the mesocarp. Stage PS 807 is characterized by an intense orange bunch coloration, with individual fruits shifting from medium orange at the base to dark orange at the apex. At this stage, fruit detachment is minimal compared to E. guineensis, and additional indicators, such as the percentage of cracking and opacity, become relevant descriptors of maturity [13].
Cracking is the percentage of fruits within the bunch exhibiting longitudinal fissures extending from the apex to the base. These cracks result from mesocarp expansion due to oil accumulation and progressive structural weakening of the exocarp. Similarly, opacity is the percentage of fruits within the bunch that showed a loss of external gloss or shine, a phenomenon linked to changes in epicuticular wax composition, microstructural disorganization, and moisture loss during ripening [13].
This study aimed to standardize and validate the optimum harvest point (OHP) for three distinct O × G cultivars, specifically Coari × La Mé (C × LM), Manaos × Compacta (M × C), and Brazil × Djongo (B × DJ). This objective was pursued by utilizing the BBCH phenological scale customized for O × G hybrids, as delineated by Hormaza et al. [13], in conjunction with a comprehensive physical, chemical, and organoleptic parameters analysis.
2. Materials and Methods
2.1. Plant Material
The O × G hybrids Coari × La Mé —C × LM (Sepalm-Palm Elite, Bogota, Colombia), Brazil × Djongo—B × DJ (Unipalma S.A., Villavicencio Colombia), and Manaos × Compacta—M × C (ASD, San Jose, Costa Rica) were evaluated in the municipality of Tumaco (Nariño), located in the southwestern region of Colombia. The research was conducted in three plantations during dry and rainy seasons (Table 1).

Table 1.
Location, age, and harvest seasons of O × G hybrids evaluated for the optimum harvest point (OHP) determination.
To determine the effect of the rain regime on the optimum harvest point, bunches were harvested in two contrasting seasons: the rainy season, which typically occurs in the first half of the year (January to July), and the dry season, which appears in the second half of the year (July to December). During the research period, the rainy season accumulated 1950 mm of rain, while the dry season accumulated 716 mm of rain.
The extended BBCH scale of the O × G hybrids [13] was used to study bunch development. Inflorescences were randomly chosen at seven different phenological stages. In every season, 29 bunches per phenological stage (PS) were evaluated in PS 709 (last PS of fruit growth) and PS 809 (last PS for fruit ripening) for the three cultivars. For PS 800, 803, 805, 806, and 807, 44 bunches were evaluated per stage. Each bunch was taken from different palms.
2.2. Bunch and Fruit Morphological Changes
The external fruit and mesocarp colors were analyzed according to Hormaza et al., [13]. Also, fruit detachment (measured as the empty locules in the bunch), fruit cracking, and fruit opacity were recorded to assess ripening in O × G hybrids. The last two parameters were evaluated using a standardized five-point scale (0–4), where:
0: No cracking or opacity observed.
1: Cracking or opacity between 0% and 25% of the fruits in the bunch.
2: Cracking or opacity between 25% and 50% of the fruits.
3: Cracking or opacity between 50% and 75% of the fruits.
4: Cracking or opacity affecting 75% to 100% of the fruits in the bunch.
These indicators serve as key descriptors for fruit maturity and contribute to optimizing harvest timing in O × G hybrids. Unlike E. guineensis, where loose fruit detachment is the primary harvest criterion, O × G hybrids exhibit delayed and reduced fruit abscission [13]. Thus, cracking and opacity provide complementary visual markers to determine the optimum harvest point (OHP).
2.3. Bunch Components and Oil Quality Analyses
Bunch analyses were conducted according to the methodology standardized by Prada and Romero [22]. The most relevant traits of the bunch were determined [23], including oil content in normal and parthenocarpic fruits, because in O × G hybrids, contrary to the African oil palm E. guineensis, the parthenocarpic fruits synthesize oil. The parameters derived from the bunch analyses were mesocarp to fruit in normal fruits, fruits to bunch, oil to dry mesocarp, and oil to bunch in both normal and parthenocarpic fruits. The selected bunches for the research were obtained from inflorescences isolated in pollination bags, hand-pollinated using a 1:9 ratio between pollen and talc in the PS 607. To determine the oil content, mesocarp of normal (with seed) and parthenocarpic (seedless) fruits were dried in an oven at 105 °C for 12 h and later packed in polyethylene bags until analysis. The samples were finely chopped in a blender and placed in a cellulose thimble to extract oil for 24 h using hexane in a Soxhlet system [24].
The oil fatty acid (FA) profile was used to determine changes in the FA during the different PS related to oil deposition in the C × LM, B × DJ, and M × C, O × G hybrids. FA profile was quantified using the methodology described by Rincon et al., [14]. In synthesis, FA methyl esters were prepared to separate saturated and unsaturated methyl esters. Then, one microliter of the methyl esters for each sample was analyzed using an Agilent 7890 gas chromatograph (Agilent Technologies, Wilmington, DE, USA), and FA mass concentration (%) was determined in the chromatographic profiles by comparing the retention time and the area under each peak generated.
2.4. Experimental Design and Statistical Analysis
The changes in the response variables during the ripening process were analyzed using an unbalanced randomized experimental design. Two-way ANOVA was performed for the response variables when the data followed a normal distribution and the variances were homogeneous. Otherwise, nonparametric tests (Kruskal-Wallis and Bonferroni) were performed. For all statistical analyses, the R Studio software version 4.1.3 was used. The following statistical approaches and packages were used:
Analysis of Variance (ANOVA) and Tukey’s Post-Hoc Test: Conducted using the stats and multcomp packages.
Principal Component Analysis (PCA): Carried out using the FactoMineR and factoextra packages.
Kruskal-Wallis Test: Conducted using the rstatix package.
Bonferroni Correction for Kruskal-Wallis Post-Hoc Comparisons: Applied using the rstatix package.
Data Visualization: Plots were generated using ggplot2 and ggpubr.
3. Results
3.1. Morphological Changes During Fruit and Bunch Morphological Development
The morphological alterations associated with fruit and bunch development from PS 709 to PS 809 are depicted in Figure 1. In the O × G hybrids, the ripening process (PS 800 to PS 809) was manifested through variations in external fruit color, mesocarp color, fruit detachment, and fruit cracking. In the initial PS stages, the O × G hybrids C × LM, B × DJ, and M × C exhibited virescent characteristics in fruit color. The immature fruits showed exocarp pigmentation ranging from dark green to light green (spanning PS 709 to PS 800 per the BBCH scale). As ripening progressed, the fruit base transitioned to yellow, followed by an orange hue in the middle section (PS 803 and PS 805), culminating in a uniform, vibrant orange shade (PS 806). Subsequently, the color darkened to an opaquer, particularly at the apex, as bunches reached PS 807 and 809. Remarkably, the alterations in color associated with ripening remained unaffected by the rainfall regime, whether during the dry or rainy seasons (Figure 1).

Figure 1.
BBCH Scale-Based Maturity Stages of Oil Palm Bunches in Oil Palm Interspecific O × G Hybrid Cultivars. B × DJ: Brazil × Djongo; C × LM: Coari × La Mé; M × C: Manaos × Compacta. PS: Phenological Stages according to the BBCH scale.
The time required to reach each phenological stage (PS), measured as Days After Anthesis (DAA), exhibited significant differences among cultivars and between seasons (Figure 2). Across all phenological stages, the Manaos × Compacta (M × C) hybrid required the most prolonged maturation period, while Brazil × Djongo (B × DJ) matured faster, followed by Coari × La Mé (C × LM). The ANOVA revealed a significant interaction between cultivar and phenological stage (p ≤ 0.001), indicating that the time to reach each stage varies depending on the genetic background. Post-hoc Tukey’s HSD tests showed that at early stages (PS 709–PS 803), the differences among cultivars were minimal, whereas at PS 805–PS 809, M × C consistently exhibited a delayed maturation compared to B × DJ and C × LM (p ≤ 0.001).
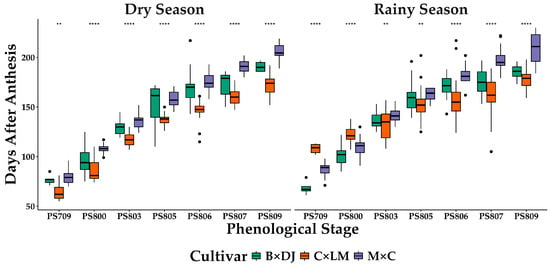
Figure 2.
Days after anthesis across phenological stages in three oil palm interspecific O × G hybrid cultivars under Dry and Rainy seasons. B × DJ: Brazil × Djongo; C × LM: Coari × La Mé; M × C: Manaos × Compacta. The bars correspond to the mean ± SD (n = 29 for PS 706 and PS 809; n = 44 for other PS). The stars correspond to the level of significance. ** p ≤ 0.01; **** p < 0.0001.
Overall, seasonal differences in maturation time were only observed in the C × LM hybrid. During the rainy season, the maturation process from PS 800 to PS 809 spanned 52 days, whereas in the dry season, it took 82 days. Despite this variation in late-stage development, the total time required for fruit growth and ripening (PS 607 to PS 809) remained unchanged across seasons, with 174 DAA in the rainy season and 172 DAA in the dry season. In contrast, B × DJ and M × C showed no significant differences in DAA between seasons, indicating that environmental factors less influence their maturation processes. (Figure 2).
Figure 3 shows the visible and identifiable changes in the bunches analyzed in this study as possible external indicators of ripeness. They are the number of fruits detached from the bunch (Figure 3A), the cracking percentage (Figure 3B), and the opacity percentage (Figure 3C). The three measured parameters showed no differences between the rainy and dry seasons. Because of this, only the results for the dry season are shown. In the case of fruit detachment (Figure 3A). The Kruskal-Wallis test showed significant differences among cultivars (Kruskal-Wallis chi-squared = 9.8414, p-value = 0.007294) and the phenological stages (Kruskal-Wallis chi-squared = 160.01, p-value < 2.2 × 10−16). The fruit detachment in the B × DJ and the C × LM hybrids was similar, with the lowest values among the cultivars. Thus, in B × DJ, an average of 5.5 ± 4.3 fruits detached at PS 807 and escalated to 15.2 ± 3.4 at PS 809. In the C × LM hybrid, at PS 807, an average of 3.6 ± 3.7 fruits detached, rising to 19.4 ± 9.6 at PS 809. The M × C hybrid detached a more significant number of fruits, averaging 20.6 ± 12.8 fruits detached at PS 807 and 80.8 ± 39.8 at PS 809.
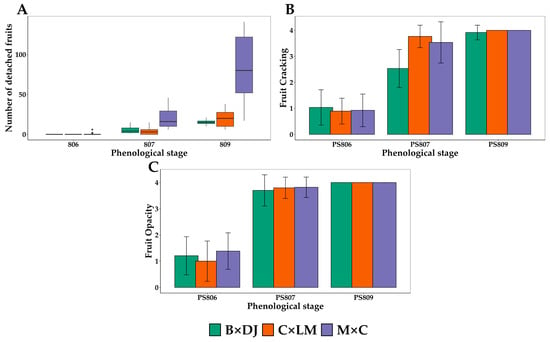
Figure 3.
External Maturity Indicators for Determining the Optimum Harvest Point in Oil Palm Interspecific O × G Hybrid Bunches at the Final Ripening Phenological Stages: (A) Number of fruits detached per bunch. (B) Percentage of cracked fruits in the bunch. (C) Percentage of fruits that lost the glossy color (opacity) per bunch. B × DJ: Brazil × Djongo; C × LM: Coari × La Mé; M × C: Manaos × Compacta. The bars correspond to the mean ± SD (n = 29 for PS 809; n = 44 for PS 806 and PS 807).
The cracking percentage differed among the cultivars (Kruskal-Wallis’s chi-squared = 9.02 and a p-value = 0.011). Also, it varied significantly among the different phenological stages (Kruskal-Wallis chi-squared = 190.83, p-value < 3.6 × 10−42). The cracking percentage was close to 25% in PS 806 and rapidly increased to 50% in B × DJ and almost 75% in C × LM and M × C at PS 807. At PS 809, the cracking percentage was above 75%, reaching 100% for all the cultivars (Figure 3B).
The other external change analyzed, the opacity percentage, did not show statistical differences among cultivars (Kruskal-Wallis chi-squared = 0.83, p-value = 0.66). However, the differences were statistically significant among the phenological stages (Kruskal-Wallis chi-squared = 208.34, p-value < 5.7 × 10−46). The fruit opacity was close to 25% at PS806 and increased to more than 75% in PS807. At PS809, all the fruits in the bunches had lost their shine in the different cultivars, with an opacity of 100% (Figure 3C).
3.2. Bunch Components
The comparison of the average fruit weight of normal fruits (AFWnf) and parthenocarpic fruits (AFWpf) across PS 806, 807, and 809 revealed a significant effect of cultivar and phenological stage but no effect of season (Figure 4A). The Kruskal-Wallis test showed no significant differences in AFWnf and AFWpf between the dry and rainy seasons (p = 0.626 and p = 0.586, respectively), indicating that seasonal variation does not strongly affect fruit weight development. However, highly significant differences among cultivars on AFWnf (p = 0.000163) and AFWpf (p = 8.42 × 10−45) suggested a strong genetic control over fruit size, with M × C consistently exhibiting the highest AFWnf and AFWpf, while B × DJ had the lowest values.
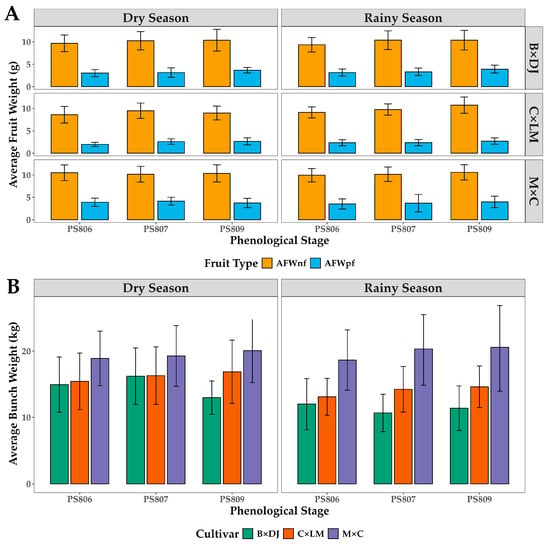
Figure 4.
Seasonal and Phenological Variations in Fruit Weight of Normal and Parthenocarpic Fruits (A) and Average Bunch (B) in Oil Palm Interspecific O × G Hybrid Cultivars. B × DJ: Brazil × Djongo; C × LM: Coari × La Mé; M × C: Manaos × Compacta. The bars correspond to the mean ± SD (n = 29 for PS 809; n = 44 for PS 806 and PS 807).
The average weight of bunches (AWB) increases in all cultivars as the phenological stage progresses (Figure 4B). Significant differences in AWB were found among cultivars, with M × C being the heaviest, with 17 kg in the dry and rainy seasons, followed by C × LM with 15 kg and 12 kg in dry and rainy seasons, respectively. B × DJ cultivar presented bunches with lower AWB with 14.5 kg and 11 kg in dry and rainy seasons, respectively. As the AWB depends on normal and parthenocarpic fruits in all cultivars, the parthenocarpic fruits increase their weight as the bunches slowly move from one stage of maturity to another. On the contrary, normal fruits did not show additional growth in the ripening period (Figure 4).
The variables related to fruit set, mesocarp to fruit, and fruit to bunch are shown in Figure 5 and Supplementary Table S1. The only statistically different variables between the two seasons were the fruit set and the fruit set of parthenocarpic fruits. Interestingly, there were no significant differences in the fruit sets of normal fruits. The fruit set (FS) is defined as the percentage of flowers that develop into fruits [25], and in the interspecific O × G hybrids, is determined by the sum of the flowers that are fertilized and develop into normal seeded fruits (FSnf) and the flowers that develop into the parthenocarpic, seedless fruits (FSpf). Thus, the results indicate that environmental conditions have a higher impact on the formation of parthenocarpic fruit than on normal fruits.
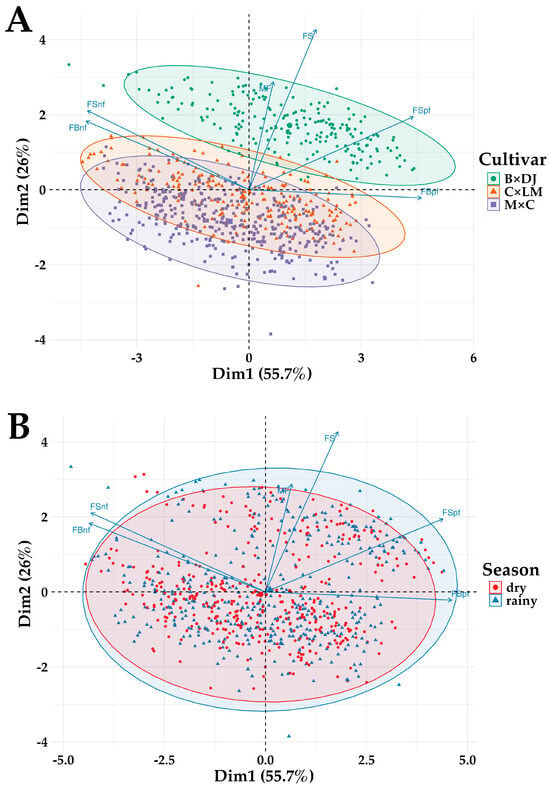
Figure 5.
Principal Component Analyses of the Seasonal and Phenological Variations in Average Bunch and Fruit Weight of Normal and Parthenocarpic Fruits in Oil Palm Interspecific O × G Hybrid Cultivars. B × DJ: Brazil × Djongo; C × LM: Coari × La Mé; M × C: Manaos × Compacta. (A). PCA biplot of fruit and bunch composition parameters by cultivar. (B). PCA biplot of fruit and bunch composition parameters by Season. MF = mesocarp-to-fruit ratio, FS = fruit set, FSnf = fruit set normal fruits, FSpf = fruit set parthenocarpic fruits, FBnf = fruit-to-bunch ratio normal fruits, FBpf = fruit-to-bunch ratio parthenocarpic fruits. in B × DJ.
In most cases, a high FS (over 65%) was observed in the three cultivars, with the highest FS reached in the PS 809 in all the hybrids. However, there were differences in the proportion of fruits. In the dry season, cultivars C × LM, and M × C, the FSpf was much higher (67.4 ± 11.2 and 49.3 ± 13.2 respectively) than the FSnf (25.0 ± 12.4 and 25.1 ± 12.8%, respectively). On the other hand, in the cultivar B × DJ, the FSnf and FSpf were similar (38.3 ± 18.8 and 42.1 ± 20.0, respectively). However, in the rainy season, the production of parthenocarpic fruits to normal fruits was very high in B × DJ (3 to 1), while in C × LM and M × C, it was 2 to 1.
Principal Component Analyses (PCA) were conducted to explore the phenotypic variation in fruit and bunch composition across the different cultivars (Figure 5). The first two principal components (PC1 and PC2) accounted for 55.7% and 26% of the total variance, respectively. The analysis by cultivar (Figure 5A) shows a clear differentiation among the three cultivars, as indicated by the 95% confidence ellipses. The B × DJ hybrid clustered distinctly from C × LM and M × C, suggesting notable differences in phenological traits. The M × C hybrid exhibited a broader dispersion along PC1, indicating more significant variability in its phenological responses. The vector projections indicate the most substantial contributors to variation among cultivars. The mesocarp-to-fruit ratio (MF%) and fruit set in normal fruits (FSnf%) had a high favorable loading on PC1, suggesting that these parameters drive most of the cultivar-level differences.
In contrast, the fruit-to-bunch ratio for normal (FBnf%) and parthenocarpic fruits (FBpf%) were more closely associated with PC2, contributing to the observed spread along this axis. Furthermore, the ellipses of C × LM and M × C overlapped, indicating that these two hybrids share more similar phenological characteristics. However, B × DJ exhibited a more substantial differentiation due to its distinct behavior in the fruit-to-bunch ratio. This separation suggests that genetic background is critical in determining the phenological profile of O × G hybrids.
Unlike the cultivar-based PCA, the dry and rainy season differentiation was less pronounced, as evidenced by the extensive overlap of the 95% confidence ellipses. While some degree of dispersion was observed along PC1, indicating minor seasonal effects, the overall phenological response appeared broadly consistent across the two seasons (Figure 5B). The vector loadings suggest that fruit set (FS%) and mesocarp-to-fruit ratio (MF%) were the most substantial contributors to variability along PC1. In contrast, the fruit-to-bunch ratio for normal (FBnf%) and parthenocarpic fruits (FBpf%) contributed more to PC2. However, their orientation and distribution suggest minimal seasonal differences in fruit development dynamics.
These results suggest that seasonal variation had a limited impact on the phenological parameters studied, with no clear distinction between dry and rainy season samples. This observation aligns with previous analyses indicating that fruit maturation and oil accumulation processes in O × G hybrids may be relatively stable across seasons.
3.3. Oil Content and Quality Analysis in O × G Interspecific Hybrids
The most critical parameters for determining oil content in a bunch (OB), are mesocarp to fruit (MF), fruit to bunch (FB), and oil to dry mesocarp (ODM). In the case of the O × G hybrid, normal and parthenocarpy fruits are quantified because both types of fruits contribute to the final oil yield.
The MF percentage showed highly significant differences among the hybrids, the phenological stages, and their interactions (Supplementary Table S1). B × DJ presented the highest MF in most phenological stages in the three cultivars in both seasons. M × C followed it, and then C × LM.
The FB was similar in both seasons, with no significant differences among the phenological stages (PS). However, there were significant differences among hybrids in the FB of parthenocarpic fruits (FBpf). No statistical differences were found among the hybrids or the phenological stages in normal fruits (Supplementary Table S1).
ODM responded to the maturity process in all cultivars: it increased during the first stages (from PS 709 to PS 803) and either increased slightly or became steady during the ripening stages. According to the type of fruit, it was found that oil synthesis occurred differently. Normal fruits showed a quick ODM increase from their unripe stages to ripe, reaching maximum PS 805–806 values (Figure 6A). Meanwhile, parthenocarpic fruits synthesize oil more slowly until the final ripening stages reach the maximum oil synthesis (Figure 6C).
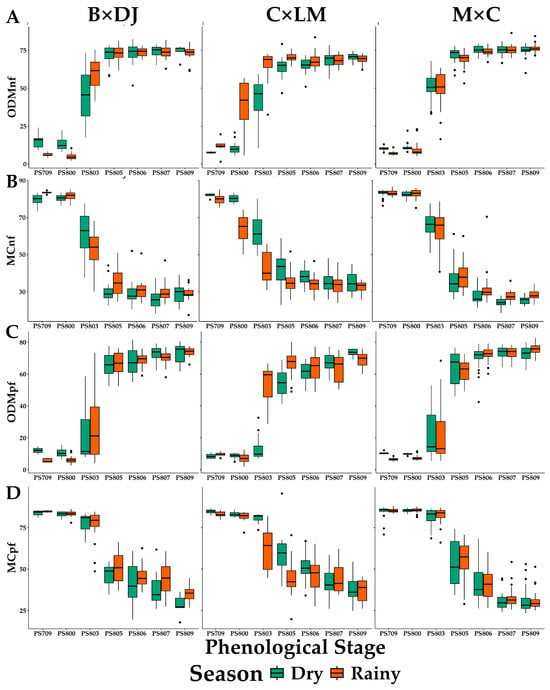
Figure 6.
Seasonal and Phenological Variations in Oil to Dry Mesocarp (A,C) and Moisture Content (B,D) of Normal (A,B) and Parthenocarpic (C,D) Fruits in Oil Palm Interspecific O × G Hybrid Cultivars. ODM = Oil to dry mesocarp, MC moisture content of normal (nf) and parthenocarpic (pf) fruits. B × DJ: Brazil × Djongo; C × LM: Coari × La Mé; M × C: Manaos × Compacta. The bars correspond to the mean ± SD (n = 29 for PS 706 and PS 809; n = 44 for other PS).
The ODM percentage of normal fruits (Figure 6A) remained similar between seasons within the different stages. Regarding oil synthesis, an increase was observed in the cultivars from PS 803, reaching the maximum content in PS 806. The lowest percentage of ODMnf was 51.8%, and the maximum was 83.5%.
Regarding the parthenocarpic fruits, the percentage of ODM in the PS 709 and PS 800 was low and had little variability between them (Figure 6C). Oil synthesis began, and the moisture content in the mesocarp exceeded 80% regardless of the cultivar and the evaluation season. In PS 803 and PS 805, there was an accelerated increase in the percentage of ODM, with a high variability within each phenological stage. However, this variation was reduced from PS 806, which is more stable in the percentages of ODM in PS 807 for the different cultivars.
The maximum fruit size is usually reached at PS 709. During the ripening processes (PS 800–809), normal fruits remain with similar weights, and parthenocarpic fruits barely increase their weight until the last stages (PS 807–809). From PS 800 to PS 809, oil synthesis occurs. The oil-to-bunch (OB) gradually increased during maturation, reaching the highest average values in PS 807 and PS 809 across all cultivars evaluated (Figure 7). In B × DJ, the maximum OB was reached at PS 809; OB synthesized by normal fruits (OBnf) was higher than OB obtained from parthenocarpic fruits (OBpf). C × LM reached the maximum OB at PS 809; normal OBnf and OBpf produced the OB in similar percentages. M × C reached the maximum OB at PS 807; likewise, C × LM oil was almost equally produced by normal and parthenocarpic fruits. Despite the minor differences in the OB reached during the rainy and dry seasons, the oil synthesis pattern was similar within the hybrids across the phenological stages (Figure 7).
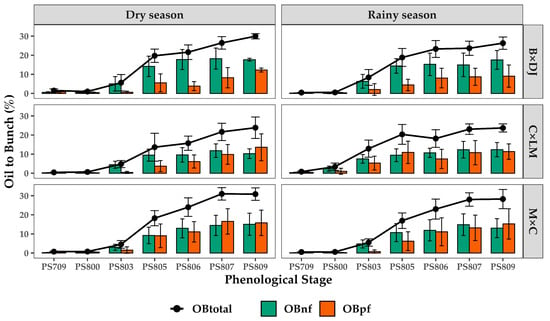
Figure 7.
Oil Accumulation Dynamics in Normal and Parthenocarpic Fruits of Oil Palm Interspecific O × G Hybrid Cultivars in the Colombian Southwest Region During the Dry and Rainy Seasons. B × DJ: Brazil × Djongo; C × LM: Coari × La Mé; M × C: Manaos × Compacta. Green Boxes: oil to bunch normal fruits; Orange boxes: oil to bunch parthenocarpic fruits; the black line corresponds to the sum of oil (total oil to bunch) of normal and parthenocarpic fruits. The bars correspond to the mean ± SD (n = 29 for PS 706 and PS 809; n = 44 for other PS).
A contrasting behavior was found between B × DJ and C × D. The normal fruits constitute about 63% of the total OB, while in C × D, this represents only 24.7% throughout the maturation stages. For the other cultivar, an equal contribution of both types of fruits was observed for the OB component (Figure 7).
The data indicate variations in fatty acid composition across phenological stages and among cultivars, highlighting the influence of genetic background and fruit maturity on oil quality. Palmitic acid (C16:0) and oleic acid (C18:1n9c) were the predominant fatty acids, with oleic acid decreasing as fruit maturity progressed, particularly in the M × C cultivar. Conversely, palmitic acid content increased at later stages, especially in M × C, suggesting a shift in lipid metabolism during ripening. Linoleic acid (C18:2n6c) and vaccenic acid (C18:1n7c) remained relatively stable across phenological stages, with minor fluctuations among cultivars. The observed variability underscores the genetic influence on fatty acid biosynthesis and the potential impact of harvest timing on oil composition. (Table 2).

Table 2.
Fatty acid composition of oil from three O × G cultivars at different maturity stages.
4. Discussion
The results obtained by Rincón et al. [14] indicated that the optimal harvest point (OHP) for the C × LM cultivar in Colombia’s Eastern region was PS 807, with a duration of 197 DAA. This differs slightly from the findings of this study, which was conducted in the country’s Southwest region. These variations suggest that environmental conditions directly influence fruit maturation. Understanding this environmental impact is crucial for future studies, especially considering the ongoing effects of climate change. Manaos × Compacta exhibited the most prolonged ripening period of the evaluated cultivars, measured from anthesis (PS 607) to harvest (PS 807–809).
Fruit growth occurs progressively, with weight gain in advanced ripening stages primarily due to mesocarp expansion and the hardening of the shell. In normal fruits of E. guineensis, this process is driven by increased cell volume and intercellular spaces [26]. However, in O × G hybrids, bunch weight is influenced by the high proportion of parthenocarpic fruits, which continue gaining weight through phenological stages [27]. The differences in reaching the maximum ODM (oil-to-dry mesocarp) percentages between normal and parthenocarpic fruits indicate that harvesting should not occur before PS 807. This is because parthenocarpic fruits require more time to reach peak oil content.
A notable change in fruit coloration throughout ripening suggests that color could be an essential criterion for harvesting. Early-ripening virescens palms undergo a pronounced color transition in African oil palms, facilitating the identification of mature bunches [28]. However, the results of this study indicate that color is not a reliable indicator of OHP in O × G cultivars. The final color did not consistently align with the maximum oil content, potentially leading to premature harvesting and reduced yield. Therefore, other traits should be prioritized for determining OHP, such as fruit opacity, which develops only in the final maturity stages.
In addition to fruit maturity parameters, this study reveals that fatty acid composition varies across phenological stages and cultivars, highlighting the importance of genetic background and harvest timing in oil quality. Palmitic acid (C16:0) and oleic acid (C18:1n9c) were the predominant fatty acids, with oleic acid content decreasing as the fruits matured, particularly in M × C. Meanwhile, palmitic acid increased at later stages, especially in M × C, suggesting shifts in lipid metabolism [29]. These changes indicate that delaying harvest beyond PS 807 could alter the balance of saturated and unsaturated fatty acids, potentially affecting oil stability and nutritional properties.
Linoleic acid (C18:2n6c) and vaccenic acid (C18:1n7c) exhibited minor fluctuations across phenological stages but remained relatively stable among cultivars, reinforcing their genetic regulation. The observed variability underscores the need to optimize OHP for oil yield and fatty acid composition, ensuring that oil extraction is performed at a stage that maximizes quality while maintaining desired lipid characteristics.
Fruit detachment and cracking are two additional key indicators of optimal harvest time. These characteristics typically appear at PS 807 or later. Furthermore, a third indicator—fruit opacity—was observed in normal and parthenocarpic fruits at full maturity.
Harvesting at PS 809 is feasible for these cultivars, as some reach their maximum oil potential at this stage, mainly due to the extended development of parthenocarpic fruits. However, high fruit detachment levels at PS 809 can result in significant oil losses in the field. This requires additional harvesting efforts and fruit collection strategies to improve yield efficiency [30,31]. The influence of harvest timing on fatty acid composition further reinforces the importance of selecting an OHP that balances oil yield and quality, preventing excessive degradation of unsaturated fatty acids that could reduce oil shelf life.
Interestingly, OHP did not vary significantly between seasons, suggesting that these harvest criteria can be applied consistently, regardless of environmental conditions. Validating OHP through multiple parameters, including color changes, fruit detachment, cracking, and opacity, can enhance crop productivity. Implementing these harvest criteria in critical cultivars has increased oil extraction rates by up to 3.6 percentage points [32].
Advancing O × G hybrid management requires optimizing OHP determination and incorporating new technologies. Several innovations can contribute to the sustainability and efficiency of the crop, such as artificial pollination, improved fruit collection tools [30], enhanced cutting instruments [33], and better harvesting practices [34].
Additionally, remote sensing methods could further refine OHP determination [35,36]. Techniques such as image analysis based on fruit coloration [37,38], spectroscopy [39], and spectral imaging [40,41] offer promising avenues for automating bunch classification. The findings from this study provide valuable input for improving these methodologies and enhancing their precision and efficiency.
This study highlights the significant variations among hybrid cultivars regarding organoleptic characteristics, bunch composition, oil content, fatty acid profile, and overall quality. These findings confirm that genetic background influences O × G cultivar behavior. Based on our results, harvest should occur at PS 807 for all cultivars, regardless of season. The selection of OHP indicators should include opacity, fruit cracking, and detachment. While at least two of these parameters should be used, growers must recognize that each cultivar exhibits unique traits, particularly regarding cracking percentages, opacity levels, and fruit detachment rates per bunch.
5. Conclusions
This study identified the optimum harvest point (OHP) for three widely cultivated Elaeis oleifera × Elaeis guineensis (O × G) hybrids—C × LM, M × C, and B × DJ—by integrating a phenological framework based on the BBCH scale with quantitative analyses of bunch and fruit characteristics. Across all hybrids, phenological stages PS807 and PS809 consistently represented the period of maximum oil accumulation, confirming their suitability as target stages for harvest under both dry and rainy season conditions.
While fruit coloration remains a traditional maturity index, it was found insufficient as a sole indicator of optimal harvest in O × G hybrids. Instead, physiological and structural descriptors such as fruit detachment, cracking, and loss of external fruit gloss or shine (opacity) proved more reliable, especially when combined. Additionally, cultivar-specific differences in ripening behavior, parthenocarpy rates, and bunch weight dynamics were statistically validated, underscoring the importance of genotype-specific harvest management.
The principal component analysis (PCA) application further emphasized the multivariate nature of ripening, supporting the use of multiple descriptors rather than relying on a single trait. These findings offer practical guidelines for optimizing harvest practices in commercial O × G plantations and highlight the utility of standardized phenological scales in improving yield consistency and oil quality.
In conclusion, by aligning harvest timing with cultivar-specific phenological and physiological markers, oil palm growers can enhance productivity, reduce losses, and implement more objective and standardized harvest protocols tailored to the unique dynamics of O × G hybrids.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/agronomy15040887/s1, Table S1: Seasonal and Phenological Variations in Fruit and Bunch Composition Parameters of Three O × G Cultivars.
Author Contributions
Conceptualization, H.M.R.; methodology, H.M.R., R.R.-R. and I.A.-D.; formal analysis, H.M.R., R.R.-R. and I.A.-D.; investigation, H.M.R., R.R.-R., I.A.-D., A.F.C.-Z. and J.L.R.; writing—original draft preparation, H.M.R., R.R.-R. and I.A.-D.; writing—review and editing, H.M.R.; supervision, H.M.R., R.R.-R. and I.A.-D.; project administration, H.M.R.; funding acquisition, H.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Colombian Oil Palm Promotion Fund (FFP) administered by Fedepalma, grant numbers MES0122, MES0323, PIV0324.
Data Availability Statement
Data is available on request from the authors.
Acknowledgments
The authors thank Palmeiras Colombia S.A. and Astorga S.A. for their important collaboration during fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alhaji, A.M.; Almeida, E.S.; Carneiro, C.R.; da Silva, C.A.S.; Monteiro, S.; Coimbra, J.S.d.R. Palm Oil (Elaeis guineensis): A Journey through Sustainability, Processing, and Utilization. Foods 2024, 13, 2814. [Google Scholar] [CrossRef]
- Corley, R.H.V.; Tinker, P.B. The Oil Palm, 5th ed.; Wiley Blackwell: Oxford, UK, 2016. [Google Scholar]
- Fedepalma. Statistical Yearbook 2023. In The Oil Palm Agroindustry in Colombia and the World 2018–2022; Fedepalma: Bogota, Colombia, 2023; p. 237. [Google Scholar]
- Foreign Agricultural Service, U.S. Department of Agriculture. Oilseeds: World Markets and Trade; Foreign Agricultural Service, U.S. Department of Agriculture: Washington, DC, USA, 2024.
- Parveez, G.K.A.; Leow, S.S.; Kamil, N.D.; Madihah, A.Z.; Ithnin, M.; Ng, M.H.; Yusof, Y.A.; Idris, Z. Oil palm economic performance in Malaysia and R&D progress in 2023. J. Oil Palm Res. 2024, 36, 171–186. [Google Scholar]
- Soh, A.C.; Mayes, S.; Roberts, J.; Barcelos, E.; Amblard, P.; Alvarado, A.; Alvarado, J.H.; Escobar, R.; Sritharan, K.; Subramaniam, M. Elaeis oleifera × Elaeis guineensis interspecific hybrid improvement. In Oil Palm Breeding; CRC Press: Boca Raton, FL, USA, 2017; pp. 283–296. [Google Scholar]
- Barcelos, E.; Rios, S.d.A.; Cunha, R.N.; Lopes, R.; Motoike, S.Y.; Babiychuk, E.; Skirycz, A.; Kushnir, S. Oil palm natural diversity and the potential for yield improvement. Front. Plant Sci. 2015, 6, 190. [Google Scholar]
- Sunilkumar, K.; Mathur, R.K.; Sparjanbabu, D.S.; Pillai, R.S.N. Evaluation of interspecific oil palm hybrids for dwarfness. J. Plant. Crops 2015, 43, 29–34. [Google Scholar]
- Mozzon, M.; Foligni, R.; Mannozzi, C. Current Knowledge on Interspecific Hybrid Palm Oils as Food and Food Ingredient. Foods 2020, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Navia, E.A.; Vila, R.A.; Daza, E.E.; Restrepo, E.F.; Romero, H.M. Assessment of tolerance to bud rot in oil palm under field conditions. Eur. J. Plant Pathol. 2014, 140, 711–720. [Google Scholar] [CrossRef]
- Sundram, S.; Intan-Nur, A.M.A. South American Bud rot: A biosecurity threat to South East Asian oil palm. Crop Protect. 2017, 101, 58–67. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; Van Den Boom, T. The BBCH system to coding the phenological growth stages of plants–history and publications. J. Kult. 2009, 61, 41–52. [Google Scholar]
- Hormaza, P.; Fuquen, E.M.; Romero, H.M. Phenology of the oil palm interspecific hybrid Elaeis oleifera × Elaeis guineensis. Sci. Agric. 2012, 69, 275–280. [Google Scholar] [CrossRef]
- Rincon, S.M.; Hormaza, P.A.; Moreno, L.P.; Prada, F.; Portillo, D.J.; García, J.A.; Romero, H.M. Use of phenological stages of the fruits and physicochemical characteristics of the oil to determine the optimal harvest time of oil palm interspecific O × G hybrid fruits. Ind. Crops Prod. 2013, 49, 204–210. [Google Scholar]
- Chang, C.; Parthiban, R.; Kalavally, V.; Hung, Y.M.; Wang, X. Unharvested palm fruit bunch ripeness detection with hybrid color correction. Smart Agric. Technol. 2024, 9, 100643. [Google Scholar]
- Forero, D.; Hormaza, P.; Romero, H. Phenological growth stages of African oil palm (Elaeis guineensis). Ann. Appl. Biol. 2012, 160, 56–65. [Google Scholar]
- Gillbanks, R.A. Standard agronomic procedures and practices. In Oil Palm. Management for Large and Sustainable Yields; Fairhurst, T., Härdter, R., Eds.; Potash & Phosphate Institute, International Potash Institute: Oxford, UK, 2003; pp. 115–150. [Google Scholar]
- Lai, J.W.; Ramli, H.R.; Ismail, L.I.; Wan Hasan, W.Z. Oil Palm Fresh Fruit Bunch Ripeness Detection Methods: A Systematic Review. Agriculture 2023, 13, 156. [Google Scholar] [CrossRef]
- Barcelos, E.; Amblard, P.; Berthaud, J.; Seguin, M. Genetic diversity and relationship in American and African oil palm as revealed by RFLP and AFLP molecular markers. Pesqui. Agropecu. Bras. 2002, 37, 1105–1114. [Google Scholar]
- Morcillo, F.; Serret, J.; Beckers, A.; Collin, M.; Tisné, S.; George, S.; Poveda, R.; Louise, C.; Tranbarger, T.J. A non-shedding fruit Elaeis oleifera palm reveals perturbations to hormone signaling, ROS homeostasis, and hemicellulose metabolism. Genes 2021, 12, 1724. [Google Scholar] [CrossRef]
- Moreno, L.P.; Romero, H.M. Phenology of the reproductive development of Elaeis oleifera (Kunth) Cortes. Agron. Colomb. 2015, 33, 29–35. [Google Scholar]
- Prada, F.; Romero, H.M. Muestreo y analisis de racimos en el cultivo de la palma de aceite. In Tecnologías para la Agroindustria de la Palma de Aceite, Guia de Facilitadores; Cenipalma: Bogotá, Colombia, 2012; p. 158. [Google Scholar]
- Widodo, P.; Nur, F.; Hafisah, E.; Forster, B.P.; Hasibuan, H.A. Bunch and Oil Analysis of Oil Palm: A Manual; CABI: Wallingford, UK, 2019; Volume 7. [Google Scholar]
- Sambanthamurthi, R.; Sundram, K.; Tan, Y.-A. Chemistry and biochemistry of palm oil. Prog. Lipid Res. 2000, 39, 507–558. [Google Scholar]
- Corley, R.H.V.; Tinker, P.B. The Oil Palm, 4th ed.; Blacwell Publishing Inc.: Oxford, UK, 2003; p. 562. [Google Scholar]
- Tranbarger, T.J.; Dussert, S.; Joet, T.; Argout, X.; Summo, M.; Champion, A.; Cros, D.; Omore, A.; Nouy, B.; Morcillo, F. Regulatory Mechanisms Underlying Oil Palm Fruit Mesocarp Maturation, Ripening, and Functional Specialization in Lipid and Carotenoid Metabolism. Plant Physiol. 2011, 156, 564–584. [Google Scholar] [CrossRef]
- González, D.A.; Cayón, G.; López, J.E.; Alarcón, W.H. Development and maturation of fruits of two Indupalma O×G hybrids (Elaeis oleifera × Elaeis guineensis). Agron. Colomb. 2013, 31, 343–351. [Google Scholar]
- Singh, R.; Low, E.-T.L.; Ooi, L.C.-L.; Ong-Abdullah, M.; Nookiah, R.; Ting, N.-C.; Marjuni, M.; Chan, P.-L.; Ithnin, M.; Manaf, M.A.A. The oil palm VIRESCENS gene controls fruit colour and encodes a R2R3-MYB. Nat. Commun. 2014, 5, 4106. [Google Scholar]
- Lieb, V.M.; Kerfers, M.R.; Kronmüller, A.; Esquivel, P.; Alvarado, A.; Jiménez, V.c.M.; Schmarr, H.-G.; Carle, R.; Schweiggert, R.M.; Steingass, C.B. Characterization of mesocarp and kernel lipids from Elaeis guineensis Jacq., Elaeis oleifera [Kunth] Cortés, and their interspecific hybrids. J. Agric. Food Chem. 2017, 65, 3617–3626. [Google Scholar]
- Castillo, E.G.; Rodríguez, C.L.F.; Páez, A.F. Evaluation of two harvesting procedures for oil palm (Elaeis guineensis Jacq.) fruits. A case study. Agron. Colomb. 2017, 35, 92–99. [Google Scholar]
- Mosquera-Montoya, M.; Ruiz-Alvarez, E.; Mesa-Fuquen, E. Economic assessment of technology adoption in oil palm plantations from Colombia. Int. J. Financ. Res. 2017, 8, 74. [Google Scholar]
- Sinisterra Ortiz, K.; Camperos, J.; Cortés, I.; Caicedo, A.; Castilla, C.; Ceballos, D. Validación a escala comercial del punto óptimo de cosecha para el cultivar híbrido interespecífico O × G Cereté x Deli. Rev. Palmas 2021, 42, 15–23. [Google Scholar]
- Ruiz Álvarez, E.; Banguera, J.; Pérez Toro, W.; Hernández Hernández, J.; Arévalo, J.; Mosquera Montoya, M. Technical and economic assessment of two harvesting tools for young Elaeis oleifera x E. guineensis oil palms. Agron. Colomb. 2020, 38, 418–428. [Google Scholar]
- Escallón-Barrios, M.; Castillo-Gomez, D.; Leal, J.; Montenegro, C.; Medaglia, A.L. Improving harvesting operations in an oil palm plantation. Ann. Oper. Res. 2022, 314, 411–449. [Google Scholar]
- Goh, J.Y.; Md Yunos, Y.; Mohamed Ali, M.S. Fresh Fruit Bunch Ripeness Classification Methods: A Review. Food Bioprocess Technol. 2025, 18, 183–206. [Google Scholar]
- You, K.; Wee, F.; Lee, Y.; Abbas, Z.; Lee, K.; Cheng, E.; Khe, C.; Jamlos, M. A review of oil palm fruit ripeness monitoring using microwave techniques in Malaysia. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Perlis, Malaysia, 23 December 2019; p. 012007. [Google Scholar]
- Ibrahim, Z.; Sabri, N.; Isa, D. Palm oil fresh fruit bunch ripeness grading recognition using convolutional neural network. J. Telecommun. Electron. Comput. Eng. (JTEC) 2018, 10, 109–113. [Google Scholar]
- Lai, J.W.; Ramli, H.R.; Ismail, L.I.; Hasan, W.Z.W. Real-Time Detection of Ripe Oil Palm Fresh Fruit Bunch Based on YOLOv4. IEEE Access 2022, 10, 95763–95770. [Google Scholar]
- Tzuan, G.T.H.; Hashim, F.H.; Raj, T.; Baseri Huddin, A.; Sajab, M.S. Oil palm fruits ripeness classification based on the characteristics of protein, lipid, carotene, and guanine/cytosine from the Raman spectra. Plants 2022, 11, 1936. [Google Scholar] [CrossRef]
- Setiawan, A.W.; Mengko, R.; Putri, A.P.H.; Danudirdjo, D.; Ananda, A.R. Classification of palm oil fresh fruit bunch using multiband optical sensors. Int. J. Electr. Comput. Eng. 2019, 9, 2386. [Google Scholar]
- Mozaffari, M.; Sadeghi, S.; Asefi, N. Prediction of the quality properties and maturity of apricot by laser light backscattering imaging. Postharvest Biol. Technol. 2022, 186, 111842. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).