Mapping the Potential Presence of the Spotted Wing Drosophila Under Current and Future Scenario: An Update of the Distribution Modeling and Ecological Perspectives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection for the Occurrence of D. suzukii
2.2. Download and Selection of WorldClim Climatic Layers
2.3. Current and Future Distribution Modeling
2.4. Model Evaluation and Importance of Each Bioclimatic Variable
3. Results
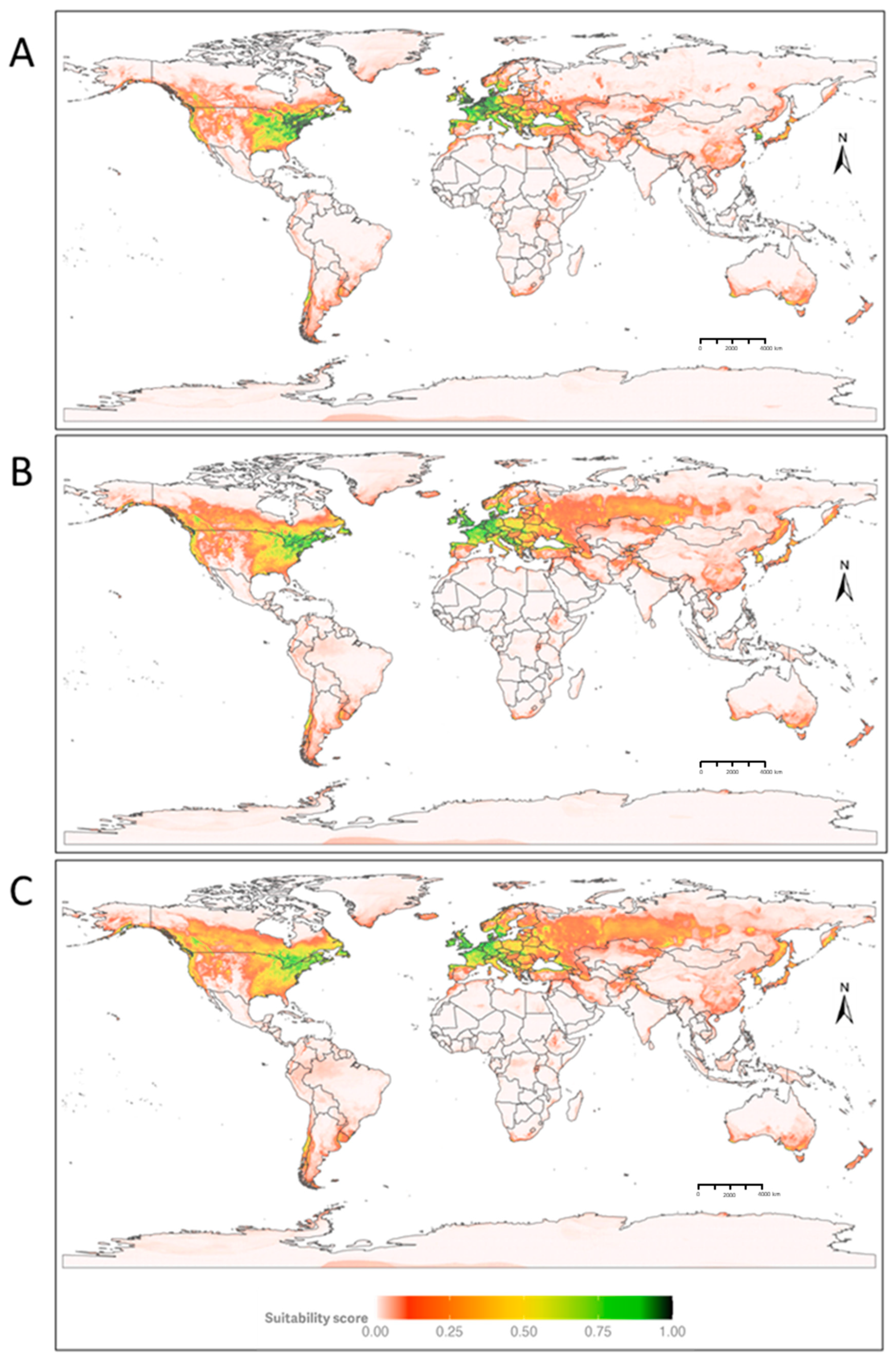
3.1. Current Distribution of D. suzukii
3.2. Future Distribution of D. suzukii
3.3. Climatic Variables Determining the Distribution of D. suzukii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion Biology of Spotted Wing Drosophila (Drosophila suzukii): A Global Perspective and Future Priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Walton, V.M.; Burrack, H.J.; Dalton, D.T.; Isaacs, R.; Wiman, N.; Ioriatti, C. Past, Present and Future of Drosophila suzukii: Distribution, Impact and Management in United States Berry Fruits. Acta Hortic. 2016, 1117, 87–94. [Google Scholar] [CrossRef]
- Funes, C.F.; Garrido, S.A.; Aquino, D.A.; Escobar, L.I.; Gomez Segade, C.; Cichón, L.I.; Ovruski, S.M.; Kirschbaum, D.S. New Records of Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae) Associated with Drosophila suzukii (Diptera: Drosophilidae) in Cherry and Berry Crops from Argentina. Rev. Soc. Entomol. Argent. 2020, 79, 39–43. [Google Scholar] [CrossRef]
- dos Santos, V.F.; Abeijon, L.M.; da Cruz Araújo, S.H.; Garcia, F.R.M.; de Oliveira, E.E. The Potential of Plant-Based Biorational Products for the Drosophila suzukii Control: Current Status, Opportunities, and Limitations. Neotrop. Entomol. 2023, 53, 236–243. [Google Scholar] [CrossRef]
- Andreazza, F.; Bernardi, D.; dos Santos, R.S.S.; Garcia, F.R.M.; Oliveira, E.E.; Botton, M.; Nava, D.E. Drosophila suzukii in Southern Neotropical Region: Current Status and Future Perspectives. Neotrop. Entomol. 2017, 46, 591–605. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M. Global Potential Distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Ometto, L.; Tait, G.; Ghirotto, S.; Kaur, R.; Drago, F.; González, J.; Walton, V.M.; Anfora, G.; Rossi-Stacconi, M.V. Distinct Genotypes and Phenotypes in European and American Strains of Drosophila suzukii: Implications for Biology and Management of an Invasive Organism. J. Pest Sci. 2020, 93, 77–89. [Google Scholar] [CrossRef]
- Boughdad, A.; Haddi, K.; El Bouazzati, A.; Nassiri, A.; Tahiri, A.; El Anbri, C.; Eddaya, T.; Zaid, A.; Biondi, A. First Record of the Invasive Spotted Wing Drosophila Infesting Berry Crops in Africa. J. Pest Sci. 2021, 94, 261–271. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Lasa, R.; Funes, C.F.; Buzzetti, K. Drosophila suzukii Management in Latin America: Current Status and Perspectives. J. Econ. Entomol. 2022, 115, 1008–1023. [Google Scholar] [CrossRef]
- Hauser, M. A Historic Account of the Invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the Continental United States, with Remarks on Their Identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Cini, A.; Ioriatti, C.; Anfora, G. A Review of the Invasion of Drosophila suzukii in Europe and a Draft Research Agenda for Integrated Pest Management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Dreves, A.J.; Walton, V.; Fisher, G.A. Oregon 2009. Available online: https://catalog.extension.oregonstate.edu/em8991 (accessed on 4 March 2022).
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding Its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Robinson, A.S.; Hendrichs, J.; Kenmore, P. Area-Wide Integrated Pest Management (AW-IPM): Principles, Practice and Prospects. In Area-Wide Control of Insect Pests; Springer: Dordrecht, The Netherlands, 2007; pp. 3–33. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization The State of Food Insecurity in the World 2013. The Multiple Dimensions of Food Security. Available online: https://www.fao.org/4/i3434e/i3434e00.htm (accessed on 31 January 2025).
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising Temperatures Reduce Global Wheat Production. Nat. Clim. Change 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Girod, P.; Lierhmann, O.; Urvois, T.; Turlings, T.C.J.; Kenis, M.; Haye, T. Host Specificity of Asian Parasitoids for Potential Classical Biological Control of Drosophila suzukii. J. Pest Sci. 2018, 91, 1241–1250. [Google Scholar] [CrossRef]
- Ziska, L.H.; Blumenthal, D.M.; Runion, G.B.; Hunt, E.R.; Diaz-Soltero, H. Invasive Species and Climate Change: An Agronomic Perspective. Clim. Change 2011, 105, 13–42. [Google Scholar] [CrossRef]
- Savary, S.; Bregaglio, S.; Willocquet, L.; Gustafson, D.; Mason D’Croz, D.; Sparks, A.; Castilla, N.; Djurle, A.; Allinne, C.; Sharma, M.; et al. Crop Health and Its Global Impacts on the Components of Food Security. Food Secur. 2017, 9, 311–327. [Google Scholar] [CrossRef]
- Dara, S.K. Integrated Insect Pest Management of Economically Important Crops. In Biopesticides in Organic Farming; CRC Press: Boca Raton, FL, USA, 2021; p. 10. [Google Scholar]
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First Records of the Potential Pest Species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L.; Dalton, D.T. Analysis of the Invasiveness of Spotted Wing Drosophila (Drosophila suzukii) in North America, Europe, and the Mediterranean Basin. Biol. Invasions 2016, 18, 3647–3663. [Google Scholar] [CrossRef]
- de la Vega, G.J.; Corley, J.C. Drosophila suzukii (Diptera: Drosophilidae) Distribution Modelling Improves Our Understanding of Pest Range Limits. Int. J. Pest Manag. 2019, 65, 217–227. [Google Scholar] [CrossRef]
- Macedo, F.L.; Ragonezi, C.; Reis, F.; de Freitas, J.G.R.; Lopes, D.H.; Aguiar, A.M.F.; Cravo, D.; Carvalho, M.A.A.P.d. Prediction of the Potential Distribution of Drosophila suzukii on Madeira Island Using the Maximum Entropy Modeling. Agriculture 2023, 13, 1764. [Google Scholar] [CrossRef]
- Nair, R.R.; Peterson, A.T. Mapping the Global Distribution of Invasive Pest Drosophila suzukii and Parasitoid Leptopilina japonica: Implications for Biological Control. PeerJ 2023, 11, e15222. [Google Scholar] [CrossRef] [PubMed]
- Ørsted, I.V.; Ørsted, M. Species Distribution Models of the Spotted Wing Drosophila (Drosophila suzukii, Diptera: Drosophilidae) in Its Native and Invasive Range Reveal an Ecological Niche Shift. J. Appl. Ecol. 2019, 56, 423–435. [Google Scholar] [CrossRef]
- Reyes, J.A.; Lira-Noriega, A. Current and Future Global Potential Distribution of the Fruit Fly Drosophila suzukii (Diptera: Drosophilidae). Can. Entomol. 2020, 152, 587–599. [Google Scholar] [CrossRef]
- Di Sora, N.; Mannu, R.; Rossini, L.; Contarini, M.; Gallego, D.; Speranza, S. Using Species Distribution Models (SDMs) to Estimate the Suitability of European Mediterranean Non-Native Area for the Establishment of Toumeyella parvicornis (Hemiptera: Coccidae). Insects 2023, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Bächli, G. TaxoDros v1.04: The Database on Taxonomy of Drosophilidae. Available online: https://www.taxodros.uzh.ch/ (accessed on 30 December 2024).
- Owens, H.; Barve, V.; Chamberlain, S. Spocc: Interface to Species Occurrence Data Sources. Available online: https://docs.ropensci.org/spocc/ (accessed on 30 March 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. 2023. Available online: https://www.r-project.org/ (accessed on 17 December 2023).
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; van Ruijven, B.J.; van Vuuren, D.P.; Birkmann, J.; Kok, K.; et al. The Roads Ahead: Narratives for Shared Socioeconomic Pathways Describing World Futures in the 21st Century. Glob. Environ. Change 2017, 42, 169–180. [Google Scholar] [CrossRef]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and Their Energy, Land Use, and Greenhouse Gas Emissions Implications: An Overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hadi, A.S. Regression Analysis by Example. In Wiley Series in Probability and Statistics, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780470055465. [Google Scholar]
- Breiman, L.; Cutler, A.; Liaw, A.; Wiener, M. RandomForest: Breiman and Cutlers Random Forests for Classification and Regression. In CRAN: Contributed Packages; University of California, Berkeley: Berkeley, CA, USA, 2002. [Google Scholar]
- Probst, P.; Boulesteix, A.-L.; Bischl, B. Tunability: Importance of Hyperparameters of Machine Learning Algorithms. J. Mach. Learn. Res. 2019, 20, 1–32. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R using the Caret Package. J. Stat. Softw. 2008, 28. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of Species–Climate Impact Models under Climate Change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Bolda, M.P.; Goodhue, R.E.; Zalom, F.G. Spotted Wing Drosophila: Potential Economic Impact of a Newly Established Pest. Giannini Found. Agric. Econ. 2010, 13, 5–8. [Google Scholar]
- Gabarra, R.; Riudavets, J.; Rodríguez, G.A.; Pujade-Villar, J.; Arnó, J. Prospects for the Biological Control of Drosophila Suzukii. BioControl 2015, 60, 331–339. [Google Scholar] [CrossRef]
- Cloonan, K.R.; Abraham, J.; Angeli, S.; Syed, Z.; Rodriguez-Saona, C. Advances in the Chemical Ecology of the Spotted Wing Drosophila (Drosophila suzukii) and Its Applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef]
- Fanning, P.D.; Grieshop, M.J.; Isaacs, R. Efficacy of Biopesticides on Spotted Wing Drosophila, Drosophila suzukii Matsumura in Fall Red Raspberries. J. Appl. Entomol. 2018, 142, 26–32. [Google Scholar] [CrossRef]
- Mi, C.; Huettmann, F.; Guo, Y.; Han, X.; Wen, L. Why Choose Random Forest to Predict Rare Species Distribution with Few Samples in Large Undersampled Areas? Three Asian Crane Species Models Provide Supporting Evidence. PeerJ 2017, 5, e2849. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; De Toni, D.C.; Valente, V.L.S. The First Records of the Invasive Pest Drosophila suzukii in the South American Continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Maino, J.L.; Schouten, R.; Umina, P. Predicting the Global Invasion of Drosophila suzukii to Improve Australian Biosecurity Preparedness. J. Appl. Ecol. 2021, 58, 789–800. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The Susceptibility of Small Fruits and Cherries to the Spotted-wing Drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche Conservatism: Integrating Evolution, Ecology, and Conservation Biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Cui, D.; Liang, S.; Wang, D.; Liu, Z. A 1 Km Global Dataset of Historical (1979–2013) and Future (2020–2100) Köppen–Geiger Climate Classification and Bioclimatic Variables. Earth Syst. Sci. Data 2021, 13, 5087–5114. [Google Scholar] [CrossRef]

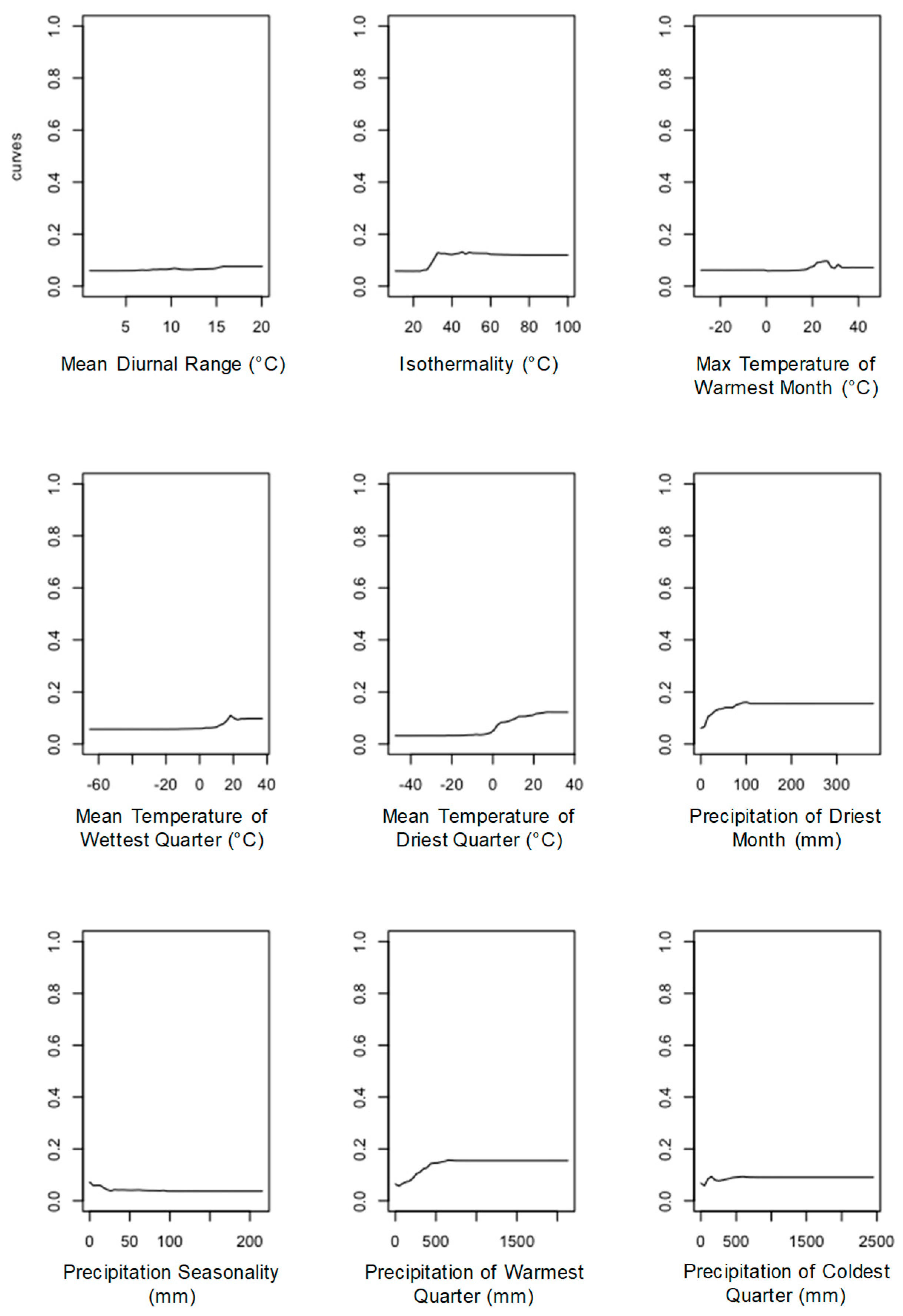
| Climatic Variables | % Increase Mean Square Error | Increase in Node Purity | % Contribution |
|---|---|---|---|
| BIO3 | 0.1258 | 113.50 | 16.45 |
| BIO14 | 0.1173 | 175.29 | 15.33 |
| BIO5 | 0.0994 | 82.39 | 13.00 |
| BIO19 | 0.0979 | 148.57 | 12.80 |
| BIO9 | 0.0843 | 65.25 | 11.02 |
| BIO15 | 0.0741 | 122.47 | 9.70 |
| BIO8 | 0.0733 | 59.13 | 9.59 |
| BIO18 | 0.0525 | 43.28 | 6.86 |
| BIO2 | 0.0401 | 26.35 | 5.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeijon, L.M.; Gómez Llano, J.H.; Robe, L.J.; Ovruski, S.M.; Garcia, F.R.M. Mapping the Potential Presence of the Spotted Wing Drosophila Under Current and Future Scenario: An Update of the Distribution Modeling and Ecological Perspectives. Agronomy 2025, 15, 838. https://doi.org/10.3390/agronomy15040838
Abeijon LM, Gómez Llano JH, Robe LJ, Ovruski SM, Garcia FRM. Mapping the Potential Presence of the Spotted Wing Drosophila Under Current and Future Scenario: An Update of the Distribution Modeling and Ecological Perspectives. Agronomy. 2025; 15(4):838. https://doi.org/10.3390/agronomy15040838
Chicago/Turabian StyleAbeijon, Lenon Morales, Jesús Hernando Gómez Llano, Lizandra Jaqueline Robe, Sergio Marcelo Ovruski, and Flávio Roberto Mello Garcia. 2025. "Mapping the Potential Presence of the Spotted Wing Drosophila Under Current and Future Scenario: An Update of the Distribution Modeling and Ecological Perspectives" Agronomy 15, no. 4: 838. https://doi.org/10.3390/agronomy15040838
APA StyleAbeijon, L. M., Gómez Llano, J. H., Robe, L. J., Ovruski, S. M., & Garcia, F. R. M. (2025). Mapping the Potential Presence of the Spotted Wing Drosophila Under Current and Future Scenario: An Update of the Distribution Modeling and Ecological Perspectives. Agronomy, 15(4), 838. https://doi.org/10.3390/agronomy15040838







