Large-Scale Plasma-Activated Water Reactor: The Differential Impact on the Growth of Tomato and Bell Pepper Plants in Nutrient-Rich and Nitrogen-Free Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Activated Water Reactor
2.2. Plasma Activated Water Treatments
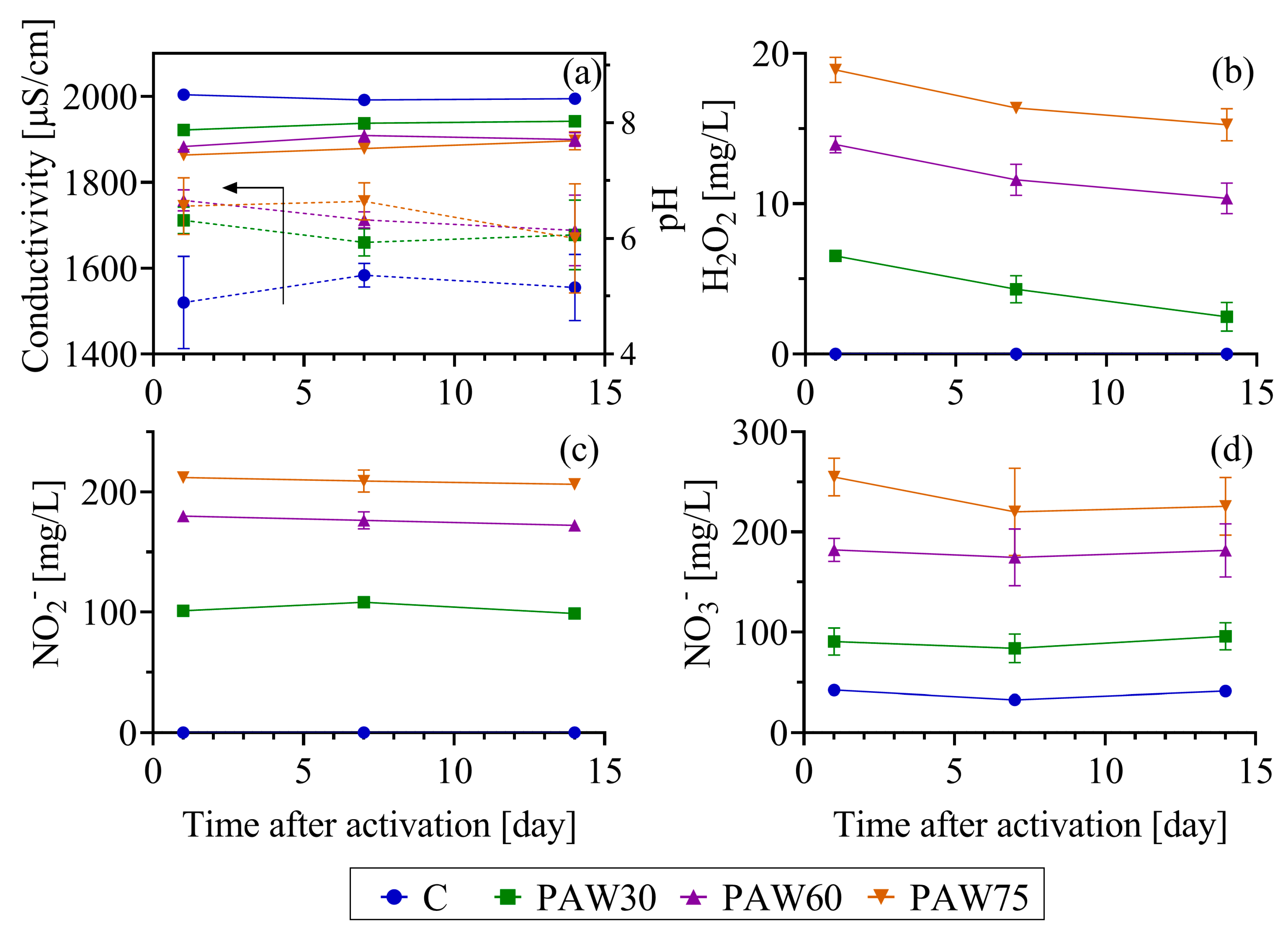
2.3. Plasma-Activated Water Physicochemical Properties
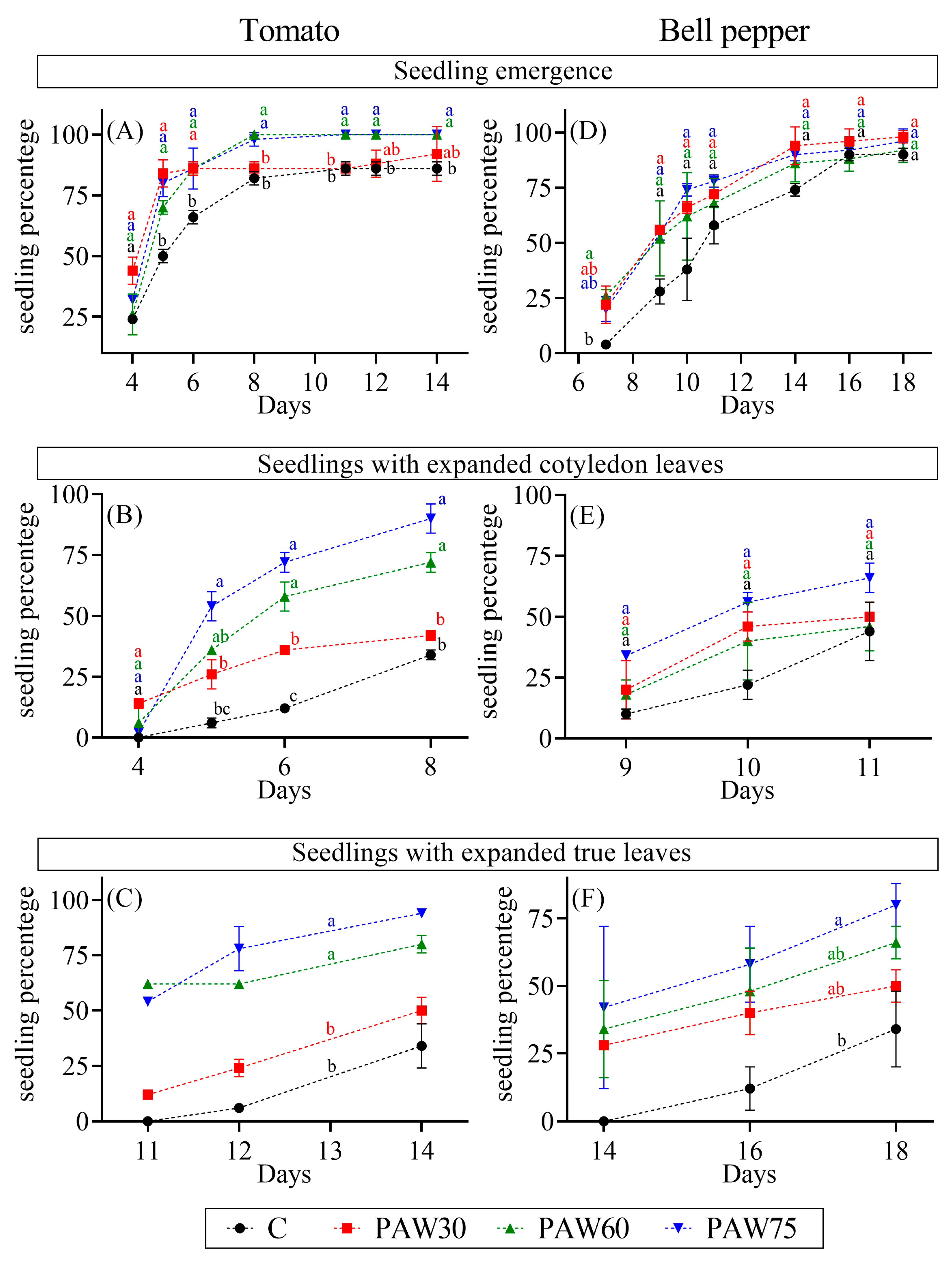
2.4. Seedling Emergence
2.5. Plant Growth
2.6. Oxidative Stress of the Plants
2.6.1. Lipid Peroxidation
2.6.2. Antioxidant Enzymes
2.7. Statistical Analysis
3. Results
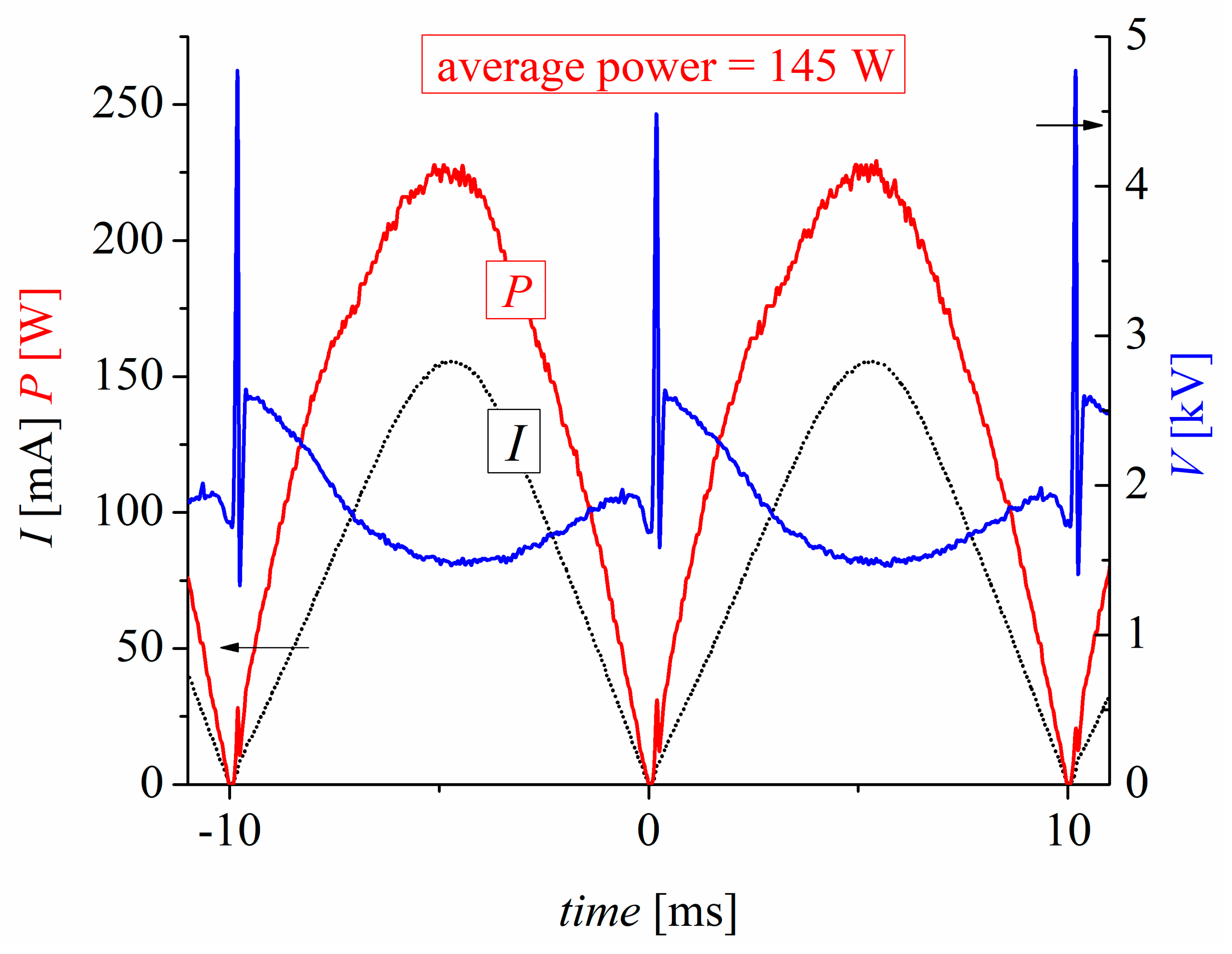
3.1. Electrical Characteristics of the Plasma Reactor and Physicochemical Properties of the PAW
3.2. Seedling Emergence
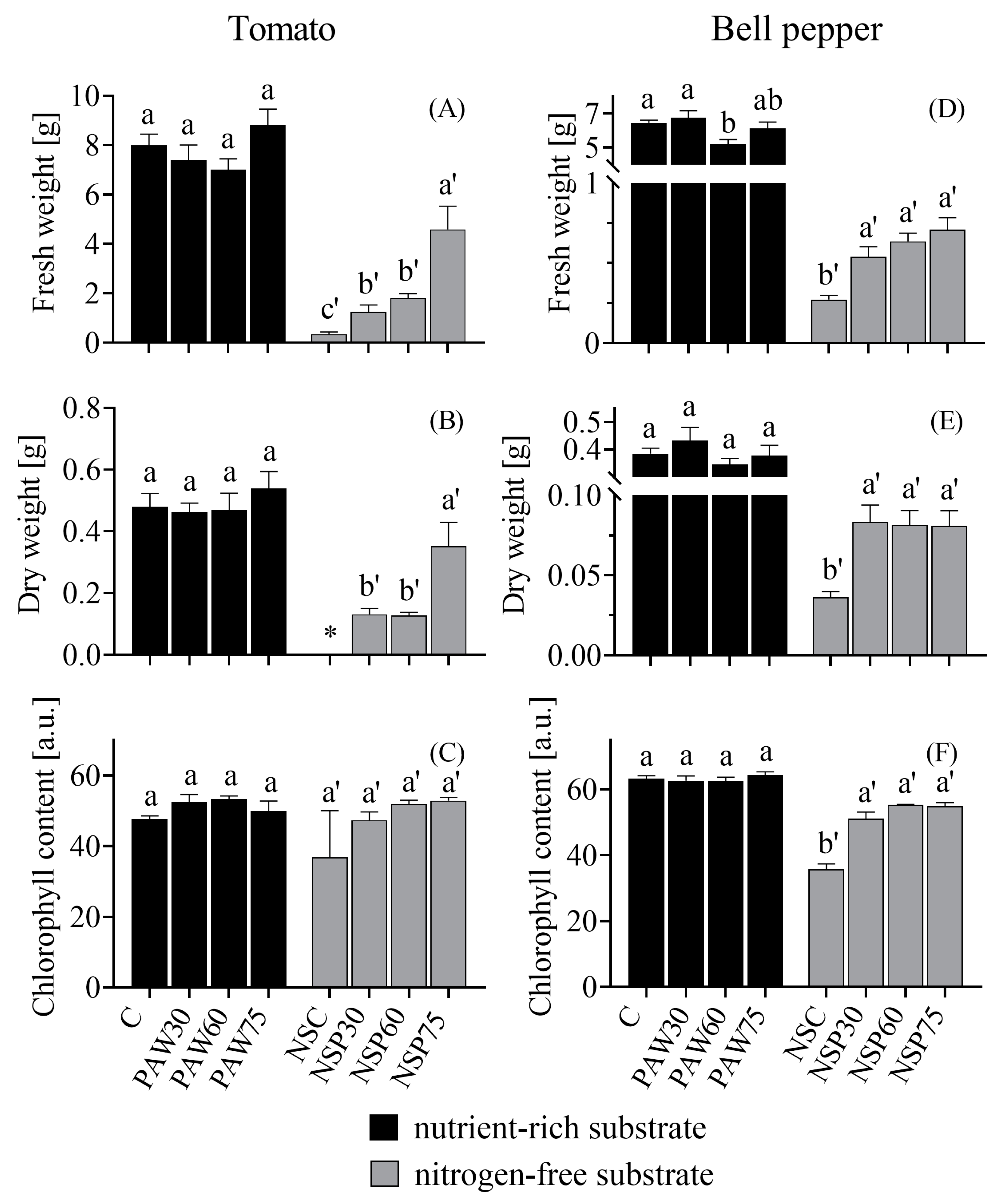
3.3. Plant Growth
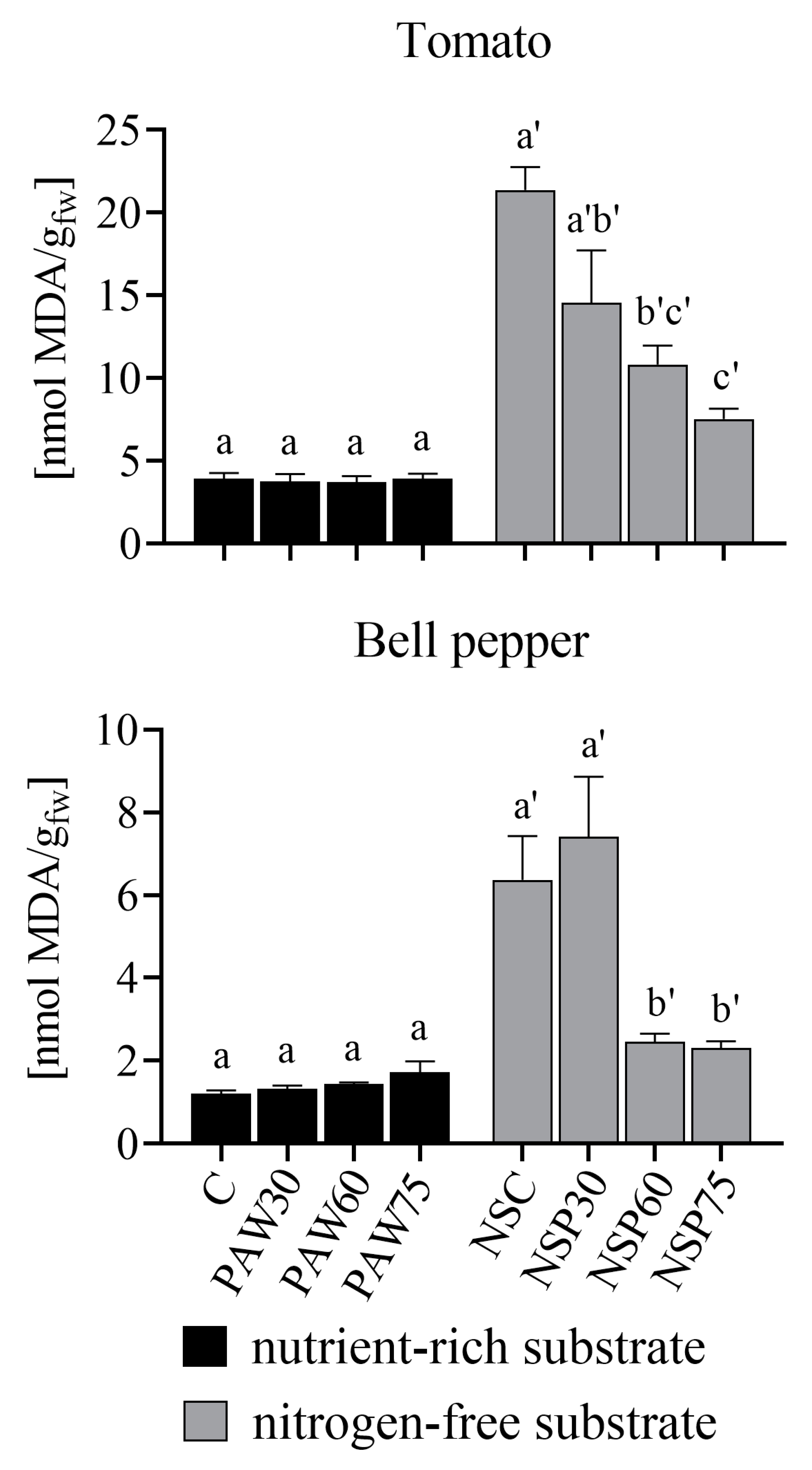
3.4. Oxidative Stress of the Plants
4. Discussion
4.1. Plasma-Activated Water and Nitrogen Fixation
4.2. Seedling Emergence
4.3. Growth and Oxidative Stress of the Plants
5. Conclusions
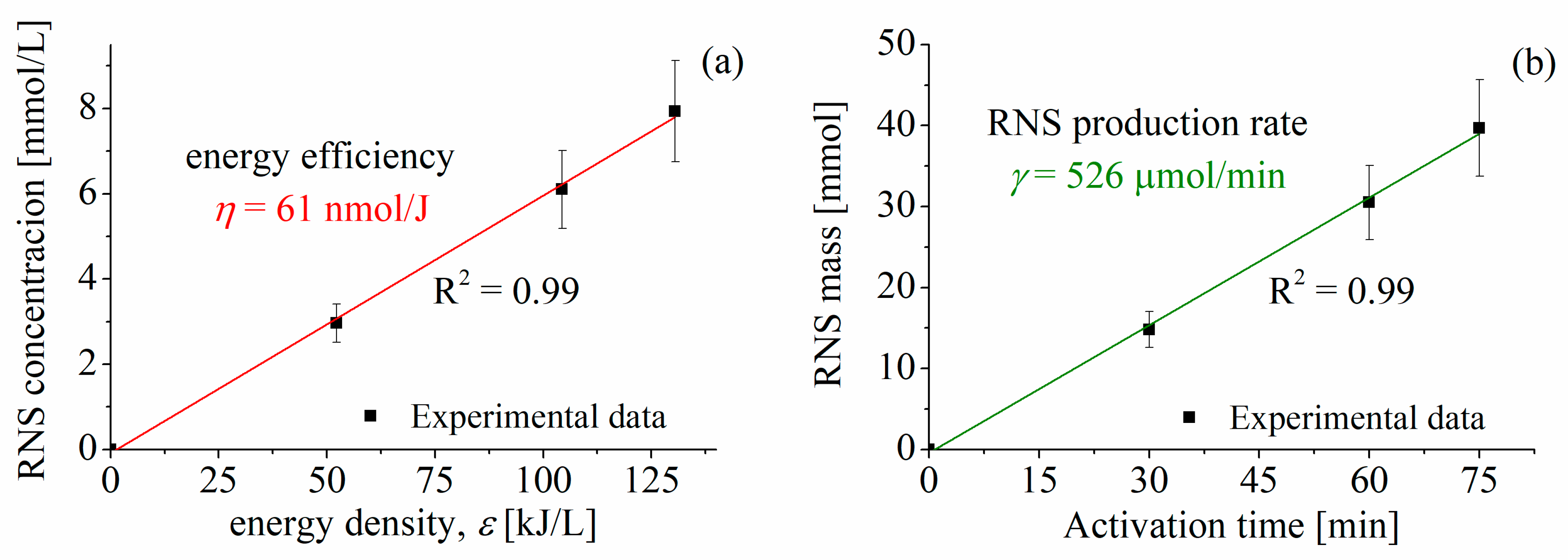
- PAW with 75 min of activation resulted in high concentrations of long-lived RONS: 212 mg/L of NO3−, 211 mg/L of NO2−, and 20 mg/L of H2O2. Notably, the RNS (NOX = NO2− + NO3−) concentration (≈8 mM) was 4 to 26 times greater than that previously found in other studies on tomato and bell pepper. Similarly, the total RNS mass synthesized in water (40 mmol) exceeded literature values by factors ranging from 10 to 1300.
- The PAW generation process demonstrated high efficiency, with an average RNS synthesis energy efficiency of 61 nmol/J and an RNS production rate of 526 μmol/min. These values rank among the highest reported for similar plasma-based systems.
- PAW significantly promoted seedling emergence and the expansion of cotyledon leaves in tomato, as well as the expansion of true leaves in both tomato and bell pepper seedlings.
- The growth-stimulating effects of PAW-derived nitrogenous compounds were pronounced in plants grown in a nitrogen-free substrate but were not observed in plants grown in a nutrient-rich substrate. In the nitrogen-free substrate, PAW irrigation resulted in a significant increase in the biomass of tomato and bell pepper plants: tomato plant fresh weight increasing by 2.6- to 13.1-fold and bell pepper plant fresh weight by 2- to 2.6-fold. Notably, at the same phenological stage, tomato plants irrigated with PAW activated for 75 min attained a dry weight comparable to that of plants cultivated in a nutrient-rich substrate.
- PAW has not induced oxidative damage in plants, indicated by the MDA values of PAW-irrigated plants being like or lower than control plants. In fact, in nitrogen-free conditions, MDA levels in PAW-irrigated plants decreased compared to the control, approaching those observed under optimal nutrient-rich conditions. Furthermore, biochemical analyses of key enzymes (superoxide dismutase, catalase, and guaiacol peroxidase) confirmed that plant resistance mechanisms respond adequately to irrigation with PAW.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilea, F.; Garcia-Vaquero, M.; Magureanu, M.; Mihaila, I.; Mildažienė, V.; Mozetič, M.; Pawłat, J.; Primc, G.; Puač, N.; Robert, E.; et al. Non-Thermal Plasma as Environmentally-Friendly Technology for Agriculture: A Review and Roadmap. Crit. Rev. Plant Sci. 2024, 43, 428–486. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma Agriculture: Review from the Perspective of the Plant and Its Ecosystem. Plasma Process. Polym. 2021, 18, 2000162. [Google Scholar] [CrossRef]
- Veerana, M.; Mumtaz, S.; Rana, J.N.; Javed, R.; Panngom, K.; Ahmed, B.; Akter, K.; Choi, E.H. Recent Advances in Non-Thermal Plasma for Seed Germination, Plant Growth, and Secondary Metabolite Synthesis: A Promising Frontier for Sustainable Agriculture. Plasma Chem. Plasma Process. 2024, 44, 2263–2302. [Google Scholar] [CrossRef]
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low Temperature Plasma Science and Technology. J. Phys. D Appl. Phys. 2022, 55, 373001. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Gusein-zade, N.; Burmistrov, D.E.; Kolik, L.V.; Dorokhov, A.S.; Izmailov, A.Y.; Shokri, B.; Gudkov, S.V. Advancements in Plasma Agriculture: A Review of Recent Studies. Int. J. Mol. Sci. 2023, 24, 15093. [Google Scholar] [CrossRef]
- Misra, N.N.; Naladala, T.; Alzahrani, K.J. Design of Systems for Plasma Activated Water (PAW) for Agri-Food Applications. J. Phys. D Appl. Phys. 2024, 57, 493003. [Google Scholar] [CrossRef]
- Wong, K.S.; Chew, N.S.L.; Low, M.; Tan, M.K. Plasma-Activated Water: Physicochemical Properties, Generation Techniques, and Applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Barjasteh, A.; Lamichhane, P.; Dehghani, Z.; Kaushik, N.; Gupta, R.; Choi, E.H.; Kaushik, N.K. Recent Progress of Non-Thermal Atmospheric Pressure Plasma for Seed Germination and Plant Development: Current Scenario and Future Landscape. J. Plant Growth Regul. 2023, 42, 5417–5432. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, D.; Wu, A.; Lin, X.; Li, X. Review of Low-Temperature Plasma Nitrogen Fixation Technology. Waste Dispos. Sustain. Energy 2021, 3, 201–217. [Google Scholar] [CrossRef]
- Robinson, C.; Stapelmann, K. Plasma Treating Water for Nitrate Based Nitrogen Fertilizer—A Review of Recent Device Designs. Curr. Opin. Green Sustain. Chem. 2024, 50, 100978. [Google Scholar] [CrossRef]
- Aceto, D.; Rotondo, P.R.; Porfido, C.; Bottiglione, B.; Paciolla, C.; Terzano, R.; Minafra, A.; Ambrico, M.; Dilecce, G.; Leoni, B.; et al. Assessing Plasma Activated Water Irrigation Effects on Tomato Seedlings. Front. Phys. 2024, 12, 1399910. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene Expression in Tomato Seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhang, X.; Xiong, C. Plasma Activated-Water Stimulates Aged Pepper Seeds and Promotes Seedling Growth. Plasma Process. Polym. 2024, 21, 2300173. [Google Scholar] [CrossRef]
- Japundžić-Palenkić, B.; Benković, R.; Benković-Lačić, T.; Antunović, S.; Japundžić, M.; Romanjek Fajdetić, N.; Mirosavljević, K. Pepper Growing Modified by Plasma Activated Water and Growth Conditions. Sustainability 2022, 14, 15967. [Google Scholar] [CrossRef]
- Lindsay, A.; Byrns, B.; King, W.; Andhvarapou, A.; Fields, J.; Knappe, D.; Fonteno, W.; Shannon, S. Fertilization of Radishes, Tomatoes, and Marigolds Using a Large-Volume Atmospheric Glow Discharge. Plasma Chem. Plasma Process. 2014, 34, 1271–1290. [Google Scholar] [CrossRef]
- Punith, N.; Harsha, R.; Lakshminarayana, R.; Hemanth, M.; Anand, M.S.; Dasappa, S. Plasma Activated Water Generation and Its Application in Agriculture. Adv. Mater. Lett. 2019, 10, 700–704. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced Seed Germination and Plant Growth by Atmospheric Pressure Cold Air Plasma: Combined Effect of Seed and Water Treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Hensel, K. Effects of Plasma Activated Water on Wheat: Germination, Growth Parameters, Photosynthetic Pigments, Soluble Protein Content, and Antioxidant Enzymes Activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- Huang, Z.; Xiao, A.; Liu, D.; Lu, X.; Ostrikov, K. Plasma-Water-Based Nitrogen Fixation: Status, Mechanisms, and Opportunities. Plasma Process. Polym. 2022, 19, 2100198. [Google Scholar] [CrossRef]
- Hoeben, W.F.L.M.; van Ooij, P.P.; Schram, D.C.; Huiskamp, T.; Pemen, A.J.M.; Lukeš, P. On the Possibilities of Straightforward Characterization of Plasma Activated Water. Plasma Chem. Plasma Process. 2019, 39, 597–626. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R., Eaton, A., Rice, E., Eds.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Balestrasse, K.B.; Gallego, S.M.; Tomaro, M.L. Oxidation of the Enzymes Involved in Nitrogen Assimilation Plays an Important Role in the Cadmium-Induced Toxicity in Soybean Plants. Plant Soil 2006, 284, 187–194. [Google Scholar] [CrossRef]
- Martiniello, P.; Paoletti, R.; Berardo, N. Effect of Phenological Stages on Dry Matter and Quality Components in Lucerne. Eur. J. Agron. 1997, 6, 79–87. [Google Scholar] [CrossRef]
- Koca, Y.O.; Erekul, O. Changes of Dry Matter, Biomass and Relative Growth Rate with Different Phenological Stages of Corn. Agric. Agric. Sci. Procedia 2016, 10, 67–75. [Google Scholar] [CrossRef][Green Version]
- Bernhard, B.J.; Below, F.E. Plant Population and Row Spacing Effects on Corn: Plant Growth, Phenology, and Grain Yield. Agron. J. 2020, 112, 2456–2465. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide Metabolism in Mammalian Organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Becana, M.; Aparicio-Tejo, P.; Irigoyen, J.J.; Sanchez-Diaz, M. Some Enzymes of Hydrogen Peroxide Metabolism in Leaves and Root Nodules of Medicago sativa. Plant Physiol. 1986, 82, 1169–1171. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Gallego, S.M.; Tomaro, M.L. Aluminium Stress Affects Nitrogen Fixation and Assimilation in Soybean (Glycine Max L.). Plant Growth Regul. 2006, 48, 271–281. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 1 February 2025).
- Bruggeman, P.; Leys, C. Non-Thermal Plasmas in and in Contact with Liquids. J. Phys. D Appl. Phys. 2009, 42, 053001. [Google Scholar] [CrossRef]
- Lukes, P.; Locke, B.R.; Brisset, J.-L. Aqueous-Phase Chemistry of Electrical Discharge Plasma in Water and in Gas–Liquid Environments. In Plasma Chemistry and Catalysis in Gases and Liquids; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2012; pp. 243–308. ISBN 978-3-527-64952-5. [Google Scholar]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Bačovčinová, M.; Hensel, K. Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters. Appl. Sci. 2021, 11, 1985. [Google Scholar] [CrossRef]
- Hunt, R. Absolute Growth Rates. In Basic Growth Analysis: Plant Growth Analysis for Beginners; Hunt, R., Ed.; Springer: Dordrecht, The Netherlands, 1990; pp. 17–24. ISBN 978-94-010-9117-6. [Google Scholar]
- Ferreyra, M.G.; Cejas, E.; Santamaría, B.; Chamorro, J.C.; Goméz, B.J.; Prevosto, L. Numerical Simulation of the Ionic Composition and Ionization Phenomena in the Positive Column of a Millisecond DC-Pulsed Glow-Type Discharge in Atmospheric Pressure Air with a Water-Cathode. Plasma Chem. Plasma Process. 2024, 44, 2199–2231. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, T. Comparative Study of Discharge Schemes for Production Rates and Ratios of Reactive Oxygen and Nitrogen Species in Plasma Activated Water. J. Phys. D Appl. Phys. 2019, 52, 385202. [Google Scholar] [CrossRef]
- Santamaría, B.; Ferreyra, M.G.; Chamorro, J.C.; Cejas, E.; Fina, B.L.; Prevosto, L. Physicochemical Properties and Time Stability of Plasma Activated Water by a Liquid-Cathode Glow-Type Discharge in Air: The Effect of Air Confinement. IEEE Trans. Plasma Sci. 2024, 52, 1923–1929. [Google Scholar] [CrossRef]
- Dinh, D.K.; Muzammil, I.; Kang, W.S.; Kim, D.; Lee, D.H. Reducing Energy Cost of in Situ Nitrogen Fixation in Water Using an Arc-DBD Combination. Plasma Sources Sci. Technol. 2021, 30, 055020. [Google Scholar] [CrossRef]
- Li, J.; Lan, C.; Nie, L.; Liu, D.; Lu, X. Distributed Plasma-Water-Based Nitrogen Fixation System Based on Cascade Discharge: Generation, Regulation, and Application. Chem. Eng. J. 2023, 478, 147483. [Google Scholar] [CrossRef]
- Rathore, V.; Tiwari, B.S.; Nema, S.K. Treatment of Pea Seeds with Plasma Activated Water to Enhance Germination, Plant Growth, and Plant Composition. Plasma Chem. Plasma Process. 2022, 42, 109–129. [Google Scholar] [CrossRef]
- Liu, B.; Honnorat, B.; Yang, H.; Arancibia, J.; Rajjou, L.; Rousseau, A. Non-Thermal DBD Plasma Array on Seed Germination of Different Plant Species. J. Phys. D Appl. Phys. 2018, 52, 025401. [Google Scholar] [CrossRef]
- Terebun, P.; Kwiatkowski, M.; Hensel, K.; Kopacki, M.; Pawłat, J. Influence of Plasma Activated Water Generated in a Gliding Arc Discharge Reactor on Germination of Beetroot and Carrot Seeds. Appl. Sci. 2021, 11, 6164. [Google Scholar] [CrossRef]
- Zhang, S.; Rousseau, A.; Dufour, T. Promoting Lentil Germination and Stem Growth by Plasma Activated Tap Water, Demineralized Water and Liquid Fertilizer. RSC Adv. 2017, 7, 31244–31251. [Google Scholar] [CrossRef]
- Ferreyra, M.G.; Caffaro, M.M.; Santamaría, B.; Zilli, C.; Hernández, A.; Fina, B.L.; Vélez, A.S.; Balestrasse, K.B.; Prevosto, L. Plasma-Activated Water Produced by a Moderately High Energy-Efficient 1-Liter Reactor: Effects on Germination and Growth of Tomato and Bell Pepper Plants. Plants 2025, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, H.J.; Zhang, X.M.; Yang, X.Y.; Zhao, W.C.; Li, Q.; Jiang, W.J. Effect of Nitrogen Deficiency on Ascorbic Acid Biosynthesis and Recycling Pathway in Cucumber Seedlings. Plant Physiol. Biochem. 2016, 108, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Xu, M.; Cheng, Z.; Yang, L.-T. Effects of Nitrogen Deficiency on the Photosynthesis, Chlorophyll a Fluorescence, Antioxidant System, and Sulfur Compounds in Oryza Sativa. Int. J. Mol. Sci. 2024, 25, 10409. [Google Scholar] [CrossRef] [PubMed]
- Ndiffo Yemeli, G.B.; Švubová, R.; Kostolani, D.; Kyzek, S.; Machala, Z. The Effect of Water Activated by Nonthermal Air Plasma on the Growth of Farm Plants: Case of Maize and Barley. Plasma Process. Polym. 2021, 18, 2000205. [Google Scholar] [CrossRef]
- Mumtaz, S.; Javed, R.; Rana, J.N.; Iqbal, M.; Choi, E.H. Pulsed High Power Microwave Seeds Priming Modulates Germination, Growth, Redox Homeostasis, and Hormonal Shifts in Barley for Improved Seedling Growth: Unleashing the Molecular Dynamics. Free Radic. Biol. Med. 2024, 222, 371–385. [Google Scholar] [CrossRef]


| Macronutrients | Micronutrients | ||
|---|---|---|---|
| Compound | cc [mM] | Compound | cc [µM] |
| KH2PO4 | 1.5 | MnSO4·H2O | 1 |
| MgSO4·7H2O | 0.8 | ZnSO4·7H2O | 1 |
| CaCl2·2H2O | 0.8 | H3BO3 | 12.5 |
| KCl | 2.5 | H2MoO4 | 0.25 |
| Fe-EDTA | 5 | CuSO4·2H2O | 0.25 |
| Reference | Discharge Type | Power [W] | Activation Time [min] | Activation Volume [L] | Maximum [RNS] [mM] | η [nmol/J] | γ [μmol/min] | RNS Mass [mmol] |
|---|---|---|---|---|---|---|---|---|
| [11] | DBD | 13 | 60 | 0.5 | 1.2 | 12 | 9.6 | 0.57 |
| [12] | Jet | 3.6 | 60 | 0.05 | 0.6 | 2.2 | 0.5 | 0.03 |
| [14] | Jet | 15 | 40 | 0.215 | 0.3 | 1.5 | 1.4 | 0.05 |
| [15] | Glow | 420 | 80 | 1.9 | 2.1 | 1.9 | 49.1 | 3.93 |
| [16] | Arc | 37 | 30 | 2 | 1.4 | 43 | 96.1 | 2.88 |
| [17] | DBD | 3 | 30 | 0.25 | 0.3 | 12 | 2.2 | 0.06 |
| This work | Glow | 145 | 75 | 5 | 8 | 61 | 526.0 | 40.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreyra, M.G.; Santamaría, B.; Caffaro, M.M.; Zilli, C.; Hernández, A.; Fina, B.L.; Balestrasse, K.B.; Prevosto, L. Large-Scale Plasma-Activated Water Reactor: The Differential Impact on the Growth of Tomato and Bell Pepper Plants in Nutrient-Rich and Nitrogen-Free Substrates. Agronomy 2025, 15, 829. https://doi.org/10.3390/agronomy15040829
Ferreyra MG, Santamaría B, Caffaro MM, Zilli C, Hernández A, Fina BL, Balestrasse KB, Prevosto L. Large-Scale Plasma-Activated Water Reactor: The Differential Impact on the Growth of Tomato and Bell Pepper Plants in Nutrient-Rich and Nitrogen-Free Substrates. Agronomy. 2025; 15(4):829. https://doi.org/10.3390/agronomy15040829
Chicago/Turabian StyleFerreyra, Matías G., Brenda Santamaría, María M. Caffaro, Carla Zilli, Alejandra Hernández, Brenda L. Fina, Karina B. Balestrasse, and Leandro Prevosto. 2025. "Large-Scale Plasma-Activated Water Reactor: The Differential Impact on the Growth of Tomato and Bell Pepper Plants in Nutrient-Rich and Nitrogen-Free Substrates" Agronomy 15, no. 4: 829. https://doi.org/10.3390/agronomy15040829
APA StyleFerreyra, M. G., Santamaría, B., Caffaro, M. M., Zilli, C., Hernández, A., Fina, B. L., Balestrasse, K. B., & Prevosto, L. (2025). Large-Scale Plasma-Activated Water Reactor: The Differential Impact on the Growth of Tomato and Bell Pepper Plants in Nutrient-Rich and Nitrogen-Free Substrates. Agronomy, 15(4), 829. https://doi.org/10.3390/agronomy15040829







