Mechanisms of Biochar in Modulating Soil Organic Selenium Transformation and Enhancing Soil Selenium Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection and Materials Pretreatment
2.1.1. Soil Sample Collection
2.1.2. Biochar Pretreatment
2.1.3. Soil Fulvic Acid Se and Humic Acid Se Pretreatment
2.2. Experiment Design and Sample Analysis
2.2.1. Pot Experiment
2.2.2. Organic Bound Selenium Transformation Culture Experiment
2.3. Sample Analysis
2.3.1. Determination of Soil and Plant Samples
2.3.2. DNA Extraction and Illumina MiSeq Sequencing
2.4. Statistical Analysis
3. Results and Discussion
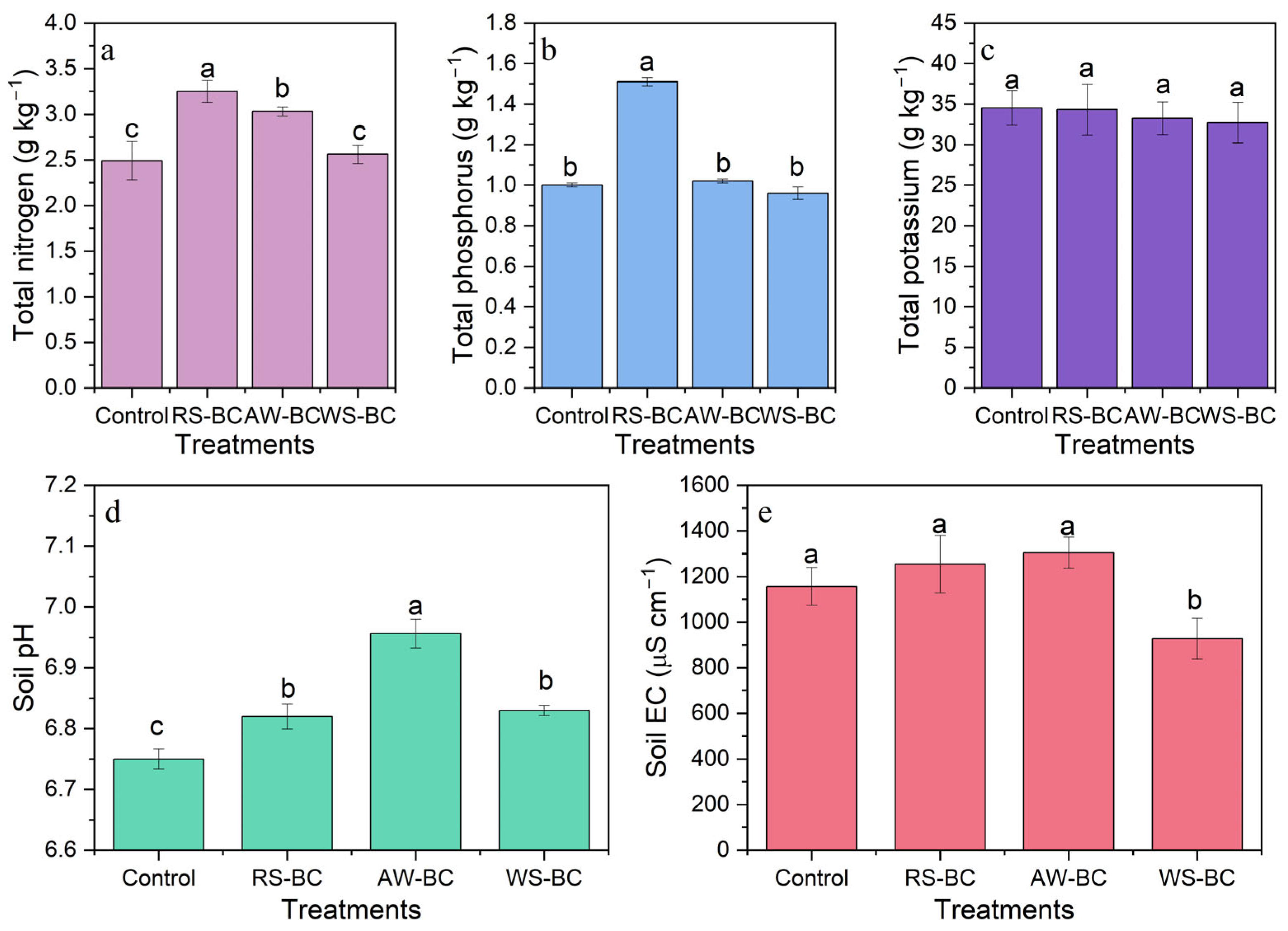
3.1. Effects of Biochar on Soil Physical and Chemical Properties
3.2. Effect of Biochar on Soil Selenium Form
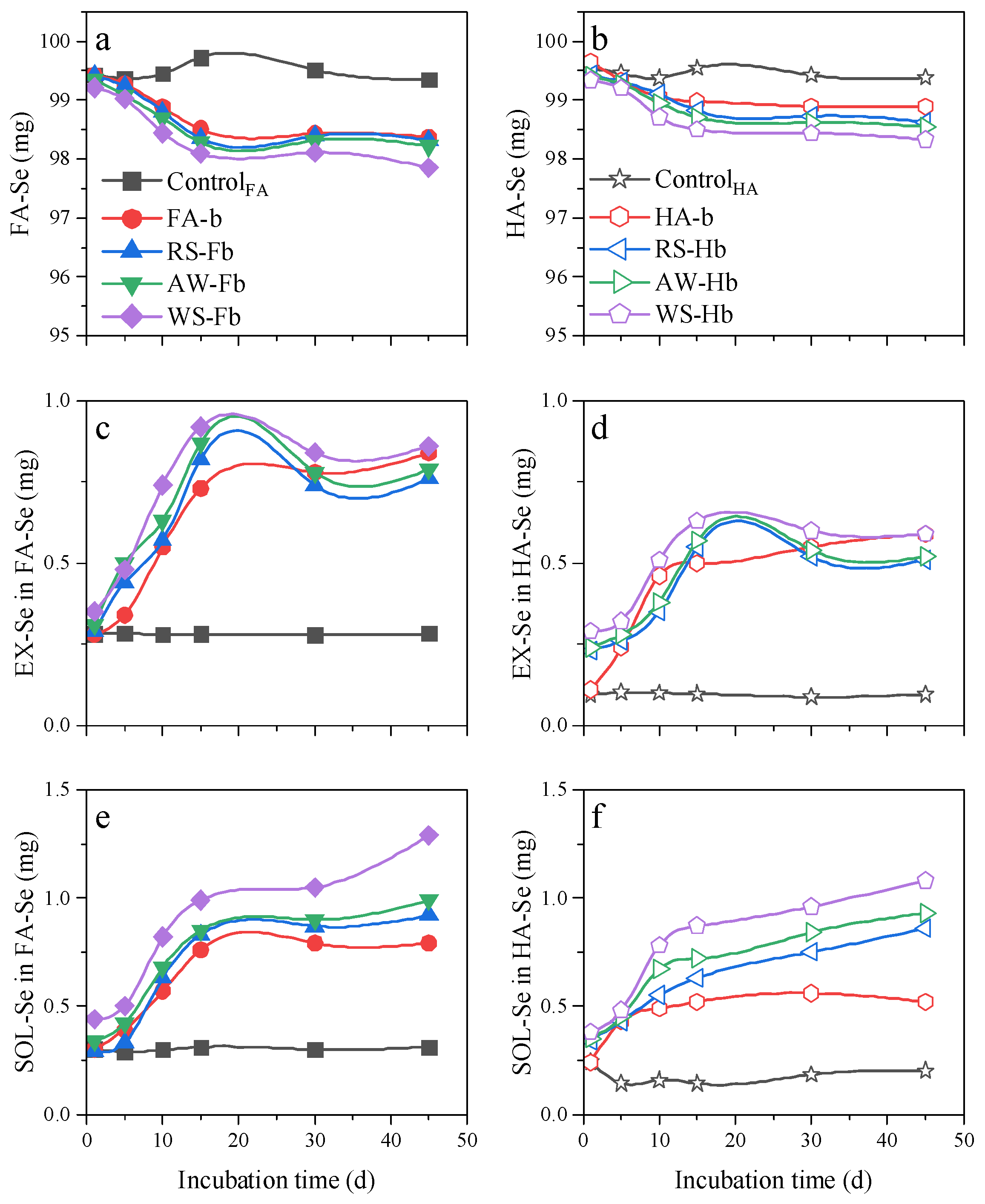
3.3. Effect of Biochar on the Transformation of Organic Bound Selenium
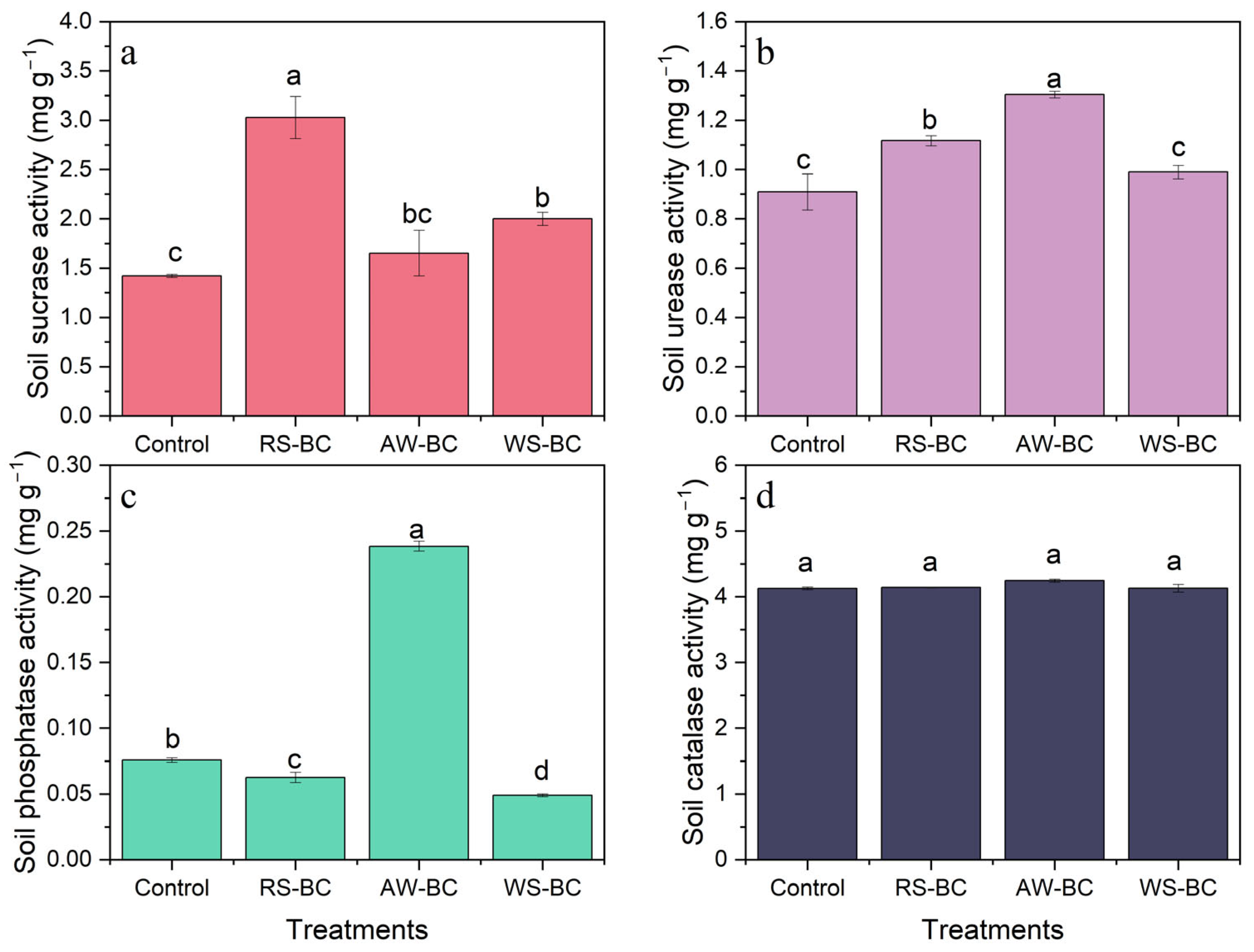
3.4. Effect of Biochar on Soil Enzyme Activity
3.5. Effects of Biochar on Bacterial Community Structure in Soil
3.6. Effects of Biochar on the Selenium Uptake of Garlic
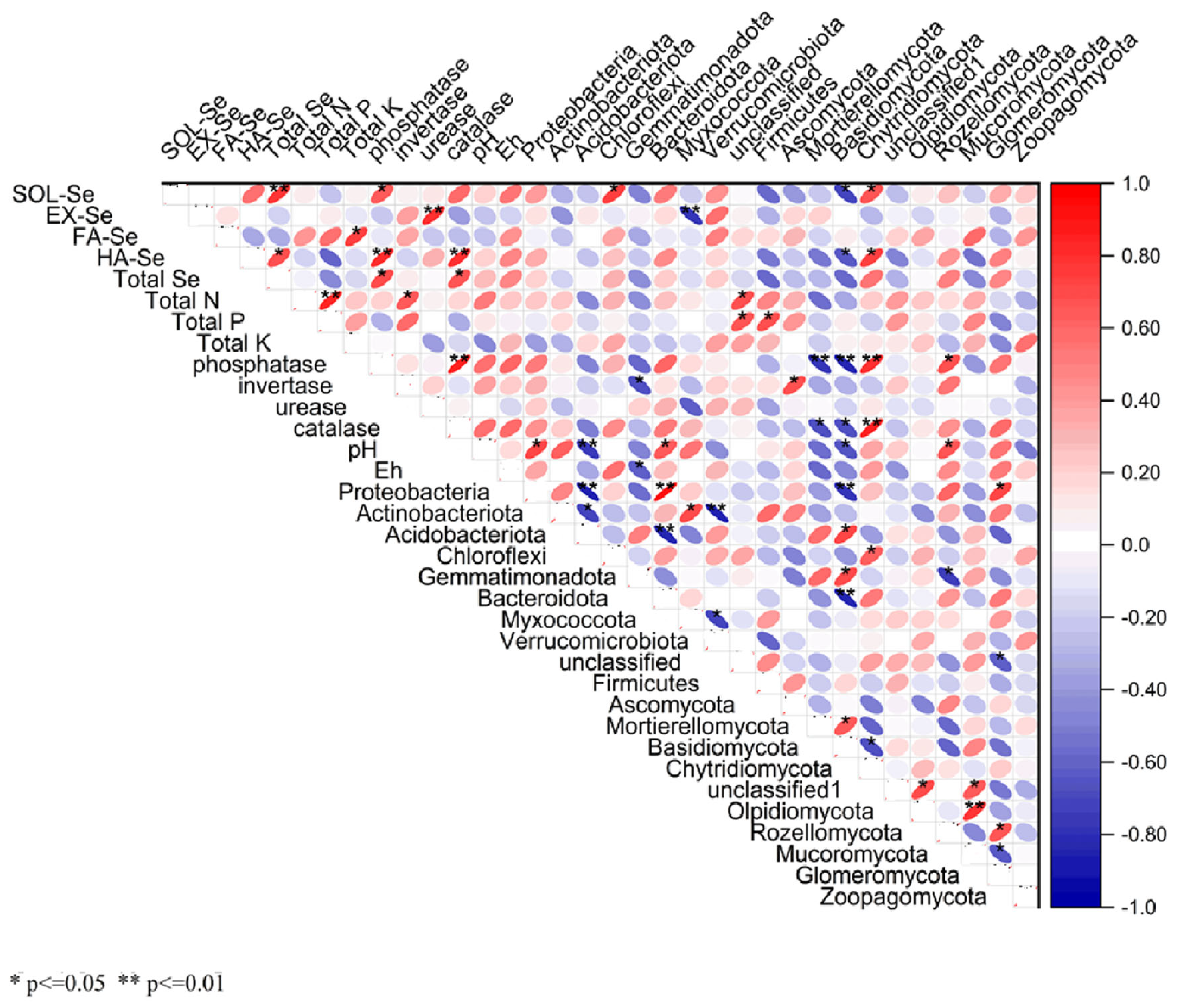
3.7. The Relationship Between Soil Biological, Physical, and Chemical Properties and Soil Selenium Forms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pachuau, L.; Dutta, R.S.; Roy, P.K.; Kalita, P.; Lalhlenmawia, H. Physicochemical and disintegrant properties of glutinous rice starch of Mizoram, India. Int. J. Biol. Macromol. 2017, 95, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Ai, Y.; Na, M.; Xu, S.; Li, X.; Zheng, X.; Zhou, J. Physiological and Biochemical Analysis of Selenium-Enriched Rice. Agronomy 2024, 14, 1715. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, G.; Du, L.; Wang, L.; Yan, H.; Yin, B.; Ou, Q. Soil-applied selenite increases selenium and reduces cadmium in roots of Moringa oleifera. Sci. Rep. 2020, 10, 20411. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. J. Trace Elem. Med. Biol. 2021, 67, 126801. [Google Scholar] [CrossRef]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and Determination of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Ajmone-Cat, M.A.; De Simone, R.; Tartaglione, A.M.; Di Biase, A.; Di Benedetto, R.; D’archivio, M.; Varì, R.; Ricceri, L.; Aureli, F.; Iacoponi, F.; et al. Critical role of maternal selenium nutrition in neurodevelopment: Effects on offspring behavior and neuroinflammatory profile. Nutrients 2022, 14, 1850. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.; Basu, A.; Wanner, C.; Sanford, R.A.; Pallud, C.; Johnson, T.M.; Mason, P.R. Mass-dependent selenium isotopic fractionation during microbial reduction of seleno-oxyanions by phylogenetically diverse bacteria. Geochim. Cosmochim. Acta 2020, 276, 274–288. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Huang, X.; Huang, C.; Wang, H.; Yin, H.; Shao, Y.; Li, P. Linking microbial community composition to farming pattern in selenium-enriched region: Potential role of microorganisms on Se geochemistry. J. Environ. Sci. 2022, 112, 269–279. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Liu, N.; Wang, M.; Zhou, F.; Zhai, H.; Qi, M.; Liu, Y.; Li, Y.; Zhang, N.; Ma, Y.; Huang, J.; et al. Selenium bioavailability in soil-wheat system and its dominant influential factors: A field study in Shaanxi province, China. Sci. Total Environ. 2021, 770, 144664. [Google Scholar] [CrossRef]
- Qin, H.-B.; Zhu, J.-M.; Su, H. Selenium fractions in organic matter from Se-rich soils and weathered stone coal in selenosis areas of China. Chemosphere 2012, 86, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Tang, J.; Chen, H.; Li, D.; Liu, Y. Impact of land use/land cover change on the topsoil selenium concentration and its potential bioavailability in a karst area of southwest China. Sci. Total Environ. 2020, 708, 135201. [Google Scholar] [CrossRef]
- Natasha; Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-B.; Zhu, J.-M.; Lin, Z.-Q.; Xu, W.-P.; Tan, D.-C.; Zheng, L.-R.; Takahashi, Y. Selenium speciation in seleniferous agricultural soils under different cropping systems using sequential extraction and X-ray absorption spectroscopy. Environ. Pollut. 2017, 225, 361–369. [Google Scholar] [CrossRef]
- Soothar, M.K.; Hamani, A.K.M.; Sootahar, M.K.; Sun, J.; Yang, G.; Bhatti, S.M.; Traore, A. Assessment of Acidic Biochar on the Growth, Physiology and Nutrients Uptake of Maize (Zea mays L.) Seedlings under Salinity Stress. Sustainability 2021, 13, 3150. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Alburquerque, J.; Sánchez-Monedero, M.; Roig, A.; Cayuela, M. Biochar accelerates organic matter degradation and enhances N mineralisation during composting of poultry manure without a relevant impact on gas emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef]
- Gong, H.; Zhai, H.; Wang, Y.; Pan, L.; Liu, Y.; Zhang, Y.; Shao, H.; Tang, G.; Ge, C.; Xu, W.; et al. Changes in selenium bioavailability in selenium-enriched paddy soils induced by different water management and organic amendments. Sci. Total Environ. 2024, 957, 177844. [Google Scholar] [CrossRef]
- Naisse, C.; Girardin, C.; Lefevre, R.; Pozzi, A.; Maas, R.; Stark, A.; Rumpel, C. Effect of physical weathering on the carbon sequestration potential of biochars and hydrochars in soil. GCB Bioenergy 2015, 7, 488–496. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, N.M.; Zhang, Y.J.; Deng, H.; Yang, H.Y. Spatial Distribution of Se Content and Its Influencing Factors in Cultivated Topsoil in Yunnan. Soils 2021, 53, 578–584. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Ohki, A.; Nakajima, T.; Hirakawa, S.; Hayashi, K.; Takanashi, H. A simple method of the recovery of selenium from food samples for the determination by ICP-MS. Microchem. J. 2016, 124, 693–698. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, F.; Wang, X.; Shi, X.; Wang, D. Understanding the effects of long-term different fertilizer applications on methylmercury accumulation in rice (Oryza sativa L.) plants. Sci. Total Environ. 2021, 777, 146125. [Google Scholar] [CrossRef]
- Probst, M.; Gómez-Brandón, M.; Herbón, C.; Barral, M.; Paradelo, R. Fungal-bacterial associations in urban allotment garden soils. Appl. Soil Ecol. 2023, 188, 104896. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Cole, M.A. Cycles of Soils: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Tolu, J.; Thiry, Y.; Bueno, M.; Jolivet, C.; Potin-Gautier, M.; Le Hécho, I. Distribution and speciation of ambient selenium in contrasted soils, from mineral to organic rich. Sci. Total Environ. 2014, 479–480, 93–101. [Google Scholar] [CrossRef]
- Xu, Y.; Hao, Z.; Li, Y.; Li, H.; Wang, L.; Zang, Z.; Liao, X.; Zhang, R. Distribution of selenium and zinc in soil-crop system and their relationship with environmental factors. Chemosphere 2020, 242, 125289. [Google Scholar] [CrossRef]
- Jing, F.; Li, H.; He, J.; Zhang, Q.; Gao, X.; Zhou, D. Application of biochar and selenium together at low dose efficiently reduces mercury and methylmercury accumulation in rice grains. Sci. Total Environ. 2024, 954, 176579. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bol, R. Sources and mechanisms of priming effect induced in two grassland soils amended with slurry and sugar. Soil Biol. Biochem. 2006, 38, 747–758. [Google Scholar] [CrossRef]
- Feng, J.; Yu, D.; Sinsabaugh, R.L.; Moorhead, D.L.; Andersen, M.N.; Smith, P.; Song, Y.; Li, X.; Huang, Q.; Liu, Y.; et al. Trade-offs in carbon-degrading enzyme activities limit long-term soil carbon sequestration with biochar addition. Biol. Rev. 2023, 98, 1184–1199. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Cheng, H.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizers activate soil enzyme activities and promote the recovery of soil beneficial microorganisms after dazomet fumigation. J. Environ. Manag. 2022, 309, 114666. [Google Scholar] [CrossRef]
- Li, K.-Y.; Song, L.-H.; Zhang, Y.; Liu, Y.; Ran, Q.-Y.; Yang, S.-W.; Chen, Z.-Y.; He, G.-D. Effects of Biochar Application Amount and Frequency on Yellow Soil Nutrients and Key Enzyme Activities. Environ. Sci. 2024, 46, 1065–1075. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Liu, Y.; Schäffer, A.; Martinez, M.; Lenz, M. Environmental selenium volatilization is possibly conferred by promiscuous reactions of the sulfur metabolism. Chemosphere 2023, 345, 140548. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- De Boer, W.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef]





| Treatments | Biochar | Soil Microbial Suspensions | Fulvic Acid Se | Huminc Acid Se |
|---|---|---|---|---|
| ControlFA † | – | – | 0.1 g | – |
| FA–b | – | 2 mL | 0.1 g | – |
| RS–Fb | 0.5 g RS | 2 mL | 0.1 g | – |
| AW–Fb | 0.5 g AW | 2 mL | 0.1 g | – |
| WS–Fb | 0.5 gWS | 2 mL | 0.1 g | – |
| ControlHA † | – | – | – | 0.1 g |
| HA–b | – | 2 mL | – | 0.1 g |
| RS–Hb | 0.5 g RS | 2 mL | – | 0.1 g |
| AW–Hb | 0.5 g AW | 2 mL | – | 0.1 g |
| WS–Hb | 0.5 gWS | 2 mL | – | 0.1 g |
| Treatments | ACE Index | Chao 1 Index | Shannon Index | Simpson Index | |
|---|---|---|---|---|---|
| Bacteria | Control | 5435 ± 43 a | 5010 ± 53 a | 6.69 ± 0.10 b | 0.0087 ± 0.0004 b |
| RS | 4550 ± 23 b | 4289 ± 84 b | 6.72 ± 0.14 a | 0.0086 ± 0.0003 b | |
| AW | 4502 ± 38 b | 4220 ± 67 b | 6.67 ± 0.17 b | 0.0091 ± 0.0003 a | |
| WS | 4310 ± 48 c | 4045 ± 64 c | 6.69 ± 0.12 b | 0.0082 ± 0.0003 b | |
| Fungi | Control | 1535 ± 36 a | 1522 ± 24 a | 4.98 ± 0.16 a | 0.0199 ± 0.0003 c |
| RS | 1537 ± 27 a | 1538 ± 32 a | 4.92 ± 0.14 a | 0.0225 ± 0.0003 b | |
| AW | 1452 ± 25 b | 1437 ± 37 c | 4.67 ± 0.17 b | 0.0326 ± 0.0002 a | |
| WS | 1535 ± 37 a | 1499 ± 31 b | 4.89 ± 0.18 a | 0.0210 ± 0.0002 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Feng, X.; Li, R.; Fan, F.; Miao, Z. Mechanisms of Biochar in Modulating Soil Organic Selenium Transformation and Enhancing Soil Selenium Availability. Agronomy 2025, 15, 701. https://doi.org/10.3390/agronomy15030701
Tang Z, Feng X, Li R, Fan F, Miao Z. Mechanisms of Biochar in Modulating Soil Organic Selenium Transformation and Enhancing Soil Selenium Availability. Agronomy. 2025; 15(3):701. https://doi.org/10.3390/agronomy15030701
Chicago/Turabian StyleTang, Zhenya, Xin Feng, Ruijiang Li, Fangling Fan, and Zhen Miao. 2025. "Mechanisms of Biochar in Modulating Soil Organic Selenium Transformation and Enhancing Soil Selenium Availability" Agronomy 15, no. 3: 701. https://doi.org/10.3390/agronomy15030701
APA StyleTang, Z., Feng, X., Li, R., Fan, F., & Miao, Z. (2025). Mechanisms of Biochar in Modulating Soil Organic Selenium Transformation and Enhancing Soil Selenium Availability. Agronomy, 15(3), 701. https://doi.org/10.3390/agronomy15030701






