Effects of High Temperature at Grain Filling Stage on Grain Quality and Gene Transcription in Heat-Sensitive Versus Heat-Tolerant Rice Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experiment Design
2.2. Determination of Milling Quality
2.3. Determination of Appearance Quality
2.4. Cooking Quality
2.5. Determination of Viscosity of Rice Starch
2.6. RNA Extraction and Sequencing
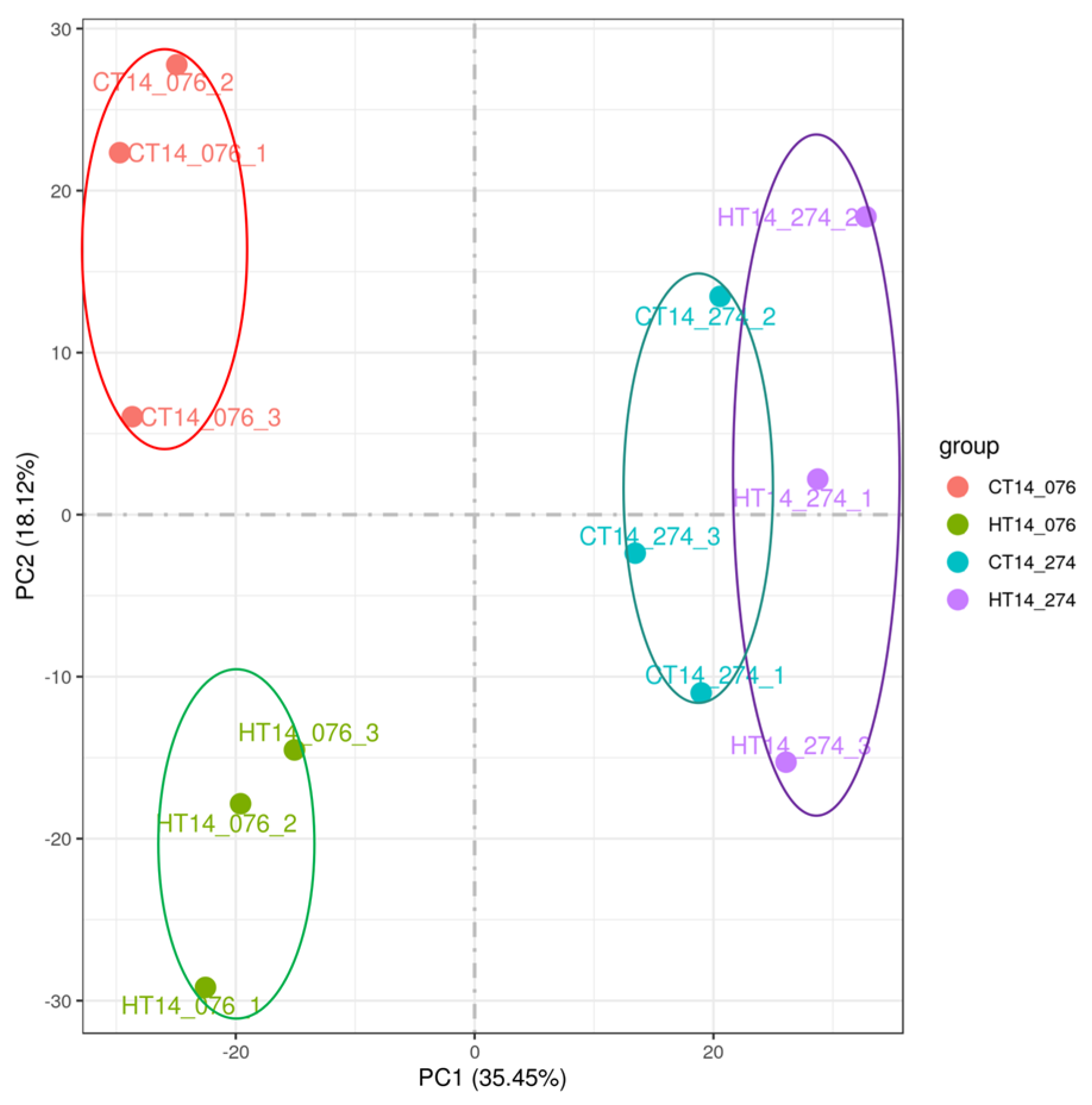
2.7. Principal Component Analysis
2.8. RNA-Seq Analysis
2.9. Real-Time Fluorescence Quantitative PCR (qRT-PCR) Validation
2.10. Data Processing and Statistical Analysis
3. Results and Discussion
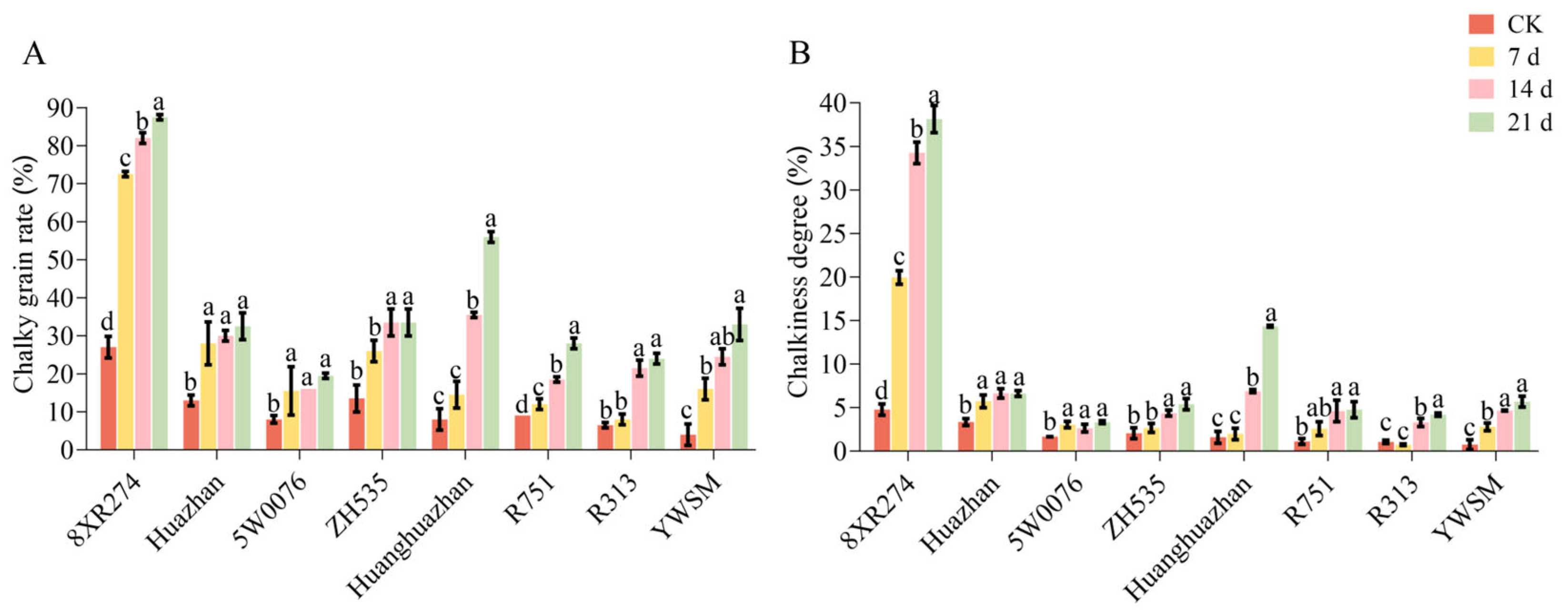
3.1. Effects of Different High-Temperature Durations on Rice Appearance Quality and Milling Quality
3.2. Effect of Different HT Treatment Time on Rice Cooking Quality
3.3. Effect of Different HT Time Treatments on Physicochemical Properties of Starch
3.4. Principal Component Analysis of Transcriptome of 8XR274 and 5W0076
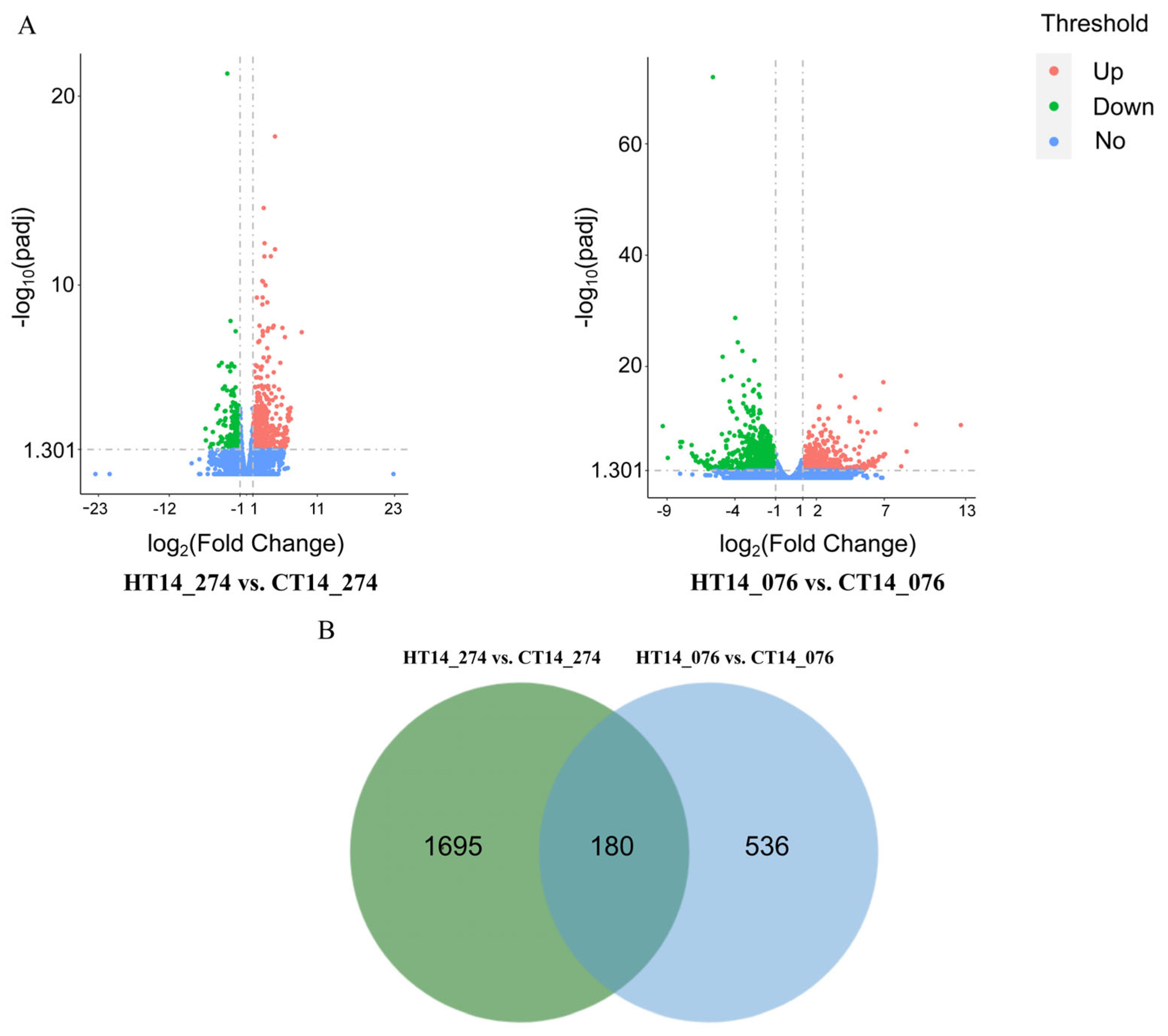
3.5. Identification of DEGs Between 8XR274 and 5W0076
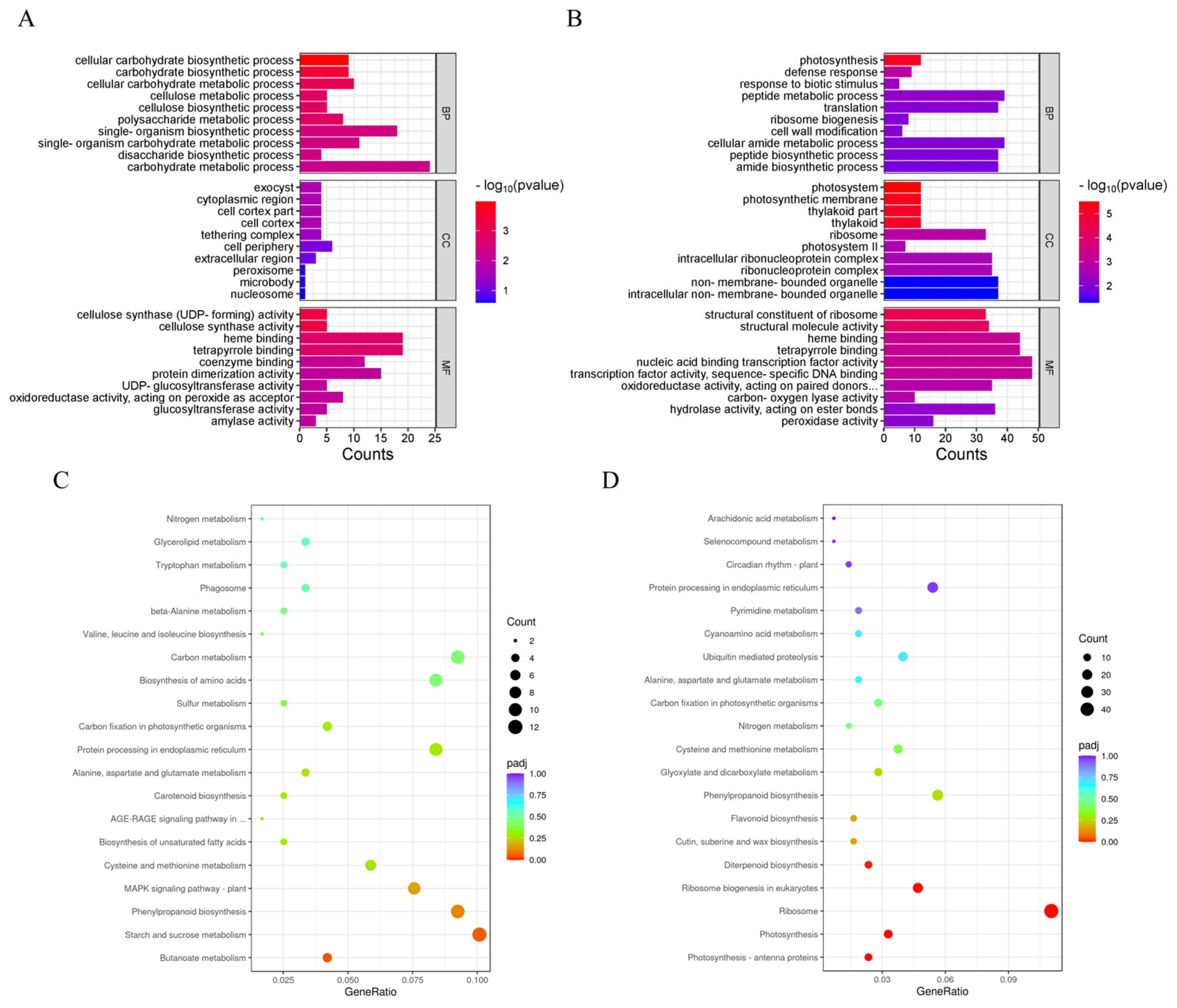
3.6. GO and KEGG Enrichment Analysis of DEGs Between 8XR274 and 5W0076
3.7. Involvement of Genes Related to “Carbohydrate” and “Photosynthesis” in Response of Rice Appearance Quality to HT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fukagawa, N.K.; Ziska, L.H. Rice: Importance for global nutrition. J. Nutr. Sci. Vitaminol. 2019, 65, S2–S3. [Google Scholar] [CrossRef]
- Verma, V.; Vishal, B.; Kohli, A.; Kumar, P.P. Systems-based rice improvement approaches for sustainable food and nutritional security. Plant Cell Rep. 2021, 40, 2021–2036. [Google Scholar] [CrossRef]
- Cheng, P.; Zhang, Y.; Liu, K.; Kong, X.; Wu, S.; Yan, H.; Jiang, P. Continuing the continuous harvests of food production: From the perspective of the interrelationships among cultivated land quantity, quality, and grain yield. Humanit. Soc. Sci. Commun. 2025, 12, 46. [Google Scholar] [CrossRef]
- Lv, Z.; Zhu, Y.; Liu, X.; Ye, H.; Tian, Y.; Li, F. Climate change impacts on regional rice production in China. Clim. Change 2018, 147, 523–537. [Google Scholar] [CrossRef]
- Hieronymus, M.; Kalén, O. Sea-level rise projections for Sweden based on the new IPCC special report: The ocean and cryosphere in a changing climate. Ambio 2020, 49, 1587–1600. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, L.; He, Z. Understanding the regulation of cereal grain filling: The way forward. J. Integr. Plant Biol. 2023, 65, 526–547. [Google Scholar] [CrossRef]
- Park, J.R.; Kim, E.G.; Jang, Y.H.; Kim, K.M. Screening and identification of genes affecting grain quality and spikelet fertility during high-temperature treatment in grain filling stage of rice. BMC Plant Biol. 2021, 21, 263. [Google Scholar] [CrossRef]
- Dou, Z.; Zhang, H.; Chen, W.; Li, G.; Liu, Z.; Ding, C.; Chen, L.; Wang, S.; Ding, Y.; Tang, S. Grain-filling of superior spikelets and inferior spikelets for japonica rice under low-amplitude warming regime in lower reaches of Yangtze River Basin. J. Agric. Sci. 2021, 159, 59–68. [Google Scholar] [CrossRef]
- Su, Q.; Rohila, J.S.; Ranganathan, S.; Karthikeyan, R. Rice yield and quality in response to daytime and nighttime temperature increase—A meta-analysis perspective. Sci. Total Environ. 2023, 1, 52–56. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, N.; Shukla, A.; Shankhdhar, S.C.; Shankhdhar, D. Impact of terminal heat stress on pollen viability and yield attributes of rice (Oryza sativa L.). Cereal Res. Commun. 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Lyman, N.B.; Jagadish, K.S.; Nalley, L.L.; Dixon, B.L.; Siebenmorgen, T. Neglecting rice milling yield and quality underestimates economic losses from high-temperature stress. PLoS ONE 2013, 8, e72157. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiong, R.; Tan, X.; Huang, S.; Pan, X.; Guo, L.; Zeng, Y.; Zhang, Z.; Zeng, Y. The impacts of post-anthesis warming on grain yield and quality of double-cropping high-quality indica rice in Jiangxi Province, China. Eur. J. Agron. 2022, 139, 126551. [Google Scholar] [CrossRef]
- Dou, Z.; Tang, S.; Chen, W.; Zhang, H.; Li, G.; Liu, Z.; Ding, C.; Chen, L.; Wang, S.; Zhang, H.; et al. Effects of open-field warming during grain-filling stage on grain quality of two japonica rice cultivars in lower reaches of Yangtze River delta. J. Cereal Sci. 2018, 81, 118–126. [Google Scholar] [CrossRef]
- Ambardekar, A.A.; Siebenmorgen, T.J.; Counce, P.A.; Lanning, S.B.; Mauromoustakos, A. Impact of field-scale nighttime air temperatures during kernel development on rice milling quality. Field Crops Res. 2011, 122, 179–185. [Google Scholar] [CrossRef]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef]
- Ward, R.; Spohr, L.; Snell, P. Rice grain quality: An Australian multi-environment study. Crop Pasture Sci. 2019, 70, 946–957. [Google Scholar] [CrossRef]
- Ahmed, F.; Abro, T.F.; Kabir, M.S.; Latif, M.A. Rice quality: Biochemical composition, eating quality, and cooking quality. In The Future of Rice Demand: Quality Beyond Productivity; Springer: Cham, Switzeland, 2020; pp. 3–24. [Google Scholar]
- Peng, B.; Liu, Y.; Qiu, J.; Peng, J.; Sun, X.; Tian, X.; Zhang, Z.; Huang, Y.; Pang, R.; Zhou, W.; et al. OsG6PGH1 affects various grain quality traits and participates in the salt stress response of rice. Front. Plant Sci. 2024, 15, 1436998. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Mazzini, F.; Pons, B.; Ntanos, D.A. Grain quality variation and relationships with morpho-physiological traits in rice (Oryza sativa L.) genetic resources in Europe. Field Crops Res. 2004, 86, 115–130. [Google Scholar] [CrossRef]
- Yao, D.; Wu, J.; Luo, Q.; Li, J.; Zhuang, W.; Xiao, G.; Deng, Q.; Lei, D.; Bai, B. Influence of high natural field temperature during grain filling stage on the morphological structure and physicochemical properties of rice (Oryza sativa L.) starch. Food Chem. 2020, 310, 125817. [Google Scholar] [CrossRef]
- Liu, W.; Yin, T.; Zhao, Y.; Wang, X.; Wang, K.; Shen, Y.; Ding, Y.; Tang, S. Effects of high temperature on rice grain development and quality formation based on proteomics comparative analysis under field warming. Front. Plant Sci. 2021, 12, 746180. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Wada, H.; Matsue, Y. Countermeasures for heat damage in rice grain quality under climate change. Plant Prod. Sci. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Singh, N.; Sodhi, N.S.; Kaur, M.; Saxena, S.K. Physico-chemical, morphological, thermal, cooking and textural properties of chalky and translucent rice kernels. Food Chem. 2003, 82, 433–439. [Google Scholar] [CrossRef]
- Cheng, F.M.; Zhong, L.J.; Wang, F.; Zhang, G.P. Differences in cooking and eating properties between chalky and translucent parts in rice grains. Food Chem. 2005, 90, 39–46. [Google Scholar] [CrossRef]
- Yang, W.; Liang, J.; Hao, Q.; Luan, X.; Tan, Q.; Lin, S.; Zhu, H.; Liu, G.; Liu, Z.; Bu, S.; et al. Fine mapping of two grain chalkiness QTLs sensitive to high temperature in rice. Rice 2021, 14, 33. [Google Scholar] [CrossRef]
- Morita, S. Prospect for developing measures to prevent high-temperature damage to rice grain ripening. Jpn. J. Crop Sci. 2008, 77, 1–12. [Google Scholar] [CrossRef]
- Fan, X.; Li, Y.; Zhang, C.; Li, E.; Chen, Z.; Li, Q.; Zhang, Z.; Zhu, Y.; Sun, X.; Liu, Q. Effects of high temperature on starch fine structure during grain-filling stages in rice: Mathematical modeling and integrated enzymatic analysis. J. Sci. Food Agric. 2019, 99, 2865–2873. [Google Scholar] [CrossRef]
- Zhang, G.; Liao, B.; Li, B.; Cai, Z. The effect of high temperature after anthesis on rice quality and starch granule structure of endosperm. Meteorol. Environ. Res. 2016, 7, 72–75. [Google Scholar]
- Zhu, L.; Shah, F.; Nie, L.; Cui, K.; Shah, T.; Wu, W.; Chen, Y.; Chen, C.; Wang, K.; Wang, Q.; et al. Efficacy of sowing date adjustment as a management strategy to cope with rice (Oryza sativa L.) seed quality deterioration due to elevated temperature. Aust. J. Crop Sci. 2013, 7, 543–549. [Google Scholar]
- Umemoto, T.; Terashima, K.; Nakamura, Y.; Satoh, H. Differences in amylopectin structure between two rice varieties in relation to the effects of temperature during grain-filling. Starch-Stärke 1999, 51, 58–62. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, D.; He, Y. Rice grain quality-traditional traits for high quality rice and health-plus substances. Mol. Breed. 2020, 40, 1. [Google Scholar] [CrossRef]
- Bao, J.; Deng, B.; Zhang, L. Molecular and genetic bases of rice cooking and eating quality: An updated review. Cereal Chem. 2023, 100, 1220–1233. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, J.; Zhang, F.; Niu, Y.; Zhao, X.; Shen, C.; Chen, K.; Teng, S.; Xu, J. Genetic dissection of rice appearance quality and cooked rice elongation by genome-wide association study. Crop J. 2021, 9, 1470–1480. [Google Scholar] [CrossRef]
- Zhong, L.J.; Cheng, F.M.; Wen, X.; Sun, Z.X.; Zhang, G.P. The deterioration of eating and cooking quality caused by high temperature during grain filling in early-season indica rice cultivars. J. Agron. Crop Sci. 2005, 191, 218–225. [Google Scholar] [CrossRef]
- Chun, A.; Lee, H.J.; Hamaker, B.R.; Janaswamy, S. Effects of ripening temperature on starch structure and gelatinization, pasting, and cooking properties in rice (Oryza sativa). J. Agric. Food Chem. 2015, 63, 3085–3093. [Google Scholar] [CrossRef]
- Xuan, Y.; Yi, Y.; Liang, H.E.; Wei, S.; Chen, N.; Jiang, L.; Ali, I.; Ullah, S.; Wu, X.; Cao, T.; et al. Amylose content and RVA profile characteristics of noodle rice under different conditions. Agron. J. 2020, 112, 117–129. [Google Scholar] [CrossRef]
- Tao, K.; Yu, W.; Prakash, S.; Gilbert, R.G. Investigating cooked rice textural properties by instrumental measurements. Food Sci. Hum. Wellness 2020, 9, 130–135. [Google Scholar] [CrossRef]
- Pang, Y.; Ali, J.; Wang, X.; Franje, N.J.; Revilleza, J.E.; Xu, J.; Li, Z. Relationship of rice grain amylose, gelatinization temperature and pasting properties for breeding better eating and cooking quality of rice varieties. PLoS ONE 2016, 11, e0168483. [Google Scholar] [CrossRef]
- Tong, C.; Chen, Y.; Tang, F.; Xu, F.; Huang, Y.; Chen, H.; Bao, J. Genetic diversity of amylose content and RVA pasting parameters in 20 rice accessions grown in Hainan, China. Food Chem. 2014, 161, 239–245. [Google Scholar] [CrossRef]
- Han, X.Z.; Hamaker, B.R. Amylopectin fine structure and rice starch paste breakdown. J. Cereal Sci. 2001, 34, 279–284. [Google Scholar] [CrossRef]
- Yan, C.J.; Li, X.; Zhang, R.; Sui, J.M.; Liang, G.H.; Shen, X.P.; Gu, S.L.; Gu, M.H. Performance and inheritance of rice starch RVA profile characteristics. Rice Sci. 2005, 12, 39–47. [Google Scholar]
- Bao, J.; Kong, X.; Xie, J.; Xu, L. Analysis of genotypic and environmental effects on rice starch. 1. Apparent amylose content, pasting viscosity, and gel texture. J. Agric. Food Chem. 2004, 52, 6010–6016. [Google Scholar] [CrossRef] [PubMed]
- GB/T 17891-2017; The National Standard for Rice Quality Evaluation, the People’s Republic of China. NBQTC (National Bureau of Quality and Technology Control): Beijing, China, 2017.
- ISO 6647-2; Rice-Determination of Amylose Content-Part 2: Spectrophotometric Routine Method without Defatting Procedure and with Calibration from Rice Standards. International Organization for Standardization: Geneva, Switzerland, 2020.
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef]
- Cao, L.; Ding, G.; Lei, L.; Zhou, J.; Bai, L.; Luo, Y.; Xia, T.; Liu, K.; Wang, X.; Yang, G.; et al. QTL analysis related to rice appearance quality and rice food quality trait using high-density SNP genetic map. Plant Growth Regul. 2024, 102, 461–470. [Google Scholar] [CrossRef]
- Ishimaru, T.; Horigane, A.K.; Ida, M.; Iwasawa, N.; San-oh, Y.A.; Nakazono, M.; Nishizawa, N.K.; Masumura, T.; Kondo, M.; Yoshida, M. Formation of grain chalkiness and changes in water distribution in developing rice caryopses grown under high-temperature stress. J. Cereal Sci. 2009, 50, 166–174. [Google Scholar] [CrossRef]
- Fitzgerald, M.A.; McCouch, S.R.; Hall, R.D. Not just a grain of rice: The quest for quality. Trends Plant Sci. 2009, 14, 133–139. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, G.; Wang, Z.; Yang, J.; Zhang, J. Ethylene and ACC levels in developing grains are related to the poor appearance and milling quality of rice. Plant Growth Regul. 2009, 58, 85–96. [Google Scholar] [CrossRef]
- Chun, A.; Song, J.; Kim, K.J.; Lee, H.J. Quality of head and chalky rice and deterioration of eating quality by chalky rice. J. Crop Sci. Biotech. 2009, 12, 239–244. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nonoue, Y.; Ebitani, T.; Suzuki, K.; Aoki, N.; Sato, H.; Ideta, O.; Hirabayashi, H.; Hirayama, M.; Ohta, H.; et al. QTL detection for eating quality including glossiness, stickiness, taste and hardness of cooked rice. Breed. Sci. 2007, 57, 231–242. [Google Scholar] [CrossRef]
- Groth, D.; Hartmann, S.; Klie, S.; Selbig, J. Principal components analysis. Methods Mol. Biol. 2013, 930, 527–547. [Google Scholar]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, C.; Li, Q.; Liu, Q. Genetic control of grain appearance quality in rice. Biotechnol. Adv. 2022, 60, 108014. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.; Prasad, P.V.; Siddique, K.H. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, L.; Shi, P.; Yang, B.; Sun, T.; Cao, W.; Zhu, Y. Effects of short-term high temperature on grain quality and starch granules of rice (Oryza sativa L.) at post-anthesis stage. Protoplasma 2017, 254, 935–943. [Google Scholar] [CrossRef]
- Glaubitz, U.; Erban, A.; Kopka, J.; Hincha, D.K.; Zuther, E. High night temperature strongly impacts TCA cycle, amino acid and polyamine biosynthetic pathways in rice in a sensitivity-dependent manner. J. Exp. Bot. 2015, 66, 6385–6397. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Haynes, P.A.; Atwell, B.J. Physiological and molecular changes in Oryza meridionalis Ng.; a heat-tolerant species of wild rice. J. Exp. Bot. 2010, 61, 191–202. [Google Scholar] [CrossRef]
- Kumar, N.; Suyal, D.C.; Sharma, I.P.; Verma, A.; Singh, H. Elucidating stress proteins in rice (Oryza sativa L.) genotype under elevated temperature: A proteomic approach to understand heat stress response. 3 Biotech 2017, 7, 205. [Google Scholar] [CrossRef]
- Liao, J.L.; Zhou, H.W.; Peng, Q.; Zhong, P.A.; Zhang, H.Y.; He, C.; Huang, Y.J. Transcriptome changes in rice (Oryza sativa L.) in response to high night temperature stress at the early milky stage. BMC Genom. 2015, 16, 18. [Google Scholar] [CrossRef]
- Miyazaki, M.; Araki, M.; Okamura, K.; Ishibashi, Y.; Yuasa, T.; Iwaya-Inoue, M. Assimilate translocation and expression of sucrose transporter, OsSUT1, contribute to high-performance ripening under heat stress in the heat-tolerant rice cultivar Genkitsukushi. J. Plant Physiol. 2013, 170, 1579–1584. [Google Scholar] [CrossRef]
- Nakata, M.; Fukamatsu, Y.; Miyashita, T.; Hakata, M.; Kimura, R.; Nakata, Y.; Kuroda, M.; Yamaguchi, T.; Yamakawa, H. High temperature-induced expression of rice α-amylases in developing endosperm produces chalky grains. Front. Plant Sci. 2017, 8, 2089. [Google Scholar] [CrossRef]
- Wang, X.; Xue, L.; Sun, J.; Zuo, J. The Arabidopsis BE1 gene, encoding a putative glycoside hydrolase localized in plastids, plays crucial roles during embryogenesis and carbohydrate metabolism. J. Integr. Plant Biol. 2010, 52, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, C.; Cai, X.; Wang, Q.; Dai, S. Heat-responsive photosynthetic and signaling pathways in plants: Insight from proteomics. Int. J. Mol. Sci. 2017, 18, 2191. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photoch. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2014, 199, 361–376. [Google Scholar] [CrossRef]
- Sailaja, B.; Subrahmanyam, D.; Neelamraju, S.; Vishnukiran, T.; Rao, Y.V.; Vijayalakshmi, P.; Voleti, S.R.; Bhadana, V.P.; Mangrauthia, S.K. Integrated physiological, biochemical, and molecular analysis identifies important traits and mechanisms associated with differential response of rice genotypes to elevated temperature. Front. Plant Sci. 2015, 6, 1044. [Google Scholar] [CrossRef]
- Hüve, K.; Bichele, I.; Rasulov, B.; Niinemets, Ü. When it is too hot for photosynthesis: Heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 2011, 34, 113–126. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef]
- Deng, F.; Li, Q.; Chen, H.; Zeng, Y.; Li, B.; Zhong, X.; Wang, L.; Ren, W. Relationship between chalkiness and the structural and thermal properties of rice starch after shading during grain-filling stage. Carbohydr. Polym. 2021, 252, 117212. [Google Scholar] [CrossRef]
- Yang, L.; Sun, M.; Zhang, P.; Bai, Y.; Xu, C.; Tang, Q.; She, D. Effect of temperature and light on grain quality of medium Japonica hybrid rice 80You121. Hybrid Rice 1998, 13, 23–28. (In Chinese) [Google Scholar]
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.M.; Jansson, S. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 2014, 26, 3646–3660. [Google Scholar] [CrossRef] [PubMed]
- Ballottari, M.; Dall’Osto, L.; Morosinotto, T.; Bassi, R. Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J. Biol. Chem. 2007, 282, 8947–8958. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Ito, H.; Tanaka, A. Chlorophyll b degradation by chlorophyll b reductase under high-light conditions. Photosyn. Res. 2015, 126, 249–259. [Google Scholar] [CrossRef] [PubMed]


| Time | Temperature (°C) | Humidity Level (%) |
|---|---|---|
| 24:00–3:00 | 29 | 65 |
| 3:00–6:00 | 30 | 65 |
| 6:00–9:00 | 32 | 65 |
| 9:00–12:00 | 36 | 65 |
| 12:00–15:00 | 38 | 65 |
| 15:00–18:00 | 36 | 65 |
| 18:00–21:00 | 31 | 65 |
| 21:00–24:00 | 30 | 65 |
| Time | Temp (°C) | Humidity Level (%) |
|---|---|---|
| 9:00–17:00 | 30 | 65 |
| 17:00–9:00 | 25 | 65 |
| Variety | High-Temperature Treatment | Peak Viscosity (cP) | Hot Paste Viscosity (cP) | Cool Paste Viscosity (cP) | Peak Time (min) | Peak Temperature (°C) | Breakdown Value (cP) | Setback Value (cP) | Recovery Value (cP) |
|---|---|---|---|---|---|---|---|---|---|
| 8XR274 | CK | 1243.0 c | 1109.0 c | 2094.5 b | 6.01 b | 88.38 a | 134.0 c | 851.5 a | 985.5 b |
| 7 d | 1885.0 b | 1533.5 b | 2686.5 ab | 5.93 a | 82.30 a | 351.5 ab | 801.5 a | 1153.0 ab | |
| 14 d | 2397.5 a | 1999.0 a | 3198.5 a | 6.10 ab | 81.55 a | 398.5 a | 801.0 a | 1199.5 a | |
| 21 d | 1924.0 b | 1735.0 b | 2822.5 a | 6.40 b | 85.63 a | 189.0 bc | 898.5 a | 1087.5 ab | |
| Huazhan | CK | 2773.0 b | 1786.5 ab | 2431.0 c | 5.54 a | 84.78 a | 986.5 b | −342.0 a | 644.5 c |
| 7 d | 3218.5 a | 1921.0 a | 3001.0 a | 5.50 a | 85.63 a | 1297.5 ab | −217.5 a | 1080.0 a | |
| 14 d | 2895.5 b | 1665.0 b | 2566.0 bc | 5.57 a | 86.85 a | 1230.5 ab | −329.5 a | 901.0 b | |
| 21 d | 3458.5 a | 1977.0 a | 2814.5 ab | 5.65 a | 74.40 a | 1481.5 a | −644.0 b | 837.5 b | |
| 5W0076 | CK | 2575.5 b | 2405.5 ab | 3549.5 b | 6.54 a | 87.50 a | 170.0 b | 974.0 a | 1144.0 c |
| 7 d | 2789.5 b | 2432.0 a | 3565.0 b | 6.60 a | 87.08 a | 357.5 b | 775.5 b | 1133.0 c | |
| 14 d | 3184.5 a | 2288.0 bc | 3849.5 a | 6.57 a | 85.63 a | 896.5 a | 665.0 b | 1561.5 a | |
| 21 d | 3326.0 a | 2257.5 c | 3567.0 b | 6.60 a | 84.80 a | 1068.5 a | 241.0 c | 1309.5 b | |
| ZH535 | CK | 2475.0 a | 1501.5 a | 2659.5 b | 6.30 a | 85.88 a | 973.5 b | 184.5 c | 1158.0 c |
| 7 d | 1745.5 c | 1059.5 b | 1866.5 c | 5.67 a | 84.00 a | 686.0 d | 121.0 c | 807.0 d | |
| 14 d | 2136.5 b | 1340.0 a | 2761.5 b | 6.17 a | 84.35 a | 796.5 c | 625.0 a | 1421.5 b | |
| 21 d | 2657.5 a | 1453.5 a | 3113.5 a | 5.83 a | 83.18 a | 1204.0 a | 456.0 b | 1660.0 a | |
| Huanghuazhan | CK | 2290.0 c | 1915.0 b | 2910.0 b | 5.84 a | 84.78 a | 375.0 b | 620.0 a | 995.0 b |
| 7 d | 3117.5 a | 2641.0 a | 3424.0 a | 5.94 a | 78.38 b | 476.5 b | 306.5 b | 783.0 b | |
| 14 d | 2755.5 b | 1877.0 b | 3335.5 a | 5.84 a | 80.40 ab | 878.5 a | 580.0 a | 1458.5 a | |
| 21 d | 2852.0 ab | 1611.0 b | 3224.0 ab | 5.87 a | 82.35 ab | 1241.0 a | 372.0 b | 1613.0 a | |
| R751 | CK | 2721.0 b | 1767.0 b | 3007.0 b | 5.77 a | 87.18 a | 954.0 b | 195.5 b | 1240.0 b |
| 7 d | 2298.0 c | 1524.0 c | 2847.0 b | 5.84 a | 85.98 a | 774.0 c | 549.0 a | 1323.0 ab | |
| 14 d | 2463.5 bc | 1692.0 b | 2968.5 b | 5.93 a | 85.98 a | 771.5 c | 523.0 a | 1276.5 ab | |
| 21 d | 3298.5 a | 2076.5 a | 3494.0 a | 5.87 a | 81.53 b | 1222.0 a | 487.0 a | 1417.5 a | |
| R313 | CK | 2716.0 b | 2061.0 b | 3367.5 b | 5.87 a | 86.83 a | 655.0 b | 651.5 a | 1306.5 ab |
| 7 d | 3048.0 a | 2524.5 a | 3630.0 a | 6.50 a | 87.05 a | 523.5 bc | 582.0 ab | 1105.5 b | |
| 14 d | 2804.0 b | 2369.5 a | 3220.5 c | 6.57 a | 85.50 a | 434.5 c | 416.5 c | 851.0 c | |
| 21 d | 3041.0 a | 2087.5 b | 3529.0 a | 6.47 a | 80.80 b | 953.5 a | 488.0 bc | 1441.5 a | |
| YWSM | CK | 3039.5 c | 2197.0 a | 3386.5 b | 6.04 a | 87.95 a | 842.5 c | 347.0 a | 1189.5 bc |
| 7 d | 3635.0 a | 2237.0 a | 3899.0 a | 6.00 a | 85.58 a | 1398.0 a | 264.0 ab | 1662.0 a | |
| 14 d | 3350.5 b | 2283.0 a | 3549.0 b | 6.17 a | 79.55 b | 1067.5 b | 198.5 bc | 1266.0 b | |
| 21 d | 2919.0 c | 2042.5 b | 3073.0 c | 6.44 a | 79.18 b | 876.5 c | 154.0 c | 1030.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Luo, H.; Yi, J.; Zhu, Y.; Ma, X.; Jiang, Y.; Peng, Y.; Xiao, Y.; Zhang, G.; Liu, X.; et al. Effects of High Temperature at Grain Filling Stage on Grain Quality and Gene Transcription in Heat-Sensitive Versus Heat-Tolerant Rice Cultivars. Agronomy 2025, 15, 668. https://doi.org/10.3390/agronomy15030668
Guo Y, Luo H, Yi J, Zhu Y, Ma X, Jiang Y, Peng Y, Xiao Y, Zhang G, Liu X, et al. Effects of High Temperature at Grain Filling Stage on Grain Quality and Gene Transcription in Heat-Sensitive Versus Heat-Tolerant Rice Cultivars. Agronomy. 2025; 15(3):668. https://doi.org/10.3390/agronomy15030668
Chicago/Turabian StyleGuo, Yujian, Hui Luo, Jiajie Yi, Yuqi Zhu, Xiaojie Ma, Yubing Jiang, Yanjiao Peng, Yunhua Xiao, Guilian Zhang, Xiong Liu, and et al. 2025. "Effects of High Temperature at Grain Filling Stage on Grain Quality and Gene Transcription in Heat-Sensitive Versus Heat-Tolerant Rice Cultivars" Agronomy 15, no. 3: 668. https://doi.org/10.3390/agronomy15030668
APA StyleGuo, Y., Luo, H., Yi, J., Zhu, Y., Ma, X., Jiang, Y., Peng, Y., Xiao, Y., Zhang, G., Liu, X., & Deng, H. (2025). Effects of High Temperature at Grain Filling Stage on Grain Quality and Gene Transcription in Heat-Sensitive Versus Heat-Tolerant Rice Cultivars. Agronomy, 15(3), 668. https://doi.org/10.3390/agronomy15030668






