Influence of Walnut Shell Biochar and Fertilizer on Lettuce Production in Hydroponic and Conventional Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar: Obtention and Characterization
2.2. Design Experiments
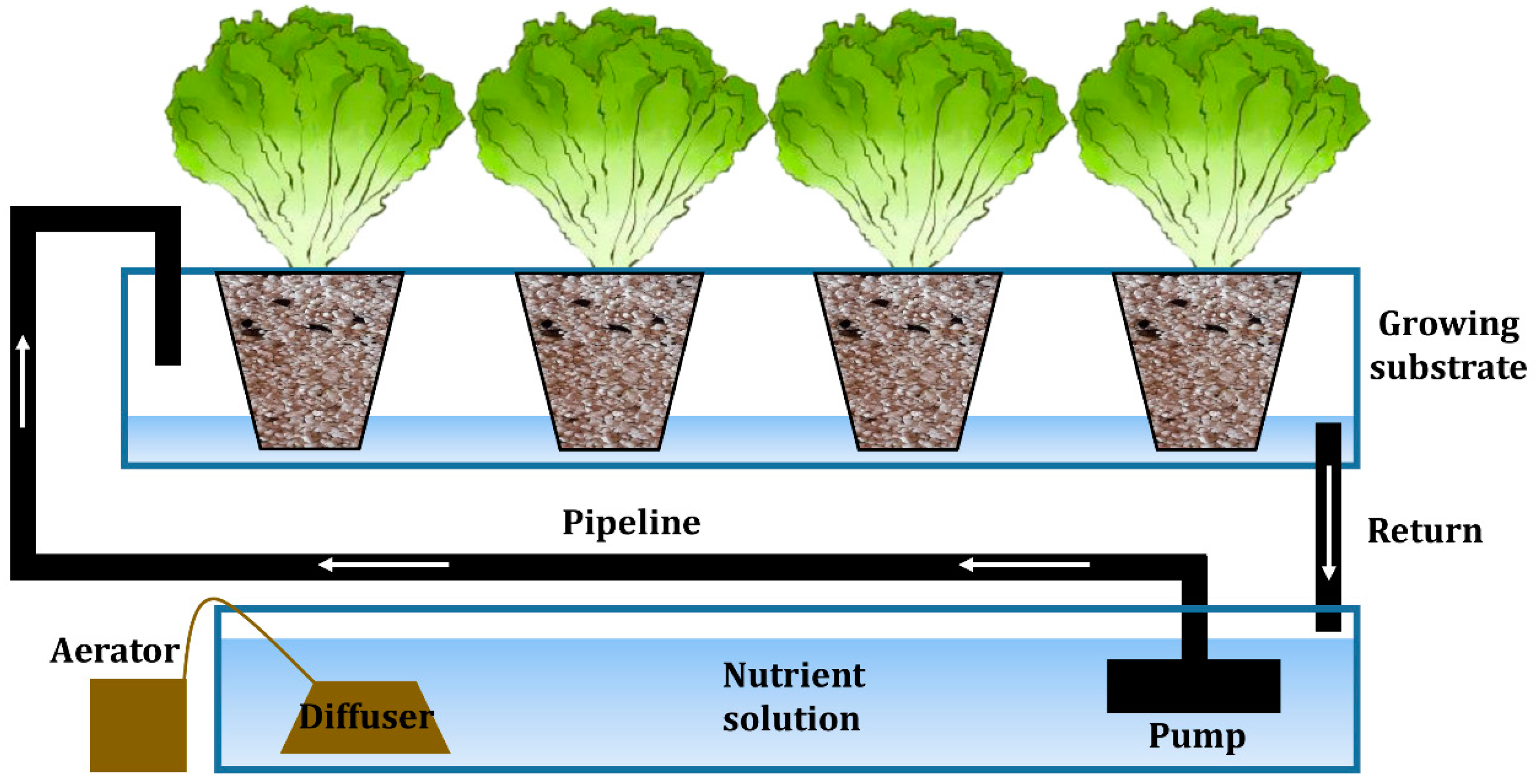
2.2.1. Description of Hydroponic System
2.2.2. Conventional System. Sampling of Soils. Description of Mixtures and Fertilizer
2.2.3. Measurement of Growth Variables
2.3. Statistical Analysis
3. Results and Discussion
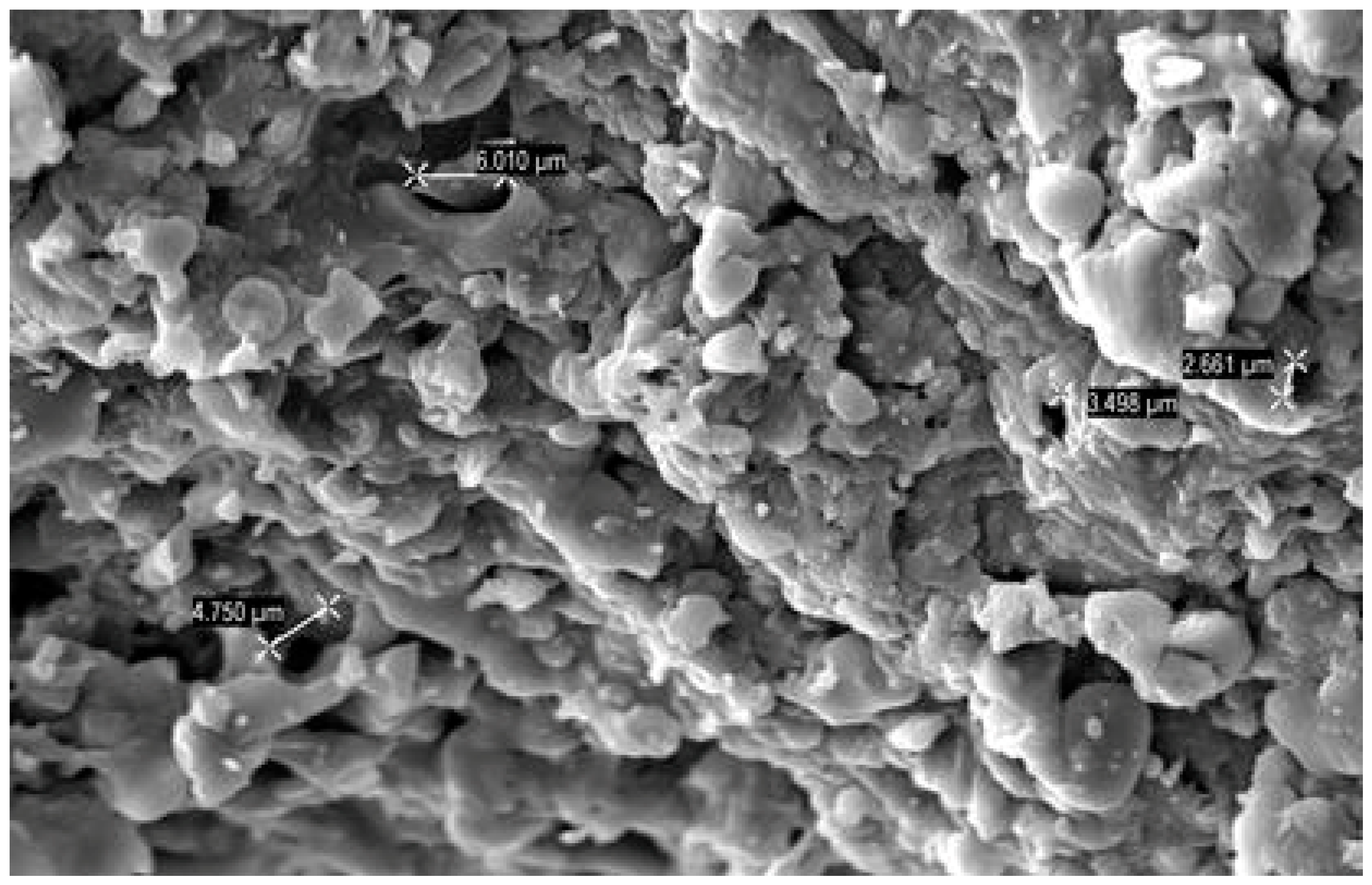
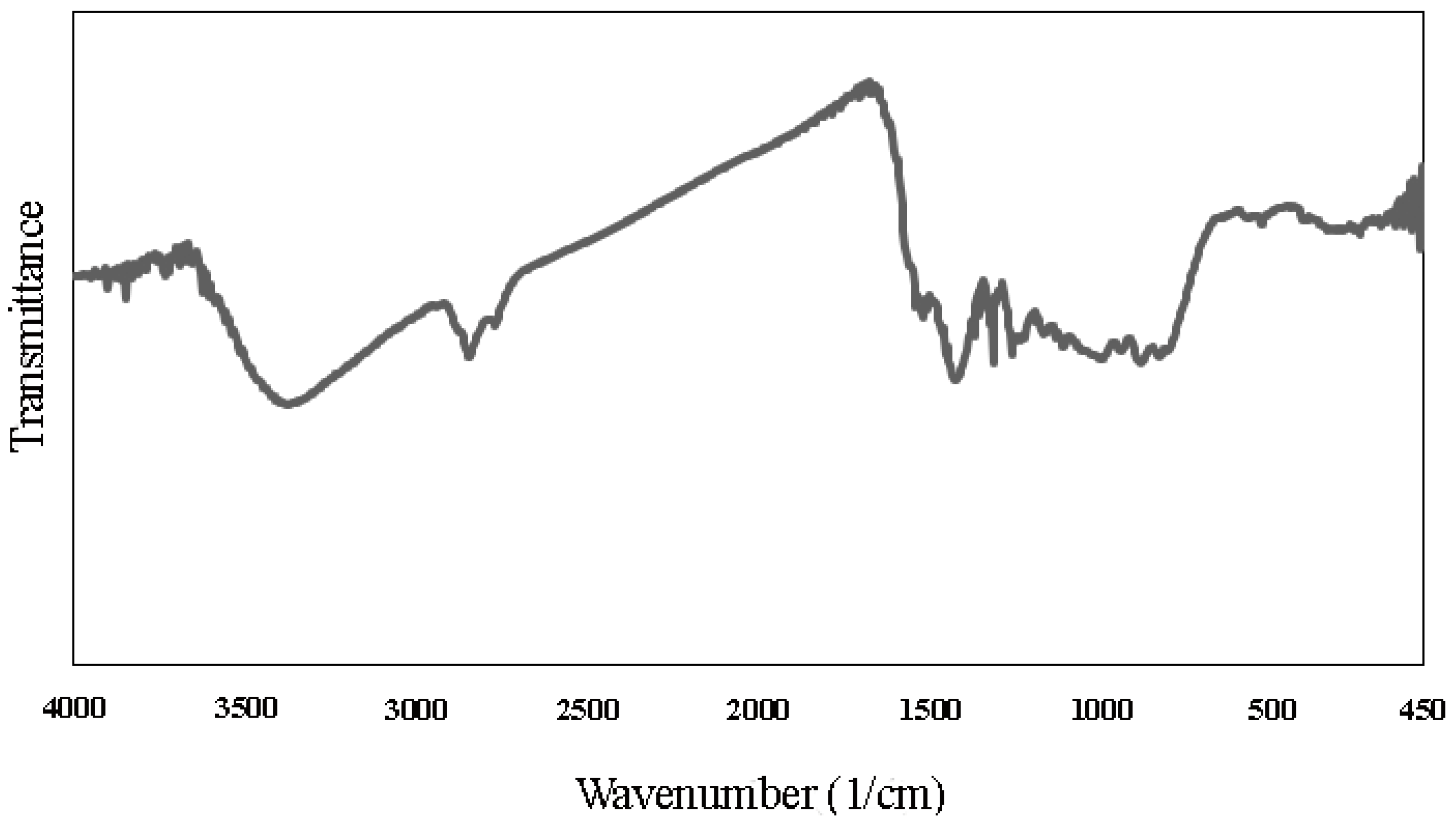
3.1. Characterization of WSB
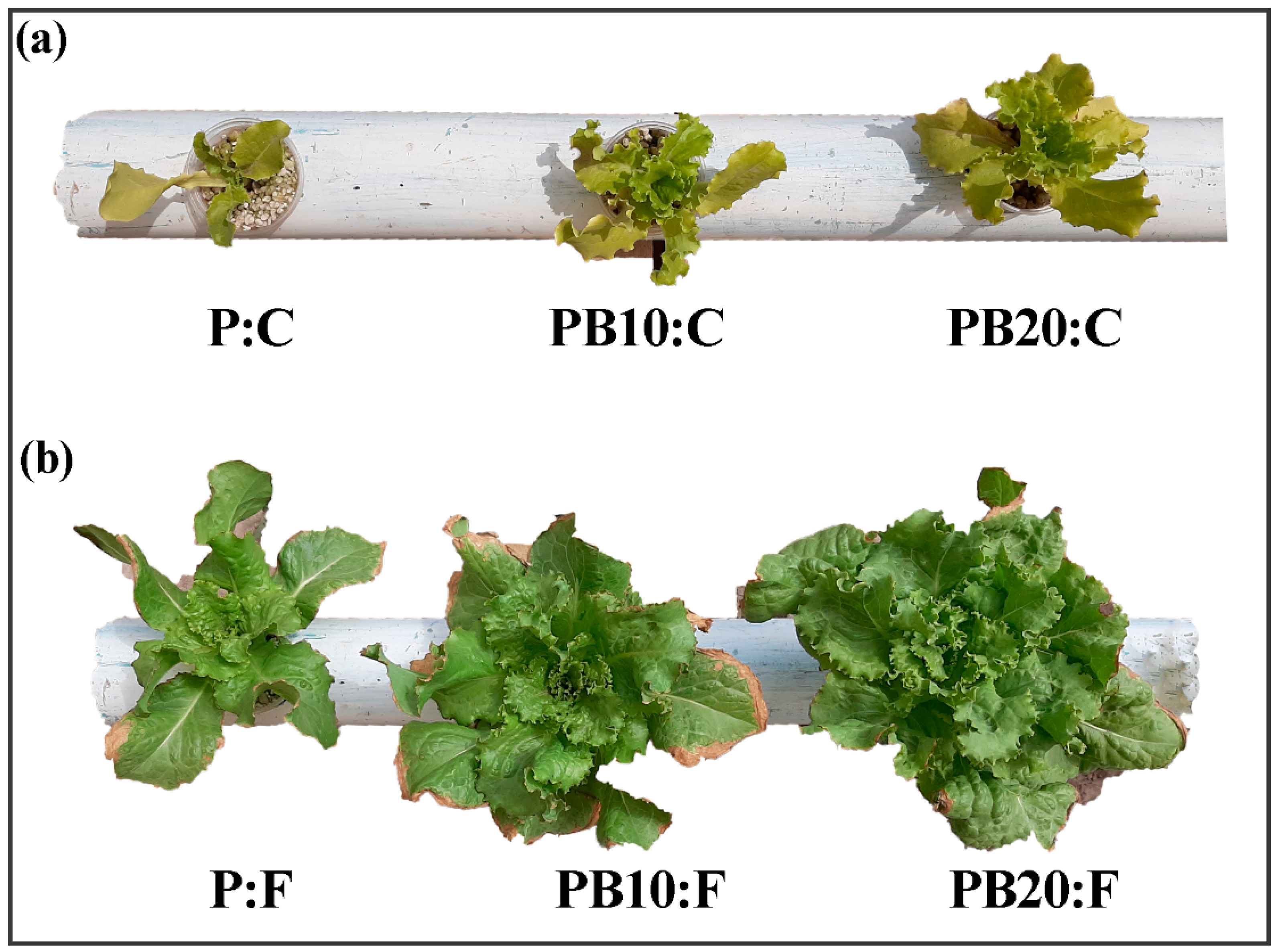
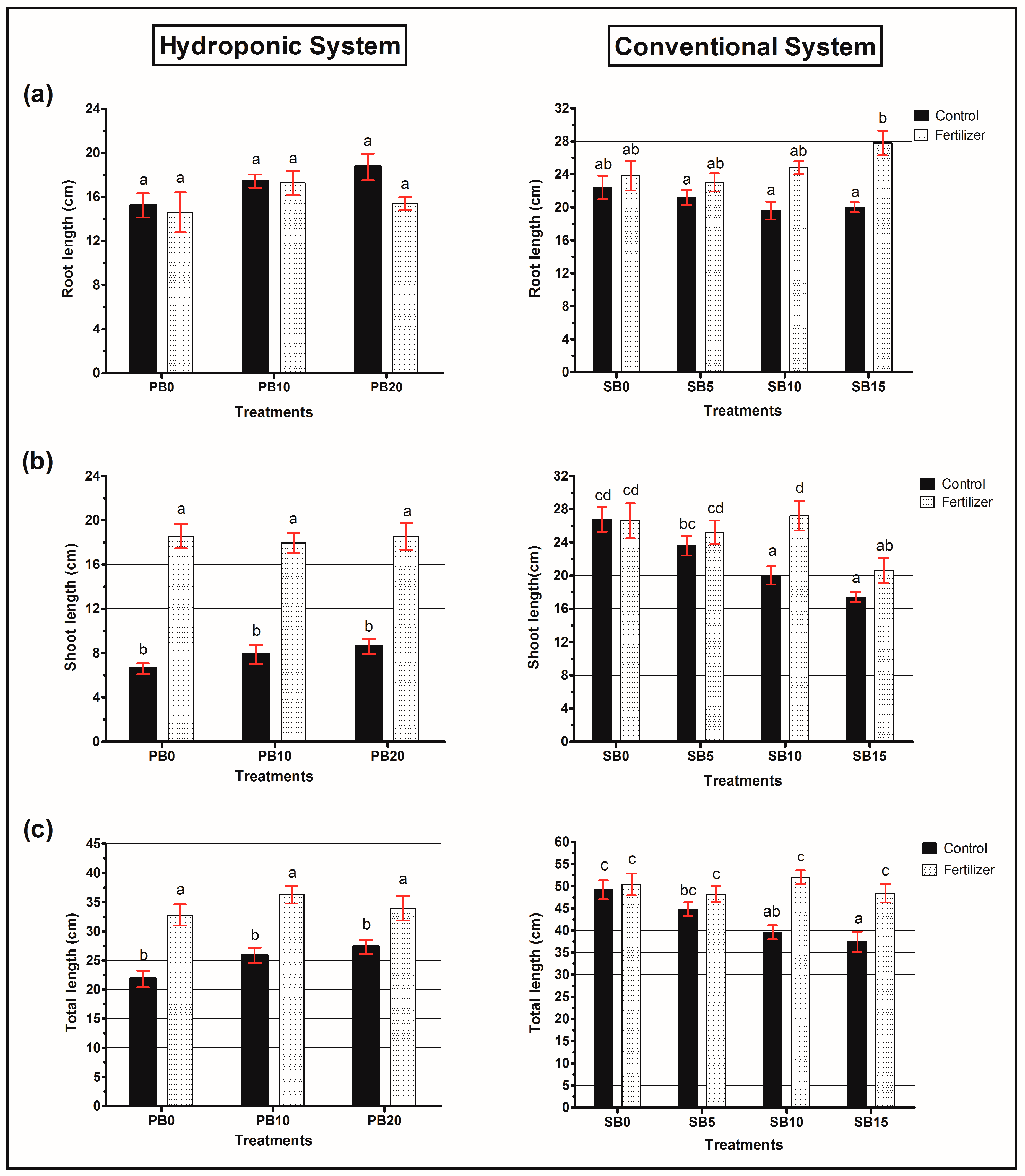
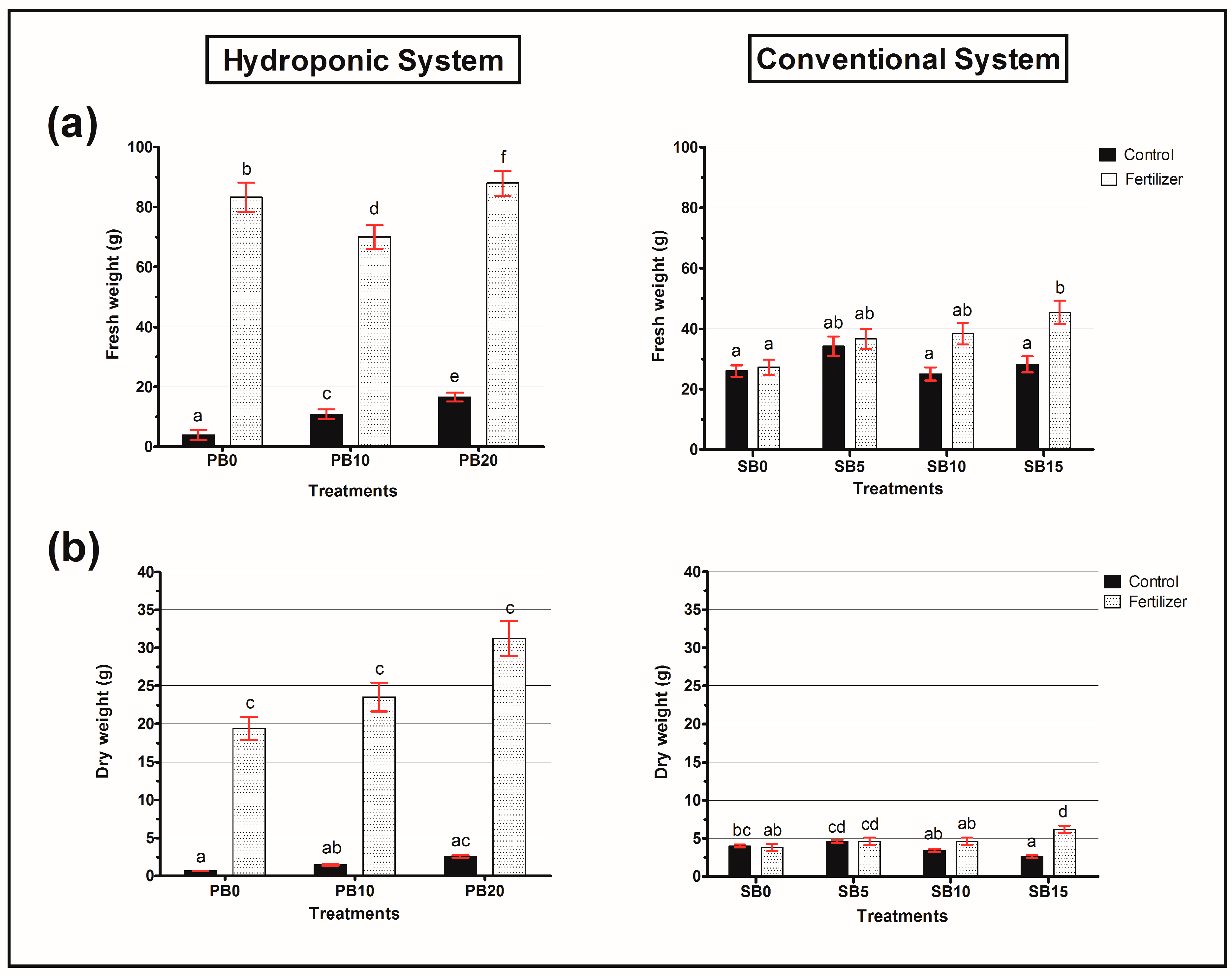
3.2. Agronomic Parameters for Both Systems
4. Conclusions
- In hydroponics, WSB alone did not enhance growth, but when combined with fertilizer, it resulted in the highest biomass production. The 20% WSB + fertilizer treatment increased fresh weight by 45% and dry weight by 38% compared to the control, demonstrating its ability to optimize nutrient availability in soilless cultivation.
- In the conventional system, WSB alone at 15% significantly improved plant growth, increasing fresh weight by 30% and leaf number by 25%, even in the absence of fertilizer. This indicates that WSB can serve as a sustainable soil amendment by enhancing water and nutrient retention.
- The highest leaf area index (LAI) was observed in conventional soil with 15% WSB and fertilizer, achieving a 1.8-fold increase compared to the control, highlighting its role in improving canopy expansion and photosynthetic efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WSB | Walnut shell biochar | |
| S | Soil | |
| P | Perlite | |
| W | Water | |
| C | Control | |
| F | Fertilizer solution recirculated | |
| NFT | Nutrient Film Technology (NFT) | |
| PB10 | Perlite + 10% Biochar | |
| PB20 | Perlite + 20% Biochar | |
| SB5 | Soil + 5% Biochar | |
| SB10 | Soil + 10% Biochar | |
| SB15 | Soil + 15% Biochar | |
| RL | Root length | |
| SL | Shoot length | |
| TL | Total length | |
| FW | Fresh weight | |
| DW | Dry weight | |
| LAI | Leaf area index | |
| EC | Electrical conductivity, (µS·cm−1) | |
| CEC | Cation exchange capacity, (mmol·Kg−1) | |
| WHC | Water holding capacity, percentage | |
| M | Moisture, percentage | |
| VM | Volatile Matter, percentage | |
| FC | Fixed Carbon, percentage | |
| C | Carbon content, percentage | |
| H | Hydrogen content, percentage | |
| O | Oxygen content, percentage | |
| N | Nitrogen content, percentage | |
| H/C | Hydrogen-Carbon molar ratio, dimensionless | |
| O/C | Oxygen-Carbon molar ratio, dimensionless | |
| R50 | Recalcitrance potential, dimensionless | |
| CS | Carbon sequestration potential, percentage | |
| MRT | Mean residence time, years | |
| by | Biochar yield, percentage | |
| CBC | Mass fraction of carbon in biochar, percentage | |
| CF | Mass fraction of carbon in feedstock, percentage | |
| BC+100 | Mass fraction of carbon that would remain after 100 years, percentage | |
References
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Lipan, L.; Issa-Issa, H.; Moriana, A.; Zurita, N.M.; Galindo, A.; Martín-Palomo, M.J.; Andreu, L.; Carbonell-Barrachina, Á.A.; Hernández, F.; Corell, M. Scheduling Regulated Deficit Irrigation with Leaf Water Potential of Cherry Tomato in Greenhouse and Its Effect on Fruit Quality. Agriculture 2021, 11, 669. [Google Scholar] [CrossRef]
- Bouabdelli, S.; Zeroual, A.; Meddi, M.; Assani, A. Impact of Temperature on Agricultural Drought Occurrence under the Effects of Climate Change. Theor. Appl. Climatol. 2022, 148, 191–209. [Google Scholar] [CrossRef]
- Shahane, A.A.; Shivay, Y.S. Soil Health and Its Improvement through Novel Agronomic and Innovative Approaches. Front. Agron. 2021, 3, 680456. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Sánchez, E.; Zabaleta, R.; Fabani, M.P.; Rodriguez, R.; Mazza, G. Effects of the Amendment with Almond Shell, Bio-Waste and Almond Shell-Based Biochar on the Quality of Saline-Alkali Soils. J. Environ. Manag. 2022, 318, 115604. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Si, B.; Wang, Y.; Chen, X.; Wang, X.; Chen, H.; Wang, H.; Zhang, F.; Bai, Y.; et al. Optimizing Biochar Application to Improve Soil Physical and Hydraulic Properties in Saline-Alkali Soils. Sci. Total Environ. 2021, 771, 144802. [Google Scholar] [CrossRef]
- FAO. Climate Change and Food Security: Risks and Responses. In The State of Food and Agriculture; FAO: Rome, Italy, 2016. [Google Scholar]
- Fontana, L.; Rossi, C.A.; Hubinger, S.Z.; Ferreira, M.D.; Spoto, M.H.F.; Sala, F.C.; Verruma-Bernardi, M.R. Physicochemical Characterization and Sensory Evaluation of Lettuce Cultivated in Three Growing Systems. Hortic. Bras. 2018, 36, 20–26. [Google Scholar] [CrossRef]
- Pomoni, D.I.; Koukou, M.K.; Vrachopoulos, M.G.; Vasiliadis, L. A Review of Hydroponics and Conventional Agriculture Based on Energy and Water Consumption, Environmental Impact, and Land Use. Energies 2023, 16, 1690. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Awad, Y.M.; Lee, S.-E.; Ahmed, M.B.M.; Vu, N.T.; Farooq, M.; Kim, I.S.; Kim, H.S.; Vithanage, M.; Usman, A.R.A.; Al-Wabel, M. Biochar, a Potential Hydroponic Growth Substrate, Enhances the Nutritional Status and Growth of Leafy Vegetables. J. Clean. Prod. 2017, 156, 581–588. [Google Scholar] [CrossRef]
- Schwarz, D.; Gross, W. Algae Affecting Lettuce Growth in Hydroponic Systems. J. Hortic. Sci. Biotechnol. 2004, 79, 554–559. [Google Scholar] [CrossRef]
- Santosh, D.T.; Gaikwad, D. Advances in Hydroponic Systems: Types and Management. In Advances in Agricultural Technology; Maitra, S., Gaikwad, D., Santosh, D.T., Eds.; Griffon: Kingston, ON, Canada, 2023; pp. 16–28. [Google Scholar]
- Malík, M.; Praus, L.; Tlustoš, P. Comparison of Recirculation and Drain-to-Waste Hydroponic Systems in Relation to Medical Cannabis (Cannabis sativa L.) Plants. Ind. Crops Prod. 2023, 202, 117059. [Google Scholar] [CrossRef]
- Djidonou, D.; Leskovar, D.I. Seasonal Changes in Growth, Nitrogen Nutrition, and Yield of Hydroponic Lettuce. HortScience 2019, 54, 76–85. [Google Scholar] [CrossRef]
- Lei, C.; Engeseth, N.J. Comparison of Growth Characteristics, Functional Qualities, and Texture of Hydroponically Grown and Soil-Grown Lettuce. LWT 2021, 150, 111931. [Google Scholar] [CrossRef]
- Maboko, M.M.; Du Plooy, C.P. Response of Hydroponically Grown Cherry and Fresh Market Tomatoes to Reduced Nutrient Concentration and Foliar Fertilizer Application under Shadenet Conditions. HortScience 2017, 52, 572–578. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Waheed, A.; Xu, H.; Qiao, X.; Aili, A.; Yiremaikebayi, Y.; Haitao, D.; Muhammad, M. Biochar in Sustainable Agriculture and Climate Mitigation: Mechanisms, Challenges, and Applications in the Circular Bioeconomy. Biomass Bioenergy 2025, 193, 107531. [Google Scholar] [CrossRef]
- Kannan, P.; Paramasivan, M.; Marimuthu, S.; Swaminathan, C.; Bose, J. Applying Both Biochar and Phosphobacteria Enhances Vigna mungo L. Growth and Yield in Acid Soils by Increasing Soil PH, Moisture Content, Microbial Growth and P Availability. Agric. Ecosyst. Environ. 2021, 308, 107258. [Google Scholar]
- Zabaleta, R.; Sánchez, E.; Fabani, P.; Mazza, G.; Rodriguez, R. Almond Shell Biochar: Characterization and Application in Soilless Cultivation of Eruca Sativa. Biomass Convers. Biorefinery 2023, 14, 18183–18200. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, C.; Srinivasakannan, C.; Wang, X. Waste Walnut Shell Valorization to Iron Loaded Biochar and Its Application to Arsenic Removal. Resour. Technol. 2017, 3, 29–36. [Google Scholar]
- Bano, A.; Aziz, M.K.; Prasad, B.; Ravi, R.; Shah, M.P.; Lins, P.V.D.S.; Meili, L.; Prasad, K.S. The Multifaceted Power of Biochar: A Review on Its Role in Pollution Control, Sustainable Agriculture, and Circular Economy. Environ. Chem. Ecotoxicol. 2025, 7, 286–304. [Google Scholar] [CrossRef]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The Effect of Sewage Sludge Biochar on Peat-Based Growing Media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Steiner, C.; Harttung, T. Biochar as a Growing Media Additive and Peat Substitute. Solid Earth 2014, 5, 995–999. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Suo, F.; You, X.; Yuan, Y.; Cheng, Y.; Zhang, C.; Li, Y. Effect of Biochar and Hydrochar from Cow Manure and Reed Straw on Lettuce Growth in an Acidified Soil. Chemosphere 2022, 298, 134191. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, Y.-F. Carbon Dynamics and Fertility in Biochar-Amended Soils with Excessive Compost Application. Agronomy 2019, 9, 511. [Google Scholar] [CrossRef]
- Boersma, M.; Wrobel-Tobiszewska, A.; Murphy, L.; Eyles, A. Impact of Biochar Application on the Productivity of a Temperate Vegetable Cropping System. N. Z. J. Crop Hortic. Sci. 2017, 45, 277–288. [Google Scholar] [CrossRef]
- Ajayi, A.E.; Holthusen, D.; Horn, R. Changes in Microstructural Behaviour and Hydraulic Functions of Biochar Amended Soils. Soil Tillage Res. 2016, 155, 166–175. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R. Influence of Pyrolysis Temperature on Physico-Chemical Properties of Corn Stover (Zea mays L.) Biochar and Feasibility for Carbon Capture and Energy Balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Sarmah, A.K. Sustainable Eco–Composites Obtained from Waste Derived Biochar: A Consideration in Performance Properties, Production Costs, and Environmental Impact. J. Clean. Prod. 2016, 129, 159–168. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Rodriguez Ortiz, L.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Influence of Pyrolysis Temperature and Bio-Waste Composition on Biochar Characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Reales, N.; Rocamundi, N.; Marvaldi, A.E.; del Carmen Fernández-Górgolas, M.; Stadler, T. Morphological and Molecular Identification of Carpophilus dimidiatus (Coleoptera: Nitidulidae) Associated with Stored Walnut in Northwestern Argentina. J. Stored Prod. Res. 2018, 76, 37–42. [Google Scholar] [CrossRef]
- Romero-Arenas, O.; López Escobedo, R.; Damián Huato, M.Á.; Hernández Treviño, I.; Parraguirre Lezama, J.F.; Huerta Lara, M. Evaluación Del Residuo de Cáscara de Nuez (Juglans regia L.) En La Producción de Plántulas de Pinus Patula, En Vivero. Agron. Costarric. 2012, 36, 103–110. [Google Scholar] [CrossRef]
- Safaei Khorram, M.; Zhang, G.; Fatemi, A.; Kiefer, R.; Mahmood, A.; Jafarnia, S.; Zakaria, M.P.; Li, G. Effect of Walnut Shell Biochars on Soil Quality, Crop Yields, and Weed Dynamics in a 4-Year Field Experiment. Environ. Sci. Pollut. Res. 2020, 27, 18510–18520. [Google Scholar] [CrossRef]
- Domingues, R.R.; Sánchez-Monedero, M.A.; Spokas, K.A.; Melo, L.C.A.; Trugilho, P.F.; Valenciano, M.N.; Silva, C.A. Enhancing Cation Exchange Capacity of Weathered Soils Using Biochar: Feedstock, Pyrolysis Conditions and Addition Rate. Agronomy 2020, 10, 824. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Simeon, V.T. Biochar and Poultry Manure Effects on Soil Properties and Radish (Raphanus sativus L.) Yield. Biol. Agric. Hortic. 2019, 35, 33–45. [Google Scholar] [CrossRef]
- Jabborova, D.; Kadirova, D.; Narimanov, A.; Wirth, S. Beneficial Effects of Biochar Application on Lettuce (Lactuca sativa L.) Growth, Root Morphological Traits and Physiological Properties. Ann. Phytomedicine 2021, 10, 93–100. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic Values of Greenwaste Biochar as a Soil Amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Baldán, Y.; Fernandez, A.; Urrutia, A.R.; Fabani, M.P.; Rodriguez, R.; Mazza, G. Non-Isothermal Drying of Bio-Wastes: Kinetic Analysis and Determination of Effective Moisture Diffusivity. J. Environ. Manag. 2020, 262, 110348. [Google Scholar] [CrossRef]
- Rodriguez, R.; Mazza, G.; Fernandez, A.; Saffe, A.; Echegaray, M. Prediction of the Lignocellulosic Winery Wastes Behavior during Gasification Process in Fluidized Bed: Experimental and Theoretical Study. J. Environ. Chem. Eng. 2018, 6, 5570–5579. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- ASTM E872-82; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. ASTM: West Conshohocken, PA, USA, 1998.
- ASTM D 1102–84; Standard Test Method for Ash in Wood. ASTM: West Conshohocken, PA, USA, 2001.
- Belda, R.M.; Lidón, A.; Fornes, F. Biochars and Hydrochars as Substrate Constituents for Soilless Growth of Myrtle and Mastic. Ind. Crops Prod. 2016, 94, 132–142. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar] [CrossRef]
- Upadhyay, V.; Choudhary, K.K.; Agrawal, S.B. Use of Biochar as a Sustainable Agronomic Tool, Its Limitations and Impact on Environment: A Review. Discov. Agric. 2024, 2, 20. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of Woodchip Biochar Additions on Greenhouse Gas Production and Sorption/Degradation of Two Herbicides in a Minnesota Soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Ferby, V.; Kopta, T.; Komorowska, M.; Fidurski, M. Evaluation of Alternative Substrates for Hydroponics Based on Biological Parameters of Leaf Lettuce (Lactuca sativa L.) and Its Stress Response. Folia Hortic. 2023, 35, 77–90. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A Review on Hydroponics and the Technologies Associated for Medium-and Small-Scale Operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Chang, Y.; Rossi, L.; Zotarelli, L.; Gao, B.; Sarkhosh, A. Greenhouse Evaluation of Pinewood Biochar Effects on Nutrient Status and Physiological Performance in Muscadine Grape (Vitis rotundifolia L.). HortScience 2021, 56, 277–285. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Sun, H.; Lü, F.; He, P. Three-Year Rice Grain Yield Responses to Coastal Mudflat Soil Properties Amended with Straw Biochar. J. Environ. Manag. 2019, 239, 23–29. [Google Scholar] [CrossRef]
- Haider, G.; Koyro, H.-W.; Azam, F.; Steffens, D.; Müller, C.; Kammann, C. Biochar but Not Humic Acid Product Amendment Affected Maize Yields via Improving Plant-Soil Moisture Relations. Plant Soil 2015, 395, 141–157. [Google Scholar] [CrossRef]
- Papathanasiou, F.; Papadopoulos, I.; Tsakiris, I.; Tamoutsidis, E. Vermicompost as a Soil Supplement to Improve Growth, Yield and Quality of Lettuce (Lactuca sativa L.). J. Food Agric. Environ. 2012, 10, 677–682. [Google Scholar]
- Rasband, W.S. ImageJ; National Institutes of Health: Bethesda, MD, USA, 2018.
- Herrera, A.L.; de la Rosa Rodríguez, R.; Téllez, L.I.T. Producción de Lechuga (Lactuca sativa L.) Con Cinco Proporciones de Macronutrientes En Solución Nutritiva. Bioagro 2023, 35, 113–122. [Google Scholar] [CrossRef]
- Yang, T.; Samarakoon, U.; Altland, J.; Ling, P. Photosynthesis, Biomass Production, Nutritional Quality, and Flavor-Related Phytochemical Properties of Hydroponic-Grown Arugula (Eruca Sativa Mill.) ‘Standard’ under Different Electrical Conductivities of Nutrient Solution. Agronomy 2021, 11, 1340. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 Meter to Measure Leaf Chlorophyll Concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat; Versión 2018; Universidad Nacional de Córdoba: Cordoba, Argentina, 2018. [Google Scholar]
- GraphPad Software. GraphPad Prism; Versión 5.00; GraphPad Software: Boston, MA, USA, 2007. [Google Scholar]
- Griffin, D.E.; Wang, D.; Parikh, S.J.; Scow, K.M. Short-Lived Effects of Walnut Shell Biochar on Soils and Crop Yields in a Long-Term Field Experiment. Agric. Ecosyst. Environ. 2017, 236, 21–29. [Google Scholar] [CrossRef]
- Rasse, D.P.; Budai, A.; O’Toole, A.; Ma, X.; Rumpel, C.; Abiven, S. Persistence in Soil of Miscanthus Biochar in Laboratory and Field Conditions. PLoS ONE 2017, 12, e0184383. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of Biochar Application to the Environment and Economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Diao, R.; Sun, M.; Huang, Y.; Zhu, X. Synergistic Effect of Washing Pretreatment and Co-Pyrolysis on Physicochemical Property Evolution of Biochar Derived from Bio-Oil Distillation Residue and Walnut Shell. J. Anal. Appl. Pyrolysis 2021, 155, 105034. [Google Scholar] [CrossRef]
- Choudhary, T.K.; Khan, K.S.; Hussain, Q.; Ahmad, M.; Ashfaq, M. Feedstock-Induced Changes in Composition and Stability of Biochar Derived from Different Agricultural Wastes. Arab. J. Geosci. 2019, 12, 617. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, J.; Tang, J.; Zhang, Q. A Study of Cadmium Remediation and Mechanisms: Improvements in the Stability of Walnut Shell-Derived Biochar. Sci. Total Environ. 2018, 636, 80–84. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar Stability in Soil: Decomposition during Eight Years and Transformation as Assessed by Compound-Specific 14C Analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L.; Smernik, R.J. Biochar Carbon Stability in a Clayey Soil as a Function of Feedstock and Pyrolysis Temperature. Environ. Sci. Technol. 2012, 46, 11770–11778. [Google Scholar] [CrossRef] [PubMed]
- Shareef, T.M.E.; Zhao, B. The Fundamentals of Biochar as a Soil Amendment Tool and Management in Agriculture Scope: An Overview for Farmers and Gardeners. J. Agric. Chem. Environ. 2016, 6, 38–61. [Google Scholar] [CrossRef]
- Everaert, K.; Baeyens, J. Removal of PCDD/F from Flue Gases in Fixed or Moving Bed Adsorbers. Waste Manag. 2004, 24, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of Pyrolysis Temperature, Heating Rate, and Residence Time on Rapeseed Stem Derived Biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Tan, Z.; Zou, J.; Zhang, L.; Huang, Q. Morphology, Pore Size Distribution, and Nutrient Characteristics in Biochars under Different Pyrolysis Temperatures and Atmospheres. J. Mater. Cycles Waste Manag. 2018, 20, 1036–1049. [Google Scholar] [CrossRef]
- Önal, E.P.; Uzun, B.B.; Pütün, A.E. Steam Pyrolysis of an Industrial Waste for Bio-Oil Production. Fuel Process. Technol. 2011, 92, 879–885. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; De Freitas, R.A.; Mangrich, A.S. Effect of Surface and Porosity of Biochar on Water Holding Capacity Aiming Indirectly at Preservation of the Amazon Biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Hall, S.; Tang, R.; Baeyens, J.; Dewil, R. Removing Polycyclic Aromatic Hydrocarbons from Water by Adsorption on Silicagel. Polycycl. Aromat. Compd. 2009, 29, 160–183. [Google Scholar] [CrossRef]
- Dávila-Jiménez, M.M.; Elizalde-González, M.P.; Hernández-Montoya, V. Performance of Mango Seed Adsorbents in the Adsorption of Anthraquinone and Azo Acid Dyes in Single and Binary Aqueous Solutions. Bioresour. Technol. 2009, 100, 6199–6206. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Walnut Shell for Ammonium and Phosphate Adsorption from Water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, A.; Singh, A. Fertilizers and Their Role in Plant Growth. Bright Sky Publ. 2021, 2, 71–88. [Google Scholar]
- Hussein, H.A. Biological Fertilizers and Their Role in Plant Growth. Int. Res. J. Adv. Sci. 2021, 2, 17–20. [Google Scholar]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef]
- Semenova, N.A.; Smirnov, A.A.; Grishin, A.A.; Pishchalnikov, R.Y.; Chesalin, D.D.; Gudkov, S.V.; Chilingaryan, N.O.; Skorokhodova, A.N.; Dorokhov, A.S.; Izmailov, A.Y. The Effect of Plant Growth Compensation by Adding Silicon-Containing Fertilizer under Light Stress Conditions. Plants 2021, 10, 1287. [Google Scholar] [CrossRef]
- Choi, H.-S.; Zhao, Y.; Dou, H.; Cai, X.; Gu, M.; Yu, F. Effects of Biochar Mixtures with Pine-Bark Based Substrates on Growth and Development of Horticultural Crops. Hortic. Environ. Biotechnol. 2018, 59, 345–354. [Google Scholar] [CrossRef]
- Carbo, X.J.M.; Uriola, R.N.C.; Delgado, I.R. Influencia de La Fertilización Orgánica En El Crecimiento y Desarrollo Del Cultivo de La Zanahoria. Rev. Científica Agroecosist. 2022, 10, 41–50. [Google Scholar]
- Hasnain, M.; Chen, J.; Ahmed, N.; Memon, S.; Wang, L.; Wang, Y.; Wang, P. The Effects of Fertilizer Type and Application Time on Soil Properties, Plant Traits, Yield and Quality of Tomato. Sustainability 2020, 12, 9065. [Google Scholar] [CrossRef]
- Dispenza, V.; De Pasquale, C.; Fascella, G.; Mammano, M.M.; Alonzo, G. Use of Biochar as Peat Substitute for Growing Substrates of Euphorbia× Lomi Potted Plants. Span. J. Agric. Res. 2016, 14, e0908. [Google Scholar] [CrossRef]


| WSB | Unit | |
|---|---|---|
| pH | 10.86 ± 0.05 | |
| EC | 561 ± 1.73 | µS·cm−1 |
| CEC | 3.13 ± 0.12 | meq·100 g−1 |
| yb | 30.00 ± 0.52 | % |
| Ash | 2.49 ± 0.12 | % |
| VM | 28.68 ± 0.62 | % |
| M | 2.11 ± 0.01 | % |
| FC | 66.82 ± 0.56 | % |
| C | 78.12 ± 0.25 | % |
| H | 2.90 ± 0.04 | % |
| O | 13.96 ± 0.28 | % |
| N | 5.01 ± 0.10 | % |
| WSB | Unit | |
|---|---|---|
| H/C | 0.037 | |
| O/C | 0.179 | |
| C/N | 15.59 | |
| Stable C mass fraction | 73.98 | % |
| R50 | 0.136 | |
| CS | 0.6 | % |
| MRT | 1157 | Years |
| BC+100 | 0.790 | |
| Specific surface area | 2.410 | m2·g−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, E.; Zabaleta, R.; Navas, A.L.; Maldonado, V.N.F.; Fabani, M.P.; Mazza, G.; Rodriguez, R. Influence of Walnut Shell Biochar and Fertilizer on Lettuce Production in Hydroponic and Conventional Systems. Agronomy 2025, 15, 658. https://doi.org/10.3390/agronomy15030658
Sanchez E, Zabaleta R, Navas AL, Maldonado VNF, Fabani MP, Mazza G, Rodriguez R. Influence of Walnut Shell Biochar and Fertilizer on Lettuce Production in Hydroponic and Conventional Systems. Agronomy. 2025; 15(3):658. https://doi.org/10.3390/agronomy15030658
Chicago/Turabian StyleSanchez, Eliana, Romina Zabaleta, Ana Laura Navas, Viviana N. Fernández Maldonado, María Paula Fabani, German Mazza, and Rosa Rodriguez. 2025. "Influence of Walnut Shell Biochar and Fertilizer on Lettuce Production in Hydroponic and Conventional Systems" Agronomy 15, no. 3: 658. https://doi.org/10.3390/agronomy15030658
APA StyleSanchez, E., Zabaleta, R., Navas, A. L., Maldonado, V. N. F., Fabani, M. P., Mazza, G., & Rodriguez, R. (2025). Influence of Walnut Shell Biochar and Fertilizer on Lettuce Production in Hydroponic and Conventional Systems. Agronomy, 15(3), 658. https://doi.org/10.3390/agronomy15030658









