Kinetic Parameters of Soil Enzymes and Temperature Sensitivity Under Different Mulching Practices in Apple Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling Method
2.3. Soil Incubation and Enzyme Assays
2.4. Data Analysis
3. Results
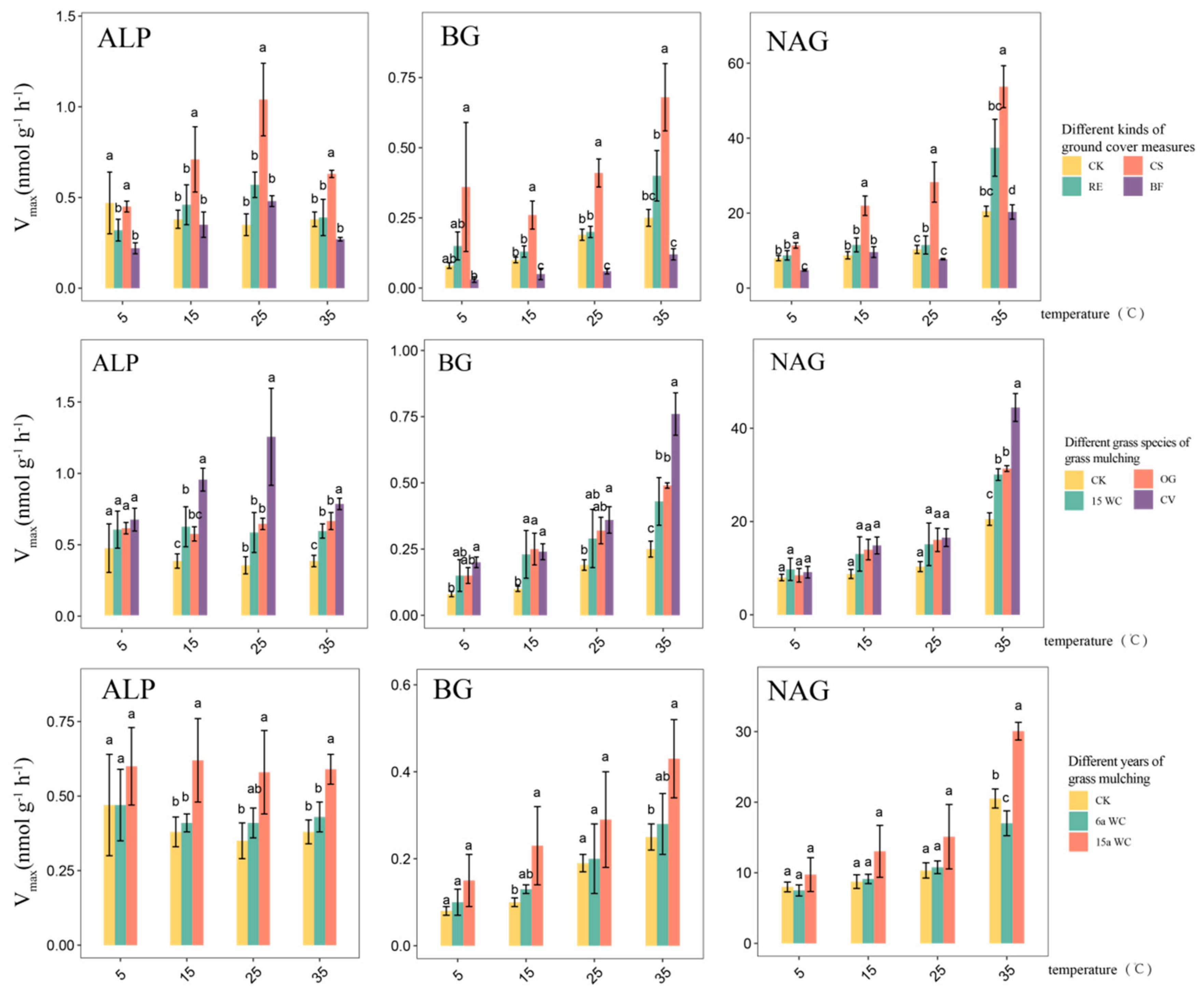
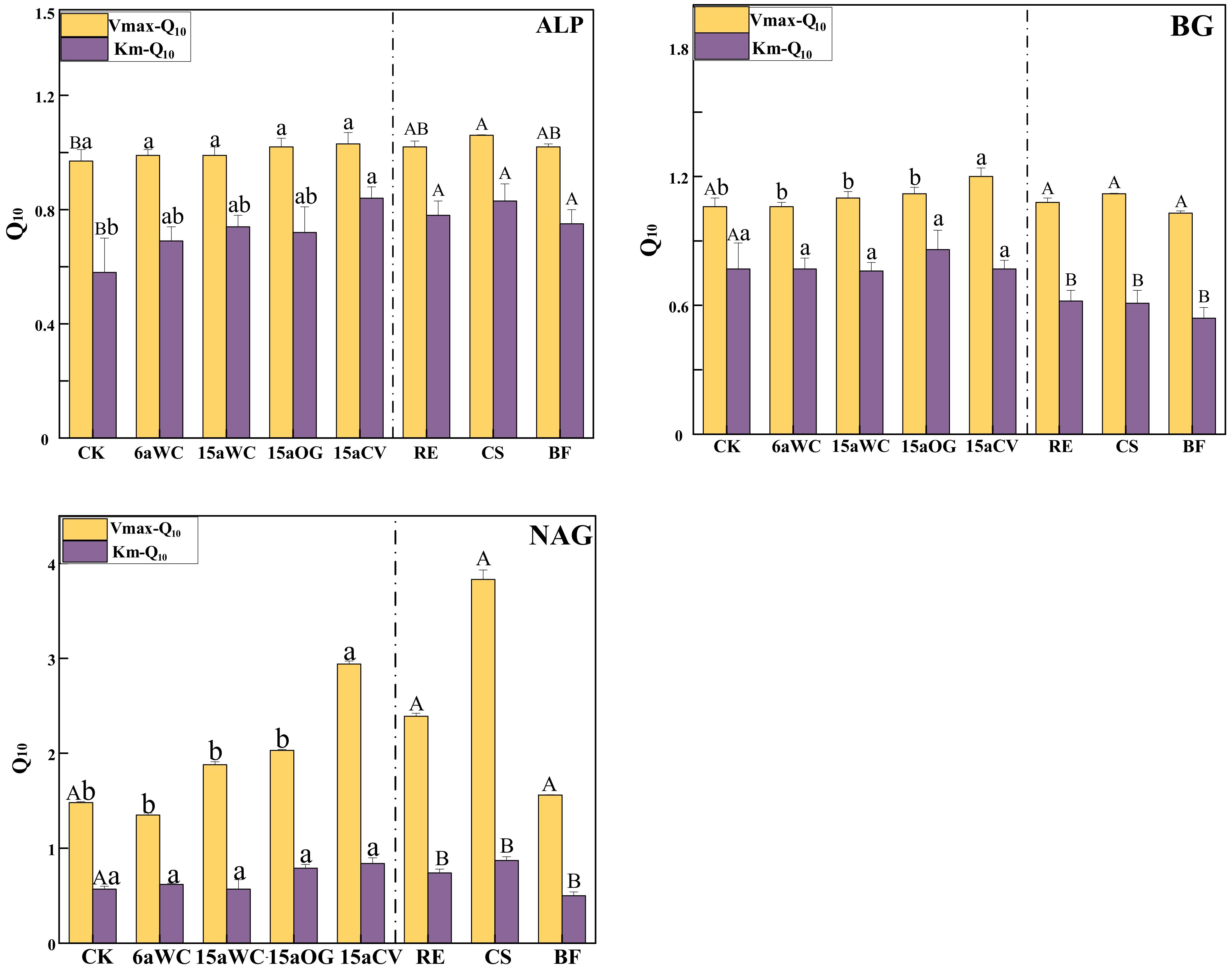
3.1. Effect of Mulching Practices on the Kinetic Parameters of Soil Enzymes Under Temperature Change
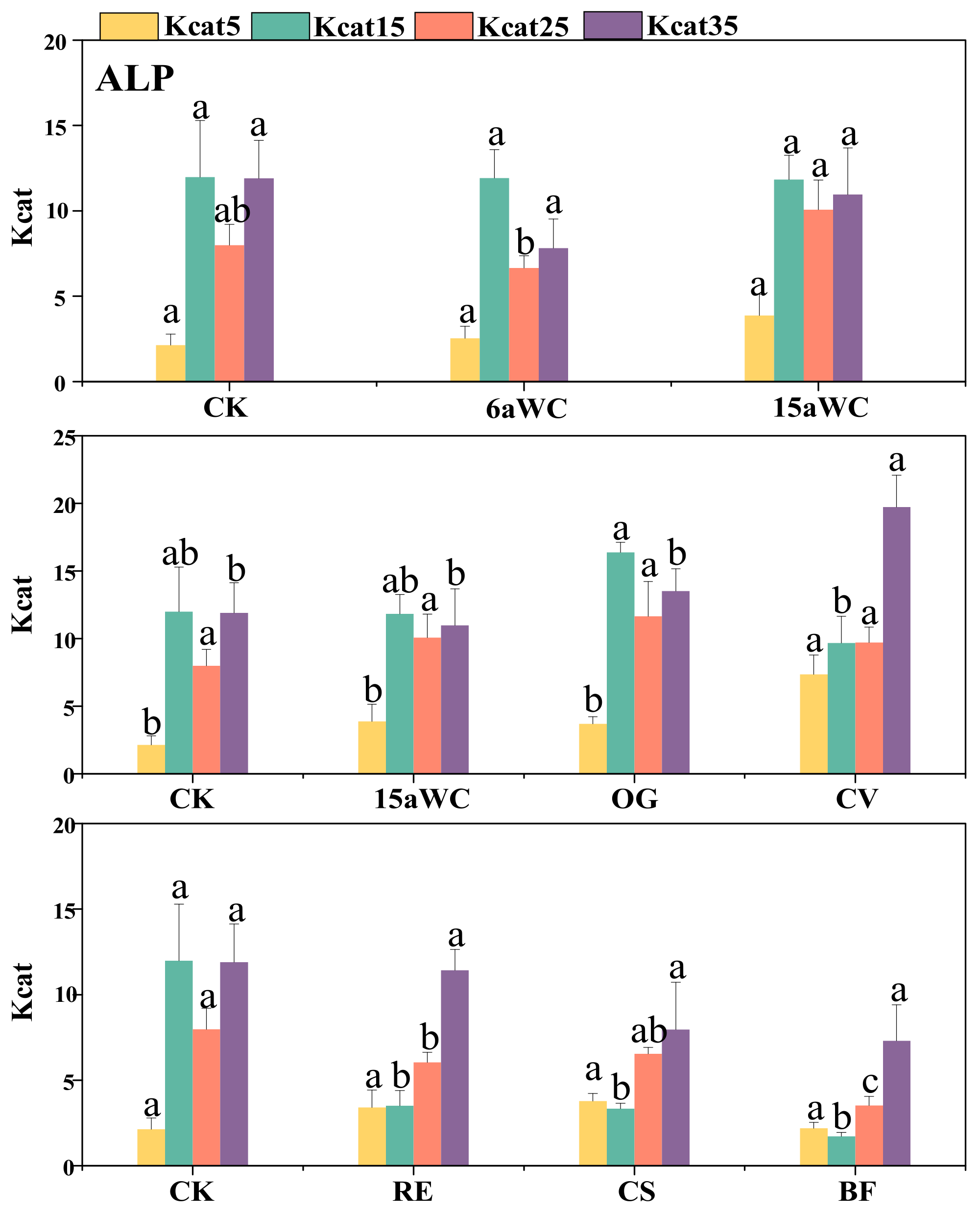
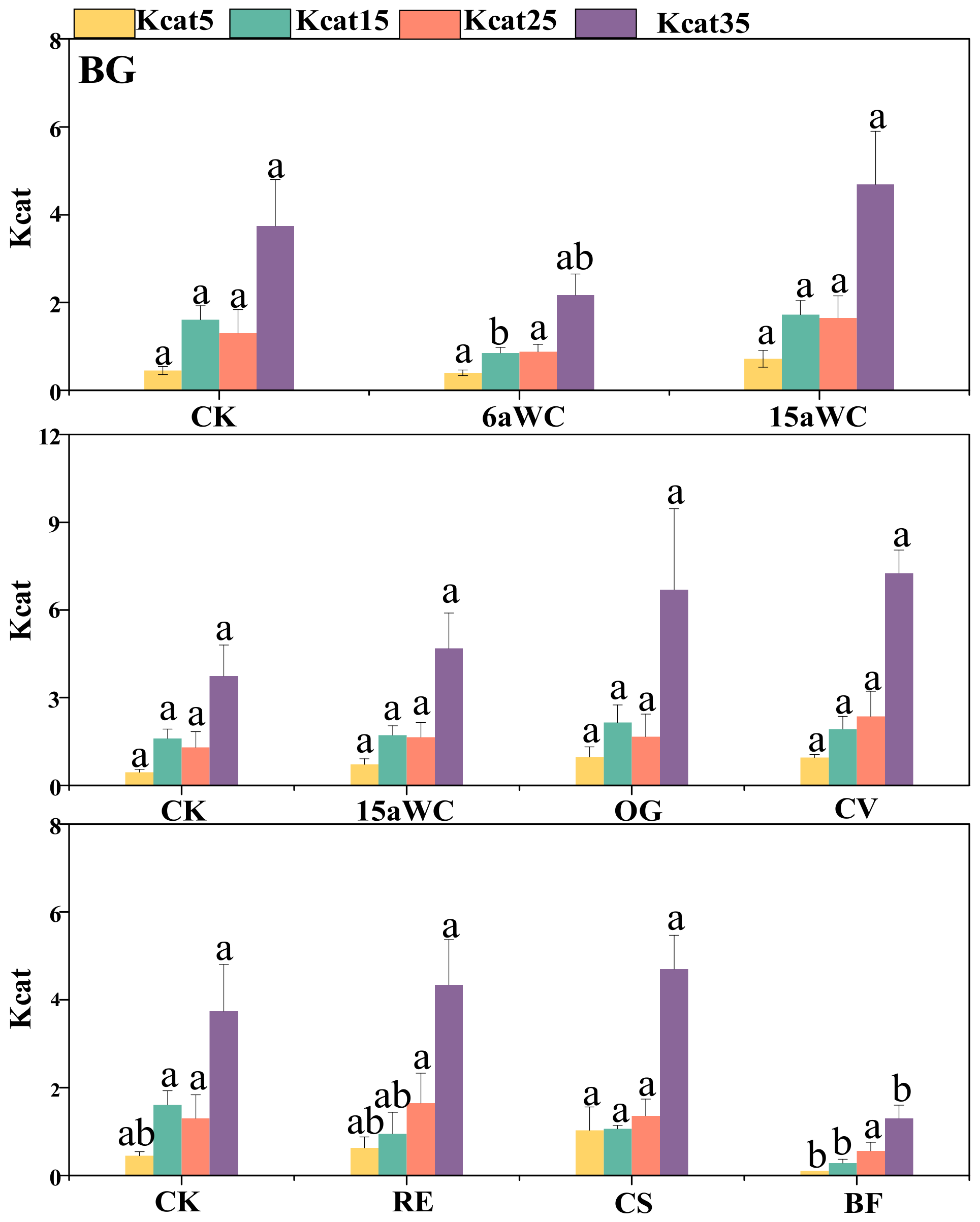
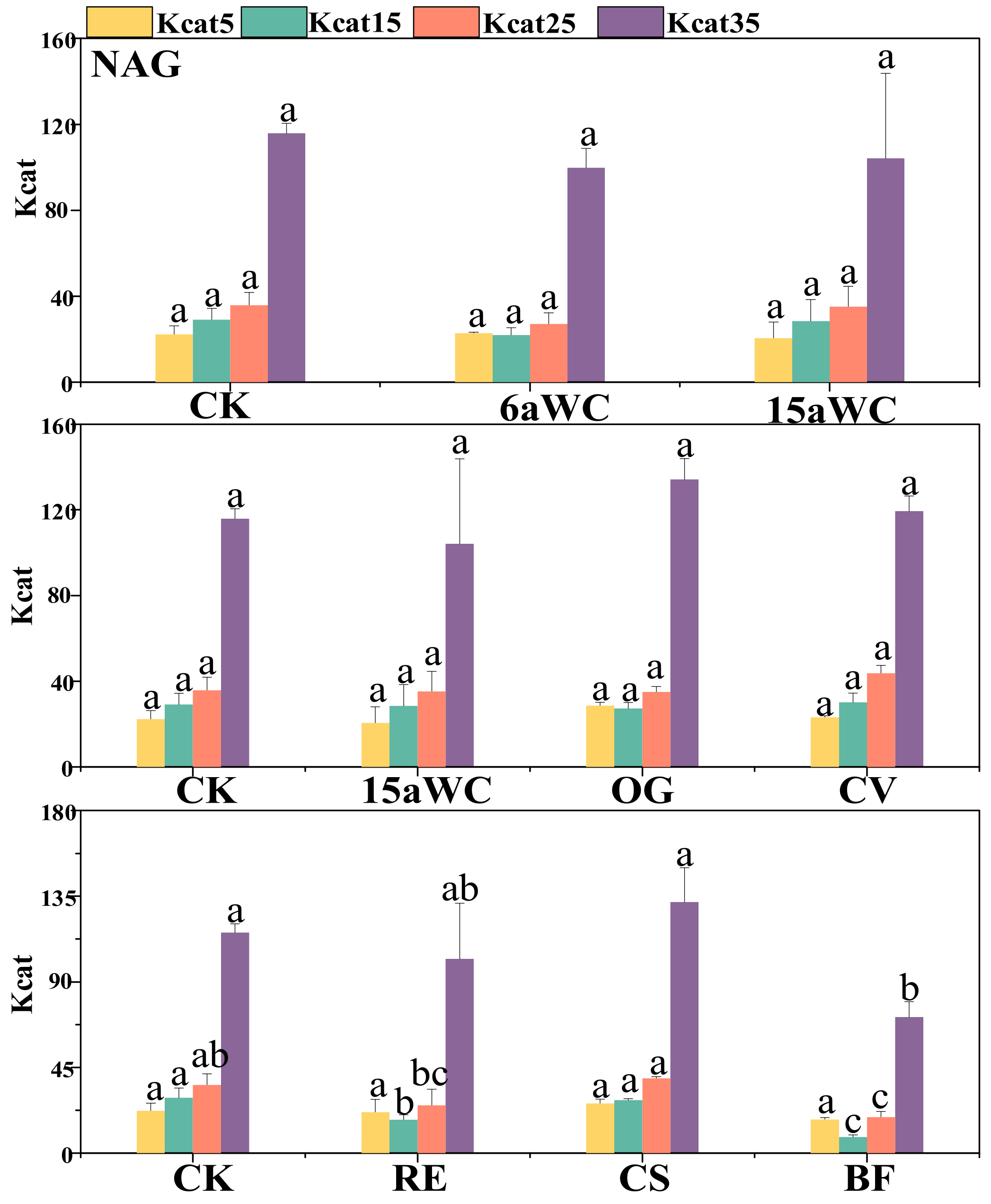
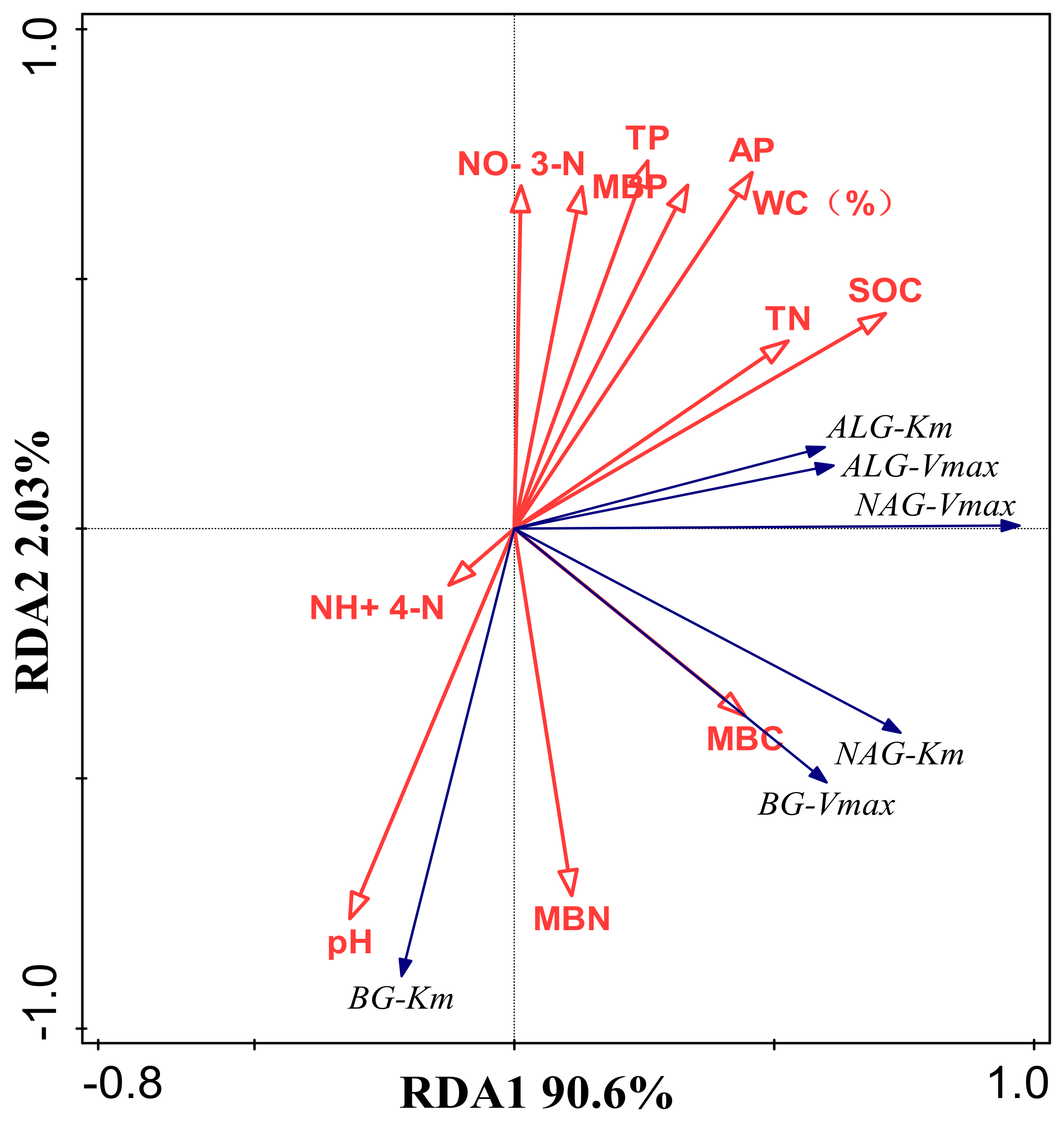
3.2. Thermodynamic Characteristics of Soil Enzyme Kinetic Parameters and Their Influencing Factors
4. Discussion
4.1. Response of Soil Enzyme Kinetic Parameters to Mulching Practices
4.2. Response of Soil Enzymes Under Coverage Treatment to Temperature Changes
4.3. Temperature Sensitivity of Soil Enzyme Kinetics in Response to Mulching Practices
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Huang, Q.; Yan, T.; Yang, J.; Zheng, Y.; Li, H.; Li, M. Effects of intercropping mulch on the content and composition of soil dissolved organic matter in apple orchard on the loess plateau. J. Environ. Manag. 2019, 250, 109531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Peng, X.; Zhang, Y.; Ning, F.; Xu, Z.; Wang, Q.; Dong, Z.; Jia, G.; Wei, L.; et al. Changes in soil organic carbon and total nitrogen in apple orchards in different climate regions on the Loess Plateau. CATENA 2021, 197, 104989. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Zhang, Y.; Wang, X.; Wang, R.; Li, J. Tillage system change affects soil organic carbon storage and benefits land restoration on loess soil in North China. Land Degrad. Dev. 2018, 29, 2880–2887. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, X.; Sun, Y.; Zhang, J.; Wu, W.; Liao, Y. Mulching practices altered soil bacterial community structure and improved orchard productivity and apple quality after five growing seasons. Sci. Hortic. 2014, 172, 248–257. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Dong, Y.; Chang, X.; Nie, X.; Liu, L.; Xiao, H.; Lu, Y.; Zeng, G. Response of soil organic carbon and nitrogen stocks to soil erosion and land use types in the Loess hilly–gully region of China. Soil Tillage Res. 2017, 166, 1–9. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, Y.; Yu, K.; Feng, H. Effects of straw mulching and plastic film mulching on improving soil organic carbon and nitrogen fractions, crop yield and water use efficiency in the Loess Plateau, China. Agric. Water Manag. 2018, 201, 133–143. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Gong, Q.; Zhai, B.; Li, Z. Effects of cover crop in an apple orchard on microbial community composition, networks, and potential genes involved with degradation of crop residues in soil. Biol. Fertil. Soils 2018, 54, 743–759. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Liu, C.; Ding, Y.; Liu, L.; Tian, Y.; Wu, X.; Li, H.; Awasthi, M.K.; Zhao, Z. Mulching practices alter soil microbial functional diversity and benefit to soil quality in orchards on the Loess Plateau. J. Environ. Manag. 2020, 271, 110985. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Luo, Y.; Awasthi, M.K.; Yang, J.; Duan, Y.; Li, H.; Zhao, Z. Mulching practices alter the bacterial-fungal community and network in favor of soil quality in a semiarid orchard system. Sci. Total Environ. 2020, 725, 138527. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, M.; Wu, W.; Tanveer, S.K.; Wen, X.; Liao, Y. The effects of conservation tillage practices on the soil water-holding capacity of a non-irrigated apple orchard in the Loess Plateau, China. Soil Tillage Res. 2013, 130, 7–12. [Google Scholar] [CrossRef]
- Zheng, W.; Gong, Q.; Zhao, Z.; Liu, J.; Zhai, B.; Wang, Z.; Li, Z. Changes in the soil bacterial community structure and enzyme activities after intercrop mulch with cover crop for eight years in an orchard. Eur. J. Soil Biol. 2018, 86, 34–41. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, H.; Fu, S.; Yao, Q. Variation in Soil Microbial Community Structure Associated with Different Legume Species Is Greater than that Associated with Different Grass Species. Front. Microbiol. 2017, 8, 1007. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, H.; Sun, W.; Zhao, Q.; Liang, M.; Chen, W.; Guo, Z.; Jiang, Y.; Jiang, Y.; Liu, G.; et al. Temperature sensitivity of soil enzyme kinetics under N and P fertilization in an alpine grassland, China. Sci. Total Environ. 2022, 838, 156042. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, Y.; Chen, W.; Sun, W.; Wang, Z.; Liu, G.; Xue, S. Soil enzyme kinetics and thermodynamics in response to long-term vegetation succession. Sci. Total Environ. 2023, 882, 163542. [Google Scholar] [CrossRef]
- Baker, N.R.; Allison, S.D. Extracellular enzyme kinetics and thermodynamics along a climate gradient in southern California. Soil Biol. Biochem. 2017, 114, 82–92. [Google Scholar] [CrossRef]
- Tian, P.; Razavi, B.S.; Zhang, X.; Wang, Q.; Blagodatskaya, E. Microbial growth and enzyme kinetics in rhizosphere hotspots are modulated by soil organics and nutrient availability. Soil Biol. Biochem. 2020, 141, 107662. [Google Scholar] [CrossRef]
- Tan, X.; Nie, Y.; Ma, X.; Guo, Z.; Liu, Y.; Tian, H.; Megharaj, M.; Shen, W.; He, W. Soil chemical properties rather than the abundance of active and potentially active microorganisms control soil enzyme kinetics. Sci. Total Environ. 2021, 770, 144500. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Lagomarsino, A.; Garzillo, A.M.V.; Pignataro, A.; Grego, S. β-Glucosidase kinetic parameters as indicators of soil quality under conventional and organic cropping systems applying two analytical approaches. Ecol. Indic. 2012, 13, 322–327. [Google Scholar] [CrossRef]
- Parvin, S.; Blagodatskaya, E.; Becker, J.N.; Kuzyakov, Y.; Uddin, S.; Dorodnikov, M. Depth rather than microrelief controls microbial biomass and kinetics of C-, N-, P- and S-cycle enzymes in peatland. Geoderma 2018, 324, 67–76. [Google Scholar] [CrossRef]
- Bradford, M. Thermal adaptation of decomposer communities in warming soils. Front. Microbiol. 2013, 4, 333. [Google Scholar] [CrossRef]
- Razavi, B.S.; Blagodatskaya, E.; Kuzyakov, Y. Temperature selects for static soil enzyme systems to maintain high catalytic efficiency. Soil Biol. Biochem. 2016, 97, 15–22. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, H.; Wang, E.; He, W.; Hao, W.; Yan, C.; Li, Y.; Mei, X.; Zhang, Y.; Sun, Z.; et al. An overview of the use of plastic-film mulching in China to increase crop yield and water-use efficiency. Natl. Sci. Rev. 2020, 7, 1523–1526. [Google Scholar] [CrossRef]
- Daryanto, S.; Fu, B.; Wang, L.; Jacinthe, P.-A.; Zhao, W. Quantitative synthesis on the ecosystem services of cover crops. Earth-Sci. Rev. 2018, 185, 357–373. [Google Scholar] [CrossRef]
- Fernandez, A.L.; Sheaffer, C.C.; Wyse, D.L.; Staley, C.; Gould, T.J.; Sadowsky, M.J. Associations between soil bacterial community structure and nutrient cycling functions in long-term organic farm soils following cover crop and organic fertilizer amendment. Sci. Total Environ. 2016, 566–567, 949–959. [Google Scholar] [CrossRef]
- Liu, C.; Tian, H.; Gu, X.; Li, N.; Zhao, X.; Lei, M.; Alharbi, H.; Megharaj, M.; He, W.; Kuzyakov, Y. Catalytic efficiency of soil enzymes explains temperature sensitivity: Insights from physiological theory. Sci. Total Environ. 2022, 822, 153365. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Zhang, J.; Li, D.; Yu, J.; Gao, H.; Li, H.; Zhao, Z. Soil phytoremediation reveals alteration in soil microbial metabolic activities along time gradient of cover crop mulching. Environ. Res. 2022, 209, 112884. [Google Scholar] [CrossRef]
- Marx, M.-C.; Kandeler, E.; Wood, M.; Wermbter, N.; Jarvis, S.C. Exploring the enzymatic landscape: Distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol. Biochem. 2005, 37, 35–48. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Mcmahon, S.K.; Schimel, J.P. Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob. Change Biol. 2009, 15, 1631–1639. [Google Scholar] [CrossRef]
- Stone, M.M.; Weiss, M.S.; Goodale, C.L.; Adams, M.B.; Fernandez, I.J.; German, D.P.; Allison, S.D. Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Glob. Change Biol. 2012, 18, 1173–1184. [Google Scholar] [CrossRef]
- Wang, Q.; He, N.; Xu, L.; Zhou, X. Microbial properties regulate spatial variation in the differences in heterotrophic respiration and its temperature sensitivity between primary and secondary forests from tropical to cold-temperate zones. Agric. For. Meteorol. 2018, 262, 81–88. [Google Scholar] [CrossRef]
- Fang, Y.; Nazaries, L.; Singh, B.K.; Singh, B.P. Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Glob. Change Biol. 2018, 24, 2775–2790. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Tonitto, C.; David, M.B.; Drinkwater, L.E. Replacing bare fallows with cover crops in fertilizer-intensive cropping systems: A meta-analysis of crop yield and N dynamics. Agric. Ecosyst. Environ. 2006, 112, 58–72. [Google Scholar] [CrossRef]
- Razavi, B.S.; Liu, S.; Kuzyakov, Y. Hot experience for cold-adapted microorganisms: Temperature sensitivity of soil enzymes. Soil Biol. Biochem. 2017, 105, 236–243. [Google Scholar] [CrossRef]
- Stone, M.M.; Plante, A.F. Changes in phosphatase kinetics with soil depth across a variable tropical landscape. Soil Biol. Biochem. 2014, 71, 61–67. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; Wang, J.; Ding, Y.; Song, X. Effects of Elevated CO2 and Drought on Plant Physiology, Soil Carbon and Soil Enzyme Activities. Pedosphere 2017, 27, 846–855. [Google Scholar] [CrossRef]
- Gerday, C.; Aittaleb, M.; Bentahir, M.; Chessa, J.-P.; Claverie, P.; Collins, T.; D’Amico, S.; Dumont, J.; Garsoux, G.; Georlette, D.; et al. Cold-adapted enzymes: From fundamentals to biotechnology. Trends Biotechnol. 2000, 18, 103–107. [Google Scholar] [CrossRef]
- Hao, D.-C.; Su, X.-Y.; Xie, H.-T.; Bao, X.-L.; Zhang, X.-D.; Wang, L.-F. Effects of tillage patterns and stover mulching on N2O production, nitrogen cycling genes and microbial dynamics in black soil. J. Environ. Manag. 2023, 345, 118458. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Yang, J.; Duan, Y.; Luo, Y.; Taherzadeh, M.J.; Li, Y.; Li, H.; Awasthi, M.K.; Zhao, Z. The diversity of microbial community and function varied in response to different agricultural residues composting. Sci. Total Environ. 2020, 715, 136983. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Upadhyaya, G.; Yuen, W.; Brown, J.; Morgenroth, E.; Raskin, L. Changes in the Structure and Function of Microbial Communities in Drinking Water Treatment Bioreactors upon Addition of Phosphorus. Appl. Environ. Microbiol. 2010, 76, 7473–7481. [Google Scholar] [CrossRef]
- Schleuss, P.-M.; Widdig, M.; Heintz-Buschart, A.; Guhr, A.; Martin, S.; Kirkman, K.; Spohn, M. Stoichiometric controls of soil carbon and nitrogen cycling after long-term nitrogen and phosphorus addition in a mesic grassland in South Africa. Soil Biol. Biochem. 2019, 135, 294–303. [Google Scholar] [CrossRef]
- Entwistle, E.M.; Zak, D.R.; Argiroff, W.A. Anthropogenic N deposition increases soil C storage by reducing the relative abundance of lignolytic fungi. Ecol. Monogr. 2018, 88, 225–244. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, H.; Wu, Y.; Wang, J.; Zhao, Z.; Li, Y.; Qiao, L.; Chen, K.; Liu, G.; Xue, S. Direct and indirect influences of long-term fertilization on microbial carbon and nitrogen cycles in an alpine grassland. Soil Biol. Biochem. 2020, 149, 107922. [Google Scholar] [CrossRef]

| Test Processing | Mulching Practices | Logogram | Number of Years | Ground Cover Material |
|---|---|---|---|---|
| Different years of grass mulching | Clear tillage control | CK | 15 years | Clearing cover |
| 6a white clover mulch | 6a WC | 6 years | White clover (Trifolium repens Linn.) | |
| 15a white clover mulch | 15a WC | 15 years | White clover (Trifolium repens Linn.) | |
| Different grass species of grass mulching | Clear tillage control | CK | 15 years | Clearing cover |
| 15a white clover mulch | 15a WC | 15 years | White clover (Trifolium repens Linn.) | |
| 15a orchard grass mulch | OG | 15 years | Orchard grass (Dactylis glomerate Linn.) | |
| 15a small crown flower mulch | CV | 15 years | Small crown flower (Coronilla varia Linn.) | |
| Different kinds of ground cover measures | Clear tillage control | CK | 15 years | Clearing cover |
| 9a ryegrass mulch | RE | 9 years | Ryegrass (Lolium perenne Linn.) | |
| 9a cornstalk mulch | CS | 9 years | Corn stalk | |
| 9a black ground fabric mulch | BF | 9 years | Polyethylene black floor cloth (1 mm) |
| Test Processing | Mulching Practices | pH | SOC (g∙kg−1) | TN (g∙kg−1) | TP (g∙kg−1) | AP (mg∙kg−1) | NO3−-N (mg∙kg−1) | NH4+-N (mg∙kg−1) | WC (%) |
|---|---|---|---|---|---|---|---|---|---|
| Different years of grass mulching | CK | 8.40 ± 0.12 a | 13.88 ± 0.91 c | 1.11 ± 0.13 b | 0.81 ± 0.07 b | 10.80 ± 2.28 b | 8.95 ± 6.14 a | 2.53 ± 0.28 b | 0.14 ± 0.01 a |
| 6a WC | 8.37 ± 0.06 a | 18.23 ± 0.64 b c | 1.13 ± 0.08 b | 1.42 ± 0.10 a | 34.83 ± 1.93 a | 23.87 ± 8.61 a | 5.49 ± 1.29 a | 0.15 ± 0.00 a | |
| 15a WC | 8.32 ± 0.03 a | 23.14 ± 1.89 a | 1.51 ± 0.11 a | 0.82 ± 0.09 b | 14.27 ± 2.81 b | 21.28 ± 6.40 a | 6.48 ± 0.73 a | 0.14 ± 0.01 a | |
| Different grass species of grass mulching | CK | 8.40 ± 0.12 a | 13.88 ± 0.91 c | 1.11 ± 0.13 c | 0.81 ± 0.07 a | 10.80 ± 2.28 a | 8.95 ± 6.14 a | 2.53 ± 0.28 c | 0.14 ± 0.01 a |
| 15a WC | 8.32 ± 0.03 a | 23.14 ± 1.89 b | 1.51 ± 0.11 b | 0.82 ± 0.09 a | 14.27 ± 2.81 a | 21.28 ± 6.40 a | 6.48 ± 0.73 a | 0.14 ± 0.01 a | |
| OG | 8.40 ± 0.09 a | 23.31 ± 1.53 b | 1.24 ± 0.07 c | 0.92 ± 0.08 a | 9.63 ± 1.07 a | 20.38 ± 21.98 a | 3.72 ± 0.53 b | 0.14 ± 0.01 a | |
| CV | 8.26 ± 0.13 b | 34.36 ± 3.74 a | 2.04 ± 0.18 a | 0.91 ± 0.05 a | 9.95 ± 2.38 a | 27.36 ± 7.92 a | 4.12 ± 0.80 b | 0.14 ± 0.02 a | |
| Different kinds of ground cover measures | CK | 8.40 ± 0.12 a | 13.88 ± 0.91d | 1.11 ± 0.13 b | 0.81 ± 0.07 c | 10.80 ± 2.28 c | 8.95 ± 6.14 b | 2.53 ± 0.28 b | 0.14 ± 0.01 b |
| RE | 8.30 ± 0.03 a | 22.57 ± 1.60 c | 1.25 ± 0.10 b | 1.02 ± 0.14 c | 20.09 ± 3.54 c | 15.00 ± 2.61 b | 4.63 ± 1.23 a | 0.15 ± 0.01 b | |
| CS | 7.79 ± 0.08 b | 40.46 ± 3.95 a | 2.13 ± 0.37 a | 3.00 ± 0.61 b | 95.64 ± 7.52 b | 41.54 ± 4.03 a | 3.88 ± 0.57 a b | 0.22 ± 0.02 a | |
| BF | 7.66 ± 0.05 c | 36.54 ± 2.09 a b | 2.00 ± 0.13 a | 3.59 ± 0.35 a | 74.30 ± 16.27 a | 58.46 ± 11.71 a | 3.79 ± 0.64 a b | 0.21 ± 0.01 a |
| Test Processing | Mulching Practices | MBC (mg∙kg−1) | MBN (mg∙kg−1) | MBP (mg∙kg−1) |
|---|---|---|---|---|
| Different years of grass mulching | CK | 148.55 ± 6.78 b | 10.42 ± 0.87 a | 3.57 ± 0.16 b |
| 6a WC | 255.27 ± 9.43 a | 9.76 ± 2.86 a | 4.31 ± 0.81 b | |
| 15a WC | 142.21 ± 6.59 b | 9.48 ± 1.36 a | 6.23 ± 2.01 a | |
| Different grass species of grass mulching | CK | 148.55 ± 6.78 c | 10.42 ± 0.87 b | 3.57 ± 0.16 c |
| 15a WC | 142.21 ± 6.59 c | 9.48 ± 1.36 b | 6.23 ± 2.01 b | |
| OG | 396.21 ± 8.18 a | 10.68 ± 0.30 b | 6.84 ± 0.39 b | |
| CV | 245.67 ± 7.58 b | 15.75 ± 1.08 a | 9.53 ± 1.19 a | |
| Different kinds of ground cover measures | CK | 148.55 ± 6.78 c | 10.42 ± 0.87 a | 3.57 ± 0.16 c |
| RE | 314.92 ± 6.91 a | 5.43 ± 1.74 c | 6.17 ± 1.14 c | |
| CS | 315.10 ± 8.56 a | 8.23 ± 0.48 b | 13.24 ± 1.95 b | |
| BF | 183.37 ± 3.71 b | 6.64 ± 2.06 b c | 21.52 ± 4.19 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Li, H.; Liang, M.; Wu, Y.; Zhao, Z.; Li, Y.; Liu, G.; Xue, S. Kinetic Parameters of Soil Enzymes and Temperature Sensitivity Under Different Mulching Practices in Apple Orchards. Agronomy 2025, 15, 617. https://doi.org/10.3390/agronomy15030617
Jiang Y, Li H, Liang M, Wu Y, Zhao Z, Li Y, Liu G, Xue S. Kinetic Parameters of Soil Enzymes and Temperature Sensitivity Under Different Mulching Practices in Apple Orchards. Agronomy. 2025; 15(3):617. https://doi.org/10.3390/agronomy15030617
Chicago/Turabian StyleJiang, Yaokun, Huike Li, Meng Liang, Yang Wu, Ziwen Zhao, Yuanze Li, Guobin Liu, and Sha Xue. 2025. "Kinetic Parameters of Soil Enzymes and Temperature Sensitivity Under Different Mulching Practices in Apple Orchards" Agronomy 15, no. 3: 617. https://doi.org/10.3390/agronomy15030617
APA StyleJiang, Y., Li, H., Liang, M., Wu, Y., Zhao, Z., Li, Y., Liu, G., & Xue, S. (2025). Kinetic Parameters of Soil Enzymes and Temperature Sensitivity Under Different Mulching Practices in Apple Orchards. Agronomy, 15(3), 617. https://doi.org/10.3390/agronomy15030617





