Abstract
The expansion of sugarcane production has led to increased nitrogen (N) fertilizer use, contributing to greenhouse gas emissions and environmental concerns. Optimizing N management is crucial for sustainable agriculture. Soil apparent electrical conductivity (ECa) has emerged as a valuable tool for mapping soil spatial variability and yield potential, potentially guiding more efficient fertilization strategies. This study evaluated sugarcane yield and N responsiveness across two areas with distinct soil types over two crop cycles. Experimental plots were classified into high (HC) and low (LC) ECa zones, with randomized blocks receiving four N rates and a control. Higher yields were generally observed in HC plots, except for the second ratoon in area 2 (Ultisol). HC plots required lower N rates to achieve maximum yield compared to LC plots. In area 1 (higher clay content), optimal N rates were lower than in area 2 (lower clay content), indicating that yield potential is linked to soil attributes and spatial variability. Although ECa alone may not define precise N doses, it effectively identifies zones with different yield potentials, supporting site-specific N management. These findings highlight the potential of ECa to improve nitrogen use efficiency and contribute to more sustainable sugarcane production.
1. Introduction
Sugarcane is one of the most important crops in tropical and subtropical regions of the planet, as it generates high value-added products considered ecologically and economically viable. In the last crop season, Brazil produced about 713 million tons of sugarcane in approximately 8.3 million hectares, with São Paulo state accounting for more than 50% of the production [1].
Proper soil management correlates with high yields and the longevity of sugarcane plantations. Physical, chemical, and biological soil attributes must provide a physical environment for roots to explore the largest volume of soil possible. This enables plants to meet their demand for water and nutrients, resulting in the full potential of their yield [2]. Nitrogen (N) stands out as one of the essential elements involved in several physiological processes closely linked to increased crop yield. However, uncertainties in quantifying N in the soil to enable the most efficient and economical way of supplying this nutrient to the plant still need to be resolved. Some authors observed some promising results when testing chemical extractants to determine N in soils in 21 sugarcane field experiments [3].
Due to the dynamics of N in the soil and the lack of extraction methods for accurate quantification of the element, the amount of N fertilizer recommended takes into account the expected crop yield [4]. To meet the nutritional needs of the plant, a significant difference in the N amounts applied to the soil (i.e., 120 to 200 kg ha−1) exists [5]. In many cases, this recommendation range leads to the application of excessive N concentrations without the expected crop response. The problem is aggravated when the N source used is urea. Under high soil moisture conditions, urea quickly volatilizes, releasing nitrous oxide (N2O) into the atmosphere [6,7,8]. Thus, in addition to the low fertilization efficiency, excess N contributes to the greenhouse effect and climate change.
Given these facts, the N fertilization recommendation should be optimized considering other sources of variation such as soil attributes (e.g., pH, cation exchange capacity, organic matter content, clay content, and soil density), climatic conditions (temperature and rainfall), and agronomic practices (soil preparation and crop rotation) [9]. Thus, being able to incorporate parameters into more efficient fertilization recommendation models will allow for the rationalization of this input. Otto et al. [10] observed that the maintenance of the remaining plant material left on the soil surface, the use of industrial residues, and rotation with legumes are factors that can reduce the response of sugarcane to N fertilization. In addition, the soil and not the fertilizer itself is the main supplier of N to sugarcane [11,12], which partially explains the low N fertilization efficiency. Only 10 to 20% of the total N accumulated by the crop comes from the fertilizer at the harvest time, but in early developmental stages, this contribution is more significant and can be above 50% [12]. As the crop develops, the contribution of N from the soil becomes the main source. Therefore, the recommended amount of N for sugarcane should focus on supporting the crop’s early developmental stages, considering that the remainder can be supplemented through the mineralization of organic matter in the soil, reducing the risk of N mining from inadequate fertilization.
As the soil is the main N supplier to plants and determining soil N contents is quite challenging, researchers have looked for tools that can help optimize N application in sugarcane plantations, including remote and proximal sensing [13]. Apparent soil electrical conductivity (ECa) values are adequate to evaluate the variability of chemical and physical soil attributes (e.g., soil moisture and temperature) [14,15,16,17,18], as well as the crop yield potential [18,19]. Although ECa alone may not provide a direct measurement of N content or determine exact fertilizer doses, its ability to identify zones with different yield potentials represents a significant step toward more targeted fertilization strategies. By mapping soil spatial variability, ECa can help delineate areas where crop responsiveness to N is likely to differ, enabling a more efficient allocation of inputs. This study seeks to highlight the role of ECa as a complementary tool in site-specific nitrogen management, bridging the gap between generalized fertilization practices and more precise, localized interventions that can enhance yield while minimizing environmental impacts.
In this context, the characterization of the soil spatial variability using ECa sensors can help to optimize the application of N in sugarcane plantations in terms of time and efficiency, ensuring an economic return to the farmers. Here, we hypothesize that the spatial variability of the ECa would allow us to define regions in the sugarcane field that could be optimized for N fertilization. Therefore, our objective was to investigate the response of sugarcane in terms of yield and N fertilization as a function of the spatial soil variability identified by the ECa. Finally, we propose a conceptual framework of future studies would investigate ECa variability for nitrogen management.
2. Materials and Methods
2.1. Study Areas
The study areas are in the municipalities of Ipaussu (area 1—23°02′30.25″ S—49°34′40.66″ W) and Monte Alto (area 2—21°13′18.20″ S—48°27′41.97″ W), São Paulo, Brazil. The soils were classified as “Latossolo Vermelho Distroférrico” (Oxisols—Soil Taxonomy) in area 1 and “Argissolo Vermelho Amarelo eutrófico” (Ultisols—Soil Taxonomy) in area 2 according to “Sistema Brasileiro de Classificação do Solo” (SiBCS), in which sugarcane varieties RB966928 and CTC 9005HP were planted, respectively. The sugarcane varieties were chosen by mill’s agronomists according to soil types. ECa was measured at a spacing of 1.0 m between soils using the electromagnetic induction sensor EM38-MK2® (Geonics, ON, Canada) pulled by a quadricycle and connected to a GPS receiver. Subsequently, the thematic map of the ECa spatial distribution of the respective areas was produced using the ordinary kriging interpolation method (Figure 1). High (values above the median value of the dataset) and low (values equal to or below the median of the data) zones were defined in both areas.
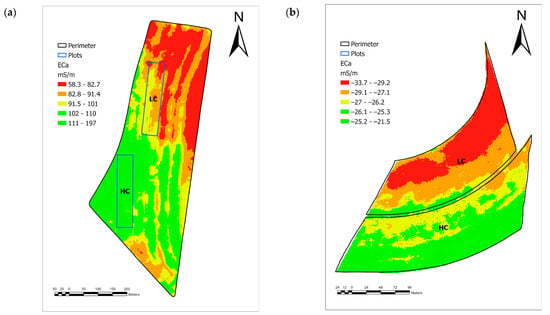
Figure 1.
Thematic map of ECa in study areas 1 (a) and 2 (b). HC and LC are sites with high and low apparent electrical conductivity, respectively.
2.2. Experimental Design
2.2.1. Area 1
In study area 1, treatments [N rates of 0 (control—no fertilization) 50, 100, 150, and 200 kg ha−1)] were established in high and low ECa zones. N was applied as ammonium nitrate (NH4NO3) in October 2019 (first ratoon) and November 2020 (second ratoon) 150 days after harvest (DAH). Treatments were arranged in randomized block design with four replications. Each plot consisted of six rows of sugarcane (40 m long, spaced 1.5 m apart), with the four central rows considered the useful plot area and the two lateral rows being the borders.
2.2.2. Area 2
In study area 2, treatments [N rates of 0 (control—no fertilization), 0, 54, 109, 163, and 218 kg ha−1 of N, respectively] followed a randomized block design treatment and four replications were established in high and low ECa zones. N was applied as NH4NO3 during 2020 (first ratoon) and 2021 (second ratoon) crop cycles. Each plot (900 m2) consisted of four sugarcane rows (150 m long, spaced 1.5 m apart), the two central rows considered the useful plot area, and the two lateral rows being the borders.
2.3. Evaluated Parameters
2.3.1. Soil
Soil samples (0.00–0.20 m and 0.20–0.40 m depths) were collected for characterizing soil chemical attributes (pH, Ca, Mg, K, P, H + Al, and organic matter) and soil granulometry. Ten randomized subsamples were collected at each plot in both areas to characterize the soil fertility. The soil cation exchange capacity (CEC), sum of bases (SB), and base saturation (BS) were calculated as described by Raij et al. [20].
2.3.2. Crop Yield
Area 1
The sugarcane yield for each plot was evaluated at the end of the crop cycle, specifically at 411 days after harvest (DAHs) during the 1st and 2nd ratoon seasons. The harvest process was carried out mechanically to ensure uniformity across all plots. After cutting, the harvested sugarcane stalks were collected and transported using a transshipment truck equipped with a calibrated load cell. The load cell measured the total mass of stalks harvested from each plot with high precision. The mass of stalks recorded by the load cell was normalized and converted into yield expressed in mega grams per hectare (Mg ha⁻−1). This approach provided an accurate and standardized measurement of sugarcane biomass accumulation across all experimental plots.
Area 2
Crop yield was estimated at five points distributed in the two central rows of each plot. At each point, stalks (1.5 m in the crop rows) were harvested and weighed, and the average mass of stalks was used to estimate crop yield (Mg ha−1) in each plot. Unlike area 1, here the evaluation was carried out at different developmental stages (at 103, 179, 220, 271, and 366 DAH in the 1st ratoon and at 88, 117, 173, and 471 DAH in the 2nd ratoon), allowing for a more detailed understanding of biomass accumulation and growth dynamics throughout the crop cycle (Figure 2).
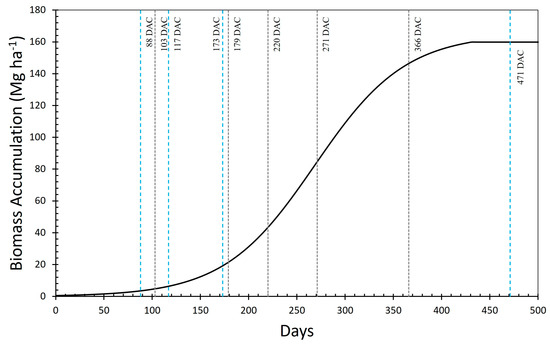
Figure 2.
Theoretical biomass accumulation curve of sugarcane in the study area 2 during 1st (black dotted lines) and 2nd (blue dotted lines) ratoons.
2.4. Data Analyses
The first step of data analysis aimed to identify outliers that could affect subsequent analyses. For all data collected in the field or laboratory, values greater or less than three standard deviations from the mean were considered outliers. After excluding outliers, data were submitted to descriptive analysis (measures of central tendency and dispersion including mean, median, standard deviation, coefficient of variation, maximum and minimum, and asymmetry and kurtosis). The effects of N fertilization on ECa, soil attributes, and crop yield were assessed by analysis of variance (ANOVA). When statistically different, the means were compared by the Tukey test (p-value < 0.05). Additionally, Pearson’s coefficient (r) was used to verify the correlation between the variables. Finally, for each experimental area, crop yield data were adjusted (α = 0.05) to regression models as a function of N rates.
3. Results
In study area 1, higher values of soil chemical attributes and soil granulometry were observed where ECa values were higher (HC plot), except for P content in the 0.00–0.20 m depth (Table 1). In contrast, the opposite was observed in study area 2, i.e., lower values of attributes were found where the ECa values were higher, except for P content in both depths and Mg content in the 0.00–0.20 m depth. In area 2, negative ECa values were observed, which according to Killick [21] are theoretically impossible under ideal conditions. However, indirect ECa measurements, such as those derived from the ratio of secondary to primary magnetic fields using electromagnetic induction (EMI) equipment, can occasionally result in negative values. These anomalies are often attributed to interference from nearby metal objects, improper calibration of the instrument, or localized environmental factors that disrupt the signal. Additionally, other authors [22,23] have documented negative ECa values in field mappings like here, where metal interference was absent, and the calibration of the EMI equipment was verified. This suggests that specific soil properties, local conditions, or unique interactions between the sensor and the soil environment might also contribute to this phenomenon.

Table 1.
Soil chemical and physical fertility and apparent electrical conductivity (ECa) of the HC and LC plots of study areas 1 and 2 in the 0.00–0.20 m and 0.20–0.40 m depths.
Soil attributes differed in absolute terms between the HC and LC plots for both study areas (Table 1), and these differences were reflected in the crop yield in the two crop seasons evaluated (Table 2). In study area 1, the HC plot produced 97.53 Mg ha−1 and 82.91 Mg ha−1 (average of 90.22 Mg ha−1 year−1) for the first and second ratoon, respectively, whereas the LC plot produced 89.63 Mg ha−1 and 71.14 Mg ha−1 (average of 80.39 Mg ha−1 year−1). In study area 2, the HC plot produced 85.18 Mg ha−1 and 86.79 Mg ha−1 (average of 85.93 Mg ha−1 year−1) for the first and second ratoon, respectively, while the LC plot produced 81.11 Mg ha−1 and 92.83 Mg ha−1 (average of 86.97 Mg ha−1 year−1). These data correspond to differences of approximately +4.9 Mg ha−1 year−1 and −0.5 Mg ha−1 year−1 between HC and LC plots for study areas 1 and 2, respectively. Thus, sugarcane yield directly correlates with soil type as a function of ECa, but high ECa patches (HC plot) have lower yields in study area 2, an Ultisol. A higher coefficient of variation (CV) was observed for plots LC compared with HC for both areas and both seasons.

Table 2.
Descriptive statistics of sugarcane yield (Mg ha−1) in study areas 1 and 2 in their plots of high (HC) and low (LC) apparent soil electrical conductivity (ECa).
In study area 1, yield was positively correlated with ECa in HC plots for the first and second ratoon cycles (r = 0.51 and 0.49, respectively—Table 3). In study area 2, this correlation was only seen in the first ratoon (r = 0.43—Table 3). The N rates statistically differed in terms of yield for the plot’s HC and LC for both areas (except for the first ratoon in the LC plot for area 1 and second ratoon in the HC plot in area 2) (Table 4). Yield differed between HC (97.53 and 82.91 Mg ha−1 for the first and second ratoons, respectively) and LC (89.63 and 71.14 Mg ha−1 for the first and second ratoons, respectively) plots in study area 1 for both ratoons. For study area 2, HC (86.79 Mg ha−1) and LC (92.83 Mg ha−1) differed only in the second ratoon. Except for the second ratoon of evaluation in study area 2, HC plots tended to have higher yield compared to LC plots. HC and LC differed for both areas in both ratoons, maintaining the same N rates (capital letters in Table 4). In study area 1, yield was higher in HC compared to the LC plot by the same N rates, while in study area 2, the second ratoon showed an inverse trend, with lower yields in the HC compared to the LC plot by the same N rates.

Table 3.
Pearson correlation coefficient (r) between the attributes evaluated in the experimental fields of high (HC plot—below the main diagonal) and low (LC plot—above the main diagonal) apparent electrical conductivity of the soil in study areas 1 and 2.

Table 4.
Analysis of variance of yield in plots of high (HC) and low (LC) apparent soil electrical conductivity (ECa) as a function of N rates applied in study areas 1 and 2.
Concerning the regression of N rates as a function of yield, HC and LC plots were adjusted to the linear and quadratic models, respectively, for study area 1 in both ratoon cycles. In the HC plot, the highest N rates led to lower yields and fitted the linear model negatively in both seasons evaluated (Figure 3). For study area 2, the N rates were adjusted to the quadratic model in the LC plot for both seasons evaluated but only in the first ratoon for the HC plot. The quadratic adjustment in the LC plots of both areas and seasons evaluated was derived to find optimal N rates to be applied, which were 109.30 kg ha−1 and 109.50 kg ha−1 (area 1) and 134.30 kg ha−1 and 185.70 kg ha−1 (area 2) for the first and second ratoon cycles, respectively. For the HC plot of study area 2 in the second ratoon, the optimal N rate was equal to 146.02 kg ha−1.
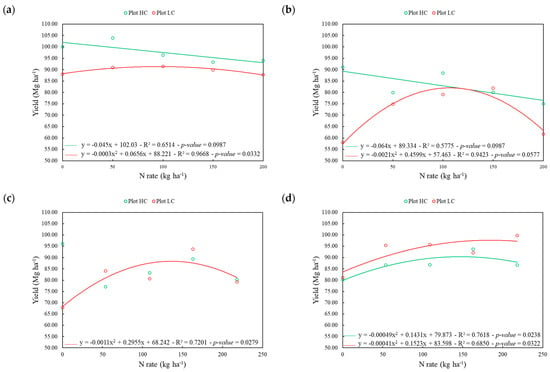
Figure 3.
Linear and quadratic fit of yield as a function of N rates for the high (HC—green) and low (LC—red) apparent soil electrical conductivity (ECa) plots in study areas 1 (a,b) and 2 (c,d) for the first (a,c) and second (b,d) ratoon. Adjustment of HC plot on area 2 in the first ratoon was not significant.
Evaluating the biomass accumulation of study area 2, the HC plot was always superior to the LC plot over time, except at 455 DAH in the second ratoon (Figure 4). The difference between HC and LC plots was small until 173 DAH and greater at 271 DAH of the first crop season (first ratoon) when an inflection in the biomass accumulation curve was observed (Figure 4).
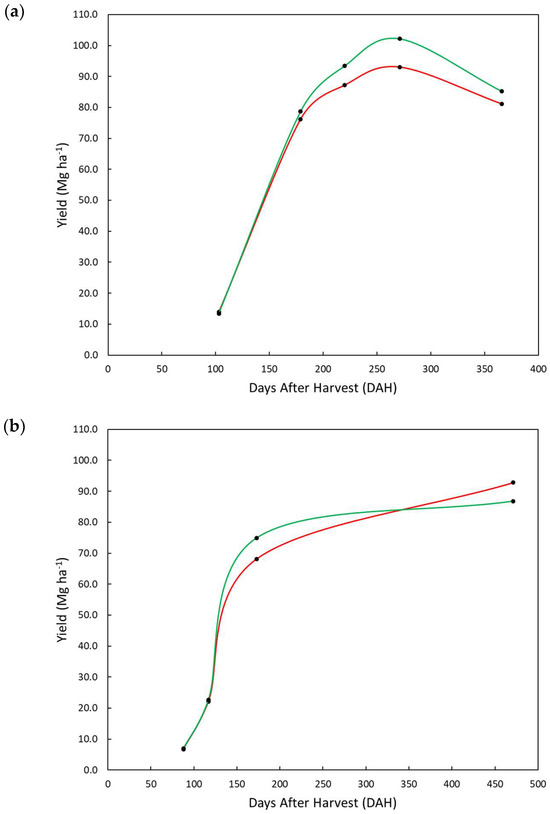
Figure 4.
Biomass accumulation curve of sugarcane in high (HC—green) and low (LC—red) apparent soil electrical conductivity (ECa) plots of study area 2 during first (a) and second (b) ratoons.
4. Discussion
ECa has been increasingly used to assess soil spatial variability in crop fields worldwide [24] and in Brazil [23], including clay content and soil organic matter [23], soil pH [25], crop yield potential [26], and for defining management zones [27]. However, the application potential of this technology associated with the crop response to N fertilization is still little explored.
Given that ECa is highly correlated with the clay content (Table 1) and, consequently, with the crop yield potential (Table 2 and Table 3), it can be an auxiliary tool for establishing N recommendations in sugarcane. Moreover, it is important to note that the soils tested in this study represent the predominant soil types in the São Paulo state, which enhances the relevance of these findings for this region [28]. However, our findings suggest that soil type and its attributes also influence ECa. As observed in study area 2, the soil clay content was higher for the low-conductivity plots (LC plot). The soil in this area, Ultisol, has higher sand content in its superficial layers and therefore lower levels of chemical attributes. This explains the fact that high-conductivity sites (HC plot) have lower fertility. Although the soil in study area 1 has a very clayey texture in both plots, a small difference in clay content (4 g kg−1 in the 0.00–0.20 m depth) was relatively detected by measuring the soil ECa by the EMI equipment. Although ECa does not directly measure clay content, these findings underscore the tool’s strong potential for delineating yield potential across variable ECa field conditions. Despite the inverse correlation between ECa and soil texture, fertility levels also differed between the plots in area 2.
One critical factor influencing ECa measurements is soil moisture, as it directly affects the conductivity of the soil. ECa measurements taken during periods of extreme soil moisture conditions, such as waterlogged or drought conditions, may obscure true variability in soil texture. In this study, we ensured measurements were conducted under non-extreme soil moisture conditions to minimize this effect. Additionally, the ECa mappings were performed in the same soil moisture condition, that is, during the same day. In qualitative terms, the ECa is an excellent parameter for distinguishing the spatial variability of crop yield within the field [23]. In quantitative terms, ECa positively correlated with yield only in HC plots, showing that in regions of low conductivity, characterized by lower relative fertility levels, other factors may govern crop yield besides soil fertility. Concerning the crop response to N fertilization, lower N rates can be applied to maintain crop yield in the HC plots (Figure 3), characterized by high conductivity and higher soil fertility levels. Our results showed that an optimal N rate can be defined in the LC plots. For the second ratoon in study area 2, where N rates were adjusted to a quadratic model in both plots, the optimal N rate was lower for the HC (146.02 kg ha−1) than LC (185.70 kg ha−1) plot. Despite the lower yields in the HC plot for study area 2 in the second ratoon (Figure 3d), the HC plot showed a lower optimal N rate than the LC plot. Also, in study area 1, characterized by high clay content, the optimal N rates are lower compared to study area 2. These findings show that high ECa sites (HC plot) tend to need lower N rates than LC plots (low ECa sites).
Historical fertilizer applications, particularly blanket N applications, may have contributed to spatial heterogeneity in nitrogen availability, influencing crop responses to N fertilization. Variations in past over- or under-fertilization likely created uneven residual N levels, which could impact nitrogen use efficiency (NUE) and crop productivity in the studied zones. Future research should include detailed assessments of residual soil nitrogen levels and historical fertilization records to better understand their influence on current crop performance. Incorporating these insights into N management strategies will allow for a more refined approach to site-specific fertilization.
The results of the present study reinforce the role of ECa as a promising tool not only for mapping soil spatial variability but also for guiding N fertilization strategies in sugarcane fields. By demonstrating the capacity of ECa to delineate zones with different yield potentials, this research provides practical evidence that ECa can be integrated into site-specific management approaches. This represents an important step toward reducing the reliance on generalized fertilization recommendations, enabling more precise and sustainable input applications. Even in the absence of direct N uptake measurements, the ability to identify areas of greater and lesser responsiveness to N through ECa mapping highlights the potential of this technique to enhance yield efficiency and optimize resource allocation in sugarcane cultivation. However, it is important to acknowledge that this study evaluated only two crop cycles, which limits the insights into the long-term effects of N fertilization strategies and soil variability. Future multi-year studies are recommended to better understand the consistency and robustness of these findings over time.
The recent study of Sanches and Otto [29], together with the present research, indicates that areas with higher yield potential are less responsive to N. If this is true for most areas cultivated with sugarcane in Brazil, the criterion of fertilization recommendation by yield expectation using a single fertilization factor for different levels of yield may not only be penalizing yield in the most restrictive environments but also oversizing the ratoon fertilization in the most productive environments. Although N fertilization recommendations based on the expected yield have been adopted for more than 50 years throughout the United States and in several other regions of the world, the definition of N use factors has been based on a few agronomic principles [30]. The main advantage of fertilizer recommendation systems based on expected yield is their ease of interpretation, which has facilitated their wide-scale adoption [31]. However, most of the time, those systems do not have a solid agronomic basis and can lead to over- or under-fertilization, as shown here. While this study was conducted in two specific areas, it is important to emphasize that these areas are representative of São Paulo’s predominant soil types, making the findings regionally relevant [28]. Future studies should focus on testing this approach in different soil types and climates to enhance the regionalization and generalizability of these findings across other sugarcane-producing regions. The same may occur indiscriminately when using the factors of 1.0 kg N Mg−1 or 1.2 kg N Mg−1 previously adopted in burnt or raw sugarcane in Brazilian conditions, respectively.
Most of the studies in the literature that assessed technologies to improve N fertilization in sugarcane fields focused on proximal [13,32] or remote [33] sensing by assessing biomass crop reflectance indexes. On the other hand, few studies in the literature proposed to assess N responsiveness assessing soil spatial variability parameters like ECa [34,35]. Although these last studies assess ECa on N responsiveness, neither of them was for sugarcane fields like the present study. So, as the soil is the main N supplier to plants and determining soil N contents is quite challenging, the present findings could help farmers to improve N fertilization by assessing the ECa spatial variability. On-farm experiments could be proposed in this sense to better advance this present topic for precision agriculture in the digital industry.
Although N recommendation methods based on soil analysis have been sought for decades with encouraging results [36,37,38], they have not been widely adopted so far. No method has identified the precise N rates for the fertilization of sugarcane ratoon cycles [3]. Even when such a method will be available in the future, advances are needed to calibrate it under field conditions. In this context, tools that assist in the optimization of N application, such as the measurement of spatial variability of ECa presented here, can improve the management of Brazilian sugarcane plantations toward low-carbon and more sustainable agriculture.
Conceptual Framework
The proposed conceptual framework (Figure 5) leverages ECa data to address the challenges of site-specific N management in agricultural systems. By systematically combining field data collection, spatial analysis, and tailored fertilization strategies, this framework provides a structured approach for improving N application. It encompasses five key steps, namely data collection, data analysis, N management recommendations, implementation, and continuous improvement. Each step builds upon the previous one, forming an iterative process that adapts to varying field conditions.
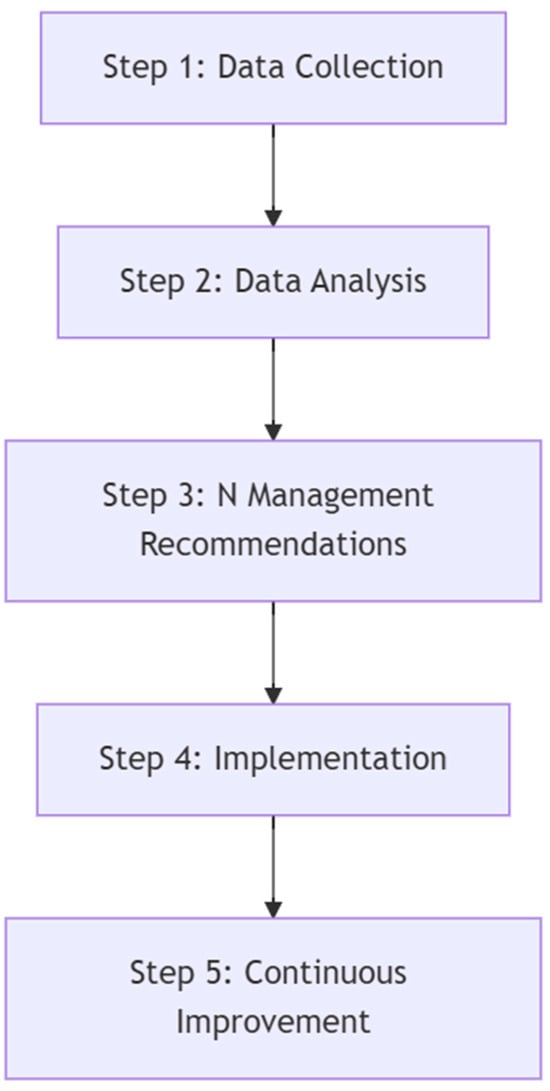
Figure 5.
Conceptual framework for using ECa data in nitrogen management.
This framework aims to bridge the gap between technological advancements in precision agriculture and practical field applications. By incorporating ECa data into decision-making processes, it empowers farmers to make informed choices about N fertilization, enhancing both productivity and environmental stewardship. The next sections describe each step of the framework, highlighting its role in achieving precise and adaptive N management strategies.
Step 1: Data Collection
The first step in the framework involves comprehensive data collection. First of all, field mapping is conducted using electromagnetic induction (EMI) tools and GPS to measure apparent electrical conductivity (ECa). After that, divide the field into two zones (quantile division) with high and low ECa values. After that, soil sampling is performed in representative areas across ECa zones to analyze critical attributes such as soil texture, organic matter, and other nutrient levels. Once the ECa zones are defined and sampled, experimental N response plots are installed across the identified zones to evaluate crop yield and response to varying N rates. These plots allow for the assessment of localized crop performance and soil behavior under different N management strategies. In addition, yield and biomass data are collected at the end of the crop cycle, with values normalized to account for plot size, providing a clear picture of yield and soil variability.
Step 2: Data Analysis
After data collection, the next step is to analyze the relationships between ECa, soil properties, and yield. Correlation analysis is employed to identify patterns and dependencies among these variables. Data from the N plots are specifically used to model crop response curves to N applications within each ECa-defined zone, providing insight into the variability of N use efficiency and yield potential across the field.
Step 3: N Management Recommendations
The third step focuses on investigating optimal N application strategies in zones characterized by high and low ECa. The results from N response plots are analyzed in conjunction with soil attributes to determine how these factors interact to influence crop yield and nitrogen use efficiency. This investigation aims to identify the best N application doses for each zone rather than providing immediate recommendations for large-scale implementation. By understanding these interactions, the framework lays the foundation for scaling validated strategies to broader areas in future applications.
Step 4: Implementation
Once insights from Step 3 are obtained, validated N management strategies are implemented in larger field areas. GPS-guided variable rate technology is employed to apply N based on the findings from previous steps. Monitoring crop performance during the growing season and after harvest is critical, as it provides feedback on the effectiveness of the implemented strategies and highlights areas for further refinement.
Step 5: Continuous Improvement
The final step emphasizes the importance of iterative improvement and regional adaptation. Data from multi-year studies and diverse fields are integrated to refine and validate the framework further. This approach ensures that N management strategies remain robust and adaptable across varying soil types, climatic conditions, and crop systems. The continuous feedback loop enhances the scalability and sustainability of precision N management using ECa.
5. Conclusions
Our results suggest that while soil apparent electrical conductivity (ECa) can reflect spatial variability in sugarcane yield, its direct use for optimizing N rates requires further investigation. Although higher yields were observed in high ECa sites (HC plots) compared to low ECa sites (LC plots) in most cases, exceptions, such as the second ratoon in study area 2, highlight the complexity of the relationship between ECa, soil attributes, and crop response to N fertilization.
For the HC plots, we observed that lower N rates are needed to reach the maximum yield potential. In study area 1, characterized by high clay content, the optimal N rates are lower compared to study area 2, which has lower clay contents. This is consistent with crop yield potential being intrinsically associated with N fertilization, in which lower N rates can be applied in areas of greater potential. Indeed, in the LC plots of study area 1 (higher clay contents), the optimal N dose was ~109 kg ha−1 against 134.30 kg ha−1 (first ratoon) and 185.70 kg ha−1 (second ratoon) in study area 2. However, the lack of consistent crop response to N across different areas and cycles indicates that other factors, such as soil N availability and plant N uptake, must be considered to refine site-specific N recommendations.
This study highlights ECa’s capacity to map soil variability and yield potential—an asset for guiding site-specific N management. While direct measurements of soil and plant N could further enhance interpretations, ECa-based zones already provide practical insights for optimizing fertilizer use. Nevertheless, broader soil types, climates, multi-year studies, and an actionable N management framework are needed and will be crucial to validate and expand these findings. Future research incorporating these elements can improve our understanding of ECa’s role in precision agriculture, advancing sustainable sugarcane management with more efficient fertilization strategies and reduced environmental impacts from excessive N applications.
Author Contributions
Conceptualization, G.M.S. and J.E.C.; methodology, G.M.S., R.O., and J.E.C.; software, G.M.S.; validation, G.M.S. and H.M.F.; formal analysis, G.M.S.; investigation, G.M.S., H.M.F., and A.S.N.; resources, R.O. and J.E.C.; data curation, G.M.S. and H.M.F.; writing—original draft preparation, G.M.S.; writing—review and editing, R.O. and J.E.C.; supervision, R.O. and J.E.C.; project administration, R.O. and J.E.C.; funding acquisition, R.O. and J.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the São Paulo Research Foundation (grants #2022/15875-1, #2016/13461-4, #2018/10225-3) and Brazilian National Council for Scientific and Technological Development (grant #314811/2023-0 to Rafael Otto).
Data Availability Statement
The data supporting the findings of this study are not publicly available due to restrictions imposed by confidentiality agreements and the proprietary nature of the data. The datasets were generated through field experiments conducted in collaboration with private agricultural partners and as such contain sensitive information that cannot be shared. Access to the data may be considered upon reasonable request subject to approval by all involved parties and the signing of a non-disclosure agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Companhia Nacional do Abastecimento (CONAB). Acompanhamento da Safra Brasileira; Cana-de-Açúcar, SAFRA 2024/25 Terceiro Levantamento Novembro/2024; CONAB: Brasília, Brazil, 2024; p. 56. [Google Scholar]
- Franco, H.C.J.; Castro, S.G.Q.; Sanches, G.M.; Kölln, O.T.; Bordonal, R.O.; Borges, B.M.M.N.; Borges, C.D. Alternatives to Increase the Sustainability of Sugarcane Production in Brazil under High Intensive Mechanization. In Sustainable Sugarcane Production; Singh, P., Tiwari, A.K., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2018; p. 426. [Google Scholar]
- Mariano, E.; Otto, R.; Montezano, Z.F.; Cantarella, H.; Trivelin, P.C.O. Soil Nitrogen Availability Indices as Predictors of Sugarcane Nitrogen Requirements. Eur. J. Agron. 2017, 89, 25–37. [Google Scholar] [CrossRef]
- Spironello, A.; Van Raij, B.; Penatti, C.P.; Cantarella, H.; Morelli, J.L.M.; Orlando Filho, J.; Landell, M.G.A.; Rossetto, R. Cultara da Cana-de-Açúcar. In Recomendação de Adubação e Calagem para o Estado de São Paulo, 2nd ed.; Van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 237–239. [Google Scholar]
- Cantarella, H.; Rossetto, R. Fertilizers for Sugarcane. In Sugarcane Bioethanol—R&D for Productivity and Sustainability; Cortez, L.A.B., Ed.; Edgard Blücher: São Paulo, Brazil, 2014; pp. 405–422. [Google Scholar]
- Crutzen, P.J.; Mosier, A.R.; Smith, K.A.; Winiwarter, W. N2O Release from Agro-Biofuel Production Negates Global Warming Reduction by Replacing Fossil Fuels. Atmos. Chem. Phys. 2008, 8, 389–395. [Google Scholar] [CrossRef]
- de Vries, F.; Bardgett, R. Plant-Microbial Linkages and Ecosystem N Retention: Lessons for Sustainable Agriculture. Front. Ecol. Environ. 2012, 10, 425–432. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Vargas, V.P.; Carmo, J.B.; Martins, A.A.; Sousa, R.M.; Andrade, C.A. Enhanced-Efficiency Fertilizers in Nitrous Oxide Emissions from Urea Applied to Sugarcane. J. Environ. Qual. 2015, 44, 423–430. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Sahrawat, K.L.; Bery, W.L.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, M. Scope and Strategies for Regulation of Nitrification in Agricultural Systems. Plant Sci. 2006, 25, 303–335. [Google Scholar]
- Otto, R.; Castro, S.A.Q.; Mariano, E.; Castro, S.G.Q.; Franco, H.C.J.; Trivelin, P.C.O. Nitrogen Use Efficiency for Sugarcane-Biofuel Production: What is the Next? BioEnergy Res. 2016, 9, 1272–1289. [Google Scholar] [CrossRef]
- Dourado-Neto, N.D.; Powlson, D.; Bakar, R.A.; Bacchi, O.O.S.; Basanta, M.V.; Cong, P.T.; Keerthisinghe, S.; Ismaili, M.; Rahman, S.M.; Reichardt, K.; et al. Multiseason Recoveries of Organic and Inorganic Nitrogen-15 in Tropical Cropping Systems. Soil Sci. Soc. Am. J. 2010, 74, 139–152. [Google Scholar] [CrossRef]
- Franco, H.C.J.; Otto, R.; Faroni, C.E.; Vitti, A.C.; Oliveira, E.C.A.; Trivelin, P.C.O. Nitrogen in Sugarcane Derived from Fertilizer under Brazilian Field Conditions. Field Crops Res. 2011, 121, 29–41. [Google Scholar] [CrossRef]
- Amaral, L.R.; Molin, J.P.; Schepers, J.S. Algorithm for Variable-Rate Nitrogen Application in Sugarcane Based on Active Crop Canopy Sensor. Agron. J. 2015, 107, 1513–1523. [Google Scholar] [CrossRef]
- Sudduth, K.A.; Kitchen, N.R.; Wiebold, W.J.; Batchelor, W.D.; Bollero, G.A.; Bullock, D.G.; Clay, D.E.; Palm, H.L.; Pierce, F.J.; Schuler, R.T.; et al. Relating Apparent Electrical Conductivity to Soil Properties across the Northcentral USA. Comput. Electron. Agric. 2005, 46, 263–283. [Google Scholar] [CrossRef]
- Molin, J.P.; Faulin, G.D.C. Spatial and Temporal Variability of Soil Electrical Conductivity Related to Soil Moisture. Sci. Agric. 2013, 70, 1–5. [Google Scholar] [CrossRef]
- Ekwue, E.; Bartholomew, J. Electrical Conductivity of Some Soils in Trinidad as Affected by Density, Water and Peat Content. Biosyst. Eng. 2011, 108, 95–103. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. Apparent Soil Electrical Conductivity Measurements in Agriculture. Comput. Electron. Agric. 2005, 46, 11–43. [Google Scholar] [CrossRef]
- McBratney, A.; Whelan, B.M.; Ancev, T.; Bouma, J. Future Directions of Precision Agriculture. Precis. Agric. 2005, 6, 7–23. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. Application of Soil Electrical Conductivity to Precision Agriculture: Theory, Principles, and Guidelines. Agron. J. 2003, 95, 455–471. [Google Scholar] [CrossRef]
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001; p. 285. [Google Scholar]
- Killick, M. An Analysis of the Relationship of Apparent Electrical Conductivity to Soil Moisture in Alluvial Recent Soils, Lower North Island, New Zealand. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2013. [Google Scholar]
- Cursi, D.E.; Gazaffi, R.; Hoffmann, H.P.; Brasco, T.L.; Amaral, L.R.; Dourado Neto, D. Novel Tools for Adjusting Spatial Variability in the Early Sugarcane Breeding Stage. Front. Plant Sci. 2021, 12, 749533. [Google Scholar] [CrossRef]
- Sanches, G.M.; Otto, R.; Adamchuk, V.; Magalhães, P.S.G. Spatial Variability of Soil Attributes by an Electromagnetic Induction Sensor: A Framework of Multiple Fields Assessment under Brazilian Soils. Biosyst. Eng. 2022, 216, 229–240. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; Bouma, J. Soil Sensing: A New Paradigm for Agriculture. Agric. Syst. 2016, 148, 71–74. [Google Scholar] [CrossRef]
- Sanches, G.M.; Magalhães, P.S.G.; Remacre, A.Z.; Franco, H.C.J. Potential of Apparent Soil Electrical Conductivity to Describe the Soil pH and Improve Lime Application in a Clayey Soil. Soil Tillage Res. 2018, 175, 217–225. [Google Scholar] [CrossRef]
- Sanches, G.M.; Franco, H.C.J.; Magalhães, P.S.G. Site-Specific Assessment of Spatial and Temporal Variability of Sugarcane Yield Related to Soil Attributes. Geoderma 2019, 334, 90–98. [Google Scholar] [CrossRef]
- Grego, C.R.; Speranza, E.A.; Rodrigues, C.A.G.; Ronquim, C.C.; Luchiari Junior, A.; Sanches, G.M.; Vaz, C.M.P.; Jorge, L.A.C.; Porto, V.H.B. Experimentação on-farm na Agricultura de Precisão. In Estudo de Caso 6—Zonas de Manejo e Adubação em Área de Cultivo de Cana-de-Açúcar na Fazenda Santa Helena, Ibaté, SP.; Pires, J.L.F., Brandão, Z.N., Eds.; Embrapa Trigo: Passo Fundo, Brazil, 2022. [Google Scholar]
- Medeiros, G.O.R.; Giarolla, A.; Sampaio, G.; Marinho, M.A. Diagnosis of the Accelerated Soil Erosion in São Paulo State (Brazil) by the Soil Lifetime Index Methodology. Rev. Bras. Cienc. Solo 2016, 40, e0150498. [Google Scholar] [CrossRef]
- Sanches, G.M.; Otto, R. A Novel Approach for Determining Nitrogen Requirement Based on a New Agronomic Principle—Sugarcane as a Crop Model. Plant Soil 2022, 472, 29–43. [Google Scholar] [CrossRef]
- Rodriguez, D.G.P.; Bullock, D.S.; Boerngen, M.A. The Origins, Implications, and Consequences of Yield-Based Nitrogen Fertilizer Management. Agron. J. 2019, 111, 725–735. [Google Scholar] [CrossRef]
- Morris, T.F.; Murrell, T.S.; Beegle, D.B.; Camberato, J.J.; Ferguson, R.B.; Grove, J.; Ketterings, Q.; Kyveryga, P.M.; Laboski, C.A.M.; McGrath, J.M.; et al. Strengths and Limitations of Nitrogen Rate Recommendations for Corn and Opportunities for Improvement. Agron. J. 2018, 110, 1–37. [Google Scholar] [CrossRef]
- Amaral, L.R.; Molin, J.P. The Effectiveness of Three Vegetation Indices Obtained from a Canopy Sensor in Identifying Sugarcane Response to Nitrogen. Agron. J. 2014, 106, 273. [Google Scholar] [CrossRef]
- Martins, D.S.; Reis, V.M.; Schultz, N.; Alves, B.J.R.; Urquiaga, S.; Pereira, W.; Sousa, J.S.; Boddey, R.B. Soil Nitrogen and Biological N2 Fixation in Sugarcane with Diazotrophic Bacteria. Plant Soil 2020, 454, 155–169. [Google Scholar] [CrossRef]
- Jaynes, D.B. Confidence Bands for Measured Economically Optimal Nitrogen Rates. Precis. Agric. 2011, 12, 196–213. [Google Scholar] [CrossRef]
- Puntel, L.A.; Pagani, A.; Archontoulis, S.V. Development of a Nitrogen Recommendation Tool for Corn Considering Static and Dynamic Variables. Eur. J. Agron. 2019, 105, 189–199. [Google Scholar] [CrossRef]
- Otto, R.; Mulvaney, R.L.; Khan, S.A.; Trivelin, P.C.O. Quantifying Soil Nitrogen Mineralization to Improve Fertilizer Nitrogen Management of Sugarcane. Biol. Fertil. Soils 2013, 49, 893–904. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Soil Test Biological Activity with the Flush of CO2: III. Corn Yield Responses to Applied Nitrogen. Soil Sci. Soc. Am. J. 2018, 82, 708–721. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Walters, D.T.; Bundy, L.G.; Li, X.; Drijber, R.A.; Sawyer, J.E.; Castellano, M.J.; Laboski, C.A.M.; Scharf, P.C.; Horwath, W.R. Combination of Biological and Chemical Soil Tests Best Predict Maize Nitrogen Response. Agron. J. 2020, 112, 1263–1278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).