Differences in Salinity Tolerance in Avena sativa and Avena nuda
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Growth Conditions, Treatment, and Sampling
2.2. Agronomic Traits
2.3. Photosynthetic and Physiological Parameters
2.4. Observation of the Anatomical Structure of the Leaf
2.5. Statistical Analysis
3. Results
3.1. Effects of Salt Stress on the Growth
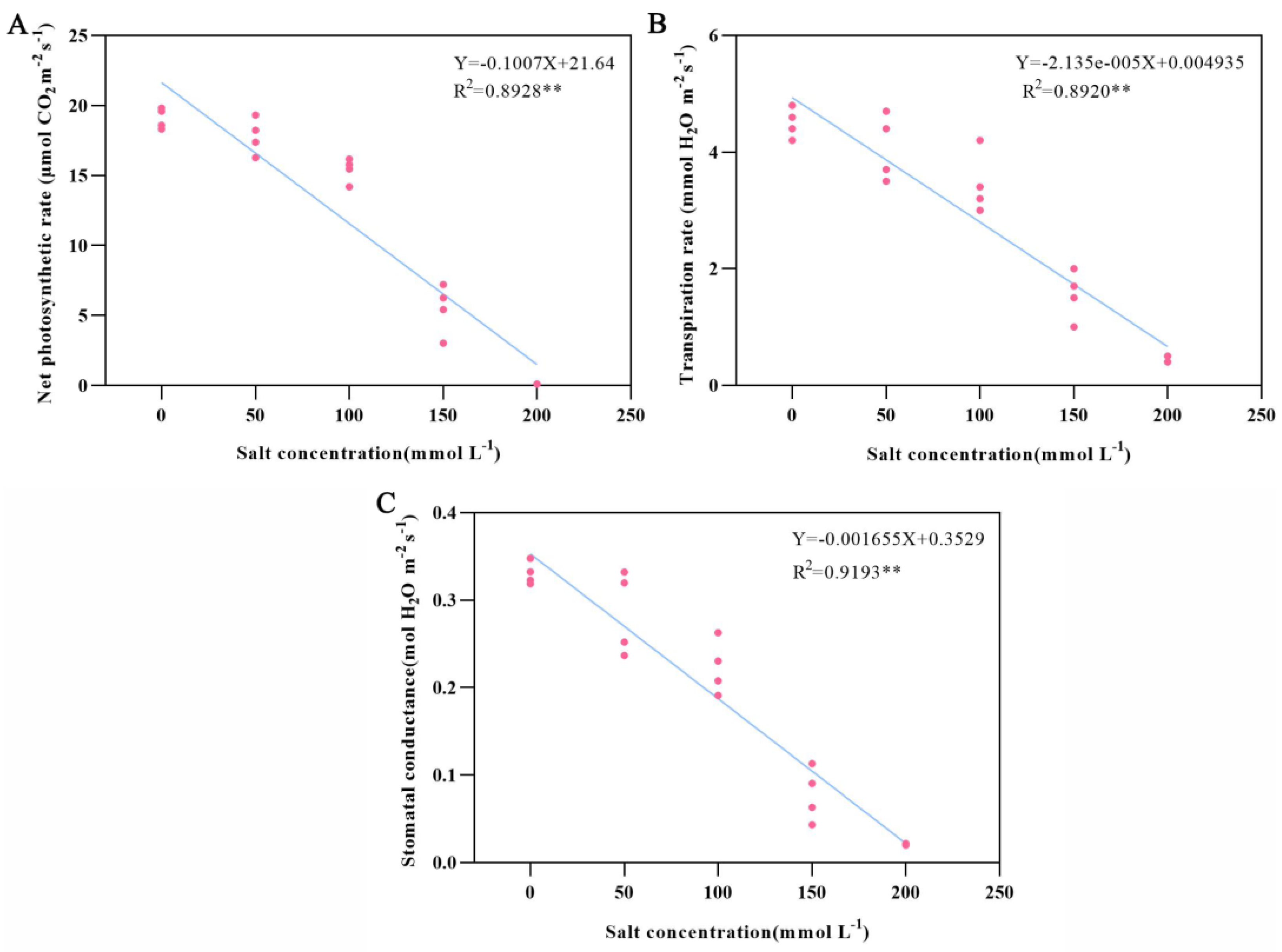
3.2. Effects of Salt Stress on Chlorophyll Content and Photosynthesis
3.3. Effects of Salt Stress on Physiological and Biochemical Properties
3.4. Effect of Salt Stress on the Microstructure of Oat Leaves
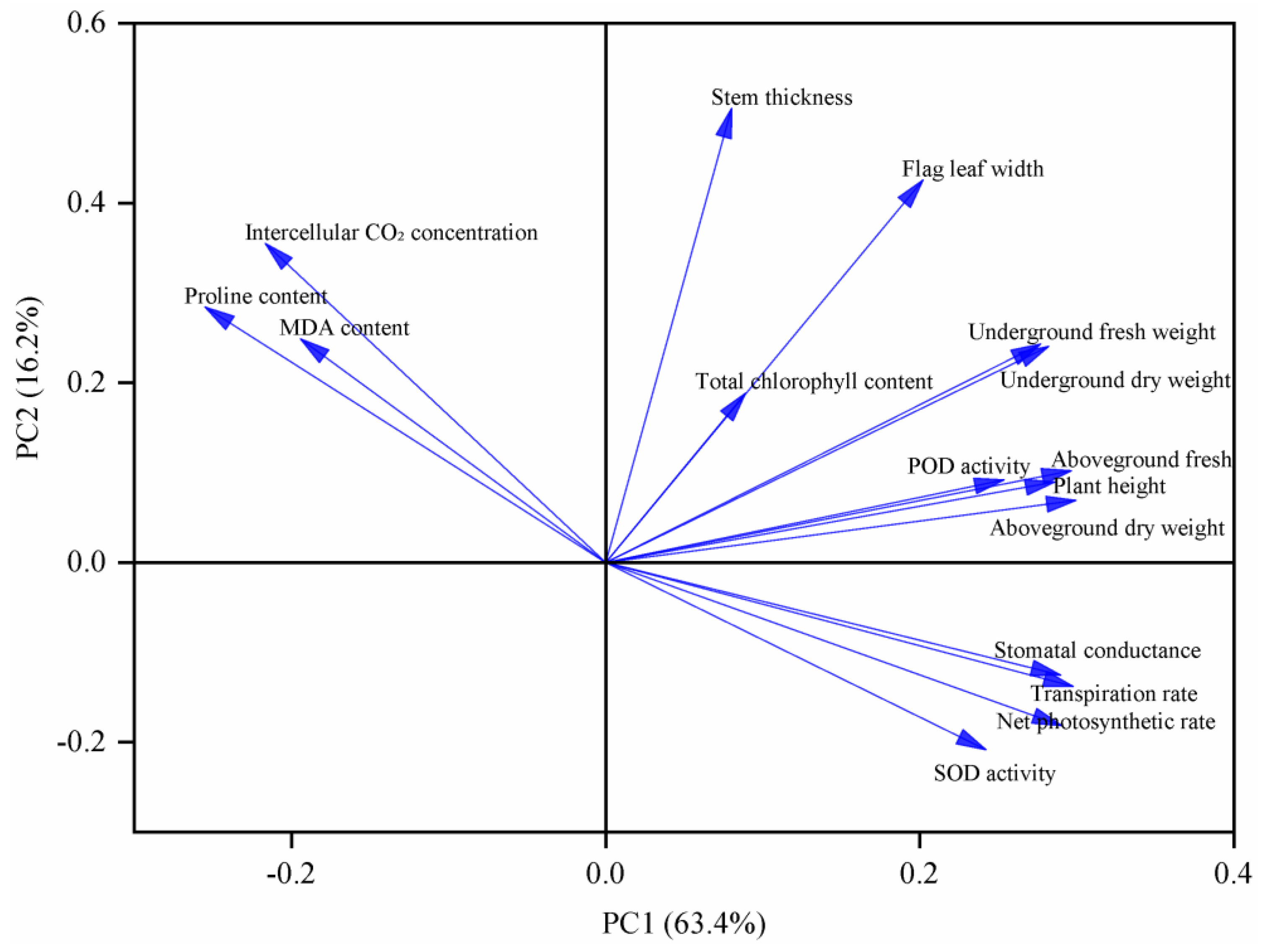
3.5. Principal Component Analysis
3.6. Correlation Analysis
3.7. Affiliation Function Analysis
4. Discussion
4.1. Effects of Different Salt Concentrations on Agronomic Traits and Physiological Characteristics in Covered Oats and Naked Oats
4.2. Effects of Salt Stress on the Cellular Structure of Leaves of in Covered Oats and Naked Oats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahid, M.; Akram, M.S.; Khan, M.A.; Zubair, M.; Shah, S.M.; Ismail, M.; Shabir, G.; Basheer, S.; Aslam, K.; Tariq, M. A phytobeneficial strain Planomicrobium sp. MSSA-10 triggered oxidative stress responsive mechanisms and regulated the growth of pea plants under induced saline environment. J. Appl. Microbiol. 2018, 124, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Nachshon, U. Cropland soil salinization and associated hydrology: Trends, processes and examples. Water 2018, 10, 1030. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Afzal, M.; Hindawi, S.E.S.; Alghamdi, S.S.; Migdadi, H.H.; Khan, M.A.; Hasnain, M.U.; Arslan, M.; Rahman, M.H.; Sohaib, M. Potential breeding strategies for improving salt tolerance in crop plants. J. Plant Growth Regul. 2023, 42, 3365–3387. [Google Scholar] [CrossRef]
- Shi, D.; Sheng, Y. Effect of various salt–alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ. Exp. Bot. 2005, 54, 8–21. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Gautam, R.K.; Sharma, P.C.; Sharma, D.K. Effect of different salt stresses on agro-morphological traits and utilisation of salt stress indices for reproductive stage salt tolerance in rice. Field Crops Res. 2016, 190, 26–33. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A New Insight of Salt Stress Signalingin Plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Udomchalothorn, T.; Plaimas, K.; Sripinyowanich, S.; Boonchai, C.; Kojonna, T.; Chutimanukul, P.; Comai, L.; Buaboocha, T.; Chadchawan, S. OsNucleolin1-L expression in Arabidopsis enhances photosynthesis via transcriptome modification under salt stress conditions. Plant Cell Physiol. 2017, 58, 717–734. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, W.; Qin, L.; Fan, D.Y.; Chow, W.S. Photoinactivation of Photosystem II in wild-type and chlorophyll b-less barley leaves: Which mechanism dominates depends on experimental circumstances. Photosynth. Res. 2015, 126, 399–407. [Google Scholar] [CrossRef]
- Hao, S.H.; Wang, Y.R.; Yan, Y.X.; Liu, Y.H.; Wang, J.Y.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132–161. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.X.; Yan, C.R.; Zhong, X.L.; Gu, F.X.; Liu, Q.; Xia, X.; Li, H.R. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Munns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Liu, J.G.; Han, X.; Yang, T.; Cui, W.H.; Wu, A.M.; Fu, C.X.; Wang, B.C.; Liu, L.J. Genome-wide transcriptional adaptation to salt stress in Populus. BMC Plant Biol. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Zolla, G.; Heimer, Y.M.; Barak, S. Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J. Exp. Bot. 2010, 61, 211–224. [Google Scholar] [CrossRef]
- Dou, L.; He, K.; Higaki, T.; Wang, X.; Mao, T. Ethylene signaling modulates cortical microtubule reassembly in response to salt stress. Plant Physiol. 2018, 176, 2071–2081. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Plant growth and development under salinity stress. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops, 1st ed.; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar]
- Alhilfi, S.K. Response of two varieties of oat plant (Avena sativa L.) to different levels of nitrogen fertilizer. Int. J. Agric. Stat. Sci. 2020, 16, 665–670. [Google Scholar]
- Ye, X.L.; Gan, Z.; Wan, Y.; Xiang, D.B.; Wu, X.Y.; Wu, Q.; Liu, C.Y.; Fan, Y.; Zou, L. Advances and perspectives in forage oat breeding. Acta Pratacult. Sin. 2023, 32, 160–177. [Google Scholar]
- Ahmed, S.; Patel, R.; Rana, M.; Kumar, N.; Choudhary, M.; Chand, S.; Singh, A.K.; Ghosh, A.; Singhal, R.K. Effect of salt, alkali and combined stresses on root system architecture and ion profiling in a diverse panel of oat (Avena spp.). Funct. Plant Biol. 2023, 51, 1–16. [Google Scholar]
- Brenes, M.; Solana, A.; Boscaiu, M.; Fita, A.; Vicente, O.; Calatayud, Á.; Prohens, J.; Plazas, M. Physiological and biochemical responses to salt stress in cultivated eggplant (Solanum melongena L.) and in S. insanum L., a close wild relative. Agronomy 2020, 10, 651–669. [Google Scholar]
- Xiao, X.L.; Duan, B.; Huang, F.Y.; Zhi, X.M.; Jiang, Z.; Ma, N. Analysis of canopy light utilization efficiency in high-yielding rapeseed varieties. Sci. Rep. 2024, 14, 31243–31255. [Google Scholar] [CrossRef]
- Wang, X.X.; Fang, Z.W.; Zhao, D.Q.; Tao, J. Effects of high-temperature stress on photosynthetic characteristics and antioxidant enzyme system of Paeonia ostii. Phyton-Int. J. Exp. Bot. 2022, 91, 599–615. [Google Scholar] [CrossRef]
- Ma, N.N.; Zuo, Y.Q.; Liang, X.Q.; Yin, B.; Wang, G.D.; Meng, Q.W. The multiple stress-responsive transcription factor SlNAC1 improves the chilling tolerance of tomato. Physiol. Plant. 2013, 149, 474–486. [Google Scholar] [CrossRef]
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Yin, Y.; Yuan, C.; Liu, Y. Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Front. Plant Sci. 2016, 7, 1387. [Google Scholar] [CrossRef]
- Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed pretreatment and foliar application of proline regulate morphological, physio-biochemical processes and activity of antioxidant enzymes in plants of two cultivars of quinoa (Chenopodium quinoa Willd.). Plants 2019, 8, 588–604. [Google Scholar] [CrossRef]
- Chen, X.R.; Gao, Z.H.; Li, Y.Y.; Nie, X.Q.; Xie, Q.L.; Chen, G.P.; Hu, Z.L. Knockdown of SlYTHDF2 Accelerates Dark–Induced Tomato Leaf Senescence by Affecting the ABA Pathway. Plants 2024, 13, 2800–2818. [Google Scholar] [CrossRef]
- Wu, J.Q.; Shu, S.; Li, C.C.; Sun, J.; Guo, S.R. Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol. Bioch. 2018, 128, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, G.L.; Liu, M.; Xue, H.T.; Zhao, P.Y.; Han, B.S.; Fan, H.P.; Wang, R.; Wang, L.X.; Guo, E.H.; et al. Study on improving yield and antioxidant enzyme activities in millet by rationing molybdenum and nitrogen. Front. Plant Sci. 2025, 15, 1524347–1524359. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.J.; Jiang, X.Y.; Wen, J.L.; Huang, M.H.; Wang, Y.Q.; Wang, M.; Dong, H.; Liu, Q.P. Disruption of the Expression of the Cinnamoyl–CoA Reductase (CCR) Gene OsCCR18 Causes Male Sterility in Rice (Oryza sativa L. japonica). Agriculture 2022, 12, 1685–1697. [Google Scholar] [CrossRef]
- Meng, C.; Lu, X.L.; Song, Y.R.; Zhang, C.S.; Li, Y.Q.; Xiang, H.Q.; Xu, Z.C. Evaluation and identification of salt tolerance of 11 Leonurus germplasm lines at the seedling stage. Acta Pratacult. Sin. 2024, 33, 196–203. [Google Scholar]
- Nakayama, H. Leaf form diversity and evolution: A never-ending story in plant biology. J. Plant Res. 2024, 137, 547–560. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.G. Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Gogna, M.; Jain, P.; Singh, N.; Mukherjee, S.; Kalra, G. Signaling mechanisms and biochemical pathways regulating pollen-stigma interaction, seed development and seedling growth in sunflower under salt stress. Plant Signal. Behav. 2021, 16, 1958129. [Google Scholar] [CrossRef]
- Liu, B.; Kang, C.; Wang, X.; Bao, G. Physiological and morphological responses of Leymus chinensis to saline-alkali stress. Grassland Sci. 2015, 61, 217–226. [Google Scholar] [CrossRef]
- Wei, J.N. Effect of Silicon Fertilizer on the Growth and Development of Early Grassland Under Salt Stress. Master’s Thesis, Lanzhou University, Lanzhou, China, 2016. [Google Scholar]
- Rosas, U.; Lara-González, J.A.; De-la-Rosa-Tilapa, A.; Terrazas, T. Persistent adventitious and basal root development during salt stress tolerance in Echinocactus platyacanthus (Cactaceae) seedlings. J. Arid Environ. 2021, 187, 104431. [Google Scholar] [CrossRef]
- Gu, J.J.; Hu, B.W.; Jia, Y.; Sha, H.J.; Li, J.W.; Ma, C.; Zhao, H.W. Effects of salt stress on root related traits and yield of rice. Crop 2019, 4, 176–182. [Google Scholar]
- Guo, H.J.; Hu, T.; Fu, J.M. Effects of saline sodic stress on growth and physiological responses of Lolium perenne. Acta Pratacult. Sin. 2012, 21, 118–125. [Google Scholar]
- Lu, C.; Qiu, N.; Lu, Q.; Wang, B.; Kuang, T. Does salt stress lead to increased susceptibility of photosystem II to photoinhibition and changes in photosynthetic pigment composition in halophyte Suaeda salsa grown outdoors? Plant Sci. 2002, 163, 1063–1068. [Google Scholar] [CrossRef]
- Winicov, I.; Button, J.D. Accumulation of photosynthesis gene transcripts in response to sodium chloride by salt-tolerant alfalfa cells. Planta 1991, 183, 478–483. [Google Scholar] [CrossRef]
- Ma, X.; Jia, Z.F.; Liu, Y. Study on photosynthesis and chlorophyll metabolism of Avena sativa L. under salt stress. Chin. Qinghai J. Animal Vet. Sci. 2021, 51, 7–14. [Google Scholar]
- Wang, M.M.; Zhou, X.R.; Liang, G.L.; Zhao, G.Q.; Jiao, R.A.; Chai, J.K.; Gao, X.M.; Li, J.N. A multi-trait evaluation of salt tolerance of 5 oat germplasm lines at the seedling stage. Acta Pratacult. Sin. 2020, 29, 143–154. [Google Scholar]
- Shabala, S.; Mackay, A. Ion transport in halophytes. Adv. Bot. Res. 2011, 57, 151–199. [Google Scholar]
- Khedr, R.A.; Sorour, S.G.R.; Aboukhadrah, S.H.; El Shafey, N.M.; Abd Elsalam, H.E.; El-Sharnouby, M.E.; El-Tahan, A.M. Alleviation of salinity stress effects on agro-physiological traits of wheat by auxin, glycine betaine, and soil additives. Saudi J. Biol. Sci. 2022, 29, 534–540. [Google Scholar] [CrossRef]
- El-Shintinawy, F.; El-Shourbagy, M.N. Alleviation of changes in protein metabolism in NaCl-stressed wheat seedlings by thiamine. Biol. Plant. 2001, 44, 541–545. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, X.H.; He, Y.L.; Zhao, X.F.; Han, T.; Yue, K.; Xin, X.Q.; Su, M.F.; Ma, W.J.; Luo, Q.J. Effects of complex saline-alkali stress on seed germination and seedling antioxidant characteristics of Chenopodium quinoa. Acta Pratacult. Sin. 2019, 28, 156–167. [Google Scholar]
- Acar, O.K.A.N.; Türkan, I.; Özdemir, F. Superoxide dismutase and peroxidase activities in drought sensitive and resistant barley (Hordeum vulgare L.) varieties. Acta Physiol. Plant. 2001, 23, 351–356. [Google Scholar] [CrossRef]
- Liu, B.S.; Kang, C.L.; Wang, X.; Bao, G.Z. Physiological and biochemical response characteristics of Leymus chinensis to saline-alkali stress. Trans. Chin. Soc. Agric. Eng. 2014, 30, 166–173. [Google Scholar]
- Ji, X.; Tang, J.; Zhang, J. Effects of salt stress on the morphology, growth and physiological parameters of Juglans microcarpa L. seedlings. Plants 2022, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Lin, S.H.; Xu, P.L.; Wang, X.J.; Bai, J.G. Effects of exogenous silicon on the activities of antioxidant enzymes and lipid peroxidation in chilling-stressed cucumber leaves. Agric. Sci. China 2009, 8, 1075–1086. [Google Scholar] [CrossRef]
- Romero-Aranda, R.; Soria, T.; Cuartero, J. Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Sci. 2001, 160, 265–272. [Google Scholar] [CrossRef]
- Lovato, M.B.; de Lemos Filho, J.P.; Martins, P.S. Growth responses of Stylosanthes humilis (Fabaceae) populations to saline stress. Environ. Exp. Bot. 1999, 41, 145–153. [Google Scholar] [CrossRef]
- Yao, X.C.; Meng, L.F.; Zhao, W.L.; Mao, G.L. Changes in the morphology traits, anatomical structure of the leaves and transcriptome in Lycium barbarum L. under salt stress. Front. Plant Sci. 2023, 14, 1090366. [Google Scholar] [CrossRef]
- Nassar, R.M.; Kamel, H.A.; Ghoniem, A.E.; Alarcón, J.J.; Sekara, A.; Ulrichs, C.; Abdelhamid, M.T. Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants 2020, 9, 237. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mittra, B. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees 2004, 18, 167–174. [Google Scholar] [CrossRef]
- Pipan, B.; Sinkovič, L.; Rutar, R.; Meglič, V. The use of DNA markers for genetic differentiation of common (Avena sativa L.) and naked oat (Avena nuda L.). J. Cent. Eur. Agric. 2021, 22, 329–340. [Google Scholar] [CrossRef]
- Ta, N.; Lu, X.P.; Zhang, Y.H.; Zhang, X.Y.; Xue, C.L.; Zhao, W.G. Character performance and genetic diversity of Avena sativa and naked oat. Inn. Mong. Agric. Sci. Technol. 2010, 38, 33–35. [Google Scholar]
- Zhao, S.F.; Cao, L.X.; Zhang, L.J.; Wu, Y.Z.; Li, Y.X. Quality and yield of different types of oat bred varieties. J. Hebei Agric. Sci. 2012, 16, 58–61. [Google Scholar]
- Wang, Q.; Li, Z.J.; Li, J.; Zhou, B.W. Evaluation of agronomic and forage quality traits of a range of oat cultivars. Acta Pratacult. Sin. 2019, 28, 149–158. [Google Scholar]
- Liu, H.; Mu, P.; Zhao, G.Q.; Zhou, X.R. The impact of herbicides concentrations on photosynthetic characters and production of covered and naked oat. Agric. Res. Arid Areas 2017, 35, 124–133. [Google Scholar]
- Tao, J.; Hou, L.Y.; Yang, J.; Song, S.H.; Bai, W.M.; Pan, Q.M.; Yin, S.Y.; Hao, J.X.; Zhang, W.H. Comparative studies on seed germination characteristics and indicators of oat varieties under salt stress. Pratacult. Sci. 2018, 35, 2414–2421. [Google Scholar]
- Yao, R.R.; Liu, H.; Zhao, G.Q.; Wang, J.L.; Wang, Q.Y.; Dong, K.; Zhang, R. Effects of seed storage time on seed germination and cytological structure of covered oats and naked oats. Acta Pratacult. Sin. 2024, 33, 154–163. [Google Scholar]
- Yao, R.; Liu, H.; Wang, J.; Shi, S.L.; Zhao, G.Q.; Zhou, X.R. Cytological structures and physiological and biochemical characteristics of covered oat (Avena sativa L.) and naked oat (Avena nuda L.) seeds during high-temperature artificial aging. BMC Plant Biol. 2024, 24, 530–542. [Google Scholar] [CrossRef]
- Nesterova, N.; Pareniuk, O.; Illienko, V.; Ruban, Y.; Shpyrka, N.; Shavanova, K. Physiological reactions in cereals family Avena sativa L. and Avena nuda L., caused by low-temperature stress factors. In Proceedings of the 2019 IEEE 39th International Conference on Electronics and Nanotechnology (ELNANO), Kyiv, Ukraine, 16–18 April 2019; pp. 502–506. [Google Scholar]



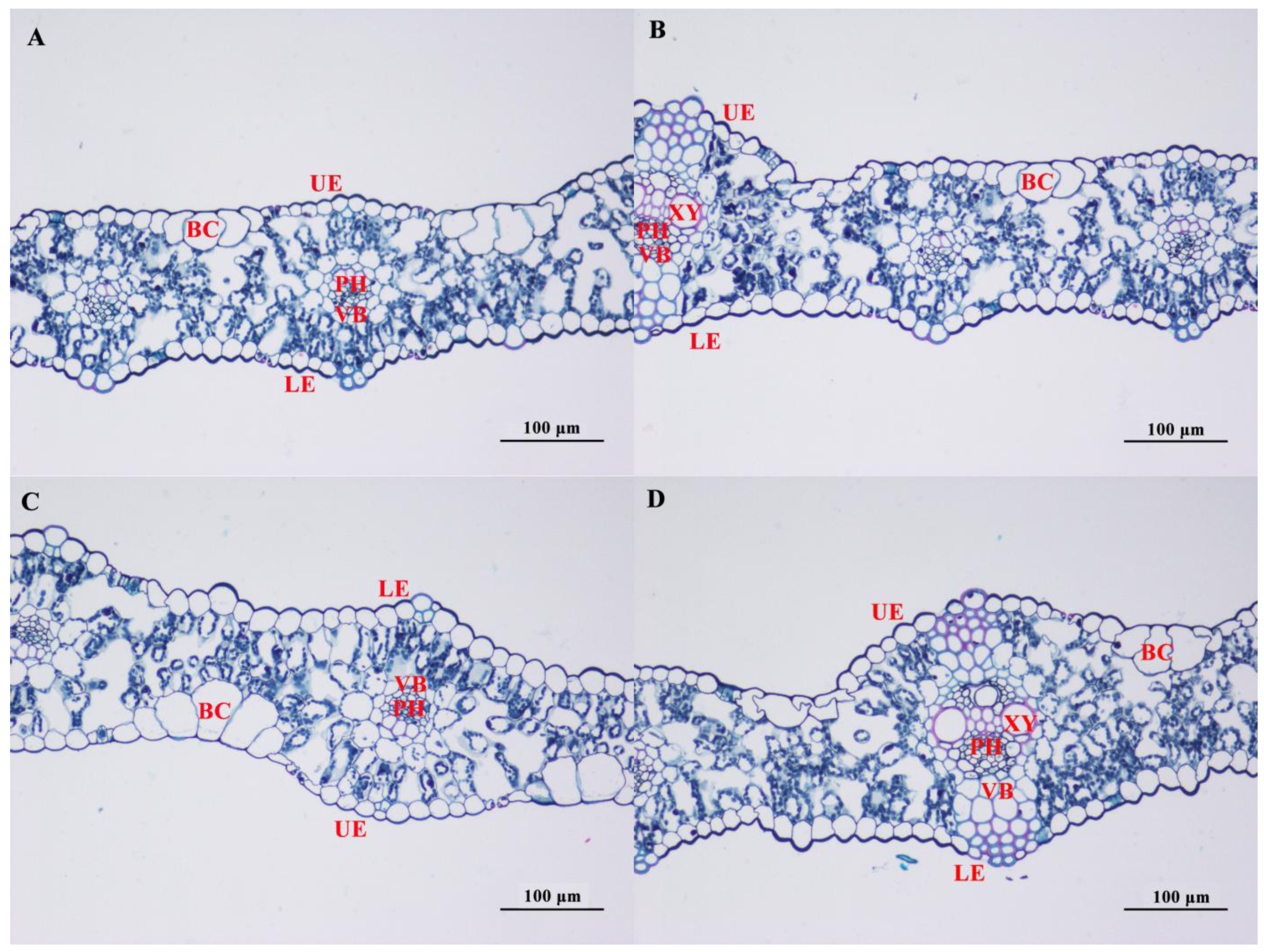
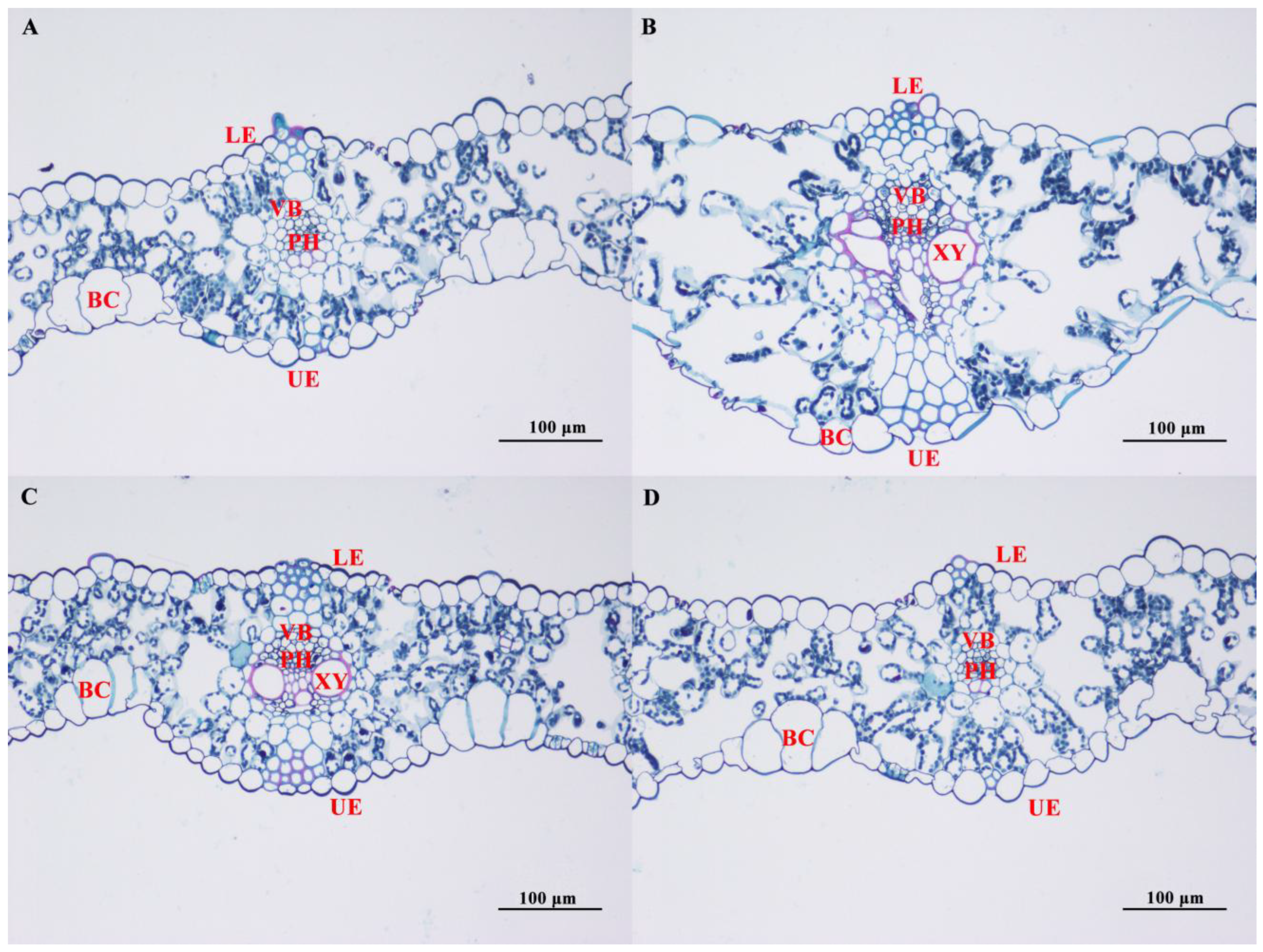
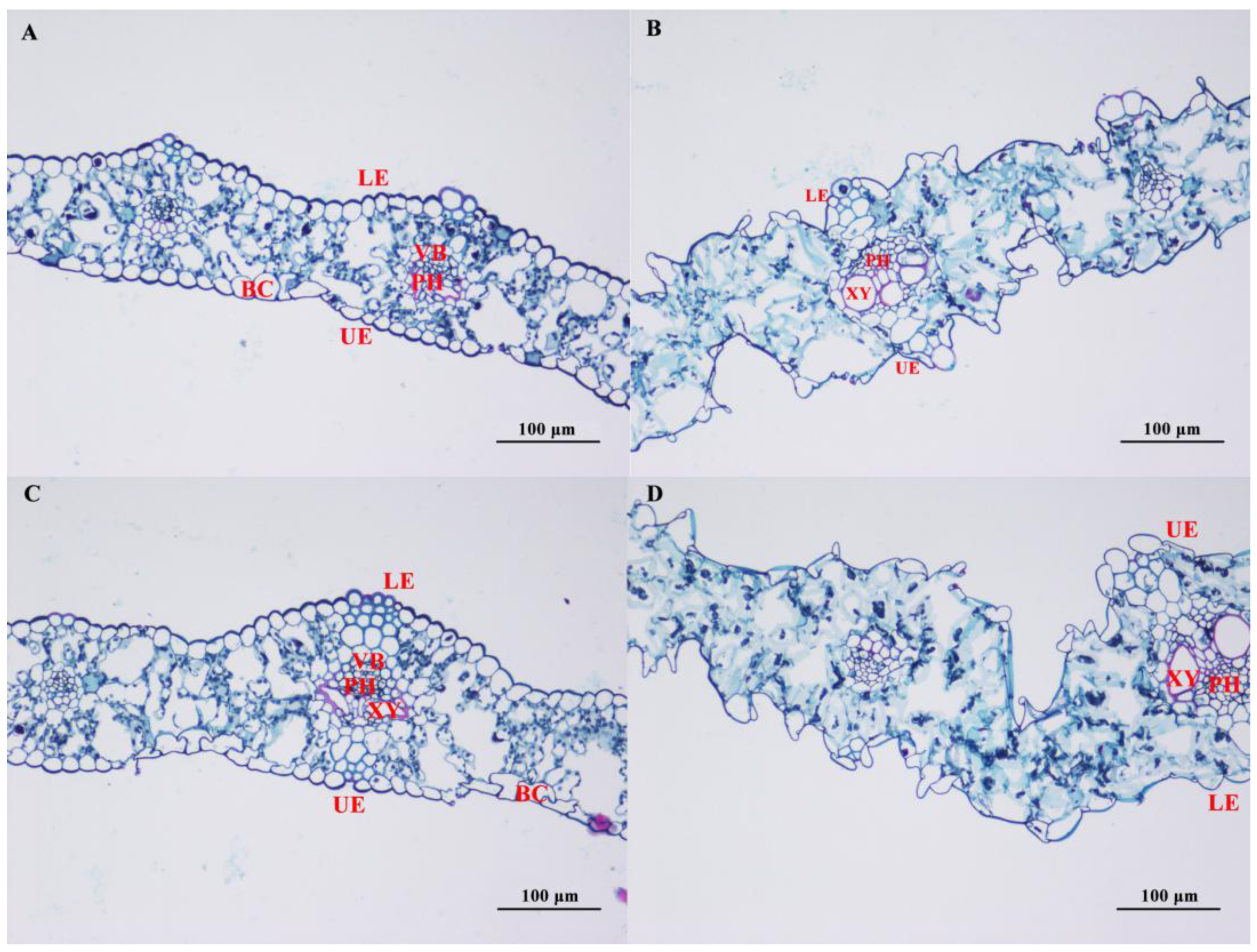

| Factor | Cultivar | Salt Concentration | Cultivar × Salt Concentration | |||
|---|---|---|---|---|---|---|
| f Value | p Value | f Value | p Value | f Value | p Value | |
| PH | 60.233 | <0.001 ** | 123.000 | <0.001 ** | 4.585 | <0.001 ** |
| FLW | 37.619 | <0.001 ** | 8.271 | <0.001 ** | 0.865 | 0.587 NS |
| ST | 62.248 | <0.001 ** | 2.186 | 0.088 NS | 2.607 | 0.012 * |
| ADW | 109.238 | <0.001 ** | 182.773 | <0.001 ** | 3.720 | 0.001 ** |
| AFW | 244.916 | <0.001 ** | 473.233 | <0.001 ** | 11.036 | <0.001 ** |
| UDW | 423.462 | <0.001 ** | 262.951 | <0.001 ** | 4.981 | <0.001 ** |
| UFW | 428.159 | <0.001 ** | 219.124 | <0.001 ** | 7.860 | <0.001 ** |
| Trait | Salt Concentration (mM) | Cultivar | |||
|---|---|---|---|---|---|
| Qinghai 444 | Baiyan No. 18 | Mengyan No. 1 | Qingyin No. 3 | ||
| PH (cm) | 0 | 64.57 ± 3.51 Aa | 54.50 ± 0.79 Ba | 64.07 ± 3.27 Aa | 53.13 ± 1.88 Ba |
| 50 | 59.20 ± 3.49 Aab | 52.87 ± 0.55 Bab | 59.13 ± 1.00 Ab | 52.70 ± 2.15 Bab | |
| 100 | 58.20 ± 2.15 Aab | 51.10 ± 0.50 Bb | 53.73 ± 0.40 Bc | 51.03 ± 1.46 Bab | |
| 150 | 53.50 ± 1.22 Ab | 42.70 ± 1.31 Cc | 49.67 ± 0.67 Bcd | 47.70 ± 2.09 Bbc | |
| 200 | 44.20 ± 0.89 Bc | 41.63 ± 1.24 Bc | 47.87 ± 0.59 Ad | 43.70 ± 2.19 Bc | |
| ST (mm) | 0 | 3.31 ± 0.10 Aa | 2.99 ± 0.20 Aa | 3.73 ± 0.56 Aa | 2.10 ± 0.25 Bb |
| 50 | 2.92 ± 0.23 BCa | 3.08 ± 0.05 ABa | 3.45 ± 0.22 Aa | 2.48 ± 0.15 Cab | |
| 100 | 2.83 ± 0.35 ABa | 2.90 ± 0.28 ABa | 3.20 ± 0.04 Aa | 2.29 ± 0.19 Bab | |
| 150 | 2.96 ± 0.07 Ba | 2.67 ± 0.12 BCa | 3.31 ± 0.12 Aa | 2.61 ± 0.14 Ca | |
| 200 | 3.28 ± 0.18 Aa | 2.95 ± 0.13 Ba | 3.32 ± 0.03 Aa | 2.38 ± 0.09 Cab | |
| ADW (g) | 0 | 0.85 ± 0.06 Aa | 0.63 ± 0.05 Ba | 0.70 ± 0.05 Ba | 0.60 ± 0.06 Ba |
| 50 | 0.77 ± 0.08 Aab | 0.54 ± 0.04 Ba | 0.62 ± 0.06 Bab | 0.51 ± 0.03 Bab | |
| 100 | 0.70 ± 0.06 Aab | 0.43 ± 0.05 Cb | 0.57 ± 0.03 Bb | 0.43 ± 0.00 Cbc | |
| 150 | 0.64 ± 0.02 Ab | 0.22 ± 0.02 Dc | 0.50 ± 0.04 Bb | 0.35 ± 0.03 Cc | |
| 200 | 0.34 ± 0.04 Ac | 0.20 ± 0.03 Bc | 0.26 ± 0.03 Bc | 0.20 ± 0.02 Bd | |
| AFW (g) | 0 | 4.82 ± 0.11 Aa | 3.64 ± 0.05 Ba | 4.82 ± 0.16 Aa | 2.51 ± 0.41 Ca |
| 50 | 4.39 ± 0.35 Aa | 3.41 ± 0.07 Ba | 3.94 ± 0.10 Ab | 2.33 ± 0.13 Ca | |
| 100 | 3.68 ± 0.15 Ab | 3.06 ± 0.09 Bb | 3.53 ± 0.04 Ac | 2.07 ± 0.09 Ca | |
| 150 | 3.29 ± 0.08 Ab | 2.08 ± 0.11 Bc | 3.24 ± 0.04 Ac | 2.06 ± 0.09 Ba | |
| 200 | 1.56 ± 0.43 Ac | 0.76 ± 0.12 Bd | 0.99 ± 0.18 ABd | 0.65 ± 0.06 Bb | |
| UDW (g) | 0 | 0.21 ± 0.00 Aa | 0.15 ± 0.01 Ba | 0.21 ± 0.00 Aa | 0.15 ± 0.00 Ba |
| 50 | 0.20 ± 0.00 Aab | 0.14 ± 0.00 Bb | 0.20 ± 0.00 Aab | 0.14 ± 0.00 Bb | |
| 100 | 0.19 ± 0.01 Ab | 0.12 ± 0.01 Bc | 0.19 ± 0.01 Ab | 0.11 ± 0.00 Bc | |
| 150 | 0.17 ± 0.01 Ac | 0.10 ± 0.00 Bd | 0.17 ± 0.01 Ac | 0.11 ± 0.00 Bc | |
| 200 | 0.12 ± 0.01 Ad | 0.09 ± 0.01 Be | 0.13 ± 0.01 Ad | 0.09 ± 0.01 Bd | |
| UFW (g) | 0 | 2.67 ± 0.05 Aa | 1.42 ± 0.10 Ca | 2.17 ± 0.23 Ba | 1.59 ± 0.06 Ca |
| 50 | 2.48 ± 0.07 Aab | 1.35 ± 0.12 Cab | 2.08 ± 0.14 Ba | 1.29 ± 0.04 Cb | |
| 100 | 2.26 ± 0.11 Ab | 1.13 ± 0.11 Bb | 2.07 ± 0.03 Aa | 1.08 ± 0.09 Bc | |
| 150 | 1.67 ± 0.05 Bc | 0.67 ± 0.05 Cc | 1.85 ± 0.06 Aa | 0.78 ± 0.06 Cd | |
| 200 | 1.13 ± 0.15 Ad | 0.53 ± 0.04 Bc | 1.36 ± 0.13 Ab | 0.56 ± 0.09 Be | |
| Factor | Cultivar | Salt Concentration | Cultivar × Salt Concentration | |||
|---|---|---|---|---|---|---|
| F Value | p Value | f Value | p Value | f Value | p Value | |
| CaC | 2830.621 | <0.001 ** | 289.704 | <0.001 ** | 187.462 | <0.001 ** |
| CbC | 1422.226 | <0.001 ** | 99.942 | <0.001 ** | 81.406 | <0.001 ** |
| TCC | 2903.200 | <0.001 ** | 215.518 | <0.001 ** | 178.899 | <0.001 ** |
| NPR | 458.832 | <0.001 ** | 40,067.878 | <0.001 ** | 106.165 | <0.001 ** |
| SC | 598.488 | <0.001 ** | 17,568.963 | <0.001 ** | 119.052 | <0.001 ** |
| TR | 2334.772 | <0.001 ** | 49,227.978 | <0.001 ** | 276.414 | <0.001 ** |
| ICDC | 558.852 | <0.001 ** | 10,428.536 | <0.001 ** | 557.590 | <0.001 ** |
| Trait | Salt Concentration (mM) | Cultivar | |||
|---|---|---|---|---|---|
| Qinghai 444 | Baiyan No. 18 | Mengyan No. 1 | Qingyin No. 3 | ||
| CaC (mg·g−1 FW) | 0 | 2.86 ± 0.05 Aa | 1.92 ± 0.03 Da | 2.68 ± 0.08 Ba | 2.36 ± 0.04 Ca |
| 50 | 2.76 ± 0.10 Aa | 1.93 ± 0.11 Ca | 2.14 ± 0.07 Bb | 2.09 ± 0.05 Bb | |
| 100 | 2.48 ± 0.10 Ab | 1.83 ± 0.04 Ca | 2.12 ± 0.06 Bb | 1.91 ± 0.04 Cc | |
| 150 | 1.97 ± 0.11 Ac | 0.99 ± 0.26 Cb | 1.79 ± 0.02 Bc | 1.80 ± 0.04 ABd | |
| 200 | 0.99 ± 0.03 Ad | 0.47 ± 0.05 Dc | 0.56 ± 0.02 Cd | 0.76 ± 0.03 Be | |
| CbC (mg·g−1 FW) | 0 | 0.90 ± 0.02 Aa | 0.65 ± 0.06 Ca | 0.82 ± 0.05 Ba | 0.76 ± 0.07 Ba |
| 50 | 0.90 ± 0.07 Aa | 0.59 ± 0.03 Cab | 0.70 ± 0.01 Bb | 0.61 ± 0.01 Cb | |
| 100 | 0.79 ± 0.06 Ab | 0.56 ± 0.01 Cb | 0.68 ± 0.00 Bb | 0.60 ± 0.03 Cb | |
| 150 | 0.60 ± 0.03 Ac | 0.31 ± 0.08 Bc | 0.55 ± 0.01 Ac | 0.56 ± 0.01 Ac | |
| 200 | 0.31 ± 0.02 Ad | 0.15 ± 0.01 Dd | 0.20 ± 0.01 Cd | 0.22 ± 0.00 Bd | |
| TCC (mg·g−1 FW) | 0 | 3.73 ± 0.08 Aa | 2.57 ± 0.08 Da | 3.50 ± 0.12 Ba | 3.12 ± 0.08 Ca |
| 50 | 3.60 ± 0.25 Aa | 2.52 ± 0.14 Ca | 2.83 ± 0.07 Bb | 2.70 ± 0.05 Bb | |
| 100 | 3.19 ± 0.33 Ab | 2.39 ± 0.04 Da | 2.80 ± 0.06 Bb | 2.51 ± 0.05 Cc | |
| 150 | 2.46 ± 0.43 Ac | 1.29 ± 0.34 Bb | 2.34 ± 0.02 Ac | 2.36 ± 0.05 Ad | |
| 200 | 1.43 ± 0.39 Ad | 0.62 ± 0.05 Dc | 0.76 ± 0.02 Cd | 0.98 ± 0.04 Be | |
| NPR (μmol CO2 m−2·s−1) | 0 | 19.82 ± 0.45 Aa | 18.33 ± 0.12 Ba | 19.60 ± 0.19 Aa | 18.60 ± 0.08 Ba |
| 50 | 19.33 ± 0.09 Ab | 16.29 ± 0.12 Db | 18.24 ± 0.07 Bb | 17.38 ± 0.31 Cb | |
| 100 | 16.19 ± 0.08 Ac | 15.47 ± 0.35 Bc | 15.78 ± 0.15 ABc | 14.19 ± 0.40 Cc | |
| 150 | 7.21 ± 0.19 Ad | 3.03 ± 0.05 Dd | 5.42 ± 0.06 Cd | 6.27 ± 0.33 Bd | |
| 200 | 0.11 ± 0.00 Ae | 0.08 ± 0.02 Be | 0.03 ± 0.02 Ce | 0.10 ± 0.00 ABe | |
| SC (mol H2O m−2·s−1) | 0 | 0.348 ± 0.003 Aa | 0.319 ± 0.005 Aa | 0.333 ± 0.001 Ba | 0.323 ± 0.000 Ba |
| 50 | 0.332 ± 0.005 Ab | 0.237 ± 0.006 Ab | 0.320 ± 0.004 Bb | 0.252 ± 0.018 Bb | |
| 100 | 0.263 ± 0.002 Ac | 0.208 ± 0.003 Bc | 0.230 ± 0.002 Cc | 0.191 ± 0.001 Dc | |
| 150 | 0.113 ± 0.004 Ad | 0.043 ± 0.003 Cd | 0.063 ± 0.001 Dd | 0.091 ± 0.001 Bd | |
| 200 | 0.022 ± 0.000 Ae | 0.020 ± 0.000 Ce | 0.020 ± 0.001 Be | 0.021 ± 0.000 Be | |
| TR (mmol H2O m−2·s−1) | 0 | 4.80 ± 0.03 Aa | 4.20 ± 0.17 Da | 4.60 ± 0.10 Ba | 4.40 ± 0.06 Ca |
| 50 | 4.70 ± 0.06 Ab | 3.70 ± 0.41 Db | 4.50 ± 0.00014 Bb | 3.70 ± 0.08 Cb | |
| 100 | 4.20 ± 0.03 Ac | 3.20 ± 0.15 Cc | 3.60 ± 0.30 Bc | 3.10 ± 0.04 Dc | |
| 150 | 2.00 ± 0.06 Ad | 1.10 ± 0.20 Cd | 1.60 ± 0.24 Bd | 1.60 ± 0.24 Bd | |
| 200 | 0.50 ± 0.01 Ae | 0.40 ± 0.01 Be | 0.40 ± 0.00002 Be | 0.40 ± 0.02 Be | |
| ICDC (μmol CO2 mol−1 air) | 0 | 287.65 ± 3.01 Bb | 293.29 ± 1.39 Ab | 289.56 ± 1.23 Bc | 288.65 ± 0.18 Bb |
| 50 | 285.91 ± 1.13 Abc | 236.20 ± 2.20 Dd | 276.62 ± 1.85 Bd | 261.77 ± 1.58 Cd | |
| 100 | 281.59 ± 1.20 Ac | 272.77 ± 1.70 Bc | 259.63 ± 1.93 De | 265.26 ± 1.50 Dc | |
| 150 | 283.06 ± 4.40 Cc | 292.46 ± 2.58 Bb | 361.64 ± 3.33 Ab | 259.96 ± 2.14 Dd | |
| 200 | 381.77 ± 2.35 Ba | 379.32 ± 2.97 Ba | 387.21 ± 2.59 Aa | 390.03 ± 3.20 Aa | |
| Factor | Cultivar | Salt Concentration | Cultivar × Salt Concentration | |||
|---|---|---|---|---|---|---|
| f Value | p Value | f Value | p Value | f Value | p Value | |
| SOD | 1615.400 | <0.001 ** | 32,932.298 | <0.001 ** | 1241.101 | <0.001 ** |
| POD | 54.327 | <0.001 ** | 126.830 | <0.001 ** | 9.129 | <0.001 ** |
| PRO | 3,932,765.060 | <0.001 ** | 322,925.780 | <0.001 ** | 227,692.589 | <0.001 ** |
| MDA | 3.362 | 0.039 * | 78.827 | <0.001 ** | 19.522 | <0.001 ** |
| Trait | Salt Concentration (mM) | Cultivar | |||
|---|---|---|---|---|---|
| Qinghai 444 | Baiyan No. 18 | Mengyan No. 1 | Qingyin No. 3 | ||
| SOD (U·g−1 FW) | 0 | 264.08 ± 2.01 Bc | 229.14 ± 1.24 Cc | 247.02 ± 42.53 Db | 300.86 ± 2.89 Ab |
| 50 | 340.80 ± 1.25 Ab | 316.89 ± 0.31 Bb | 290.42 ± 57.81 Db | 304.42 ± 1.39 Cb | |
| 100 | 367.16 ± 2.85 Ca | 416.56 ± 0.13 Aa | 381.58 ± 30.39 Ba | 343.16 ± 0.52 Da | |
| 150 | 246.20 ± 0.38 Ad | 148.94 ± 1.88 Cd | 161.20 ± 53.82 Bd | 132.43 ± 0.06 Dc | |
| 200 | 87.76 ± 1.96 Be | 101.95 ± 0.39 Ae | 88.32 ± 16.62 Be | 63.22 ± 0.59 Cd | |
| POD (U·g−1 FW) | 0 | 14.21 ± 0.07 Aa | 11.09 ± 0.81 Ba | 10.75 ± 0.17 Bb | 10.89 ± 0.13 Bb |
| 50 | 15.29 ± 0.31 Aa | 11.24 ± 0.02 Ba | 14.69 ± 1.45 Aa | 11.31 ± 0.03 Ba | |
| 100 | 11.44 ± 0.84 Ab | 9.39 ± 1.06 Aab | 10.62 ± 0.65 Ab | 11.85 ± 0.13 Aa | |
| 150 | 11.79 ± 0.19 Ab | 7.69 ± 0.37 Cb | 8.30 ± 0.13 Cbc | 9.78 ± 0.15 Bc | |
| 200 | 8.90 ± 0.47 Ac | 7.88 ± 0.03 Bb | 6.93 ± 0.10 Bc | 6.90 ± 0.14 Bd | |
| PRO (μg·g−1 FW) | 0 | 43.88 ± 0.00 Dd | 61.42 ± 0.11 Ad | 45.88 ± 0.02 Cd | 47.64 ± 0.02 Be |
| 50 | 52.92 ± 0.11 Cc | 67.63 ± 0.02 Ac | 53.69 ± 0.04 Bc | 51.70 ± 0.07 Dd | |
| 100 | 53.66 ± 0.37 Cc | 62.90 ± 0.09 Ad | 49.78 ± 0.10 Dc | 60.32 ± 0.13 Bc | |
| 150 | 151.27 ± 0.10 Cb | 178.21 ± 0.55 Ab | 153.28 ± 0.18 Bb | 86.97 ± 0.16 Db | |
| 200 | 627.20 ± 0.94 Ba | 578.16 ± 0.81 Ca | 709.63 ± 0.69 Aa | 627.01 ± 0.18 Ba | |
| MDA (μmol·g−1 FW) | 0 | 14.51 ± 0.27 Ab | 12.39 ± 0.11 Bc | 13.17 ± 0.24 Aa | 12.85 ± 0.13 Ab |
| 50 | 20.73 ± 5.54 Aab | 18.24 ± 0.69 Ab | 14.22 ± 0.33 Aa | 13.69 ± 0.03 Ab | |
| 100 | 14.40 ± 0.65 Ab | 12.42 ± 0.10 Ac | 16.90 ± 4.44 Aa | 13.15 ± 0.87 Ab | |
| 150 | 19.20 ± 2.23 ABab | 17.15 ± 0.17 ABb | 20.13 ± 0.47 Aa | 14.43 ± 0.88 Bb | |
| 200 | 26.86 ± 0.95 Ba | 23.15 ± 0.48 Ba | 19.05 ± 0.50 Ba | 42.83 ± 5.79 Aa | |
| Salt Concentration | Oat Cultivars | D Value | Rank |
|---|---|---|---|
| 50 mmol/L | Qinghai 444 | 0.921 | 1 |
| Mengyan No. 1 | 0.561 | 2 | |
| Baiyan No. 18 | 0.250 | 3 | |
| Qingyin No. 3 | 0.049 | 4 | |
| 100 mmol/L | Qinghai 444 | 1.000 | 1 |
| Mengyan No. 1 | 0.670 | 2 | |
| Baiyan No. 18 | 0.413 | 3 | |
| Qingyin No. 3 | 0.124 | 4 | |
| 150 mmol/L | Mengyan No. 1 | 0.829 | 1 |
| Qinghai 444 | 0.808 | 2 | |
| Baiyan No. 18 | 0.264 | 3 | |
| Qingyin No. 3 | 0.123 | 4 | |
| 200 mmol/L | Qinghai 444 | 0.925 | 1 |
| Mengyan No. 1 | 0.604 | 2 | |
| Baiyan No. 18 | 0.371 | 3 | |
| Qingyin No. 3 | 0.284 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Pu, D.; Yang, C.; Wang, P.; Zhao, Y.; Zhou, Q. Differences in Salinity Tolerance in Avena sativa and Avena nuda. Agronomy 2025, 15, 594. https://doi.org/10.3390/agronomy15030594
Zhang J, Pu D, Yang C, Wang P, Zhao Y, Zhou Q. Differences in Salinity Tolerance in Avena sativa and Avena nuda. Agronomy. 2025; 15(3):594. https://doi.org/10.3390/agronomy15030594
Chicago/Turabian StyleZhang, Junchao, Dan Pu, Chenxi Yang, Pei Wang, Yuanyuan Zhao, and Qingping Zhou. 2025. "Differences in Salinity Tolerance in Avena sativa and Avena nuda" Agronomy 15, no. 3: 594. https://doi.org/10.3390/agronomy15030594
APA StyleZhang, J., Pu, D., Yang, C., Wang, P., Zhao, Y., & Zhou, Q. (2025). Differences in Salinity Tolerance in Avena sativa and Avena nuda. Agronomy, 15(3), 594. https://doi.org/10.3390/agronomy15030594





