Development of Male and Female Gametophytes in Cannabis sativa L. cv. Helena (Cannabaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Embriologycal Analyses
2.1.1. Male and Female Structures
2.1.2. Pollen Viability
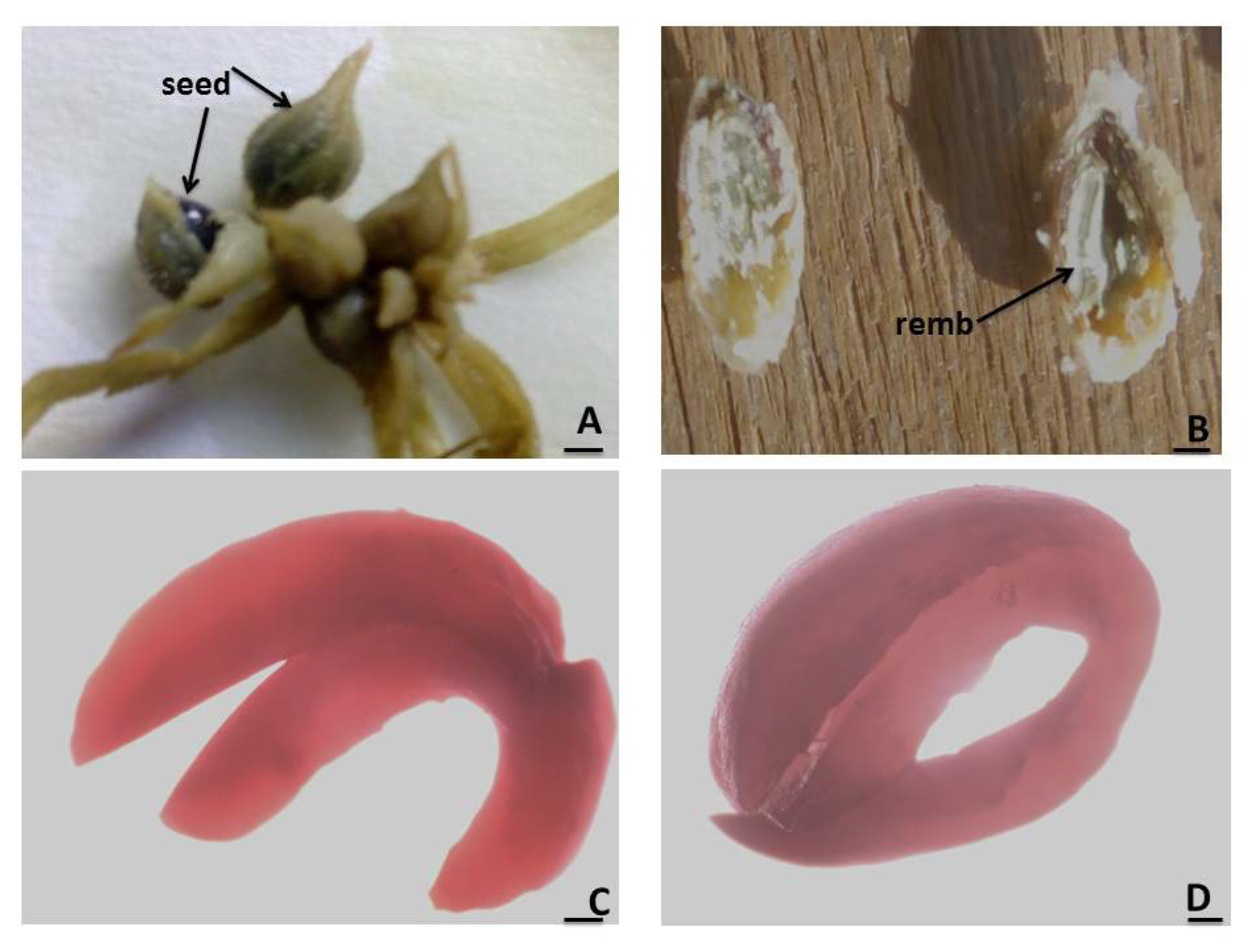
2.1.3. Seed Viability
2.2. Statistical Analysis
3. Results
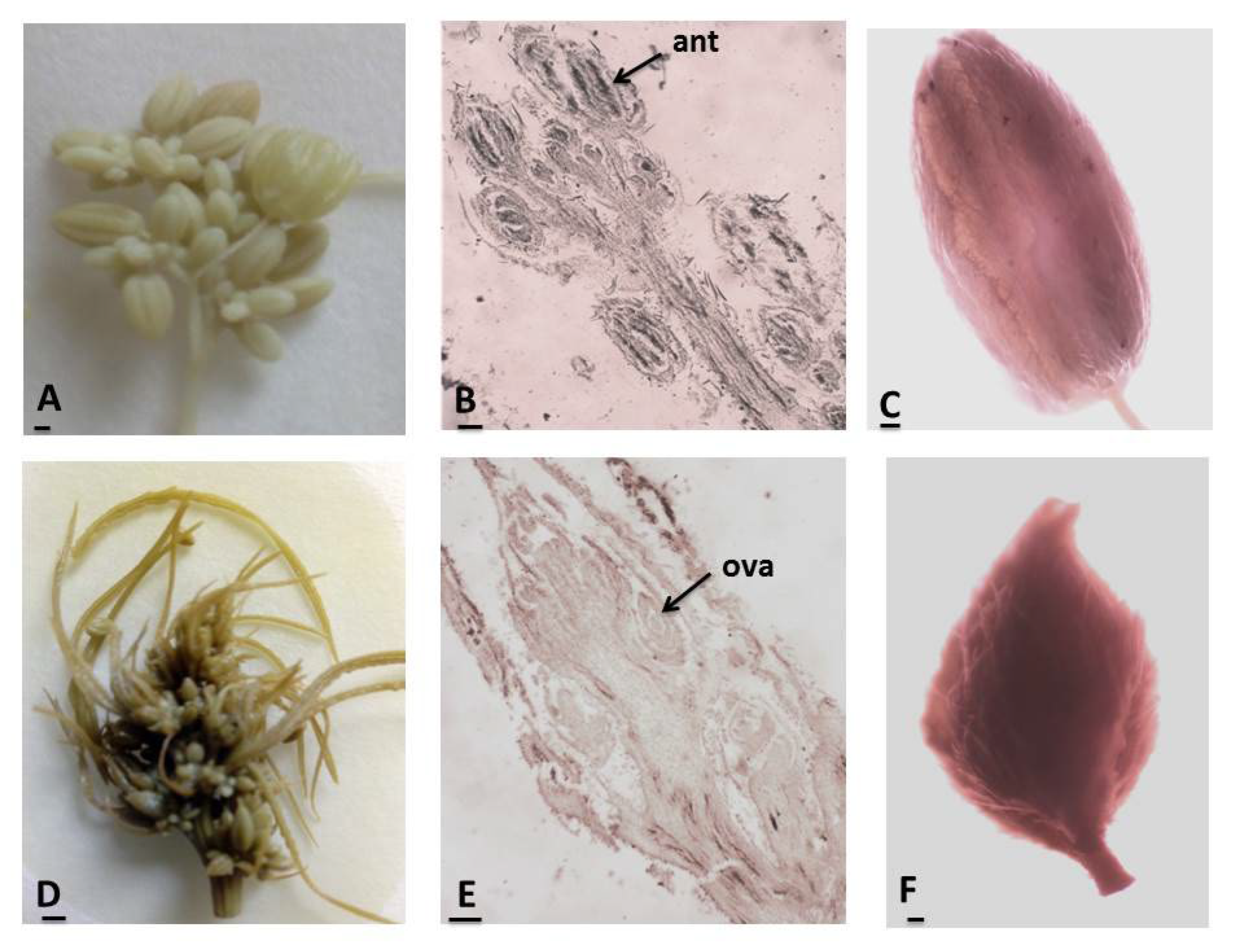
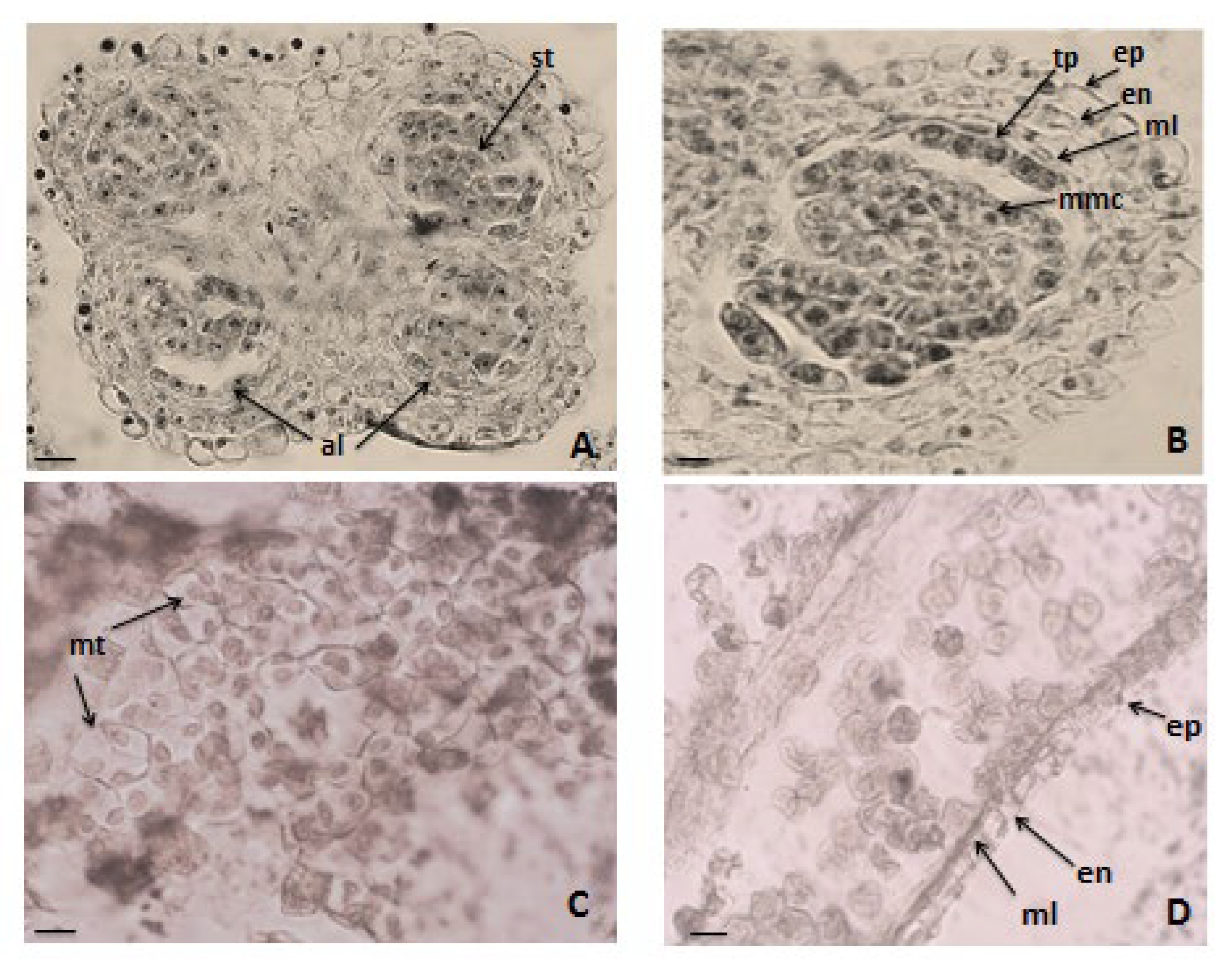
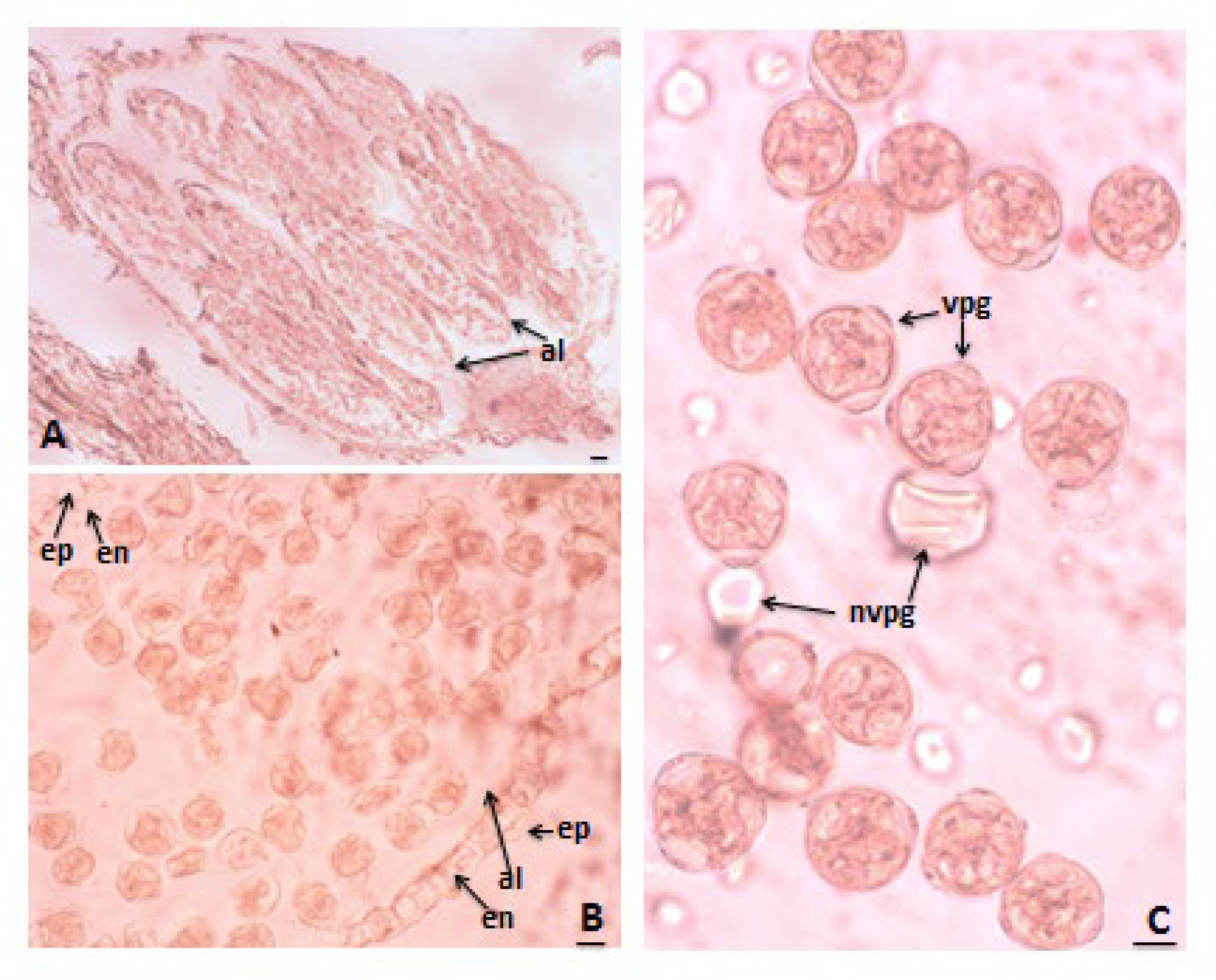
3.1. Anther and Development of Male Gametophyte
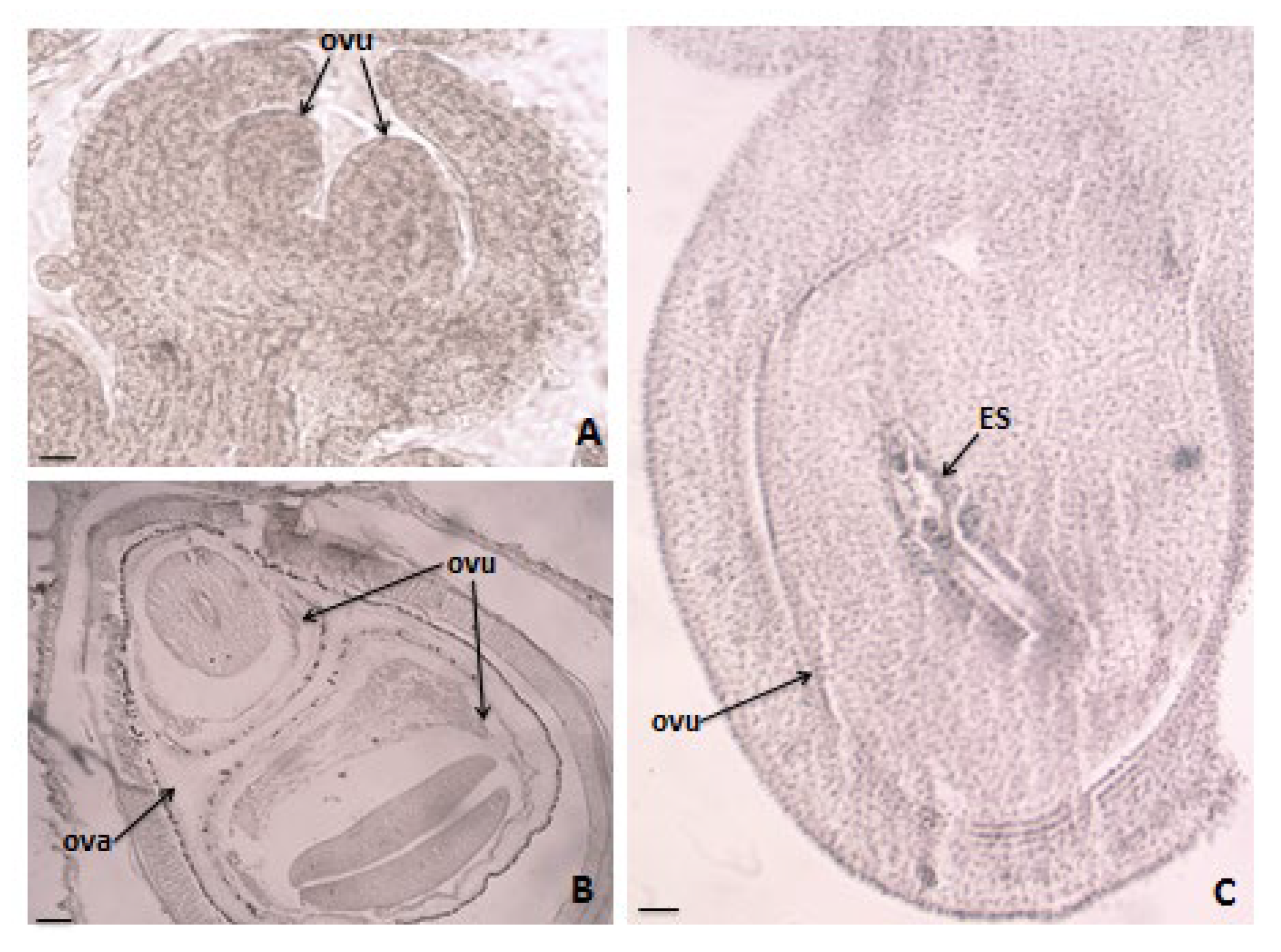
3.2. Ovule and Development of Female Gametophyte
3.3. Pollen Viability
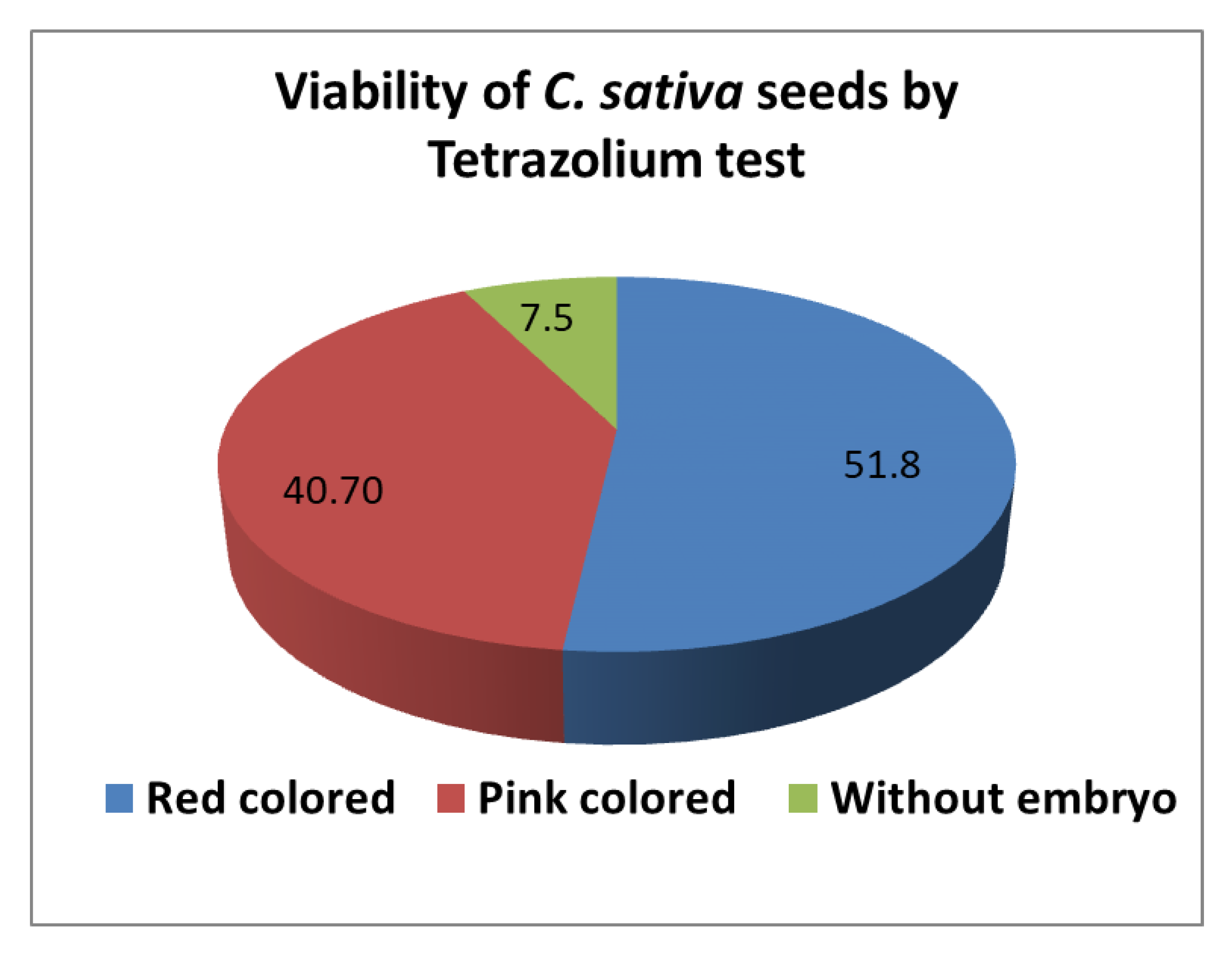
3.4. Seed Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haberlandt, F. Welche Einflusse bedingen das Geschlecht der HanfpflanZen? Fuhlings landw Ztg. 1877, 881. In Morphology of Cannabis sativa L.; State University of Iowa: Iowa City, IA, USA, 1914; Available online: https://www.scribd.com/document/400629834/Morphology-of-Cannabis-Sativa-L (accessed on 11 February 2025).
- Saccardo, P.A. Sulle cause determinanti la sessualita nslla Canapa. Bul. Soc. Veneto-Trent. Sei. Nat. Padova. 1879. In Morphology of Cannabis sativa L.; State University of Iowa: Iowa City, IA, USA, 1914; Available online: https://www.scribd.com/document/400629834/Morphology-of-Cannabis-Sativa-L (accessed on 11 February 2025).
- Heyer, F. Untersuchungen uber das Verhaltniss des geschlechtes bei einhausigen und Zweihausigen Pflanzen. Ber. aus d. physiolog. Laborat. d. landw. Instituts d. Universitat Halle. 1 Bd. 5. Heft. 1884. In Morphology of Cannabis sativa L.; State University of Iowa: Iowa City, IA, USA, 1914; Available online: https://www.scribd.com/document/400629834/Morphology-of-Cannabis-Sativa-L (accessed on 11 February 2025).
- Dusing, C.A. Die experimsntelle Prufung der Theorie von der Regulierung des Geschlechtsverbaltnisse. Jenaiehe Ztschr. f. naturwiss. 1885 19. Suppl. II. In Morphology of Cannabis sativa L.; State University of Iowa: Iowa City, IA, USA, 1914; Available online: https://www.scribd.com/document/400629834/Morphology-of-Cannabis-Sativa-L (accessed on 11 February 2025).
- Hoffman, H. Ueber Sexualitat. Bot. Ztg. 1885, 43. In Morphology of Cannabis sativa L.; State University of Iowa: Iowa City, IA, USA, 1914; Available online: https://www.scribd.com/document/400629834/Morphology-of-Cannabis-Sativa-L (accessed on 11 February 2025).
- Fisch, C. Ueber die Zahlenverhaltnisse des Geschlechtes beim Hanf. Ber. d. d. bot. Bd. V. 1887. In Morphology of Cannabis sativa L.; State University of Iowa: Iowa City, IA, USA, 1914; Available online: https://www.scribd.com/document/400629834/Morphology-of-Cannabis-Sativa-L (accessed on 11 February 2025).
- Noll, F. Vorlaufiger Abschluss der Versuche uber die Bestummung des Geschlechts bei dioechen Pflanzen. Sitzungsber der niederrh. Gesellsch. f. Natur-U. Heilkunde. Bonn. 1907. In Morphology of Cannabis sativa L.; State University of Iowa: Iowa City, IA, USA, 1914; Available online: https://www.scribd.com/document/400629834/Morphology-of-Cannabis-Sativa-L (accessed on 11 February 2025).
- Strasburger, E. Üeber geschlechbestummende Ursachen. Jahrb. Wiss. Bot. 1910, 48, 427–520. [Google Scholar]
- Galoch, E. The hormonal control of sex differentiation in dioecious plants of hemp (Cannabis sativa). Acta Physiol Plant 1980, 2, 31–39. [Google Scholar]
- Lacombe, J.P. Discrimination des sexes en fonction de caractères végétatifs précoces chez le Chanvre dioïque (Cannabis sativa L.). Physiol Vég 1980, 18, 419–430. [Google Scholar]
- Spitzer-Rimon, B.; Shai Duchin, S.; Bernstein, N.; Kamenetsky, R. Architecture and Florogenesis in Female Cannabis sativa Plants. Front Plant Sci. 2019, 10, 350. [Google Scholar] [CrossRef]
- Faeti, V.G.; Mandolino, V.G.; Ranalli, P. Genetic diversity of Cannabis sativa germplasm based on RAPD markers. Plant Breed. 1996, 115, 367–370. [Google Scholar] [CrossRef]
- Forapani, S.; Carboni, A.; Paoletti, C.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. Comparison of hemp varieties using random amplified polymorphic DNA markers. Crop Sci. 2001, 41, 1682–1688. [Google Scholar] [CrossRef]
- Moliterni, V.M.C.; Cattivelli, L.; Ranalli, P.; Mandolino, G. The sexual differentiation of Cannabis sativa L.: A morphological and molecular study. Euphytica 2004, 140, 95–106. Available online: https://www.scribd.com/document/454759367/Euphitica2005hemp (accessed on 11 February 2025). [CrossRef]
- Hammond, C.T.; Mahlberg, P.G. Morphogenesis of capitate glandular hairs of Cannabis sativa (Cannabaceae). Am. J. Bot. 1977, 64, 1023–1031. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Cannabis sativa L.: Botany and horticulture In Cannabis sativa L.–Botany and Biotechnology; Springer: New York, NY, USA, 2017; pp. 79–100. Available online: https://www.scribd.com/document/450709197/Cannabis-sativa-L-Botany-Biotechnology-pdf# (accessed on 11 February 2025).
- Raman, V.; Lata, H.; Chandra, S.; Khan, I.A.; ElSohly, M.A. Morpho-anatomy of marijuana (Cannabis sativa L.). In Cannabis sativa L.–Botany and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Hemphill, J.; Turner, J.; Mahlberg, P. Cannabinoid content of individual plant organs from different geographical strain of Cannabis sativa L. J. Nat. Prod. 1980, 43, 112–121. [Google Scholar] [CrossRef]
- Punja, Z.K.; Ni, L.; Lung, S.; Buirs, L. Total yeast and mold levels in high THC-containing cannabis (Cannabis sativa L.) inflorescences are influenced by genotype, environment, and pre-and post-harvest handling practices. Front Microbiol. 2023, 14, 1192035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheljazkov, V.D.; Noller, J.S.; Dale, R.; Maggi, F. Terpenes and cannabinoids yields and profile from direct-seeded and transplanted CBD-Cannabis sativa. J. Agric. Food Chem. 2022, 70, 10417–10428. [Google Scholar] [CrossRef] [PubMed]
- Zinger, N. Beitrage zur Kenntniss der weiblichen Bluthen und Inflorescenzen bei Cannabineen. Flora 1898, 85, 189–253. [Google Scholar]
- Briosi, G.; Tognini, F. 1894–96. Intorno alla anatomia della canapa (Cannabis sativa L.); Tip. Bernardoni di C. Rebeschini e c.: Milano, Italy, 1894. [Google Scholar]
- Montemartini, L. Sul valore morfologico dell r ovario e del 1 ovulo della Canapa; Rendiconti Congresso Botanica di Palermo: Palermo, Italy, 1902; 8vo br.; p. VIII-224. [Google Scholar]
- Prain, D. On the Morphology, Teratology, and Diclinism of the Flowers of Cannabis, No, 12; Scientific Memoirs; Medical and Sanitary Departments: New Delhi, India, 1904. [Google Scholar]
- Modilewsky, J. Zur Samenbildung einiger Urticifloren. Flora 1908, 98, 423–470. [Google Scholar] [CrossRef]
- Reed, J. Morphology of Cannabis sativa L. Master’s Thesis, State University of Iowa, Ames, IA, USA, 1914. [Google Scholar] [CrossRef]
- Ram, M. Occurrence of endosperm haustorium in Cannabis sativa L. Ann. Bot. 1960, 24, 79–82. [Google Scholar] [CrossRef]
- Davis, G. Systematic Embryology of the Angiosperms; John Wiley & Sons, Inc.: New York, NY, USA, 1966. [Google Scholar]
- Poddubnaya-Arnoldi, V.A. Cannabaceae. In Characteristics of Angiosperms Families According to Cytoembryological Features; Nauka: Moskow, Russia, 1982; pp. 51–52. (In Russian) [Google Scholar]
- McPhee, H.C. Meiotic cytokinesis of Cannabis. Botan. Gazette 1924, 78, 335–341. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. Cytoplasmic connections between angiosperm meiocytes. Annal. Bot. 1966, 30, 221–230. [Google Scholar] [CrossRef]
- Asanova, D.K. Specific features of microsporogenesis in the hemp of the Shu valley. Biol. Bull. Rus. Acad. Sci. 2002, 29, 545–550. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. Origin of exine. Nature 1962, 195, 1069–1071. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. The Pollen Wall: Structure and Development: Pollen; Butterworth-Heinemann: Oxford, UK, 1971; pp. 75–98. [Google Scholar]
- Bradley, D.E. The study of pollen grain surfaces in the electron microscope. New Phytol. 1958, 57, 226–229. [Google Scholar] [CrossRef]
- Punt, W.; Malotaux, M. Cannabaceae, moraceae and urticaceae. Rev. Palaeobot. Palynol. 1984, 42, 23–44. [Google Scholar] [CrossRef]
- Galán-Ávila, A.; García-Fortea, E.; Prohens, J.; Herraiz, F.J. Microgametophyte Development in Cannabis sativa L. and First Androgenesis Induction Through Microspore Embryogenesis. Front Plant Sci. 2021, 25, 669424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, N.G. The genera of the Cannabaceae in the southeastern United States. J. Arnold Arboret. 1970, 51, 185–203. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Z.; Bartholomew, B. Cannabaceae. Flora China 2003, 5, 74–75. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC). Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products: Manual for Use by National Drug Testing Laboratories; United Nations Publications: New York, NY, USA, 2009. [Google Scholar]
- Hammond, C.T.; Mahlberg, P.G. Morphology of glandular hairs of Cannabis sativa from scanning electron microscopy. Am. J. Bot. 1973, 60, 524–528. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Joerg Bohlmann, J.; Page, J.E.; Samuelset, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- Mandolino, G.; Carboni, A.; Forapani, S.; Faeti, V.; Ranalli, P. Identification of DNA markers linked to the male sex in dioecious hemp (Cannabis sativa L.). Theor. Appl. Genet. 1999, 98, 86–92. [Google Scholar] [CrossRef]
- Sakamoto, K.; Akiyama, Y.; Fukui, K.; Kamada, H.; Satoh, S. Characterization; Genome Sizes and Morphology of Sex Chromosomes in Hemp (Cannabis sativa L.). Cytologia 1998, 63, 459–464. [Google Scholar] [CrossRef]
- Kaushal, S. Impact of physical and chemical mutagens on sex expression in Cannabis sativa. Indian J. Fundam. Appl. Life Sci. 2012, 2, 97–103. [Google Scholar]
- Shao, H.; Song, S.-J.; Clarke, R.C. Female-Associated DNA Polymorphisms of Hemp (Cannabis sativa L.). J. Ind. Hemp. 2003, 8, 5–9. [Google Scholar] [CrossRef]
- Mendel, P.; Bharat Lalge, A.; Vyhnanek, T.; Trojan, V.; Kalousek, P.; Maassen, H.; Havel, L. Progress in early sex determination of cannabis plant by DNA markers. MendelNet 2016, 1, 731–735. [Google Scholar]
- Araméndiz-Tatis, H.; Cardona-Ayala, C.E.; Espitia-Camacho, M.; Herrera-Contreras, A.C.; Villalba-Soto, A.L. Agronomic evaluation of Cannabis sativa (L.) cultivars in northern Colombia. Rev. Colomb. Cienc. Hortícolas 2023, 17, e15695. [Google Scholar] [CrossRef]
- Campbell, L.G.; Peach, K.; Wizenberg, S.B. Dioecious hemp (Cannabis sativa L.) plants do not express significant sexually dimorphic morphology in the seedling stage. Sci. Rep. 2021, 11, 16825. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.; Kayser, O. The cannabis plant: Botanical aspects. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Preedy, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–12. Available online: https://www.scribd.com/document/403631308/The-Cannabis-Plant-Botanical-Aspects-pdf (accessed on 11 February 2025).
- Mishchenko, S.; Mokher, J.; Laiko, I.; Burbulis, N.; Kyrychenko, H.; Dudukova, S. Phenological growth stages of hemp (Cannabis sativa L.): Codification and description according to the BBCH scale. Žemės ūkio mokslai 2017, 24. [Google Scholar] [CrossRef]
- Heidenhain, R. Eine neue Verwendung des haematoxylin. Archiv. Mikrosk. Anat. 1885, 24, 468–470. Available online: https://link.springer.com/article/10.1007/BF02960389 (accessed on 11 February 2025). [CrossRef]
- Poddubnaja-Arnoldi, V.A. Cytoembryology of Angiosperms; Nauka: Moscow, Russia, 1976. [Google Scholar]
- Batygina, T.B. Embryology of Floering Plants. Terminology and Concepts, Volume 1: Generative Organs of Flower; Science Publishers, Inc.: New York, NY, USA, 2002; p. 421. [Google Scholar]
- Singh, R.J. Plant Cytogenetics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; p. 488. Available online: https://scholar.google.com/scholar_lookup?title=Plant+Cytogenetics&author=Singh,+R.J.&publication_year=2003 (accessed on 11 February 2025).
- Peters, J. Tetrazolium Testing Handbook; Association of Official Seed Analysts: Ithaca, NY, USA, 2007; p. 88. Available online: https://scholar.google.com/scholar_lookup?title=Tetrazolium+Testing+Handbook&author=Peters,+J.&publication_year=2007 (accessed on 11 February 2025).
- Moore, R.P. Handbook on Tetrazolium Testing; International Seed Testing Association: Zurich, Switzerland, 1985. [Google Scholar]
- Schnarf, K. Embryologie der Angiospermen; Gebruder Bontraeger: Berlin, Germany, 1929; p. 689. [Google Scholar]
- Maheshwari, P. Recent Advances in the Embryology of Angiosperms; International Society of Plant Morphologists, University of Delhi: New Delhi, India, 1963; 467p. [Google Scholar]
- Tursin, G. Study on the Generative Organ of Salvia Officinalis. Materials All-Union Symposium “Sexual Development and Embryogenesis in Plants”; Moscow State University Publishing House: Moscow, Russia, 1973. (In Russian) [Google Scholar]



| Authors | Data |
|---|---|
| Zinger [22] | Morphology of the pistillate flower and inflorescence of Cannabaceae; |
| Briosi and Tognini [23] | C. sativa inflorescence, organography, organogeny, structure of megasporangium, female gametophyte, microsporangium and male gametophyte; |
| Montemartini [24] | Morphology of the ovary and ovule of C. sativa, including their vascular supply and discussion on the origin of these parts; |
| Prain [25] | Morphology, teratology, and diclinism of the C. sativa flowers, describing abnormalities, nature of ovary, and origin of ovule; |
| Modilewsky [26] | Female gametophyte, fertilization, embryo development in C. sativa; |
| Reed [27] | Morphological descriptions of C. sativa floral organs and their development; |
| Ram [28] | Development and structure of endosperm in C. sativa; |
| Davis [29] | Onagrad-type embryo with two-celled suspensor; suspected apomixis; |
| Poddubnaya-Arnoldy [30] | Double fertilization of premitotic type; polyembryony; |
| McPhee [31]; Heslop-Harrison [32]; Asanova [33] | Meiosis in the microsporangium; |
| Heslop-Harrison [34,35] | Development and structure of tapetum anther layer; |
| Bradley [36]; Punt and Malotaux [37] | Exine formation and characteristics of pollen grains; |
| Galán-Ávila et al. [38] | Microsporogenesis, and microgametogenesis and correlated microgametophyte developmental stages; |
| Miller [39]; Wu et al. [40]; United Nations Office on Drugs and Crime (UNODC) [41]; Raman et al. [18] | Anatomy of male and female flowers; |
| Spitzer-Rimon et al. [11] | Architecture and timing of initiation and differentiation of the inflorescence and individual flowers of different C. sativa cultivars; |
| Hammond and Mahlberg [42]; Spitzer-Rimon et al. [11]; Livingston et al. [43] | Morphology of male flowers; |
| Mandolino et al. [44]; Sakamoto et al. [45]; Kausal [46] | Genetic sex determination; |
| Shao et al. [47]; Mendel et al. [48] | Development of molecular markers for sex expression in C. sativa; |
| Araméndiz-Tatis et al. [49] | Genetic variability in vegetative and reproductive characteristics, sexual dimorphism in juvenile plants; |
| Farag and Kayser [51]; Mishchenko et al. [52]; Raman et al. [18] | Terminology for phenological stages of C. sativa development; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yankova-Tsvetkova, E.; Semerdjieva, I.; Sikora, V.; Zheljazkov, V.D. Development of Male and Female Gametophytes in Cannabis sativa L. cv. Helena (Cannabaceae). Agronomy 2025, 15, 474. https://doi.org/10.3390/agronomy15020474
Yankova-Tsvetkova E, Semerdjieva I, Sikora V, Zheljazkov VD. Development of Male and Female Gametophytes in Cannabis sativa L. cv. Helena (Cannabaceae). Agronomy. 2025; 15(2):474. https://doi.org/10.3390/agronomy15020474
Chicago/Turabian StyleYankova-Tsvetkova, Elina, Ivanka Semerdjieva, Vladimir Sikora, and Valtcho D. Zheljazkov. 2025. "Development of Male and Female Gametophytes in Cannabis sativa L. cv. Helena (Cannabaceae)" Agronomy 15, no. 2: 474. https://doi.org/10.3390/agronomy15020474
APA StyleYankova-Tsvetkova, E., Semerdjieva, I., Sikora, V., & Zheljazkov, V. D. (2025). Development of Male and Female Gametophytes in Cannabis sativa L. cv. Helena (Cannabaceae). Agronomy, 15(2), 474. https://doi.org/10.3390/agronomy15020474








