ZmC2GnT Positively Regulates Maize Seed Rot Resistance Against Fusarium verticillioides
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Plasmid Construction
2.3. Seed Inoculation
2.4. Gene Expression Analysis
2.5. GUS Staining
3. Results
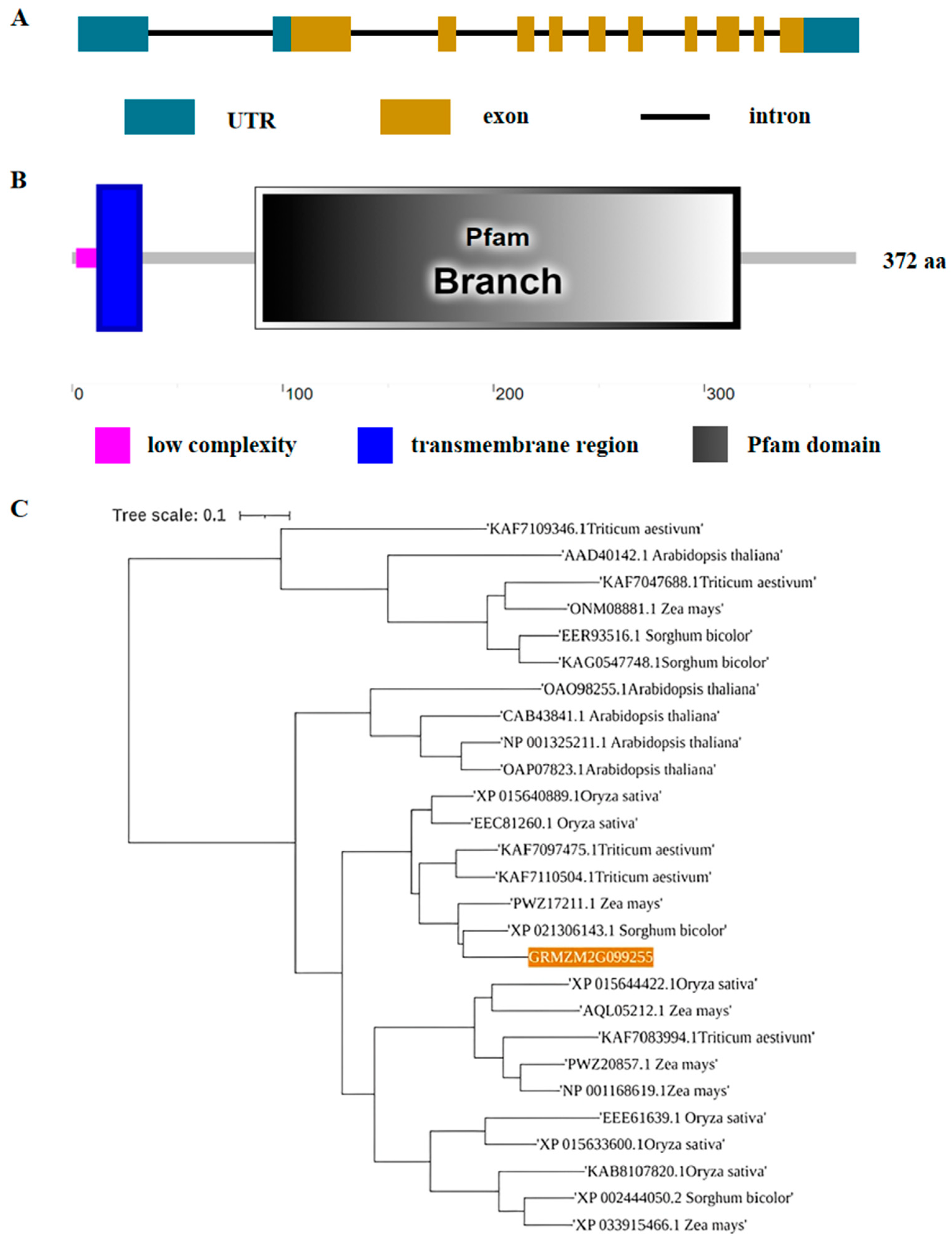
3.1. ZmC2GnT Encodes a Putative β-1,6-N-Acetylglucosaminyltransferase Protein
3.2. ZmC2GnT Helps Seeds Resist F. verticillioides
3.3. The Expression of ZmC2GnT Increases in Response to F. verticillioides Infection
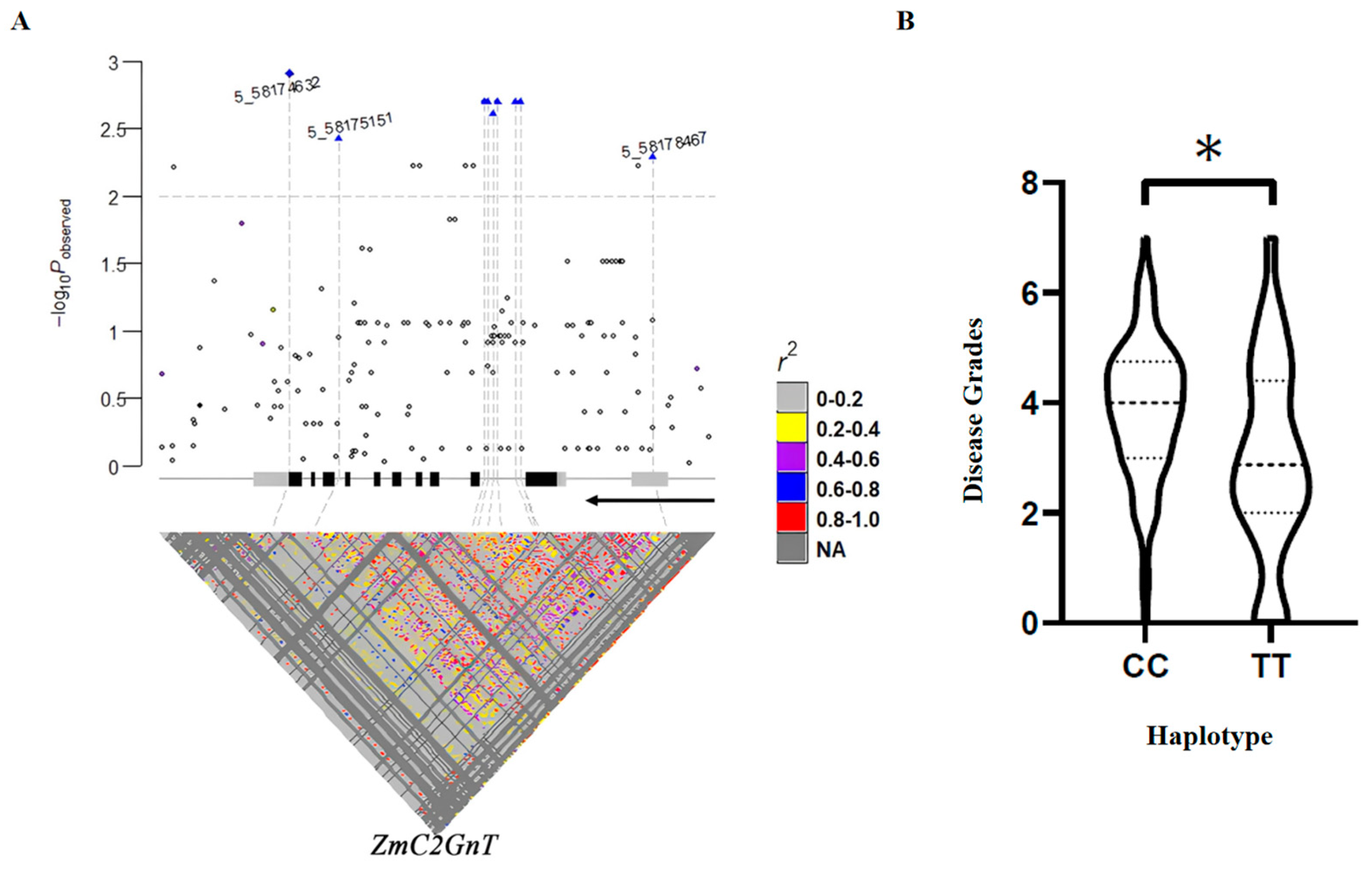
3.4. The Cis-Element on the ZmC2GnT Promoter Is Responsible for Resistance Against F. verticillioides
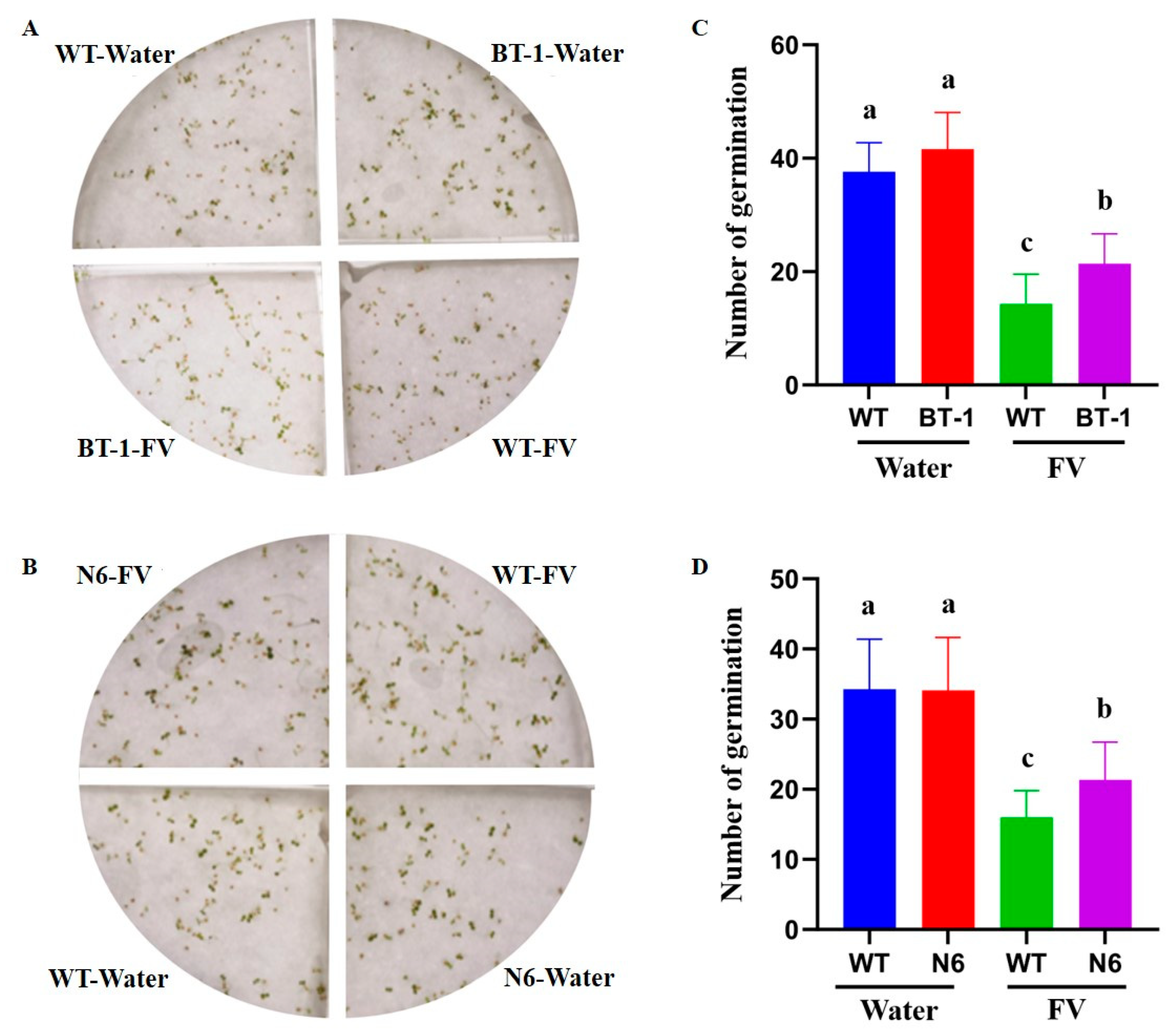
3.5. ZmC2GnT Promotes Plant Immunity to F. verticillioides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilke, A.L.; Bronson, C.R.; Tomas, A.; Munkvold, G.P. Seed Transmission of Fusarium verticillioides in Maize Plants Grown Under Three Different Temperature Regimes. Plant Dis. 2007, 91, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; Hinton, D.M. Fusaric Acid and Pathogenic Interactions of Corn and Non-Corn Isolates of Fusarium moniliforme, a Nonobligate Pathogen of Corn. In Fumonisins in Food; Jackson, L.S., DeVries, J.W., Bullerman, L.B., Eds.; Springer: Boston, MA, USA, 1996; pp. 175–191. [Google Scholar]
- Munkvold, G.P.; McGee, D.C.; Carlton, W.M. Importance of Different Pathways for Maize Kernel Infection by Fusarium moniliforme. Phytopathology 1997, 87, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P. Epidemiology of Fusarium Diseases and their Mycotoxins in Maize Ears. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Zitomer, N.C.; Riley, R.T. Extraction and Analysis of Fumonisins and Compounds Indicative of Fumonisin Exposure in Plant and Mammalian Tissues and Cultured Cells. In Microbial Toxins: Methods and Protocols; Holst, O., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 171–185. [Google Scholar]
- Czembor, E.; Waśkiewicz, A.; Piechota, U.; Puchta, M.; Czembor, J.H.; Stȩpień, Ł. Differences in Ear Rot Resistance and Fusarium verticillioides-Produced Fumonisin Contamination Between Polish Currently and Historically Used Maize Inbred Lines. Front. Microbiol. 2019, 10, 449. [Google Scholar] [CrossRef]
- Gaikpa, D.S.; Miedaner, T. Genomics-assisted breeding for ear rot resistances and reduced mycotoxin contamination in maize: Methods, advances and prospects. Theor. Appl. Genet. 2019, 132, 2721–2739. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. The fate of mycotoxins during the primary food processing of maize. Food Control 2021, 121, 107651. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular Basis of Resistance to Fusarium Ear Rot in Maize. Front. Plant Sci. 2017, 8, 1774. [Google Scholar] [CrossRef] [PubMed]
- de Jong, G.; Pamplona, A.K.A.; Von Pinho, R.G.; Balestre, M. Genome-wide association analysis of ear rot resistance caused by Fusarium verticillioides in maize. Genomics 2018, 110, 291–303. [Google Scholar] [CrossRef]
- Samayoa, L.F.; Cao, A.; Santiago, R.; Malvar, R.A.; Butrón, A. Genome-wide association analysis for fumonisin content in maize kernels. BMC Plant Biol. 2019, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.B.; Marino, T.P.; Manching, H.C.; Wisser, R.J. Genomic prediction for resistance to Fusarium ear rot and fumonisin contamination in maize. Crop Sci. 2020, 60, 1863–1875. [Google Scholar] [CrossRef]
- Zila, C.T.; Samayoa, L.F.; Santiago, R.; Butrón, A.; Holland, J.B. A Genome-Wide Association Study Reveals Genes Associated with Fusarium Ear Rot Resistance in a Maize Core Diversity Panel. G3 Genes|Genomes|Genet. 2013, 3, 2095–2104. [Google Scholar] [CrossRef]
- Chen, J.; Shrestha, R.; Ding, J.; Zheng, H.; Mu, C.; Wu, J.; Mahuku, G. Genome-Wide Association Study and QTL Mapping Reveal Genomic Loci Associated with Fusarium Ear Rot Resistance in Tropical Maize Germplasm. G3 Genes|Genomes|Genet. 2016, 6, 3803–3815. [Google Scholar] [CrossRef]
- Guo, Z.; Zou, C.; Liu, X.; Wang, S.; Li, W.-X.; Jeffers, D.; Fan, X.; Xu, M.; Xu, Y. Complex Genetic System Involved in Fusarium Ear Rot Resistance in Maize as Revealed by GWAS, Bulked Sample Analysis, and Genomic Prediction. Plant Dis. 2020, 104, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, G.; Zhang, A.; Loladze, A.; Hu, Y.; Wang, H.; Qu, J.; Zhang, X.; Olsen, M.; San Vicente, F.; et al. Genome-wide association study and genomic prediction of Fusarium ear rot resistance in tropical maize germplasm. Crop J. 2021, 9, 325–341. [Google Scholar] [CrossRef]
- Butrón, A.; Reid, L.M.; Santiago, R.; Cao, A.; Malvar, R.A. Inheritance of maize resistance to gibberella and fusarium ear rots and kernel contamination with deoxynivalenol and fumonisins. Plant Pathol. 2015, 64, 1053–1060. [Google Scholar] [CrossRef]
- Giomi, G.M.; Kreff, E.D.; Iglesias, J.; Fauguel, C.M.; Fernandez, M.; Oviedo, M.S.; Presello, D.A. Quantitative trait loci for Fusarium and Gibberella ear rot resistance in Argentinian maize germplasm. Euphytica 2016, 211, 287–294. [Google Scholar] [CrossRef]
- Kebede, A.Z.; Woldemariam, T.; Reid, L.M.; Harris, L.J. Quantitative trait loci mapping for Gibberella ear rot resistance and associated agronomic traits using genotyping-by-sequencing in maize. Theor. Appl. Genet. 2016, 129, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kuska, M.T.; Mahlein, A.K. Aiming at decision making in plant disease protection and phenotyping by the use of optical sensors. Eur. J. Plant Pathol. 2018, 152, 987–992. [Google Scholar] [CrossRef]
- Gao, X.; Shim, W.-B.; Göbel, C.; Kunze, S.; Feussner, I.; Meeley, R.; Balint-Kurti, P.; Kolomiets, M. Disruption of a Maize 9-Lipoxygenase Results in Increased Resistance to Fungal Pathogens and Reduced Levels of Contamination with Mycotoxin Fumonisin. Mol. Plant-Microbe Interact. 2007, 20, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Borrego, E.; Shim, W.-B.; Isakeit, T.; Kolomiets, M. Quantification of Fungal Colonization, Sporogenesis, and Production of Mycotoxins Using Kernel Bioassays. J. Vis. Exp. 2012, e3727. [Google Scholar] [CrossRef]
- Zhou, G.; Li, S.; Ma, L.; Wang, F.; Jiang, F.; Sun, Y.; Ruan, X.; Cao, Y.; Wang, Q.; Zhang, Y.; et al. Mapping and Validation of a Stable Quantitative Trait Locus Conferring Maize Resistance to Gibberella Ear Rot. Plant Dis. 2021, 105, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Zhou, Z.; Mu, C.; Zhang, X.; Gao, J.; Liang, Y.; Chen, J.; Wu, Y.; Li, X.; Wang, S.; et al. Dissecting the genetic architecture of Fusarium verticillioides seed rot resistance in maize by combining QTL mapping and genome-wide association analysis. Sci. Rep. 2017, 7, 46446. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, H.; Liu, E.; He, K.; Song, Y.; Dong, C.; Wang, Z.; Zhang, X.; Zhou, Z.; Xu, Y.; et al. Evaluation and Identification of Resistance Lines and QTLs of Maize to Seedborne Fusarium verticillioides. Plant Dis. 2022, 106, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, M.; Liu, X.; Zhang, B.; Cui, Y.; Cao, X.; Zhang, Z.; Shi, C.; Wei, H.; He, H.; et al. RBB1 negatively regulates rice disease resistance by modulating protein glycosylation. J. Integr. Plant Biol. 2024. early view. [Google Scholar] [CrossRef]
- Kreppel, L.K.; Blomberg, M.A.; Hart, G.W. Dynamic Glycosylation of Nuclear and Cytosolic Proteins: CLONING And CHARACTERIZATION Of A UNIQUE O-GlcNAc TRANSFERASE With MULTIPLE TETRATRICOPEPTIDE REPEATS*. J. Biol. Chem. 1997, 272, 9308–9315. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lu, Q.; Xu, J.; Shi, J. Cloning and expression analysis of two salt and Fusarium graminearum stress associated UDP-glucosyltransferases genes in wheat. Yichuan Hered. 2008, 30, 1608–1614. [Google Scholar]
- Wang, C.; Li, J.; Liu, L.; Zeng, L.; Xue, L. Characterization of the functional domain of STT3a of oligosaccharyltransferase from Dunaliella salina. Shengwu Gongcheng Xuebao (Chin. J. Biotechnol.) 2010, 26, 760–766. [Google Scholar]
- Li, H.; Yin, H.; Zhang, X.; Yang, A. Enhancement of drought resistance in transgenic tobacco expressing sucrose: Sucrose 1-fructosyltransferase gene from Lactuca sativa. Shandong Daxue Xuebao (Shandong Univ.) 2007, 42, 1–6. [Google Scholar]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium Mycotoxin Deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana*. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Song, L.; Wang, J.; Wang, D.; Fan, B.; Wang, S.; Ye, W. Cloning and Salt-tolerance Analysis of N-acetylglucosaminyltransferase Gene (GhGnT) from Gossypium hirsutum L. Mol. Plant Breed. 2011, 9, 309–317. [Google Scholar]
- Zhang, M.; Wei, F.; Guo, K.; Hu, Z.; Li, Y.; Xie, G.; Wang, Y.; Cai, X.; Peng, L.; Wang, L. A Novel FC116/BC10 Mutation Distinctively Causes Alteration in the Expression of the Genes for Cell Wall Polymer Synthesis in Rice. Front. Plant Sci. 2016, 7, 1366. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Z.; Dong, C.; Chen, J.; Ding, J.; Zhang, X.; Mu, C.; Chen, Y.; Li, X.; Li, H.; et al. Linkage mapping and genome-wide association study reveals conservative QTL and candidate genes for Fusarium rot resistance in maize. BMC Genom. 2020, 21, 357. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-C.; Ko, W.-H. A simple method for obtaining single-spore isolates of fungi. Bot. Bull. Acad. Sin. 1997, 38, 41–44. [Google Scholar]
- Mu, C.; Gao, J.; Zhou, Z.; Wang, Z.; Sun, X.; Zhang, X.; Dong, H.; Han, Y.; Li, X.; Wu, Y.; et al. Genetic analysis of cob resistance to F. verticillioides: Another step towards the protection of maize from ear rot. Theor. Appl. Genet. 2019, 132, 1049–1059. [Google Scholar] [CrossRef]
- Bierhuizen, M.F.; Mattei, M.G.; Fukuda, M. Expression of the developmental I antigen by a cloned human cDNA encoding a member of a beta-1,6-N-acetylglucosaminyltransferase gene family. Genes Dev. 1993, 7, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.A.; Davies, G.J.; Bulone, V.; Henrissat, B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 1997, 326, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-C.; Ong, E.; Fukuda, M. Molecular Cloning and Expression of a Novel beta-1,6-N-Acetylglucosaminyltransferase That Forms Core 2, Core 4, and I Branches *. J. Biol. Chem. 1999, 274, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” Browser for Exploring and Analyzing Large-Scale Biological Data Sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R. Seed inoculation with endophytic fungal entomopathogens promotes plant growth and reduces crown and root rot (CRR) caused by Fusarium culmorum in wheat. Planta 2018, 248, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, H.S.; Shoaib, A.; Anwar, A.; Perveen, S.; Javed, S.; Mehnaz, S. Seed biopriming with Ochrobactrum ciceri mediated defense responses in Zea mays (L.) against Fusarium rot. Physiol. Mol. Biol. Plants 2024, 30, 49–66. [Google Scholar] [CrossRef]
- Dinango, V.N.; Dhouib, H.; Wakam, L.N.; Kouokap, L.K.; Youmbi, D.Y.; Eke, P.; Driss, F.; Tounsi, S.; Boyom, F.F.; Frikha-Gargouri, O. Bacterial endophytes inhabiting desert plants provide protection against seed rot caused by Fusarium verticillioides and promote growth in maize. Pest Manag. Sci. 2024, 80, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, T.W.; Parekh, R.B.; Dwek, R.A. Glycobiology. Annu. Rev. Biochem. 1988, 57, 785–838. [Google Scholar] [CrossRef]
- Zachara, N.E.; O’Donnell, N.; Cheung, W.D.; Mercer, J.J.; Marth, J.D.; Hart, G.W. Dynamic O-GlcNAc Modification of Nucleocytoplasmic Proteins in Response to Stress: A SURVIVAL RESPONSE of MAMMALIAN CELLS*. J. Biol. Chem. 2004, 279, 30133–30142. [Google Scholar] [CrossRef] [PubMed]
- Zachara, N.E.; Hart, G.W. Cell signaling, the essential role of O-GlcNAc! Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2006, 1761, 599–617. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Li, H.; Ye, W.; Song, Z.; Zhou, Z.; Jing, P.; Chen, J.; Wu, J. ZmC2GnT Positively Regulates Maize Seed Rot Resistance Against Fusarium verticillioides. Agronomy 2025, 15, 461. https://doi.org/10.3390/agronomy15020461
Sun D, Li H, Ye W, Song Z, Zhou Z, Jing P, Chen J, Wu J. ZmC2GnT Positively Regulates Maize Seed Rot Resistance Against Fusarium verticillioides. Agronomy. 2025; 15(2):461. https://doi.org/10.3390/agronomy15020461
Chicago/Turabian StyleSun, Doudou, Huan Li, Wenchao Ye, Zhihao Song, Zijian Zhou, Pei Jing, Jiafa Chen, and Jianyu Wu. 2025. "ZmC2GnT Positively Regulates Maize Seed Rot Resistance Against Fusarium verticillioides" Agronomy 15, no. 2: 461. https://doi.org/10.3390/agronomy15020461
APA StyleSun, D., Li, H., Ye, W., Song, Z., Zhou, Z., Jing, P., Chen, J., & Wu, J. (2025). ZmC2GnT Positively Regulates Maize Seed Rot Resistance Against Fusarium verticillioides. Agronomy, 15(2), 461. https://doi.org/10.3390/agronomy15020461






