Selenium Biofortification with Se-Enriched Urea and Se-Enriched Ammonium Sulfate Fertilization in Different Common Bean Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Treatments
2.2. Method for Preparing Fertilizer and Characterization of Nitrogen Fertilizers
2.3. Gas Exchanges, Fluorometry, and Soil Plant Analysis Development (SPAD) Index
2.4. Seed Yield
2.5. The Total Concentration of Selenium and Sulfur
2.6. Centesimal Composition (Biomolecules)
2.7. Statistical Analysis
3. Results
3.1. Gas Exchanges, Fluorometry, and SPAD Index
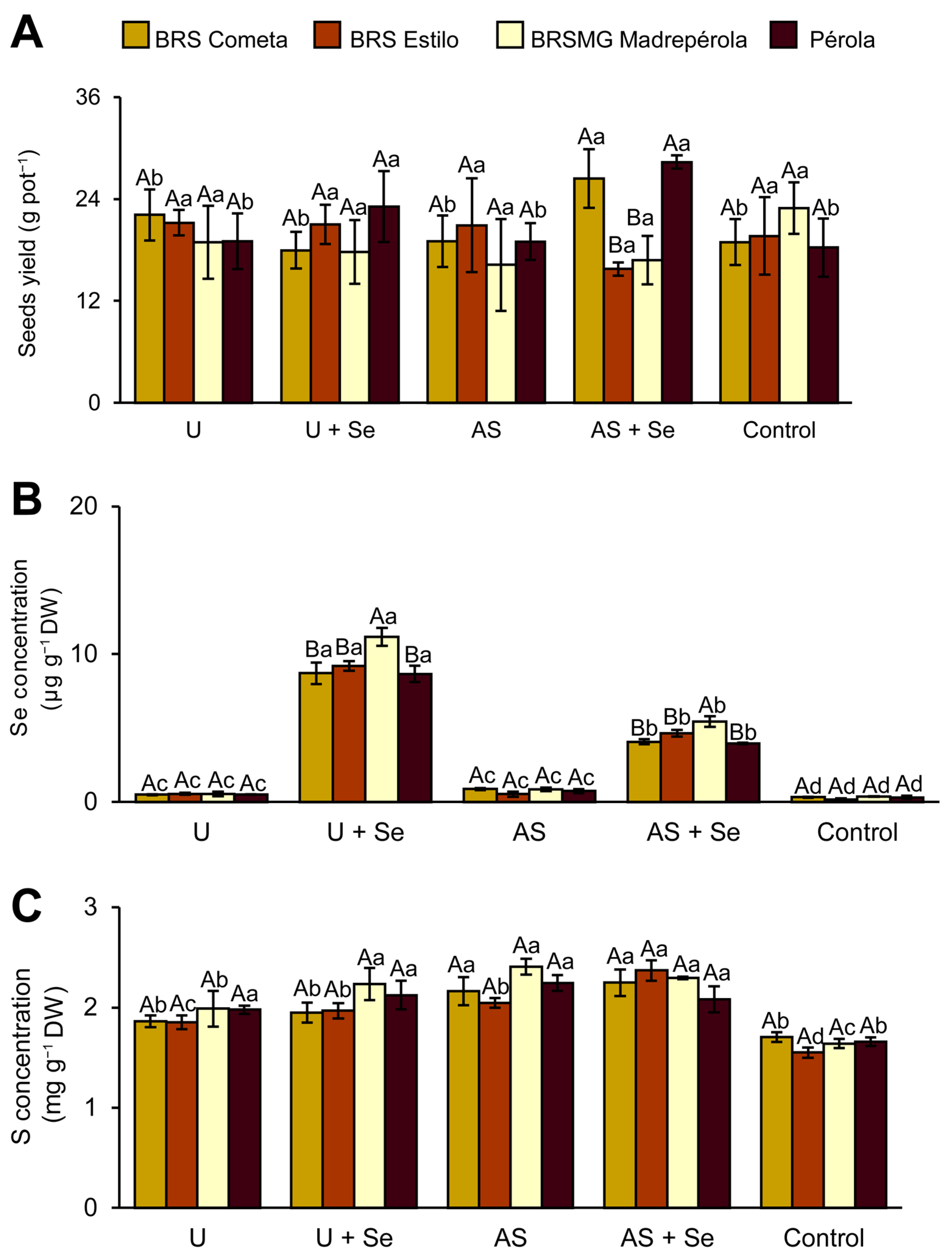
3.2. Seed Yield and Selenium and Sulfur Concentration in the Seeds
3.3. Centesimal Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nassary, E.K.; Baijukya, F.; Ndakidemi, P.A. Intensification of Common Bean and Maize Production through Rotations to Improve Food Security for Smallholder Farmers. J. Agric. Food Res. 2020, 2, 100040. [Google Scholar] [CrossRef]
- CIAT—Centro Internacional de Agricultura Tropical. Beans. Available online: https://ciat.cgiar.org/what-we-do/breeding-better-crops/beans/ (accessed on 21 December 2024).
- Torres, A.R.; Brito, B.; Imperial, J.; Palacios, J.M.; Ciampitti, I.A.; Ruiz-Argüeso, T.; Hungria, M. Hydrogen-Uptake Genes Improve Symbiotic Efficiency in Common Beans (Phaseolus vulgaris L.). Antonie Van Leeuwenhoek 2020, 113, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Justino, L.F.; Heinemann, A.B.; Matta, D.H.; Stone, L.F.; Gonçalves, P.A.O.; Silva, S.C. Characterization of common bean production regions in Brazil using machine learning techniques. Agric. Syst. 2025, 224, 104237. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant Proteins as High-Quality Nutritional Source for Human Diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Grela, E.R.; Samolińska, W.; Kiczorowska, B.; Klebaniuk, R.; Kiczorowski, P. Content of Minerals and Fatty Acids and Their Correlation with Phytochemical Compounds and Antioxidant Activity of Leguminous Seeds. Biol. Trace Elem. Res. 2017, 180, 338–348. [Google Scholar] [CrossRef]
- USDA—United States Department of Agriculture (Ed.) Food Data Central; United States Department of Agriculture: Washington, DC, USA, 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 2 February 2024).
- Natasha, S.M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Ritchie, H.; Reay, D.S.; Higgins, P. Quantifying, Projecting, and Addressing ‘India’s Hidden Hunger. Front. Sustain. Food Syst. 2018, 2, 11. [Google Scholar] [CrossRef]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Bela, K.; Horváth, E.; Gallé, Á.; Szabados, L.; Tari, I.; Csiszár, J. Plant Glutathione Peroxidases: Emerging Role of the Antioxidant Enzymes in Plant Development and Stress Responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dinh, Q.T.; Qi, M.; Wang, M.; Yang, W.; Zhou, F.; Liang, D. Radicular and foliar uptake, and xylem- and phloem-mediated transport of selenium in maize (Zea mays L.): A comparison of five Se exogenous species. Plant Soil 2020, 446, 111–123. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing Selenium Functional Foods and Beverages: A Review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.J.; Ortiz-Monasterio, I.; Yazici, A.; Kaur, C.; Mahmood, K.; et al. Simultaneous biofortification of wheat with zinc, iodine, selenium, and iron through foliar treatment of a micronutrient cocktail in six countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Vermeiren, C.; Ceulemans, J.; Thiry, Y.; Smolders, E. Increased soil pH and enhanced microbial activity stimulate the gradual immobilization of selenate added to soils. Soil Biol. Biochem. 2025, 202, 109688. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Yuan, Z.; Cai, S.; Yan, C.; Rao, S.; Cheng, S.; Xu, F.; Liu, X. Research Progress on the Physiological Mechanism by Which Selenium Alleviates Heavy Metal Stress in Plants: A Review. Agronomy 2024, 14, 1787. [Google Scholar] [CrossRef]
- Silva, M.A.; de Sousa, G.F.; Corguinha, A.P.B.; Lessa, J.H.d.L.; Dinali, G.S.; Oliveira, C.; Lopes, G.; Amaral, D.; Brown, P.; Guilherme, L.R.G. Selenium Biofortification of Soybean Genotypes in a Tropical Soil via Se-Enriched Phosphate Fertilizers. Front. Plant Sci. 2022, 13, 988140. [Google Scholar] [CrossRef] [PubMed]
- Nothstein, A.K.; Eiche, E.; Riemann, M.; Nick, P.; Winkel, L.H.E.; Göttlicher, J.; Steininger, R.; Brendel, R.; von Brasch, M.; Konrad, G.; et al. Tracking Se Assimilation and Speciation through the Rice Plant—Nutrient Competition, Toxicity and Distribution. PLoS ONE 2016, 11, e0152081. [Google Scholar] [CrossRef]
- Rao, S.; Yu, T.; Cong, X.; Lai, X.; Xiang, J.; Cao, J.; Liao, X.; Gou, Y.; Chao, W.; Xue, H.; et al. Transcriptome, Proteome, and Metabolome Reveal the Mechanism of Tolerance to Selenate Toxicity in Cardamine Violifolia. J. Hazard. Mater. 2021, 406, 124283. [Google Scholar] [CrossRef]
- Kolbert, Z.; Molnár, Á.; Feigl, G.; Van Hoewyk, D. Plant Selenium Toxicity: Proteome in the Crosshairs. J. Plant Physiol. 2019, 232, 291–300. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioaccessibility of Selenium, Selenomethionine and Selenocysteine from Foods and Influence of Heat Processing on the Same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- WHO—World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.S.; Oliveira, J.R.; Curi, N.; Schulze, D.G.; Marques, J.J. Selenium and Mercury in Brazilian Cerrado Soils and Their Relationships with Physical and Chemical Soil Characteristics. Chemosphere 2019, 218, 412–415. [Google Scholar] [CrossRef]
- Leite, G.d.S.d.O.; Nascimento, C.W.A.D.; Nascimento, R.C.; Boechat, C.L.; Morais, P.G.C.; Saraiva, P.C.; Luz, S.L.; da Silva, Y.J.A.B. Geochemical background and geopedological interactions of selenium in soils from Piauí state, Northeastern Brazil. Rev. Bras. Cienc. Solo 2024, 48, e0230110. [Google Scholar] [CrossRef]
- Söderlund, M.; Virkanen, J.; Holgersson, S.; Lehto, J. Sorption and speciation of selenium in boreal forest soil. J. Environ. Radioact. 2016, 164, 220–231. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, S.N.; Raymundo, J.F.; Costa, F.F.R.; Lessa, J.H.d.L.; Guilherme, L.R.G.; Lopes, G. Selenium Application Methods and Rates for Biofortification of Common Bean and Their Residual Effects on Mombaça Grass. Crop Pasture Sci. 2022, 73, 792–803. [Google Scholar] [CrossRef]
- Klikocka, H.; Klikocka, H.; Barczak, B.; Szostak, B.; Kobiałka, A. Effect of Sulphur and Nitrogen Fertilization on the Selenium Content and Uptake by Grain of Spring Wheat. J. Elem. 2017, 22, 985–994. [Google Scholar] [CrossRef]
- Kim, H.; Hirai, M.Y.; Hayashi, H.; Chino, M.; Naito, S.; Fujiwara, T. Role of O-Acetyl-l-Serine in the Coordinated Regulation of the Expression of a Soybean Seed Storage-Protein Gene by Sulfur and Nitrogen Nutrition. Planta 1999, 209, 282–289. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, D.; Song, W.; Lei, L.; Yu, D.; Miao, S. Effects of Nitrogen Application on Selenium Accumulation, Translocation and Distribution of Winter Wheat at Different Growth Periods. J. Plant Nutr. Fertil. 2016, 22, 395–402. [Google Scholar]
- Oumer, A.; Joy, E.J.M.; De Groote, H.; Broadley, M.R.; Gashu, D. Burden of selenium deficiency and cost-effectiveness of selenium agronomic biofortification of staple cereals in Ethiopia. Br. J. Nutr. 2024, 132, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, P.F.; Faquin, V.; Ramos, S.J.; Guilherme, L.R.G.; Bastos, C.E.A.; Carvalho, G.S.; Costa, E.T. de S. Selenato e Selenito na Produção e Biofortificação Agronômica com Selênio em Arroz. Pesqui. Agropecuária Bras. 2012, 47, 831–837. [Google Scholar] [CrossRef]
- Reis, H.P.G.; Barcelos, J.P.D.Q.; Furlani, E., Jr.; Santos, E.F.; Silva, V.M.; Moraes, M.F.; Putti, F.F.; Reis, A.R. Agronomic Biofortification of Upland Rice with Selenium and Nitrogen and Its Relation to Grain Quality. J. Cereal Sci. 2018, 79, 508–515. [Google Scholar] [CrossRef]
- Lessa, J.H.d.L.; Araujo, A.M.; Ferreira, L.A.; Júnior, E.C.d.S.; de Oliveira, C.; Corguinha, A.P.B.; Martins, F.A.D.; de Carvalho, H.W.P.; Guilherme, L.R.G.; Lopes, G. Agronomic Biofortification of Rice (Oryza sativa L.) with Selenium and Its Effect on Element Distributions in Biofortified Grains. Plant Soil 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Félix, M.R.; Zauza, S.B.; Namorato, F.A.; Castro, D.G.; Martins, F.A.D.; de Lima Lessa, J.H.; Guilherme, L.R.G.; Botelho, F.B.S. Biofortification of Upland Rice Using Selenium-Enriched Urea: Evaluation of Potential Genotypes. J. Food Compos. Anal. 2023, 122, 105409. [Google Scholar] [CrossRef]
- Ravello, R.A.V.; de Oliveira, C.; Lessa, J.; Boas, L.V.V.; de Castro, E.M.; Guilherme, L.R.G.; Lopes, G. Selenium Application Influenced Selenium Biofortification and Physiological Traits in Water-Deficit Common Bean Plants. Crop Pasture Sci. 2021, 73, 44–55. [Google Scholar] [CrossRef]
- Cipriano, P.E.; da Silva, R.F.; de Lima, F.R.D.; de Oliveira, C.; de Lima, A.B.; Celante, G.; Dos Santos, A.A.; Archilha, M.V.L.R.; Pinatto-Botelho, M.F.; Faquin, V.; et al. Selenium Biofortification via Soil and Its Effect on Plant Metabolism and Mineral Content of Sorghum Plants. J. Food Compos. Anal. 2022, 109, 104505. [Google Scholar] [CrossRef]
- Cipriano, P.E.; da Silva, R.F.; de Oliveira, C.; de Lima, A.B.; Martins, F.A.D.; Celante, G.; dos Santos, A.A.; Archilha, M.V.L.R.; Pinatto Botelho, M.F.; Faquin, V.; et al. Sodium Selenate, Potassium Hydroxy-Selenide, Acetylselenide and Their Effect on Antioxidant Metabolism and Plant Nutrition and Yield in Sorghum Genotypes. Foods 2023, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Lara, T.S.; Lessa, J.H.d.L.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium Biofortification of Wheat Grain via Foliar Application and Its Effect on Plant Metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Paciolla, C.; de Leonardis, S.; Dipierro, S. Effects of selenite and selenate on the antioxidant systems in Senecio scandens L. Plant Biosyst. 2011, 145, 253–259. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Siuéia Junior, M.; Silva, D.F.; Cipriano, P.E.; Souza, R.R.; Faquin, V.; Silva, M.L.S.; Guilherme, L.R.G. Residual selenate as selenite in the soil: Effect on the accumulation of selenium, macronutrients, and gas exchange in arugula. Sci. Hortic. 2024, 337, 113569. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S. Selenium biofortification: Roles, mechanisms, responses and prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- dos Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.D.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa Solos: Brasilia, Brazil, 2018. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, updated the 2015 international soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service, Department of Agriculture: Washington, DA, USA, 1999. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Pelotas, Brasília, 2017. [Google Scholar]
- USEPA—United States Environmental Protection Agengy. Microwave Assisted Acid Digestion of Sediments Sludge, Soils, and Oils. EPA SW 846:3051A; United States Environmental Protection Agengy: Washington, DC, USA, 2007. [Google Scholar]
- Malavolta, E. Manual de Química Agrícola: Adubos e Adubação, 3rd ed.; Editora Agronômica Ceres: São Paulo, Brazil, 1981. [Google Scholar]
- AOAC—Association of Official Analysis Chemists. Official Methods of Analysis of AOAC International; Association of Official Analysis Chemists International: Washington, DC, USA, 2016. [Google Scholar]
- de Carvalho, A.M.X.; Mendes, F.Q.; Mendes, F.Q.; Tavares, L.d.F. SPEED Stat: A Free, Intuitive, and Minimalist Spreadsheet Program for Statistical Analyses of Experiments. Crop Breed. Appl. Biotechnol. 2020, 20, e327420312. [Google Scholar] [CrossRef]
- da Cruz Ferreira, R.L.; de Mello Prado, R.; de Souza Junior, J.P.; Gratão, P.L.; Tezotto, T.; Cruz, F.J.R. Oxidative Stress, Nutritional Disorders, and Gas Exchange in Lettuce Plants Subjected to Two Selenium Sources. J. Soil Sci. Plant Nutr. 2020, 20, 1215–1228. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Moller, I.S.; White, P. Functions of Macronutrients. In ‘Marschner’s mineral nutrition of higher plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar]
- Lee, B.-R.; Zaman, R.; Avice, J.-C.; Ourry, A.; Kim, T.-H. Sulfur Use Efficiency Is a Significant Determinant of Drought Stress Tolerance in Relation to Photosynthetic Activity in Brassica Napus Cultivars. Front. Plant Sci. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings Under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2022, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Sager, M. Selenium in Agriculture, Food, and Nutrition. Pure Appl. Chem. 2006, 78, 111–133. [Google Scholar] [CrossRef]
- Torralbo, F.; Vicente, R.; Morcuende, R.; González-Murua, C.; Aranjuelo, I. C and N Metabolism in Barley Leaves and Peduncles Modulates Responsiveness to Changing CO2. J. Exp. Bot. 2019, 70, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium Uptake, Dynamic Changes in Selenium Content and Its Influence on Photosynthesis and Chlorophyll Fluorescence in Rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Treml, V.; Tumajer, J.; Jandová, K.; Oulehle, F.; Rydval, M.; Čada, V.; Treydte, K.; Mašek, J.; Vondrovicová, L.; Lhotáková, Z.; et al. Increasing Water-Use Efficiency Mediates Effects of Atmospheric Carbon, Sulfur, and Nitrogen on Growth Variability of Central European Conifers. Sci. Total Environ. 2022, 838, 156483. [Google Scholar] [CrossRef]
- Coppa, E.; Celletti, S.; Sestili, F.; Mimmo, T.; Garcia Molina, M.D.; Cesco, S.; Astolfi, S. Interaction between Sulfate and Selenate in Tetraploid Wheat (Triticum turgidum L.) Genotypes. Int. J. Mol. Sci. 2023, 24, 5443. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.-R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The Role of Selenium and Nano Selenium on Physiological Responses in Plant: A Review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Premarathna, L.; McLaughlin, M.J.; Kirby, J.K.; Hettiarachchi, G.M.; Stacey, S.; Chittleborough, D.J. Selenate-Enriched Urea Granules Are a Highly Effective Fertilizer for Selenium Biofortification of Paddy Rice Grain. J. Agric. Food Chem. 2012, 60, 6037–6044. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Li, J.; Xu, Z.; Qian, C.; Xia, X.; Liu, Y.; Feng, Y. Tofu By-Product Soy Whey Substitutes Urea: Reduced Ammonia Volatilization, Enhanced Soil Fertility and Improved Fruit Quality in Cherry Tomato Production. Environ. Res. 2023, 226, 115662. [Google Scholar] [CrossRef]
- Drahoňovský, J.; Száková, J.; Mestek, O.; Tremlová, J.; Kaňa, A.; Najmanová, J.; Tlustoš, P. Selenium Uptake, Transformation and Inter-Element Interactions by Selected Wildlife Plant Species after Foliar Selenate Application. Environ. Exp. Bot. 2016, 125, 12–19. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Lee, J.; Sonne, C.; Brown, R.J.C.; Kim, K.-H. Selenium in Soil-Microbe-Plant Systems: Sources, Distribution, Toxicity, Tolerance, and Detoxification. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2383–2420. [Google Scholar] [CrossRef]


| Genotype | Acronyms | Fertilizer Type | Addition of Selenium |
|---|---|---|---|
| BRS Cometa | U | Urea | − |
| BRS Cometa | U + Se | Urea | + |
| BRS Cometa | AS | Ammonium sulfate | − |
| BRS Cometa | AS + Se | Ammonium sulfate | + |
| BRS Cometa | Control | - | − |
| BRS Estilo | U | Urea | − |
| BRS Estilo | U + Se | Urea | + |
| BRS Estilo | AS | Ammonium sulfate | − |
| BRS Estilo | AS + Se | Ammonium sulfate | + |
| BRS Estilo | Control | - | − |
| BRSMG Madrepérola | U | Urea | − |
| BRSMG Madrepérola | U + Se | Urea | + |
| BRSMG Madrepérola | AS | Ammonium sulfate | − |
| BRSMG Madrepérola | AS + Se | Ammonium sulfate | + |
| BRSMG Madrepérola | Control | - | − |
| Pérola | U | Urea | − |
| Pérola | U + Se | Urea | + |
| Pérola | AS | Ammonium sulfate | − |
| Pérola | AS + Se | Ammonium sulfate | + |
| Pérola | Control | - | − |
| Nitrogen Fertilizers a | Expected | Obtained b |
|---|---|---|
| Urea | 0.00 | <DL |
| Se-enriched urea | 573.600 | 671.240 |
| Ammonium sulfate | 0.00 | <DL |
| Se-enriched ammonium sulfate | 260.800 | 332.805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namorato, F.A.; Cipriano, P.E.; Zauza, S.B.; Benevenute, P.A.N.; Araújo, S.N.d.; Correia, R.F.R.; Ribeiro, I.C.A.; Morais, E.G.d.; Martins, F.A.D.; Silva, M.L.d.S.; et al. Selenium Biofortification with Se-Enriched Urea and Se-Enriched Ammonium Sulfate Fertilization in Different Common Bean Genotypes. Agronomy 2025, 15, 440. https://doi.org/10.3390/agronomy15020440
Namorato FA, Cipriano PE, Zauza SB, Benevenute PAN, Araújo SNd, Correia RFR, Ribeiro ICA, Morais EGd, Martins FAD, Silva MLdS, et al. Selenium Biofortification with Se-Enriched Urea and Se-Enriched Ammonium Sulfate Fertilization in Different Common Bean Genotypes. Agronomy. 2025; 15(2):440. https://doi.org/10.3390/agronomy15020440
Chicago/Turabian StyleNamorato, Filipe Aiura, Patriciani Estela Cipriano, Stefânia Barros Zauza, Pedro Antônio Namorato Benevenute, Suellen Nunes de Araújo, Raphael Felipe Rodrigues Correia, Ivan Célio Andrade Ribeiro, Everton Geraldo de Morais, Fábio Aurélio Dias Martins, Maria Ligia de Souza Silva, and et al. 2025. "Selenium Biofortification with Se-Enriched Urea and Se-Enriched Ammonium Sulfate Fertilization in Different Common Bean Genotypes" Agronomy 15, no. 2: 440. https://doi.org/10.3390/agronomy15020440
APA StyleNamorato, F. A., Cipriano, P. E., Zauza, S. B., Benevenute, P. A. N., Araújo, S. N. d., Correia, R. F. R., Ribeiro, I. C. A., Morais, E. G. d., Martins, F. A. D., Silva, M. L. d. S., & Guilherme, L. R. G. (2025). Selenium Biofortification with Se-Enriched Urea and Se-Enriched Ammonium Sulfate Fertilization in Different Common Bean Genotypes. Agronomy, 15(2), 440. https://doi.org/10.3390/agronomy15020440










