Study on the Automatic Selection of Sensitive Hyperspectral Bands for Rice Nitrogen Retrieval Based on a Maximum Inscribed Rectangle
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Study Area
2.1.2. Hyperspectral Remote Sensing Images
2.1.3. Measurement of Rice Plant Nitrogen Content
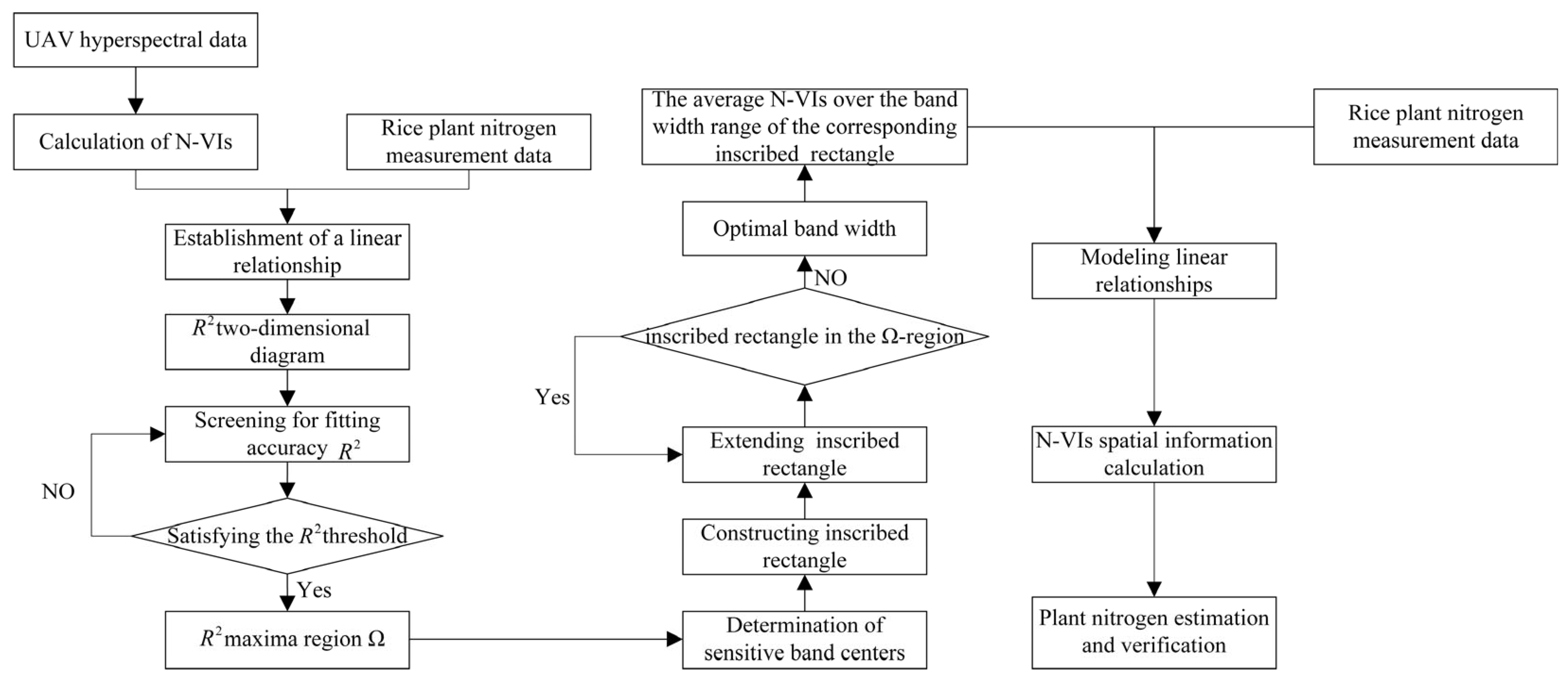
2.2. Methods
2.2.1. Vegetation Index Selection
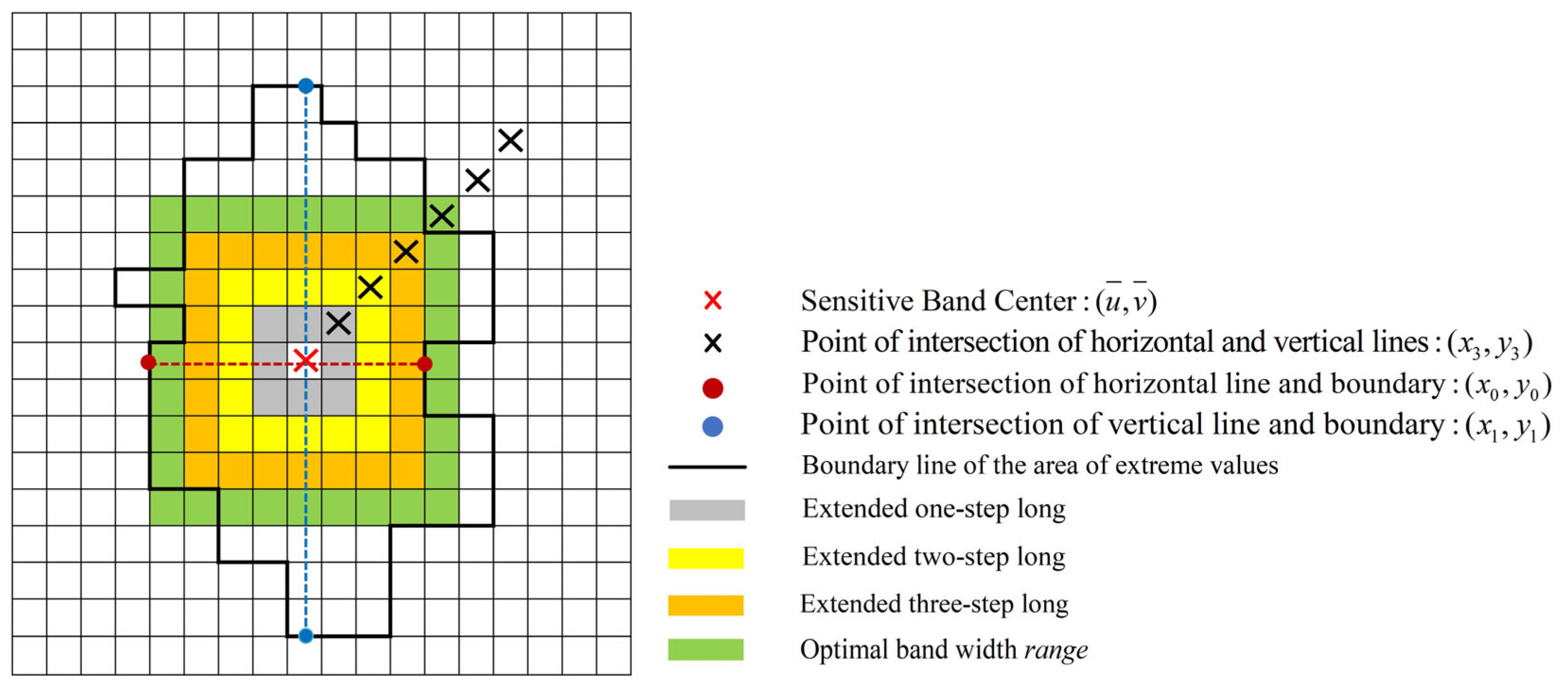
2.2.2. Determination of the Optimal Bandwidth for Rice Plant Nitrogen
2.2.3. Evaluating the Accuracy of Rice Plant Nitrogen Estimation Models
3. Results
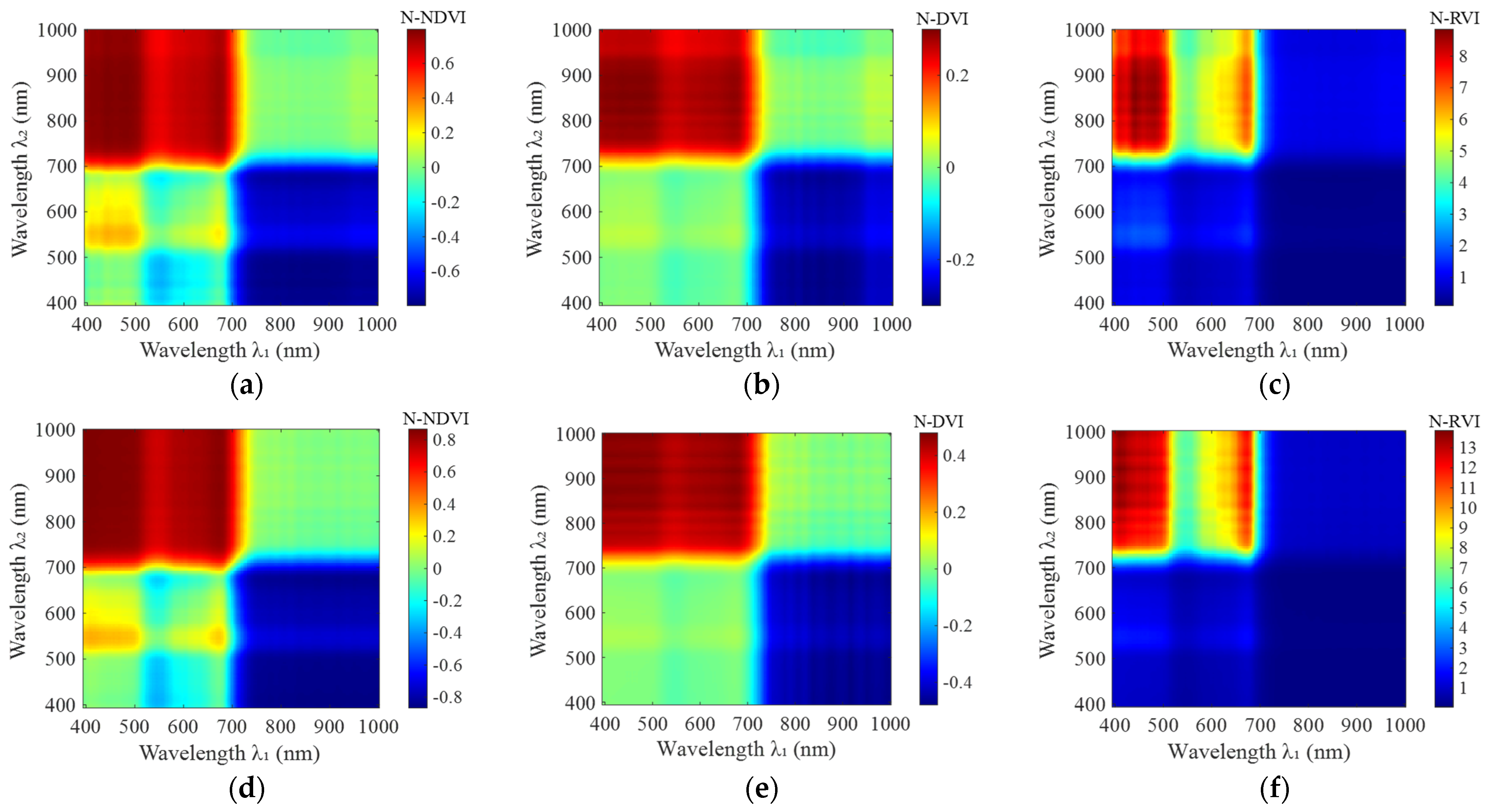
3.1. Narrowband Vegetation Index Calculations
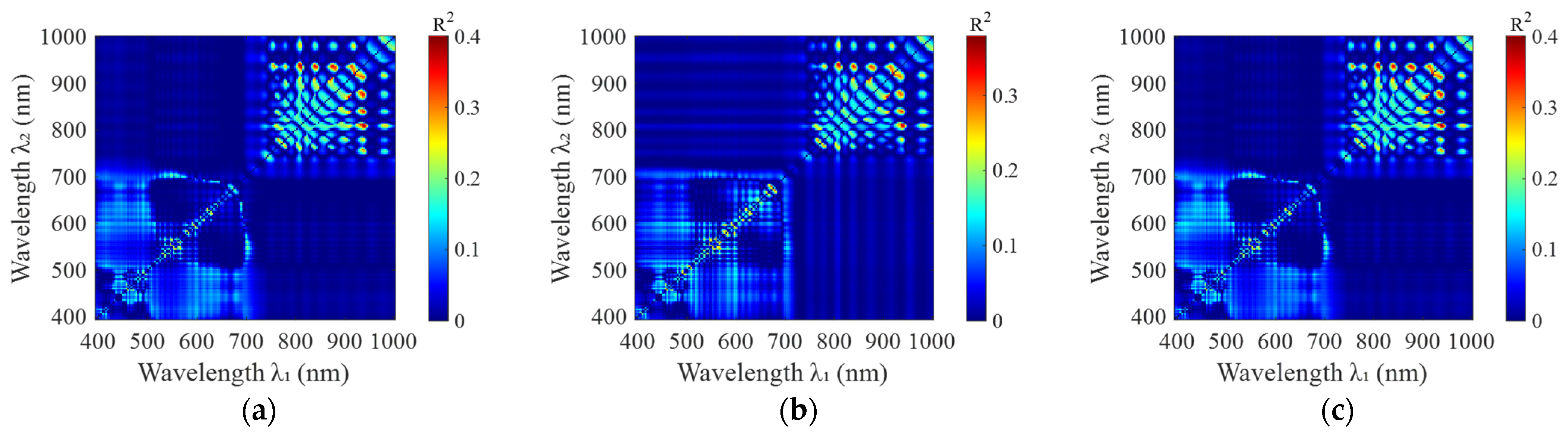
3.2. Determination of Rice Plant Nitrogen-Sensitive Centers
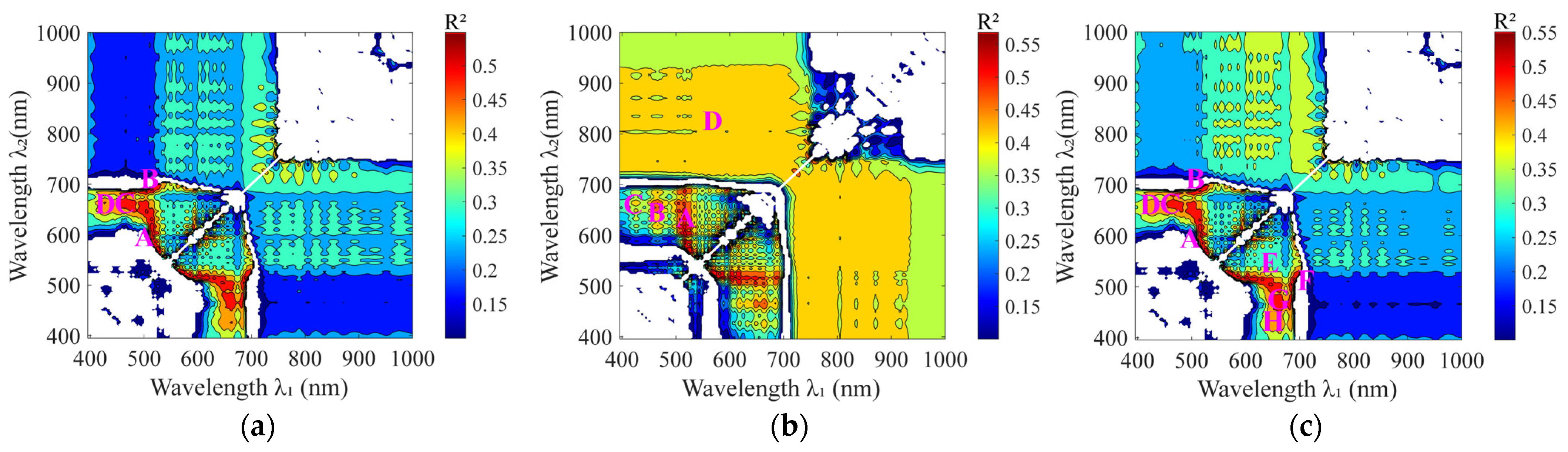
3.3. Selection of the Optimal Bandwidth for Rice Plant Nitrogen
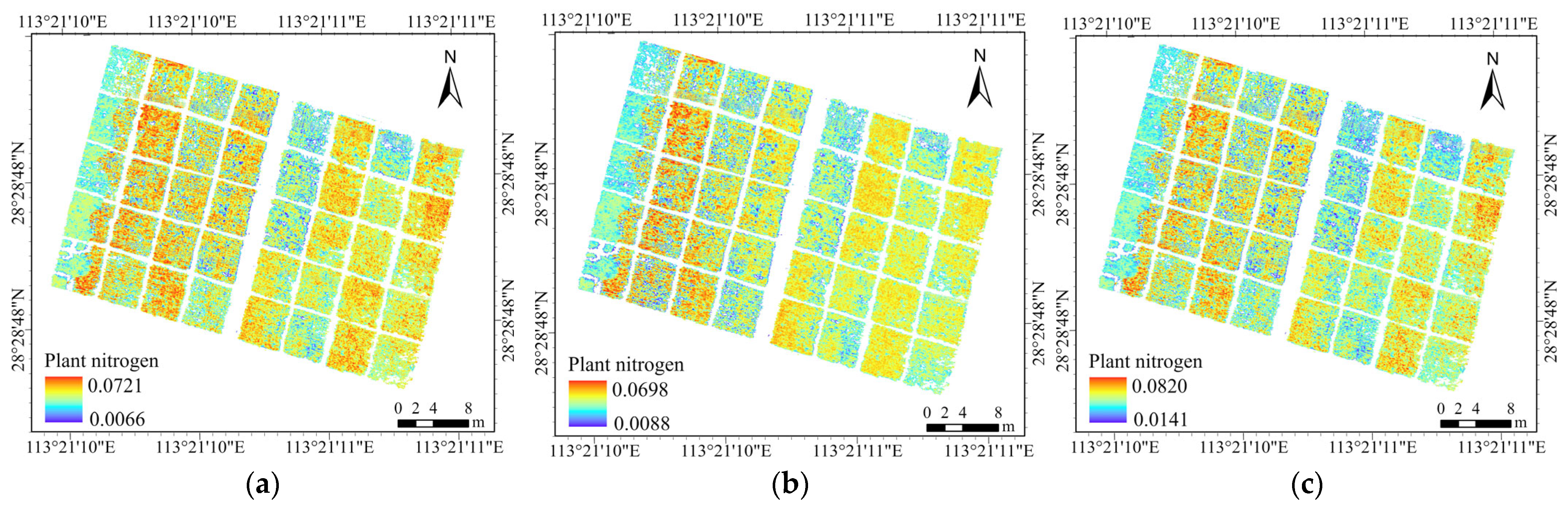
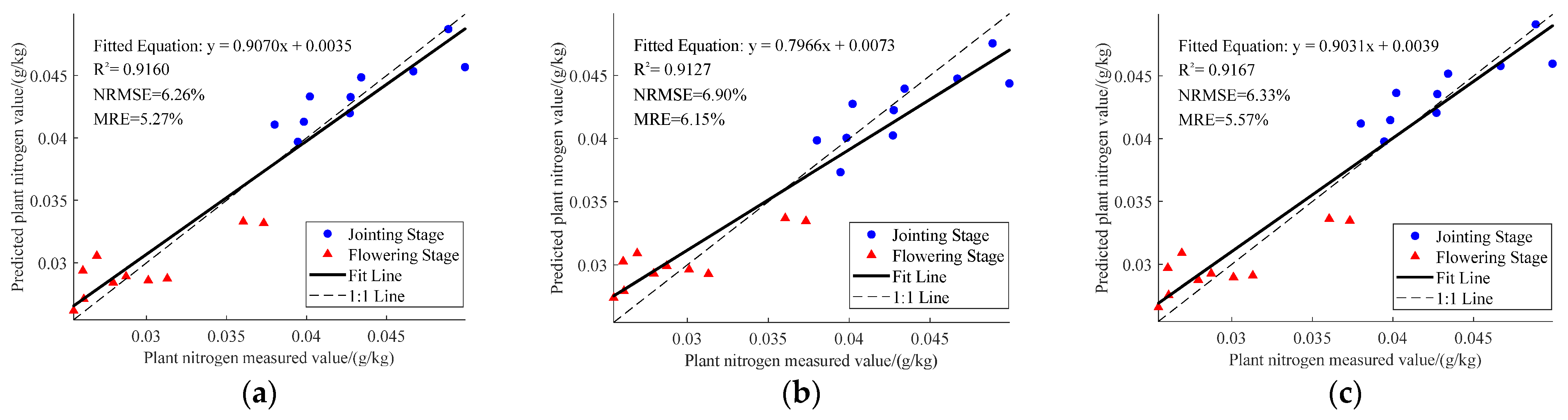
3.4. Verification of Accuracy of Plant Nitrogen Estimation with Optimal Bandwidth
3.4.1. Validating the Accuracy of Plant Nitrogen at the Jointing Stage
3.4.2. Validating the Accuracy of Plant Nitrogen at the Flowering Stage
4. Discussion
4.1. Nitrogen Inversion Models Based on Different Narrow-Band Vegetation Indices
4.2. Automatic Determination of Sensitive Bandwidths on the Basis of the Maximum Inscribed Rectangle
4.3. Limitations of This Study and Future Directions for Improvement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alam, I.; Zhang, H.Y.; Du, H.; Rehman, N.U.; Manghwar, H.; Lei, X.; Khan, Z.; Batool, K.; Ge, L.F. Bioengineering Techniques to Improve Nitrogen Transformation and Utilization: Implications for Nitrogen Use Efficiency and Future Sustainable Crop Production. J. Agric. Food Chem. 2023, 71, 3921–3939. [Google Scholar] [CrossRef]
- Maltese, N.E.; Maddonni, G.A.; Melchiori RJ, M.; Caviglia, O.P. Plant nitrogen status at flowering and kernel set efficiency in early- and late-sown maize crops. Field Crop. Res. 2021, 270, 108216. [Google Scholar] [CrossRef]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Marcotuli, I.; Gadaleta, A.; De Pinto, M.C. The Role of Glutamine Synthetase (GS) and Glutamate Synthase (GOGAT) in the Improvement of Nitrogen Use Efficiency in Cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef]
- Ertekin, I. influence of nitrogen rose and plant density of the yield and quality properties of dual purpose barley grown under the mediterranean climatic aonditions. J. Elem. 2022, 27, 113–126. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Cang, L.; Wang, Y.J.; Zhou, D.M. Effects of soil properties, nitrogen application, plant phenology, and their interactions on plant uptake of cadmium in wheat. J. Hazard. Mater. 2020, 384, 121452. [Google Scholar] [CrossRef] [PubMed]
- Vigneau, N.; Ecarnot, M.; Rabatel, G.; Roumet, P. Potential of field hyperspectral imaging as a non destructive method to assess leaf nitrogen content in Wheat. Field Crop. Res. 2011, 122, 25–31. [Google Scholar] [CrossRef]
- Konara, B.; Krishnapillai, M.; Galagedara, L. Recent Trends and Advances in Utilizing Digital Image Processing for Crop Nitrogen Management. Remote Sens. 2024, 16, 4514. [Google Scholar] [CrossRef]
- Shahi, T.B.; Xu, C.Y.; Neupane, A.; Guo, W. Recent Advances in Crop Disease Detection Using UAV and Deep Learning Techniques. Remote Sens. 2023, 15, 2450. [Google Scholar] [CrossRef]
- Narmilan, A.; Gonzalez, F.; Salgadoe, A.S.A.; Kumarasiri, U.; Weerasinghe, H.A.S.; Kulasekara, B.R. Predicting Canopy Chlorophyll Content in Sugarcane Crops Using Machine Learning Algorithms and Spectral Vegetation Indices Derived from UAV Multispectral Imagery. Remote Sens. 2022, 14, 1140. [Google Scholar] [CrossRef]
- Tian, F.K.; Ransom, C.J.; Zhou, J.F.; Wilson, B.; Sudduth, K.A. Assessing the impact of soil and field conditions on cotton crop emergence using UAV-based imagery. Comput. Electron. Agric. 2024, 218, 108738. [Google Scholar] [CrossRef]
- Maimaitijiang, M.; Ghulam, A.; Sidike, P.; Hartling, S.; Maimaitiyiming, M.; Peterson, K.; Shavers, E.; Fishman, J.; Peterson, J.; Kadam, S.; et al. Unmanned Aerial System (UAS)-based phenotyping of soybean using multisensor data fusion and extreme learning machine. ISPRS-J. Photogramm. Remote Sens. 2017, 134, 43–58. [Google Scholar] [CrossRef]
- Blekanov, I.; Molin, A.; Zhang, D.; Mitrofanov, E.; Mitrofanova, O.; Li, Y. Monitoring of grain crops nitrogen status from uav multispectral images coupled with deep learning approaches. Comput. Electron. Agric. 2023, 212, 108047. [Google Scholar] [CrossRef]
- Gallo, I.; Boschetti, M.; Rehman, A.U.; Candiani, G. Self-Supervised Convolutional Neural Network Learning in a Hybrid Approach Framework to Estimate Chlorophyll and Nitrogen Content of Maize from Hyperspectral Images. Remote Sens. 2023, 15, 4765. [Google Scholar] [CrossRef]
- Colovic, M.; Yu, K.; Todorovic, M.; Cantore, V.; Hamze, M.; Albrizio, R.; Stellacci, A.M. Hyperspectral Vegetation Indices to Assess Water and Nitrogen Status of Sweet Maize Crop. Agronomy 2022, 12, 2181. [Google Scholar] [CrossRef]
- Berger, K.; Verrelst, J.; Féret, J.B.; Wang, Z.H.; Wocher, M.; Strathmann, M.; Danner, M.; Mauser, W.; Hank, T. Crop nitrogen monitoring: Recent progress and principal developments in the context of imaging spectroscopy missions. Remote Sens. Environ. 2020, 242, 111758. [Google Scholar] [CrossRef] [PubMed]
- Vaddi, R.; Kumar, B.; Manoharan, P.; Agilandeeswari, L.; Sangeetha, V. Strategies for dimensionality reduction in hyperspectral remote sensing: A comprehensive overview. Egypt. J. Remote Sens. Space Sci. 2024, 27, 82–92. [Google Scholar] [CrossRef]
- Macfarlane, F.; Murray, P.; Marshall, S.; White, H. Investigating the Effects of a Combined Spatial and Spectral Dimensionality Reduction Approach for Aerial Hyperspectral Target Detection Applications. Remote Sens. 2021, 13, 1647. [Google Scholar] [CrossRef]
- Moharram, M.A.; Sundaram, D.M. Dimensionality reduction strategies for land use land cover classification based on airborne hyperspectral imagery: A survey. Environ. Sci. Pollut. Res. 2023, 30, 5580–5602. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.H.; Feng, S.; Du, W.; Wang, D.K.; Guo, Z.H.; Xing, S.M.; Jin, Z.Y.; Cao, Y.L.; Xu, T.Y. A Study of Nitrogen Deficiency Inversion in Rice Leaves Based on the Hyperspectral Reflectance Differential. Front. Plant Sci. 2020, 11, 573272. [Google Scholar] [CrossRef]
- Stellacci, A.M.; Castrignanò, A.; Troccoli, A.; Basso, B.; Buttafuoco, G. Selecting optimal hyperspectral bands to discriminate nitrogen status in durum wheat: A comparison of statistical approaches. Environ. Monit. Assess. 2016, 188, 4. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Liang, K.M.; Zhu, F.F.; Zhong, X.H.; Lu, Z.H.; Chen, Y.B.; Pan, J.F.; Lu, C.S.; Huang, J.C.; Ye, Q.H.; et al. Differential Study on Estimation Models for Indica Rice Leaf SPAD Value and Nitrogen Concentration Based on Hyperspectral Monitoring. Remote Sens. 2024, 16, 4604. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Pandey, R.N.; Sahoo, R.N.; Gupta, V.K.; Datta, S.C.; Kumar, D. Monitoring nitrogen, phosphorus and sulphur in hybrid rice (Oryza sativa L.) using hyperspectral remote sensing. Precis. Agric. 2017, 18, 736–761. [Google Scholar] [CrossRef]
- Tian, T.; Wang, J.L.; Tao, Y.Y.; Ji, F.F.; He, Q.Q.; Sun, C.M.; Zhang, Q. Estimating Rice Leaf Nitrogen Content and Field Distribution Using Machine Learning with Diverse Hyperspectral Features. Agronomy 2024, 14, 2760. [Google Scholar] [CrossRef]
- Yu, F.H.; Bai, J.C.; Jin, Z.Y.; Zhang, H.G.; Yang, J.X.; Xu, T.Y. Estimating the rice nitrogen nutrition index based on hyperspectral transform technology. Front. Plant Sci. 2023, 14, 1118098. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Xu, T.Y.; Yu, F.H.; Chen, C.L. Measurement of nitrogen content in rice by inversion of hyperspectral reflectance data from an unmanned aerial vehicle. Cienc. Rural 2018, 48, e20180008. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.S.; Li, D.; Wang, C.Y.; Jiang, H.; Zheng, Q.; Peng, Z.P. Estimation of Paddy Rice Nitrogen Content and Accumulation Both at Leaf and Plant Levels from UAV Hyperspectral Imagery. Remote Sens. 2021, 13, 2956. [Google Scholar] [CrossRef]
- Peng, Y.P.; Zhong, W.L.; Peng, Z.P.; Tu, Y.T.; Xu, Y.G.; Li, Z.X.; Liang, J.Y.; Huang, J.C.; Liu, X.; Fu, Y.Q. Enhanced Estimation of Rice Leaf Nitrogen Content via the Integration of Hybrid Preferred Features and Deep Learning Methodologies. Agronomy 2024, 14, 1248. [Google Scholar] [CrossRef]
- Lai, J.K.; Lin, W.S. Real-Time Detection of Rice Growth Phase Transition for Panicle Nitrogen Application Timing Assessment. Agronomy 2021, 11, 2465. [Google Scholar] [CrossRef]
- Hu, T.; Liu, Z.H.; Hu, R.; Tian, M.; Wang, Z.W.; Li, M.; Chen, G.H. Convolutional Neural Network-Based Estimation of Nitrogen Content in Regenerating Rice Leaves. Agronomy 2024, 14, 1422. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Abdalla, A.; Tang, Z.; Cen, H.Y. Improving rice nitrogen stress diagnosis by denoising strips in hyperspectral images via deep learning. Biosyst. Eng. 2022, 219, 165–176. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, Y.; Tian, Y.C.; Feng, W.; Cao, W.X. Exploring hyperspectral bands and estimation indices for leaf nitrogen accumulation in wheat. Int. J. Appl. Earth Obs. Geoinf. 2010, 12, 89–100. [Google Scholar] [CrossRef]
- Wang, W.; Yao, X.; Yao, X.F.; Tian, Y.C.; Liu, X.J.; Ni, J.; Cao, W.D.; Zhu, Y. Estimating leaf nitrogen concentration with three-band vegetation indices in rice and wheat. Field Crop. Res. 2012, 129, 90–98. [Google Scholar] [CrossRef]
- Hasituya; Li, F.; Elsayed, S.; Hu, Y.C.; Schmidhalter, U. Passive reflectance sensing using optimized two- and three-band spectral indices for quantifying the total nitrogen yield of maize. Comput. Electron. Agric. 2020, 173, 105403. [Google Scholar] [CrossRef]
- Liang, L.; Di, L.P.; Huang, T.; Wang, J.H.; Lin, L.; Wang, L.J.; Yang, M.H. Estimation of Leaf Nitrogen Content in Wheat Using New Hyperspectral Indices and a Random Forest Regression Algorithm. Remote Sens. 2018, 10, 1940. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, B.W.; Fan, J.H.; Ma, Y.C.; Wang, Y.; Zhang, Z. A Systematic Study of Estimating Potato N Concentrations Using UAV-Based Hyper and Multi-Spectral Imagery. Agronomy 2022, 12, 2533. [Google Scholar] [CrossRef]
- Burns, B.W.; Green, V.S.; Hashem, A.A.; Massey, J.H.; Shew, A.M.; Adviento-Borbe, M.A.A.; Milad, M. Determining nitrogen deficiencies for maize using various remote sensing indices. Precis. Agric. 2022, 23, 791–811. [Google Scholar] [CrossRef]
- Holzhauser, K.; Räbiger, T.; Rose, T.; Kage, H.; Kühling, I. Estimation of Biomass and N Uptake in Different Winter Cover Crops from UAV-Based Multispectral Canopy Reflectance Data. Remote Sens. 2022, 14, 4525. [Google Scholar] [CrossRef]
- Jiang, R.; Sanchez-Azofeifa, A.; Laakso, K.; Wang, P.; Xu, Y.; Zhou, Z.Y.; Luo, X.W.; Lan, Y.B.; Zhao, G.P.; Chen, X. UAV-based partially sampling system for rapid NDVI mapping in the evaluation of rice nitrogen use efficiency. J. Clean Prod. 2021, 289, 125705. [Google Scholar] [CrossRef]
- Liu, S.S.; Li, L.T.; Fan, H.Y.; Guo, X.Y.; Wang, S.Q.; Lu, J.W. Real-time and multistage recommendations for nitrogen fertilizer topdressing rates in winter oilseed rape based on canopy hyperspectral data. Ind. Crop. Prod. 2020, 154, 112699. [Google Scholar] [CrossRef]
- Yao, L.L.; Wang, Q.; Yang, J.B.; Zhang, Y.; Zhu, Y.; Cao, W.X.; Ni, J. UAV-Borne Dual-Band Sensor Method for Monitoring Physiological Crop Status. Sensors 2019, 19, 816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Cheng, T.; Shi, L.; Wang, W.W.; Niu, Z.; Guo, W.; Ma, X.M. Combining spectral and texture features of UAV hyperspectral images for leaf nitrogen content monitoring in winter wheat. Int. J. Remote Sens. 2022, 43, 2335–2356. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.Y.; Cao, Q.; Liang, Y.; Krienke, B.; Tian, Y.C.; Zhu, Y.; Cao, W.X.; Liu, X.J. Use of an Active Canopy Sensor Mounted on an Unmanned Aerial Vehicle to Monitor the Growth and Nitrogen Status of Winter Wheat. Remote Sens. 2020, 12, 3684. [Google Scholar] [CrossRef]
- Bai, H.Z.; Xiao, D.P. Spatiotemporal changes of rice phenology in China during 1981-2010. Theor. Appl. Climatol. 2020, 140, 1483–1494. [Google Scholar] [CrossRef]
- Gobbo, S.; Migliorati, M.D.; Ferrise, R.; Morari, F.; Furlan, L.; Sartori, L. Evaluation of different crop model-based approaches for variable rate nitrogen fertilization in winter wheat. Precis. Agric. 2022, 23, 1922–1948. [Google Scholar] [CrossRef]
- Inoue, Y. Synergy of Remote Sensing and Modeling for Estimating Ecophysiological Processes in Plant Production. Plant Prod. Sci. 2003, 6, 3–16. [Google Scholar] [CrossRef]
- Zhang, N.D.; Liu, X.R.; Ren, J.Q.; Wu, S.R.; Li, F.J. Estimating the winter wheat harvest index with canopy hyperspectral remote sensing data based on the dynamic fraction of postanthesis phase biomass accumulation. Int. J. Remote Sens. 2022, 43, 2029–2058. [Google Scholar] [CrossRef]
- Colorado, J.D.; Cera-Bornacelli, N.; Caldas, J.S.; Petro, E.; Rebolledo, M.C.; Cuellar, D.; Calderon, F.; Mondragon, I.F.; Jaramillo-Botero, A. Estimation of Nitrogen in Rice Crops from UAV-Captured Images. Remote Sens. 2020, 12, 3396. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, H.C.; Li, Z.H.; Yang, G.J. Quantitative analysis and hyperspectral remote sensing of the nitrogen nutrition index in winter wheat. Int. J. Remote Sens. 2020, 41, 858–881. [Google Scholar] [CrossRef]
- Muhammad, S.; Kumazawa, K.J.S.S.; Nutrition, P. Use of optical spectrograpiuc 15N-analyses to trace nitrogen applied at tile heading stage of rice. Soil Sci. Plant Nutr. 1972, 18, 143–146. [Google Scholar] [CrossRef]
- Muhammad, S.; Kumazawa, K.J.S.S.; Nutrition, P. The absorption, distribution, and redistribution of 15N-labelled ammonium and nitrate nitrogen administered at different growth stages of rice. Soil Sci. Plant Nutr. 1974, 20, 47–55. [Google Scholar] [CrossRef]
- Wada, G.; Shoji, S.; Takahashi, J. The fate of fertilizer nitrogen applied to the paddy field and its absorption by rice plant: 4. Distribution of basal and top-dressed nitrogen in rice plant. Jpn. J. Crop Sci. 1973, 42, 84–90. [Google Scholar] [CrossRef]
- Verrelst, J.; Malenovsky, Z.; Van Der Tol, C.; Camps-Valls, G.; Gastellu-Etchegorry, J.P.; Lewis, P.; North, P.; Moreno, J. Quantifying Vegetation Biophysical Variables from Imaging Spectroscopy Data: A Review on Retrieval Methods. Surv. Geophys. 2019, 40, 589–629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.H.; Huang, L.M.; Li, J.L.; Qi, J.G. A comparative analysis of broadband and narrowband derived vegetation indices in predicting LAI and CCD of a cotton canopy. ISPRS-J. Photogramm. Remote Sens. 2007, 62, 25–33. [Google Scholar] [CrossRef]
- Liang, L.; Huang, T.; Di, L.P.; Geng, D.; Yan, J.; Wang, S.G.; Wang, L.J.; Li, L.; Chen, B.Q.; Kang, J.R. Influence of Different Bandwidths on LAI Estimation Using Vegetation Indices. IEEE J. Sel. Top. Appl. Earth Observ. Remote Sens. 2020, 13, 1494–1502. [Google Scholar] [CrossRef]
- Goswami, S.; Choudhary, S.S.; Chatterjee, C.; Mailapalli, D.R.; Mishra, A.; Raghuwanshi, N.S. Estimation of nitrogen status and yield of rice crop using unmanned aerial vehicle equipped with multispectral camera. J. Appl. Remote Sens. 2021, 15, 042407. [Google Scholar] [CrossRef]
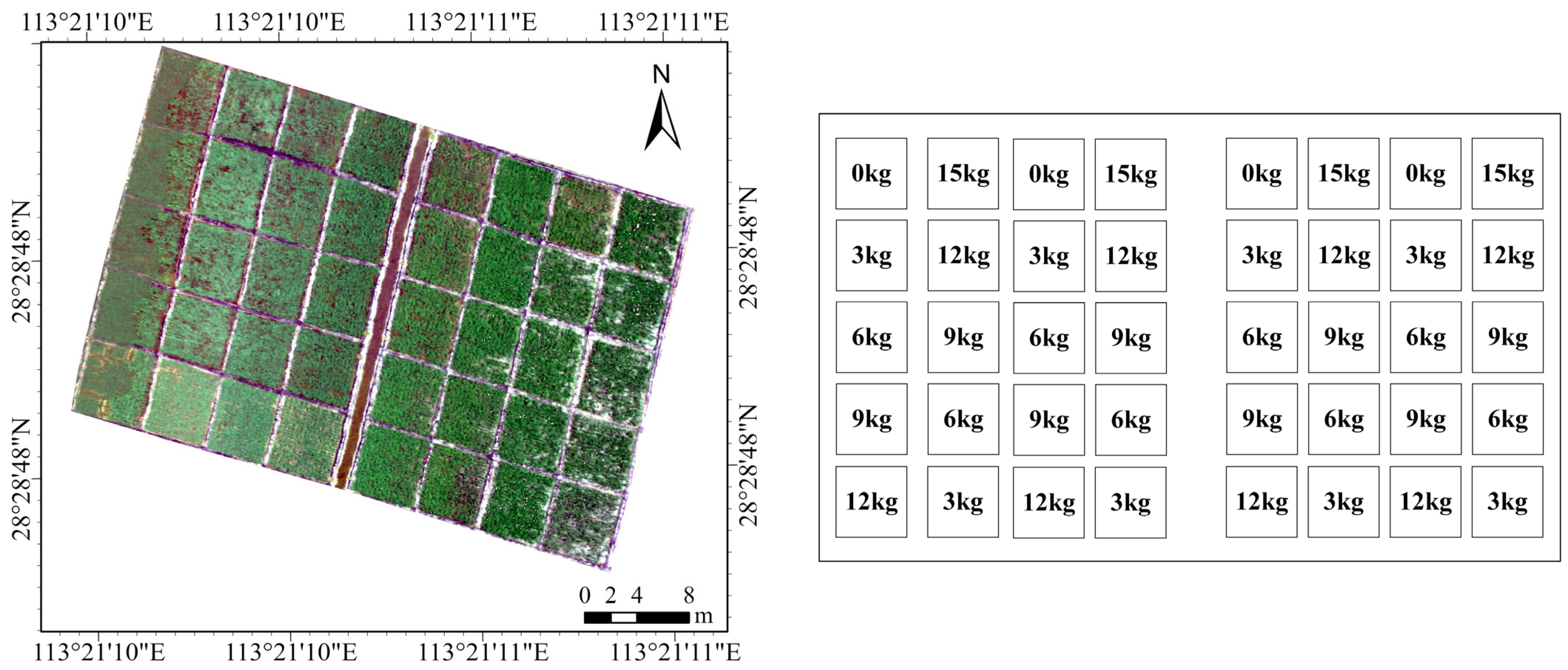



| Parameter | Detail |
|---|---|
| Location | Rice Experimental Base, Gaoqiao Town, Changsha County, Hunan Provincial Academy of Agricultural Sciences. |
| Latitude | 28°35′ N |
| Longitude | 113°14′ E |
| Elevation | 57 m |
| Cropping System | Biannual |
| Rice Varieties | Zhongzao 39 (Hybrid), Xiangzaoxian 24 (Conventional) |
| Number of Plots per Variety | 20 |
| Total Number of Plots | 40 |
| Plot Area | 15 m2 |
| Nitrogen Application Levels | 0, 3, 6, 9, 12, 15 kg |
| Fertilizer Application Ratio | Base fertilizer: Additional fertilizer = 6:4 |
| Narrowband Vegetation Index | Axis | Jointing Stage | Flowering Stage | |||||
|---|---|---|---|---|---|---|---|---|
| X-Axis | Y-Axis | Max | Min | Mean | Max | Min | Mean | |
| N-NDVI | 395–720 | 680–1001 | 0.80 | 0.48 | 0.71 | 0.87 | 0.53 | 0.80 |
| 395–510 | 520–680 | 0.33 | 0.02 | 0.21 | 0.36 | 0.01 | 0.20 | |
| 520–580 | 640–690 | −0.08 | −0.22 | −0.16 | −0.11 | −0.30 | −0.22 | |
| N-DVI | 395–720 | 710–1001 | 0.30 | 0.07 | 0.26 | 0.48 | 0.10 | 0.43 |
| N-RVI | 395–510 | 740–1001 | 8.85 | 6.30 | 7.95 | 13.86 | 8.70 | 12.40 |
| 610–690 | 730–1001 | 7.11 | 4.05 | 5.92 | 12.14 | 5.44 | 9.08 | |
| Growth Phases | N-VIs Indicators | Sensitive Band Center Wavelength/nm | Optimal Bandwidth | |
|---|---|---|---|---|
| Jointing stage | N-NDVI | 510.32 | 623.00 | ±3 |
| 513.72 | 688.19 | ±3 | ||
| 469.60 | 660.72 | ±6 | ||
| 483.16 | 650.43 | ±12 | ||
| N-DVI | 517.11 | 633.28 | ±9 | |
| 466.22 | 653.86 | ±9 | ||
| 425.70 | 664.15 | ±3 | ||
| 575.09 | 818.77 | ±33 | ||
| N-RVI | 510.32 | 623.00 | ±3 | |
| 469.60 | 660.72 | ±6 | ||
| 513.72 | 688.19 | ±3 | ||
| 483.16 | 650.43 | ±12 | ||
| 623.00 | 510.32 | ±3 | ||
| 660.72 | 469.60 | ±6 | ||
| 688.19 | 513.72 | ±3 | ||
| 650.43 | 483.16 | ±12 | ||
| Flowering stage | N-NDVI | 914.77 | 931.87 | ±3 |
| 877.11 | 935.28 | ±3 | ||
| 839.38 | 935.28 | ±3 | ||
| 808.47 | 935.28 | ±3 | ||
| N-DVI | 914.77 | 931.87 | ±3 | |
| 877.11 | 935.28 | ±3 | ||
| 839.38 | 935.28 | ±3 | ||
| 808.47 | 935.28 | ±3 | ||
| N-RVI | 914.77 | 931.87 | ±3 | |
| 877.11 | 935.28 | ±3 | ||
| 839.38 | 935.28 | ±3 | ||
| 808.47 | 935.28 | ±3 | ||
| 931.87 | 914.77 | ±3 | ||
| 935.28 | 877.11 | ±3 | ||
| 935.28 | 839.38 | ±3 | ||
| 935.28 | 808.47 | ±3 | ||
| Growth Phases | Band Center | Optimal Bandwidth | Modeling Set Accuracy Evaluation (n = 25) | |||
|---|---|---|---|---|---|---|
| λ1/nm | λ2/nm | R2 | NRMSE/% | MRE/% | ||
| Jointing stage | 517.11 | 633.28 | ±6 | 0.5108 ** | 7.4408 | 5.7564 |
| ±9 | 0.5101 ** | 7.4461 | 5.7604 | |||
| ±12 | 0.5089 ** | 7.4548 | 5.7654 | |||
| 466.22 | 653.86 | ±6 | 0.4769 ** | 7.6948 | 6.2468 | |
| ±9 | 0.4690 ** | 7.7528 | 6.2946 | |||
| ±12 | 0.4540 ** | 7.8611 | 6.3830 | |||
| 425.70 | 664.15 | ±0 | 0.4497 ** | 7.8923 | 6.5407 | |
| ±3 | 0.4330 ** | 8.0110 | 6.6303 | |||
| ±6 | 0.4138 ** | 8.1449 | 6.7307 | |||
| 575.09 | 818.77 | ±30 | 0.4355 ** | 7.9937 | 6.2000 | |
| ±33 | 0.4356 ** | 7.9925 | 6.2012 | |||
| ±36 | 0.4353 ** | 7.9948 | 6.2031 | |||
| Flowering stage | 914.77 | 931.87 | ±0 | 0.2952 ** | 12.3120 | 10.6291 |
| ±3 | 0.2941 ** | 12.3214 | 10.6503 | |||
| ±6 | 0.2586 ** | 12.6280 | 10.8877 | |||
| 877.11 | 935.28 | ±0 | 0.3096 ** | 12.1854 | 10.5608 | |
| ±3 | 0.2995 ** | 12.2741 | 10.6372 | |||
| ±6 | 02616** | 12.6022 | 10.8363 | |||
| 839.38 | 935.28 | 0 | 0.3182 ** | 12.1090 | 10.3255 | |
| ±3 | 0.2951 ** | 12.3136 | 10.4368 | |||
| ±6 | 0.2328 ** | 12.8458 | 10.8436 | |||
| 808.47 | 935.28 | 0 | 0.2963 ** | 12.3031 | 10.8818 | |
| ±3 | 0.3029 ** | 12.2439 | 10.7597 | |||
| ±6 | 0.2921 ** | 12.3384 | 10.5477 | |||
| N-VIs | Sensitive Band Center | Optimum Bandwidth | Fitting Equations Between N-VIs and Plant Nitrogen | Results of Validation of Plant Nitrogen Estimation Accuracy n = 10 | |||
|---|---|---|---|---|---|---|---|
| λ1/nm | λ2/nm | R2 | NRMSE/% | MRE /% | |||
| N-NDVI | 510.32 | 623.00 | ±3 | y = 0.05031 + 0.13084*x | 0.7656 ** | 4.84 | 3.79 |
| 469.60 | 660.72 | ±6 | y = 0.04985 + 0.08360*x | 0.7043 ** | 5.38 | 4.29 | |
| 513.72 | 688.19 | ±3 | y = 0.05001 + 0.10144*x | 0.7149 ** | 5.10 | 3.96 | |
| 483.16 | 650.43 | ±12 | y = 0.05316 + 0.09278*x | 0.6721 ** | 5.55 | 4.47 | |
| N-DVI | 517.11 | 633.28 | ±9 | y = 0.03941 + 0.93806*x | 0.6807 ** | 5.52 | 4.31 |
| 466.22 | 653.86 | ±9 | y = 0.05072 + 0.91247*x | 0.5627 ** | 6.07 | 5.21 | |
| 425.70 | 664.15 | ±3 | y = 0.04531 + 0.80783*x | 0.5364 ** | 6.23 | 5.28 | |
| 575.09 | 818.77 | ±33 | y = 0.02477−0.05313*x | 0.4939 * | 7.07 | 4.98 | |
| N-RVI | 510.32 | 623.00 | ±3 | y = −0.02209 + 0.07230*x | 0.7593 ** | 4.93 | 3.96 |
| 469.60 | 660.72 | ±6 | y = 0.00101 + 0.04930*x | 0.7041 ** | 5.92 | 4.99 | |
| 513.72 | 688.19 | ±3 | y = −0.00571 + 0.05519*x | 0.7208 ** | 5.03 | 3.87 | |
| 483.16 | 650.43 | ±12 | y = −0.00301 + 0.05705*x | 0.6708 ** | 5.60 | 5.10 | |
| 623.00 | 510.32 | ±3 | y = 0.10757 − 0.05730*x | 0.7401 ** | 4.94 | 3.74 | |
| 660.72 | 469.60 | ±6 | y = 0.08383 − 0.03449*x | 0.7105 ** | 4.99 | 4.17 | |
| 688.19 | 513.72 | ±3 | y = 0.09449 − 0.04416*x | 0.7455 ** | 5.10 | 3.87 | |
| 650.43 | 483.16 | ±12 | y = 0.08837 − 0.03619*x | 0.6769 ** | 5.24 | 4.18 | |
| N-VIs | Sensitive Band Center | Optimum Bandwidth | Fitting Equations Between N-VIs and Plant Nitrogen | Results of Validation of Plant Nitrogen Estimation Accuracy n = 10 | |||
|---|---|---|---|---|---|---|---|
| λ1/nm | λ2/nm | R2 | NRMSE /% | MRE /% | |||
| N-NDVI | 914.77 | 931.87 | ±3 | y = 0.02365 + 0.65680*x | 0.6379 ** | 10.43 | 9.41 |
| 877.11 | 935.28 | ±3 | y = 0.02351 + 0.48638*x | 0.6989 ** | 8.28 | 6.75 | |
| 839.38 | 935.28 | ±3 | y = 0.02686 + 0.41471*x | 0.7056 ** | 8.84 | 7.12 | |
| 808.47 | 935.28 | ±3 | y = 0.03554 + 0.33679*x | 0.7312 ** | 9.99 | 9.08 | |
| N-DVI | 914.77 | 931.87 | ±3 | y = 0.02432 + 0.53284*x | 0.6252 ** | 11.05 | 10.35 |
| 877.11 | 935.28 | ±3 | y = 0.02437 + 0.38232*x | 0.6995 ** | 8.90 | 7.99 | |
| 839.38 | 935.28 | ±3 | y = 0.02710 + 0.34652*x | 0.7117 ** | 9.40 | 8.45 | |
| 808.47 | 935.28 | ±3 | y = 0.03520−0.29567*x | 0.7266 ** | 12.03 | 11.21 | |
| N-RVI | 914.77 | 931.87 | ±3 | y = −0.29774 + 0.32143*x | 0.6406 ** | 10.90 | 10.02 |
| 877.11 | 935.28 | ±3 | y = −0.21282 + 0.23641*x | 0.7003 ** | 8.33 | 7.18 | |
| 839.38 | 935.28 | ±3 | y = −0.17749 + 0.20435*x | 0.7063 ** | 8.84 | 7.51 | |
| 808.47 | 935.28 | ±3 | y = −0.13896 + 0.17459*x | 0.7314 ** | 10.28 | 9.40 | |
| 931.87 | 914.77 | ±3 | y = 0.35896 − 0.33535*x | 0.6371 ** | 10.13 | 8.95 | |
| 935.28 | 877.11 | ±3 | y = 0.27344 − 0.25001*x | 0.6976 ** | 8.39 | 6.41 | |
| 935.28 | 839.38 | ±3 | y = 0.23719 − 0.21032*x | 0.7044 ** | 8.97 | 6.80 | |
| 935.28 | 808.47 | ±3 | y = 0.19771 − 0.16225*x | 0.7310 ** | 9.73 | 8.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Chen, Y.; Wu, S.; Kuang, W.; Tan, J.; Zha, Y.; Fang, B.; Yang, P. Study on the Automatic Selection of Sensitive Hyperspectral Bands for Rice Nitrogen Retrieval Based on a Maximum Inscribed Rectangle. Agronomy 2025, 15, 406. https://doi.org/10.3390/agronomy15020406
Fan Y, Chen Y, Wu S, Kuang W, Tan J, Zha Y, Fang B, Yang P. Study on the Automatic Selection of Sensitive Hyperspectral Bands for Rice Nitrogen Retrieval Based on a Maximum Inscribed Rectangle. Agronomy. 2025; 15(2):406. https://doi.org/10.3390/agronomy15020406
Chicago/Turabian StyleFan, Yaobing, Youxing Chen, Shangrong Wu, Wei Kuang, Jieyang Tan, Yan Zha, Baohua Fang, and Peng Yang. 2025. "Study on the Automatic Selection of Sensitive Hyperspectral Bands for Rice Nitrogen Retrieval Based on a Maximum Inscribed Rectangle" Agronomy 15, no. 2: 406. https://doi.org/10.3390/agronomy15020406
APA StyleFan, Y., Chen, Y., Wu, S., Kuang, W., Tan, J., Zha, Y., Fang, B., & Yang, P. (2025). Study on the Automatic Selection of Sensitive Hyperspectral Bands for Rice Nitrogen Retrieval Based on a Maximum Inscribed Rectangle. Agronomy, 15(2), 406. https://doi.org/10.3390/agronomy15020406






