Optimizing Cassava Growth with Localized Struvite Application: Root Proliferation and Fertilization Efficiency
Abstract
1. Introduction
2. Materials and Methods
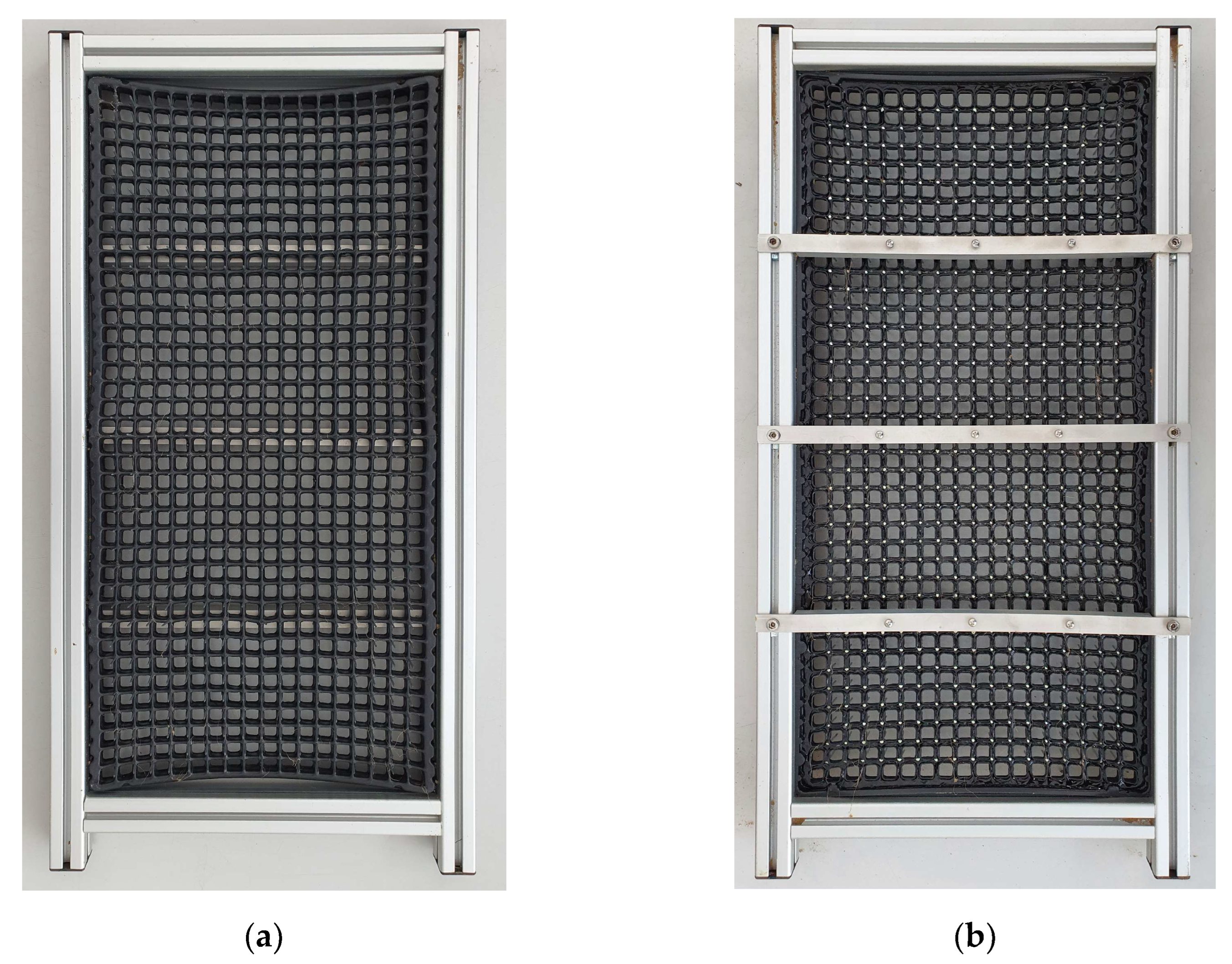
2.1. Rhizobox Preparation and Experimental Set-Up
2.2. Plant Material and Planting
2.3. Growth Conditions
2.4. Shoot Measurements
2.5. Substrate Nutrient Concentrations
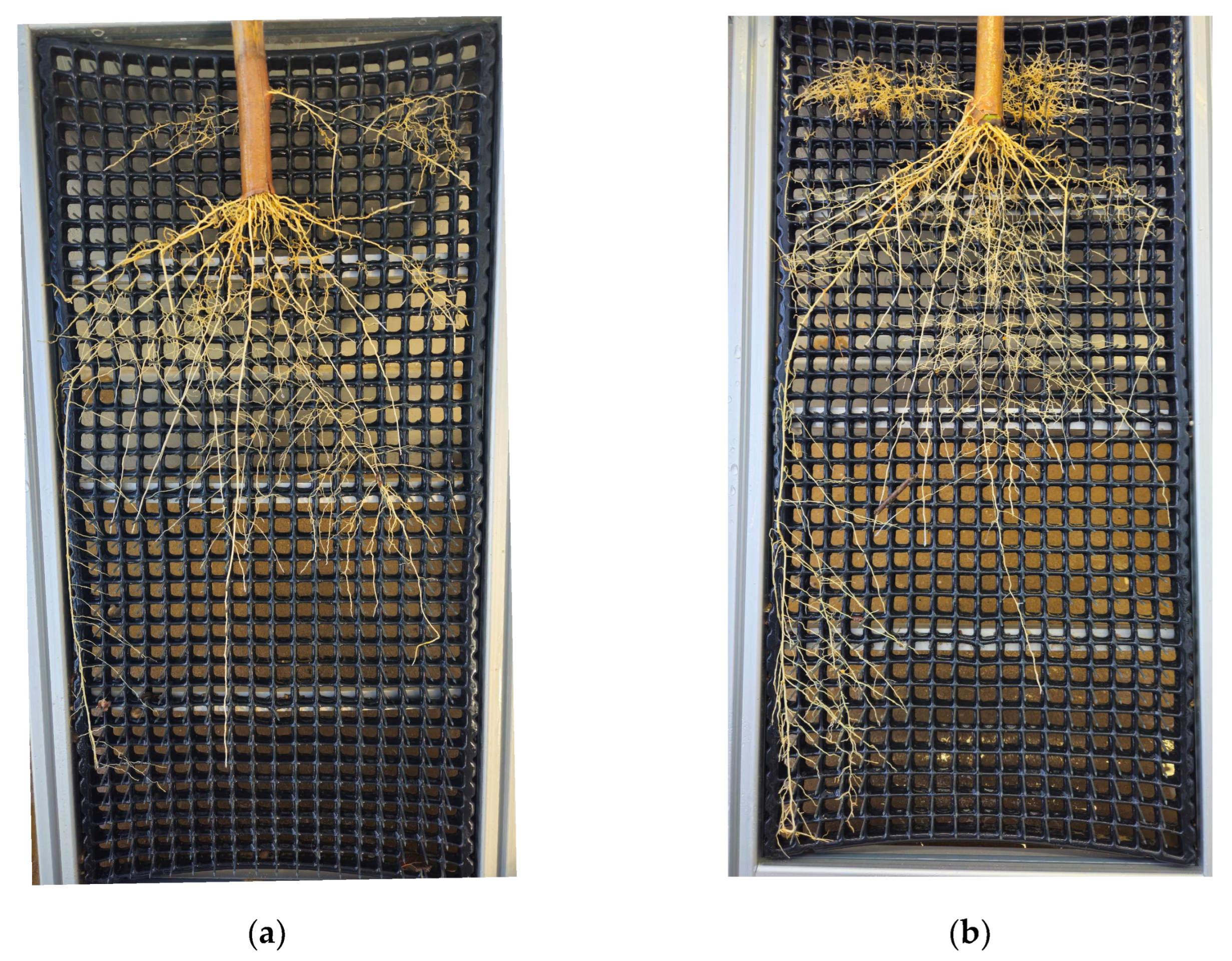
2.6. Root-Washing and Measurements
2.7. Plant Nutrient Analysis
2.8. Roots Length Determination
2.9. Lateral Root Traits Using WinRHIZO
2.10. Calculations
2.11. Statistical Analysis
3. Results and Discussion
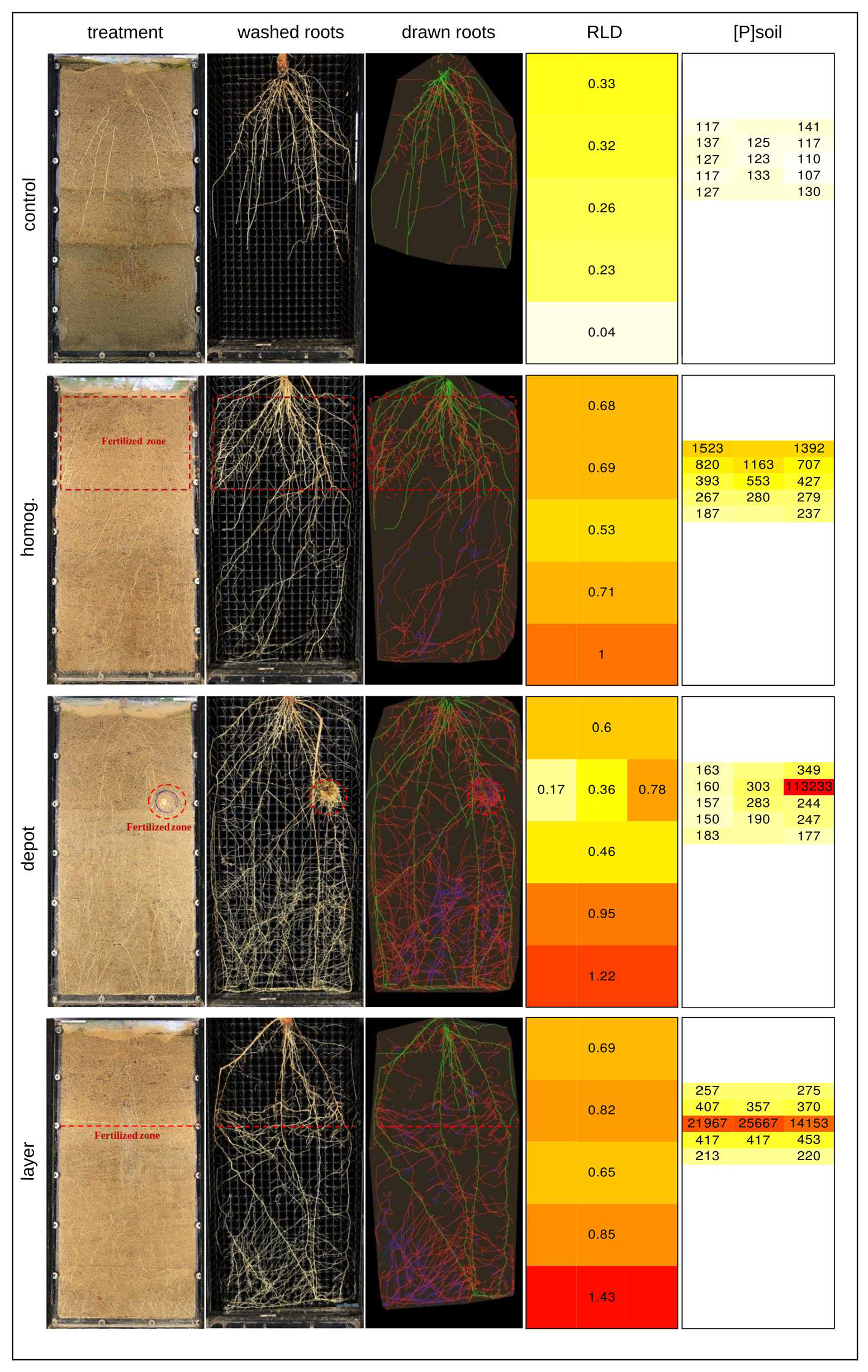
3.1. Root Length Density Distribution Is Associated with Nutrient Placement
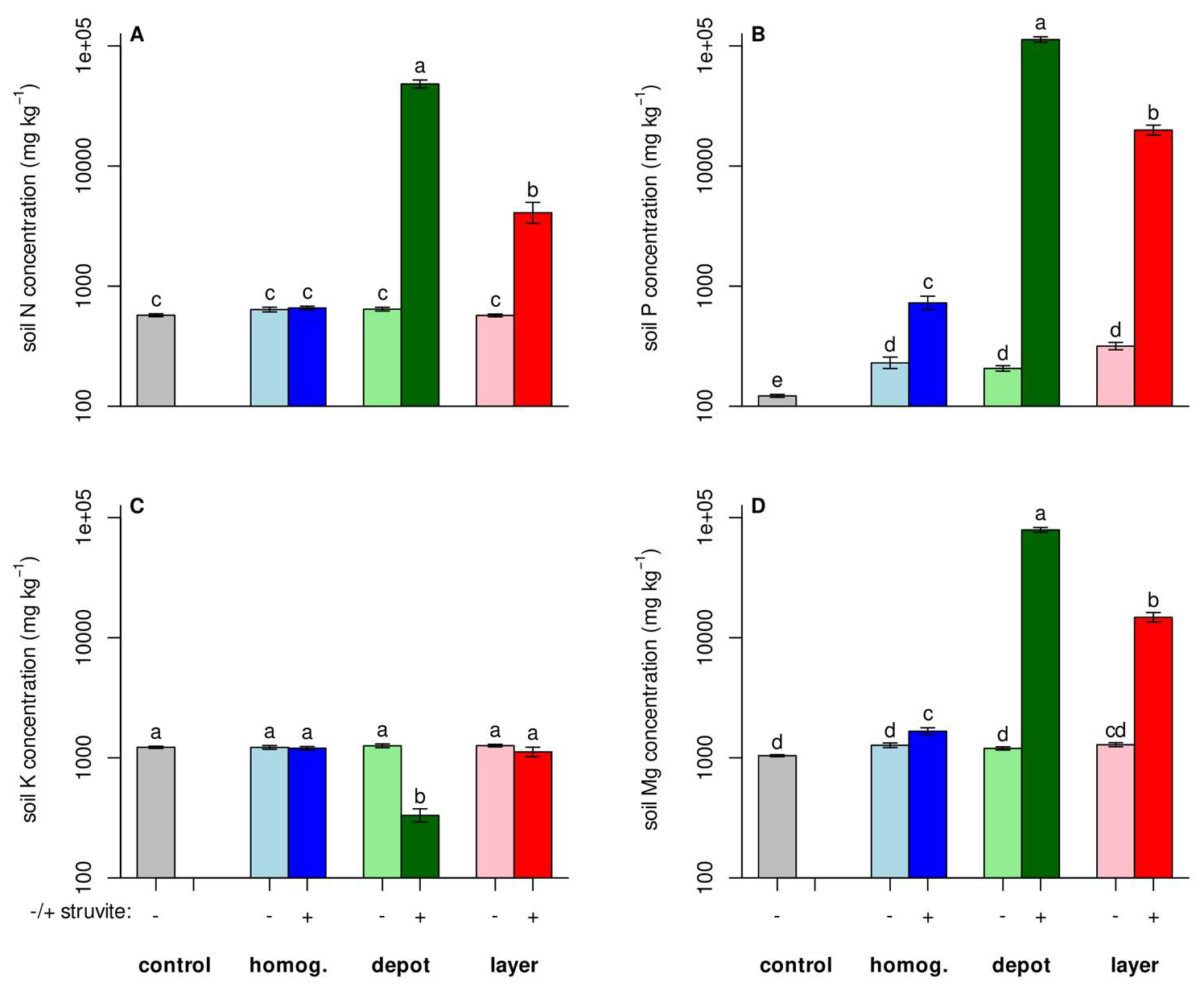
3.2. Nutrients Did Not Spread Much
3.3. Fertilization Increased Nutrient Concentrations in the Biomass
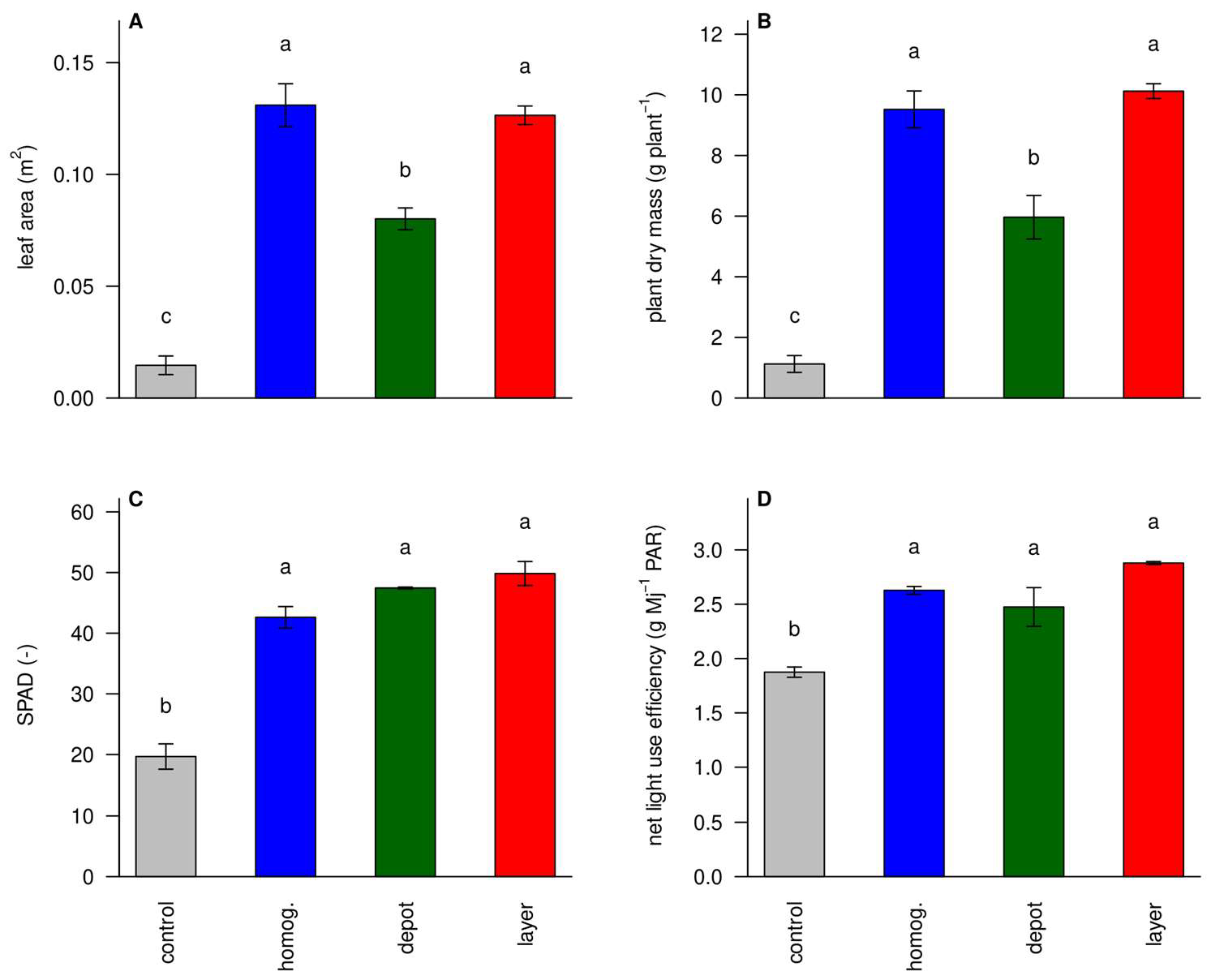
3.4. Unfertilized Plants Were Small and Had Signs of N Deficiency
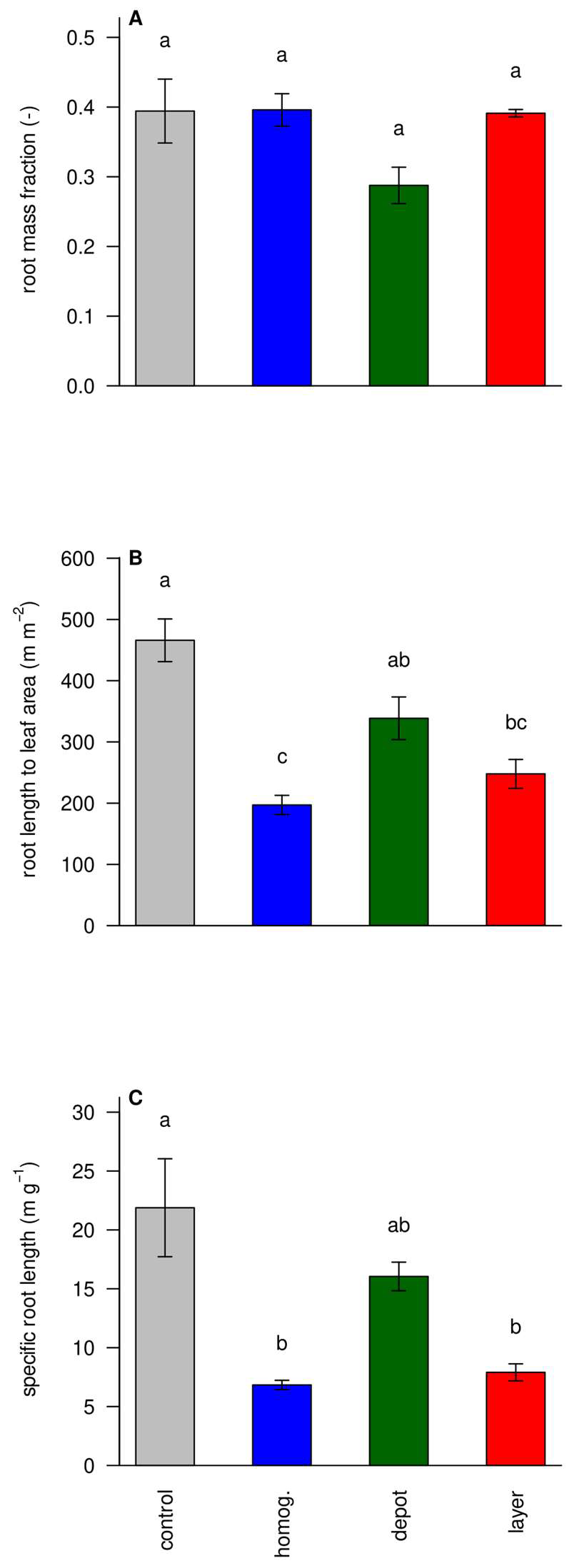
3.5. Functional Ratios Show Less Stress in the ‘Homogenized’ and ‘Layer’ Treatments
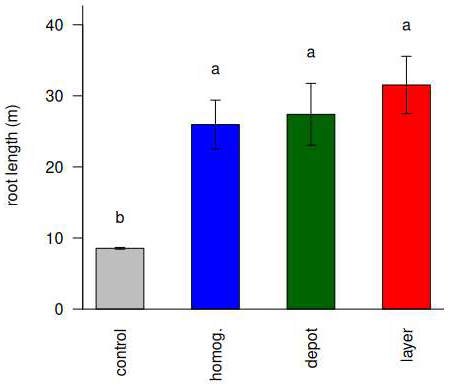
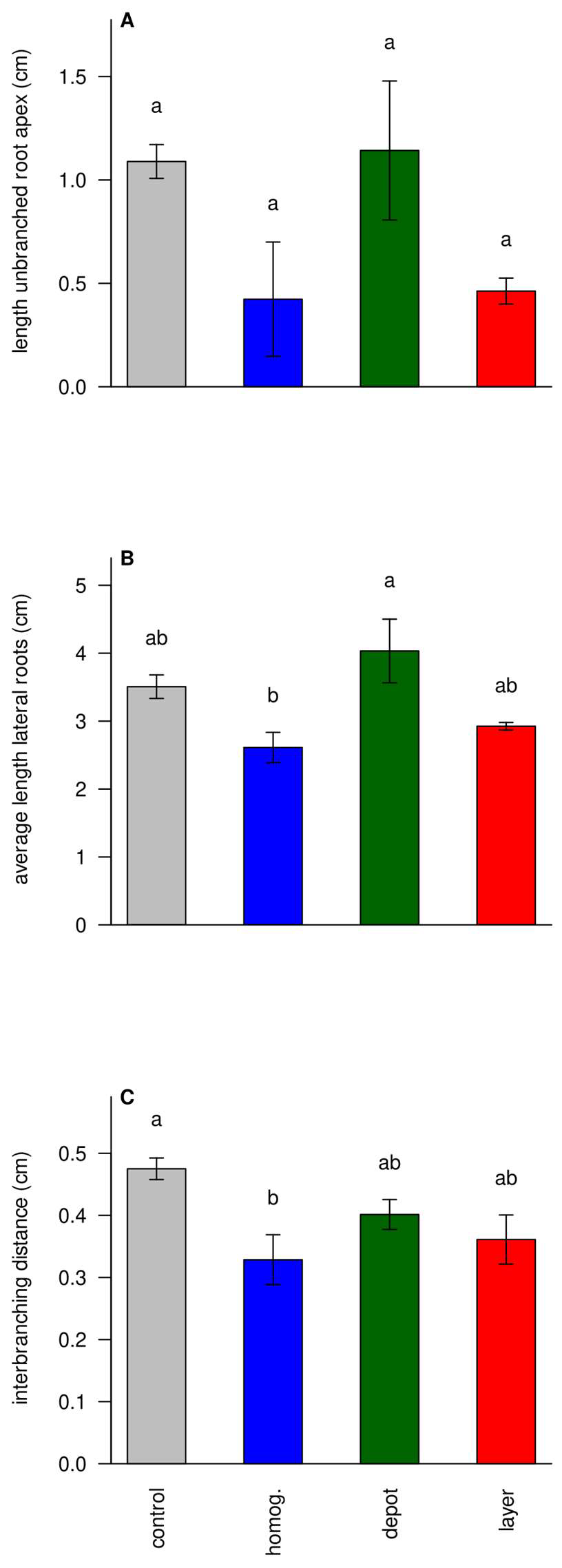
3.6. Struvite Increases Foraging Intensity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Substrate Sampling Scheme in the Rhizotrons

Appendix B. Root-Washing Device

References
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer Placement to Improve Crop Nutrient Acquisition and Yield: A Review and Meta-Analysis. Field Crops Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Drew, M.C. Comparison of the Effects of a Localized Supply of Phosphate, Nitrate, Ammonium and Potassium on the Growth of the Seminal Root System, and the Shoot, in Barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- van Vuuren, M.M.I.; Robinson, D.; Griffiths, B.S. Nutrient Inflow and Root Proliferation during the Exploitation of a Temporally and Spatially Discrete Source of Nitrogen in Soil. Plant Soil 1996, 178, 185–192. [Google Scholar] [CrossRef]
- Robinson, D.; Hodge, A.; Griffiths, B.S.; Fitter, A.H. Plant Root Proliferation in Nitrogen–Rich Patches Confers Competitive Advantage. Proc. R. Soc. London. Ser. B Biol. Sci. 1999, 266, 431–435. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Weiner, J.; Li, F.-M. Root Proliferation in Response to Neighbouring Roots in Wheat (Triticum aestivum). Basic Appl. Ecol. 2019, 39, 10–14. [Google Scholar] [CrossRef]
- Schneider, H.M.; Lynch, J.P. Should Root Plasticity Be a Crop Breeding Target? Front. Plant Sci. 2020, 11, 546. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dunbabin, V.M.; Postma, J.A.; Diggle, A.J.; Siddique, K.H.; Rengel, Z. Modelling Root Plasticity and Response of Narrow-Leafed Lupin to Heterogeneous Phosphorus Supply. Plant Soil 2013, 372, 319–337. [Google Scholar] [CrossRef]
- Robinson, D. Root Proliferation, Nitrate Inflow and Their Carbon Costs during Nitrogen Capture by Competing Plants in Patchy Soil. Plant Soil 2001, 232, 41–50. [Google Scholar] [CrossRef]
- Gleeson, S.K.; Fry, J.E. Root Proliferation and Marginal Patch Value. Oikos 1997, 79, 387–393. [Google Scholar] [CrossRef]
- Croft, S.A.; Hodge, A.; Pitchford, J.W. Optimal Root Proliferation Strategies: The Roles of Nutrient Heterogeneity, Competition and Mycorrhizal Networks. Plant Soil 2012, 351, 191–206. [Google Scholar] [CrossRef]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus Recovery as Struvite: Recent Concerns for Use of Seed, Alternative Mg Source, Nitrogen Conservation and Fertilizer Potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Wollmann, I.; Gauro, A.; Müller, T.; Möller, K. Phosphorus Bioavailability of Sewage Sludge-Based Recycled Fertilizers. J. Plant Nutr. Soil Sci. 2018, 181, 158–166. [Google Scholar] [CrossRef]
- de Sousa, R.; Alleoni, L. Performance of Struvite and Organomineral Fertilizers Compared to Traditional Source of Phosphorus in Maize Cultivation on Tropical Soils. J. Soil Sci. Plant Nutr. 2024, 24, 5250–5271. [Google Scholar] [CrossRef]
- Pérez-Piqueres, A.; Ribó, M.; Rodríguez-Carretero, I.; Quiñones, A.; Canet, R. Struvite as a Sustainable Fertilizer in Mediterranean Soils. Agronomy 2023, 13, 1391. [Google Scholar] [CrossRef]
- Omidire, N.; Brye, K. Wastewater-Recycled Struvite as a Phosphorus Source in a Wheat-Soybean Double-Crop Production System in Eastern Arkansas. Agrosyst. Geosci. Environ. 2022, 5, e20271. [Google Scholar] [CrossRef]
- Leon, P.; Nakayama, Y.; Margenot, A. Field-Scale Evaluation of Struvite Phosphorus and Nitrogen Leaching Relative to Monoammonium Phosphate. J. Environ. Qual. 2024, 53, 23–34. [Google Scholar] [CrossRef]
- Kokulan, V.; Schneider, K.; Macrae, M.; Wilson, H. Struvite Application to Field Corn Decreases the Risk of Environmental Phosphorus Loss While Maintaining Crop Yield. Agric. Ecosyst. Environ. 2024, 366, 108936. [Google Scholar] [CrossRef]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A Review and Meta-Analysis of the Agricultural Potential of Struvite as a Phosphorus Fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A Slow-Release Fertiliser for Sustainable Phosphorus Management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Robles-Aguilar, A.A.; Pang, J.; Postma, J.A.; Schrey, S.D.; Lambers, H.; Jablonowski, N.D. The Effect of pH on Morphological and Physiological Root Traits of Lupinus angustifolius Treated with Struvite as a Recycled Phosphorus Source. Plant Soil 2019, 434, 65–78. [Google Scholar] [CrossRef]
- Fathima, A.A.; Sanitha, M.; Tripathi, L.; Muiruri, S. Cassava (Manihot esculenta) Dual Use for Food and Bioenergy: A Review. Food Energy Secur. 2023, 12, e380. [Google Scholar] [CrossRef]
- Omondi, J.O.; Yermiyahu, U.; Omondi, J.O.; Yermiyahu, U. Improvement in Cassava Yield per Area by Fertilizer Application. In Cassava—Biology, Production, and Use; IntechOpen: London, UK, 2021 ISBN 978-1-83968-909-3.
- Munyahali, W.; Birindwa, D.; Pypers, P.; Swennen, R.; Vanlauwe, B.; Merckx, R. Increased Cassava Growth and Yields through Improved Variety Use and Fertilizer Application in the Highlands of South Kivu, Democratic Republic of Congo. Field Crops Res. 2023, 302, 109056. [Google Scholar] [CrossRef]
- Connor, D.J.; Cock, J.H.; Parra, G.E. Response of Cassava to Water Shortage I. Growth and Yield. Field Crops Res. 1981, 4, 181–200. [Google Scholar] [CrossRef]
- Adiele, J.G.; Schut, A.G.T.; van den Beuken, R.P.M.; Ezui, K.S.; Pypers, P.; Ano, A.O.; Egesi, C.N.; Giller, K.E. A Recalibrated and Tested LINTUL-Cassava Simulation Model Provides Insight into the High Yield Potential of Cassava under Rainfed Conditions. Eur. J. Agron. 2021, 124, 126242. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H. Response of Cassava to Water Stress. Plant Soil 1987, 100, 345–360. [Google Scholar] [CrossRef]
- Lal, R.; Maurya, P.R. Root Growth of Some Tropical Crops in Uniform Columns. Plant Soil 1982, 68, 193–206. [Google Scholar] [CrossRef]
- Biratu, G.K.; Elias, E.; Ntawuruhunga, P. Does the Application of Mineral and Organic Fertilizer Affect Cassava Tuber Quality? An Evidence from Zambia. J. Agric. Food Res. 2022, 9, 100339. [Google Scholar] [CrossRef]
- Birindwa, D.; Van Laere, J.; Munyahali, W.; De Bauw, P.; Dercon, G.; Kintche, K.; Merckx, R. Early Planting of Cassava Enhanced the Response of Improved Cultivars to Potassium Fertilization in South Kivu, Democratic Republic of Congo. Field Crops Res. 2023, 296, 108903. [Google Scholar] [CrossRef]
- Imakumbili, M.L.E.; Semu, E.; Semoka, J.M.R.; Abass, A.; Mkamilo, G. Managing Cassava Growth on Nutrient Poor Soils under Different Water Stress Conditions. Heliyon 2021, 7, e07331. [Google Scholar] [CrossRef] [PubMed]
- Riewklang, K.; Polprasert, C.; Nakason, K.; Polprasert, S.; Kwonpongsagoon, S.; Mahasandana, S.; Panyapinyopol, B. Enhancing Chemical Phosphorus Precipitation from Tapioca Starch Anaerobic Digestion Effluent in a Modified Pilot-Scale Fluidized Bed Reactor. Environ. Res. 2023, 231, 116277. [Google Scholar] [CrossRef]
- Punyasu, N.; Thaiprasit, J.; Kalapanulak, S.; Saithong, T.; Postma, J.A. Modeling Cassava Root System Architecture and the Underlying Dynamics in Shoot–Root Carbon Allocation during the Early Storage Root Bulking Stage. Plant Soil 2024, 1–18. [Google Scholar] [CrossRef]
- Thanni, B.; Merckx, R.; Hauser, S.; Soretire, A.; Honnay, O. Multiple Taxa Inoculants of Arbuscular Mycorrhizal Fungi Enhanced Colonization Frequency, Biomass Production, and Water Use Efficiency of Cassava (Manihot esculenta). Int. Microbiol. 2024, 27, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Nagel, K.A.; Temperton, V.M.; Dietrich, C.C.; Briese, C.; Jablonowski, N.D. Coming Late for Dinner: Localized Digestate Depot Fertilization for Extensive Cultivation of Marginal Soil with Sida hermaphrodita. Front. Plant Sci. 2018, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Valle, S.F.; Giroto, A.S.; Guimarães, G.G.F.; Nagel, K.A.; Galinski, A.; Cohnen, J.; Jablonowski, N.D.; Ribeiro, C. Co-Fertilization of Sulfur and Struvite-Phosphorus in a Slow-Release Fertilizer Improves Soybean Cultivation. Front. Plant Sci. 2022, 13, 861574. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Schrey, S.D.; Postma, J.A.; Temperton, V.M.; Jablonowski, N.D. Phosphorus Uptake from Struvite Is Modulated by the Nitrogen Form Applied. J. Plant Nutr. Soil Sci. 2020, 183, 80–90. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Grunert, O.; Hernandez-Sanabria, E.; Mysara, M.; Meers, E.; Boon, N.; Jablonowski, N.D. Effect of Applying Struvite and Organic N as Recovered Fertilizers on the Rhizosphere Dynamics and Cultivation of Lupine (Lupinus angustifolius). Front. Plant Sci. 2020, 11, 572741. [Google Scholar] [CrossRef] [PubMed]
- Valle, S.F.; Giroto, A.S.; Dombinov, V.; Robles-Aguilar, A.A.; Jablonowski, N.D.; Ribeiro, C. Struvite-Based Composites for Slow-Release Fertilization: A Case Study in Sand. Sci. Rep. 2022, 12, 14176. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Murakami, S.; Karasawa, T.; Ejiri, M.; Shiono, K. Complete Root Specimen of Plants Grown in Soil-Filled Root Box: Sampling, Measuring, and Staining Method. Plant Methods 2021, 17, 97. [Google Scholar] [CrossRef]
- van der Bom, F.J.T.; Williams, A.; Borrell, A.K.; Raymond, N.; Bell, M.J. Phosphorus Management Is Key to Effective Deployment of Root Ideotypes in Complex Soil Environments. Plant Soil 2023, 489, 323–340. [Google Scholar] [CrossRef]
- Nagel, K.A.; Putz, A.; Gilmer, F.; Heinz, K.; Fischbach, A.; Pfeifer, J.; Faget, M.; Blossfeld, S.; Ernst, M.; Dimaki, C.; et al. GROWSCREEN-Rhizo Is a Novel Phenotyping Robot Enabling Simultaneous Measurements of Root and Shoot Growth for Plants Grown in Soil-Filled Rhizotrons. Funct. Plant Biol. 2012, 39, 891–904. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardising Root Classification, Sampling, Processing and Trait Measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- Pei, Y.; Dong, J.; Zhang, Y.; Yuan, W.; Doughty, R.; Yang, J.; Zhou, D.; Zhang, L.; Xiao, X. Evolution of Light Use Efficiency Models: Improvement, Uncertainties, and Implications. Agric. For. Meteorol. 2022, 317, 108905. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2024. Available online: https://rvlenth.github.io/emmeans/authors.html#citation (accessed on 1 December 2024).
- Hodge, A. The Plastic Plant: Root Responses to Heterogeneous Supplies of Nutrients. New Phytol 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Rech, I.; Withers, P.J.A.; Jones, D.L.; Pavinato, P.S. Solubility, Diffusion and Crop Uptake of Phosphorus in Three Different Struvites. Sustainability 2019, 11, 134. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot Size Matters: A Meta-Analysis of the Effects of Rooting Volume on Plant Growth. Funct. Plant Biol. 2012, 39, 839. [Google Scholar] [CrossRef] [PubMed]
- Yesigat, A.; Worku, A.; Mekonnen, A.; Bae, W.; Feyisa, G.L.; Gatew, S.; Han, J.-L.; Liu, W.; Wang, A.; Guadie, A. Phosphorus Recovery as K-Struvite from a Waste Stream: A Review of Influencing Factors, Advantages, Disadvantages and Challenges. Environ. Res. 2022, 214, 114086. [Google Scholar] [CrossRef] [PubMed]
- Nansahwang, A.; Leksungnoen, P.; Armatmontree, C.; Aramrak, S.; Kongsil, P.; Wisawapipat, W. Phosphate Mineral Solubility Controls on Cassava Root Exudates, Rhizosphere Nutrient Availability, and Plant Nutrient Accumulation. Rhizosphere 2022, 23, 100575. [Google Scholar] [CrossRef]
- Adiele, J.G.; Schut, A.G.T.; Ezui, K.S.; Pypers, P.; Giller, K.E. Dynamics of N-P-K Demand and Uptake in Cassava. Agron. Sustain. Dev. 2020, 41, 1. [Google Scholar] [CrossRef]
- Spear, S.N.; Edwards, D.G.; Asher, C.J. Response of Cassava, Sunflower, and Maize to Potassium Concentration in Solution III. Interactions between Potassium, Calcium, and Magnesium. Field Crops Res. 1978, 1, 375–389. [Google Scholar] [CrossRef]
- Bray, R.H. A Nutrient Mobility Concept of Soil-Plant Relationships. Soil Sci. 1954, 78, 9. [Google Scholar] [CrossRef]
- Chua, M.F.; Youbee, L.; Oudthachit, S.; Khanthavong, P.; Veneklaas, E.J.; Malik, A.I. Potassium Fertilisation Is Required to Sustain Cassava Yield and Soil Fertility. Agronomy 2020, 10, 1103. [Google Scholar] [CrossRef]
- Hertzberger, A.; Cusick, R.; Margenot, A. Maize and Soybean Response to Phosphorus Fertilization with Blends of Struvite and Monoammonium Phosphate. Plant Soil 2021, 461, 547–563. [Google Scholar] [CrossRef]
- Ceballos, I.; Ruiz, M.; Fernández, C.; Peña, R.; Rodríguez, A.; Sanders, I. The In Vitro Mass-Produced Model Mycorrhizal Fungus, Rhizophagus Irregularis, Significantly Increases Yields of the Globally Important Food Security Crop Cassava. PLoS ONE 2013, 8, e70633. [Google Scholar] [CrossRef] [PubMed]
- Di Tomassi, I.; Chatterjee, N.; Barrios-Masias, F.; Zhou, Q.; Gu, C.; Margenot, A. Arbuscular Mycorrhizae Increase Biomass and Nutrient Uptake of Tomato Fertilized with Struvite Compared to Monoammonium Phosphate. Plant Soil 2021, 464, 321–333. [Google Scholar] [CrossRef]
- Mahakosee, S.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P.; Holbrook, C.C.; Kvien, C.K.; Banterng, P. Light Interception and Radiation Use Efficiency of Cassava under Irrigated and Rainfed Conditions and Seasonal Variations. Agriculture 2022, 12, 725. [Google Scholar] [CrossRef]
- Lopez, G.; Ahmadi, S.H.; Amelung, W.; Athmann, M.; Ewert, F.; Gaiser, T.; Gocke, M.I.; Kautz, T.; Postma, J.; Rachmilevitch, S.; et al. Nutrient Deficiency Effects on Root Architecture and Root-to-Shoot Ratio in Arable Crops. Front. Plant Sci. 2023, 13, 1067498. [Google Scholar] [CrossRef]
- Körner, C.; Renhardt, U. Dry Matter Partitioning and Root Length/Leaf Area Ratios in Herbaceous Perennial Plants with Diverse Altitudinal Distribution. Oecologia 1987, 74, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ötvös, K.; Benková, E. Spatiotemporal Mechanisms of Root Branching. Curr. Opin. Genet. Dev. 2017, 45, 82–89. [Google Scholar] [CrossRef]
- Motte, H.; Beeckman, T. The Evolution of Root Branching: Increasing the Level of Plasticity. J. Exp. Bot. 2019, 70, 785–793. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, B. Exploring the Importance of Root Architecture Plasticity in Plant Adaptation to Environmental Constraints. Plant Species Biol. 2023, 38, 234–244. [Google Scholar] [CrossRef]
- IBG-2 Optimizing Cassava Growth with Localized Struvite Application: Root Proliferation and Fertilization Efficiency. 2025; Available online: https://data.fz-juelich.de/dataset.xhtml?persistentId=doi:10.26165/JUELICH-DATA/KVUOSI (accessed on 1 December 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, R.; Giroto, A.S.; Ohrem, B.; Beckmann, S.; Ademi, A.; Boeckem, V.; Bochmann, H.; Müller-Linow, M.; Lenz, H.; Ribeiro, C.; et al. Optimizing Cassava Growth with Localized Struvite Application: Root Proliferation and Fertilization Efficiency. Agronomy 2025, 15, 353. https://doi.org/10.3390/agronomy15020353
Borges R, Giroto AS, Ohrem B, Beckmann S, Ademi A, Boeckem V, Bochmann H, Müller-Linow M, Lenz H, Ribeiro C, et al. Optimizing Cassava Growth with Localized Struvite Application: Root Proliferation and Fertilization Efficiency. Agronomy. 2025; 15(2):353. https://doi.org/10.3390/agronomy15020353
Chicago/Turabian StyleBorges, Roger, Amanda S. Giroto, Benedict Ohrem, Silas Beckmann, Ali Ademi, Vera Boeckem, Helena Bochmann, Mark Müller-Linow, Henning Lenz, Caue Ribeiro, and et al. 2025. "Optimizing Cassava Growth with Localized Struvite Application: Root Proliferation and Fertilization Efficiency" Agronomy 15, no. 2: 353. https://doi.org/10.3390/agronomy15020353
APA StyleBorges, R., Giroto, A. S., Ohrem, B., Beckmann, S., Ademi, A., Boeckem, V., Bochmann, H., Müller-Linow, M., Lenz, H., Ribeiro, C., Wojciechowski, T., Jablonowski, N. D., & Postma, J. A. (2025). Optimizing Cassava Growth with Localized Struvite Application: Root Proliferation and Fertilization Efficiency. Agronomy, 15(2), 353. https://doi.org/10.3390/agronomy15020353







