Abstract
The development of plant-growth-promoting bacteria (PGPB) is one of the important research topics in agricultural microbiology. Four bacterial isolates that exhibited nitrogen fixation, phosphate and potassium solubilization, as well as indole-3-acetic acid (IAA) and siderophore production abilities, were selected from biogas residue, namely BR-1, BR-16, BR-17, and BR-44. According to morphological and molecular biological identification, BR-1, BR-16, BR-17, and BR-44 belonged to Bacillus subtilis, Bacillus cereus, Bacillus megaterium, and Bacillus subtilis, respectively. The four functional strains were combined into a composite microbial community. The optimal media were peptone (15 g/L), sucrose (10 g/L), and KCl (2 g/L); the optimal incubation conditions were an initial pH of 7.0, a volume of 47 mL/250 mL, an inoculum size of 6% v/v, an incubation temperature of 31 °C, a shaking speed of 205 r/min, and an incubation period of 20 h, as determined through a single factor test and the response surface methodology (RSM). In the optimized process, a liquid bacterial inoculant with an OD600 of 1.866 was obtained, with an effective viable count of 8.955 × 109 CFU/mL. A solid bacterial inoculant was prepared by using bran as a substrate, and its effective viable count was 1.11 × 109 CFU/g. The application of the bacterial inoculants promoted the growth of watermelon, increased the photosynthetic efficiency and yield, and improved fruit quality. This study provides a theoretical basis and technical support for the development and application of microbial inoculants.
1. Introduction
Plant-growth-promoting bacteria (PGPB) can regulate soil nutrient cycling by fixing nitrogen, solubilizing phosphorus, solubilizing potassium, and producing indole-3-acetic acid (IAA) and siderophores [1,2,3,4,5]. These bacteria can improve plants’ nutrient uptake and provide a favorable root zone environment for their growth. Isolating and selecting high-efficiency functional strains and constructing multi-functional microbial communities that are adapted to different environmental requirements are important tasks in agricultural microbiology research. Some microorganisms have multiple potential functions [6]. Walia et al. [7] showed that Bacillus subtilis CKT1 had the capacity for phosphate solubilization, IAA production, siderophore production, and HCN production. After inoculation with CKT1, the shoot length, root length, shoot dry weight, and root dry weight of tomato seedlings increased significantly, i.e., by 5.22%, 21.12%, 63.50%, and 54.08%, respectively. Muthuraja et al. [8] isolated three strains with the capacity for potassium solubilization and IAA and siderophore production; these could significantly improve maize plants’ growth and K uptake. Therefore, actively developing and utilizing multi-functional PGPB is beneficial for the development of high-quality agricultural products.
To date, there has been great progress in research on the preparation of microbial inoculant technology at home and abroad, and researchers have successfully developed microbial inoculants and formulas that are suitable for the growth and reproduction of specific microorganisms. Liquid inoculants have the advantages of good water solubility and simplicity in application, and they are widely used in production. In the process of preparing such inoculants, by optimizing components such as the nitrogen source, carbon source, and mineral salts, nutrients can be provided that enable strains to grow and reproduce so as to improve the effectiveness of the inoculants [9,10]. In the liquid fermentation process, changes in the environmental fermentation conditions affect the reproductive capacity and functional sizes of strains. Some studies have evaluated the effects of different initial pH values, inoculum sizes, volumes, incubation temperatures, shaking speeds, and incubation periods on the production of cell biomass and secondary metabolites [11,12,13,14]. Compared with liquid inoculants, solid inoculants are easy to store, convenient to transport, and have a long shelf life, thus becoming a major research focus in the field of inoculants. When preparing solid microbial inoculants, it is often necessary to add substrates; the selection of appropriate substrates can ensure the successful reproduction and metabolism of microorganisms, improve the survival rates of strains, and improve the performance of such inoculants [15,16]. Therefore, by optimizing the medium and the environmental fermentation conditions and adding a suitable substrate, the preparation of liquid and solid microbial inoculants can be achieved.
Small watermelon (Citrullus lanatus L.) is a popular fruit in the market due to its characteristics of small fruit type, excellent quality; fruit can be produced out of season, short growing period, and high economic benefit [17]. However, the unreasonable application of chemical fertilizers and pesticides will lead to the decline of watermelon quality and a series of environmental pollution problems [18]. In order to improve the yield and quality of watermelon and promote its sustainable production, adding bio-fertilizers is an effective way to optimize measures [19]. Beneficial microorganisms exist widely in different ecological environments in nature. PGPB have been screened in various plant rhizospheres and habitats, such as saline–alkali land and alpine land [7,20,21,22], and they have been applied to crops. Biogas residue is the product of the anaerobic fermentation of organic waste such as livestock manure and straw, and it is rich in essential nutrients (N, P, K), trace elements, humic acid, and cellulose. It also contains a variety of bioactive substances and is often used as an organic fertilizer in agricultural production [23,24]. Previous studies mostly focused on the use of biogas residue to promote crop growth and development [25,26]; however, few studies have been conducted on whether it contains beneficial microorganisms and whether their mechanism of action can promote the growth of plants. Therefore, it is worth exploring whether beneficial bacterial isolates can be screened from agricultural waste, such as biogas residue, and effectively applied. In this study, we screened the dominant functional strains present in biogas residue, focusing on those with the capacity for nitrogen fixation, phosphate and potassium solubilization, and IAA and siderophore production. The dominant strains were combined into a complex flora; the medium formula and the culture conditions of the liquid inoculant were optimized, and the best method for the preparation of a microbial inoculant was obtained via the RSM. A solid inoculant was prepared by selecting a microbial substrate with a good curing effect. Through a pot experiment, the potential of these inoculants to promote the growth and development of watermelon was examined. The purposes of this study were to develop a complete system for the preparation of composite inoculants, to create high-quality strain resources for the development of inoculants, and to provide a theoretical basis and technical support for the effective application of inoculants.
2. Materials and Methods
2.1. Medium
Nutrient agar (NA): 3 g of beef paste, 10 g of peptone, 5 g of NaCl, 18 g of agar, and 1 L of distilled water. Beef extract peptone medium (BPM): 3 g of beef paste, 10 g of peptone, 5 g of NaCl, and 1 L of distilled water. The Ashby nitrogen-free medium: 0.2 g of KH2PO4, 0.2 g of MgSO4·7H2O, 0.2 g of NaCl, 5 g of CaCO3, 10 g of glucose, 0.1 g of CaSO4·2H2O, and 1 L of distilled water. The soluble inorganic phosphorus medium: 10 g of C6H12O6·H2O, 0.3 g of NaCl, 0.3 g of KCl, 0.5 g of [(NH4)2SO4], 0.3 g of MgSO4·7H2O, 0.03 g of MnSO4·4H2O, 0.03 g of FeSO4·7H2O, 10 g of Ca3(PO4)2, and 1 L of distilled water. The IAA-producing medium: 10 g of peptone, 5 g of yeast extract, 10 g of NaCl, 0.2 g of L-tryptophan, and 1 L of distilled water. Chrome azurol S (CAS) agar: 60.5 mg of CAS, 72.9 mg of hexadecyltrimethylammonium (HDTMA) bromide, 2.645 mg of FeCl3·6H2O, 295.25 mg of NaH2PO4·2H2O, 1213.5 g of Na2HPO4·12H2O, 125 mg of NH4Cl, 37.5 mg of KH2PO4, 62.5 mg of NaCl, 9000 mg agar, 1 L of distilled water (All glassware used in the experiment was soaked in 6 mol/L HCl and rinsed several times with distilled water for iron removal treatment). CAS test-fluid: 0.079 g CAS was dissolved in 50 mL of deionized water, 10 mL of 1 mmol/L FeCl3 solution (dissolved in HCl) was added, and then slowly added into 0.069 g HDTMA solution dissolved in 40 mL of deionized water. Note: 18 g agar was added to the solid medium based on the above formula.
2.2. Strains
We weighed 10.0 g of the biogas residue and added 90 mL of sterile water. After shaking at 30 °C, 170 rpm for 30 min, diluted to a gradient of 10−5. Then, 200 μL of the diluted fluid was spread onto nutrient agar (NA), and incubated upside down at 28 °C for 24 h. Different morphological colonies were picked and streaked on new media for purification. A total of 45 bacterial strains were isolated and inoculated onto the agarslant culture medium, and were stored at 4 °C in the Microbiology Laboratory of Shanxi Institute of Organic Dryland Farming, Shanxi Agricultural University.
2.3. Screening of Functional Strains
All 45 strains were inoculated on the Ashby nitrogen-free medium, the soluble inorganic phosphorus medium, or the soluble potassium solid medium. After being incubated at 28 °C for 7 d, 4 d, or 4 d, respectively, the strains with a good growth status were inoculated in BPM. After shaking at 28 °C and 170 r/min for 48 h, a seed liquid was prepared, which was injected into the Ashby nitrogen-free medium, the soluble inorganic phosphorus medium, and the soluble potassium liquid medium at a 1% inoculation size. They were then cultured at 28 °C and 170 r/min for 7 d, 4 d, and 4 d, respectively. The nitrogen content of the bacterial solution was determined via the semi-micro-Kjeldahl method, the phosphorus content was determined via Mo-Sb colorimetry, and the potassium content was determined via atomic absorption spectrophotometry [27].
Salkowski colorimetry was used to determine the IAA production capacity of the strains [28]. Different strains were inoculated in the IAA-producing medium, shaken at 28 °C, 170 r/min for 48 h, and then centrifuged at 4000 r/min for 10 min. The supernate was mixed with the Salkowski coloritic solution in equal amounts, and then left for 30 min to observe the degree of redness of the mixture. Strains with a higher degree of redness were judged as those with higher IAA production capacity, and their OD530 was detected. OD530 mixed with distilled water and Salkowski colorimetric solution was used as the blank, and the IAA production content of strains was calculated according to the standard curve.
The CAS detection method was used to determine the siderophore synthesis rates of the strains [29]. The strains were inoculated on a CAS agar and cultured at 28 °C for 48 h. The ratio of the orange halo diameter (D) around the colony-to-colony diameter (d) was calculated. The strains with higher D/d were inoculated in BPM at 1% of the inoculum size, respectively, and cultured at 28 °C, 170 r/min for 48 h, then centrifuged at 4000 r/min for 10 min, diluted in the supernate 10 times, mixed with CAS test-fluid in equal quantity, left for 60 min to detect OD630 (AS), using OD630 mixed with CAS test-fluid and distilled in water as reference ratio (Ar). The siderophore synthesis rate was calculated.
Siderophore synthesis rates (%) = [(Ar − As)/Ar] × 100
2.4. Strain Identification
The screened strains were inoculated on NA and cultured inversely at 30 °C for 24 h. The colony color, surface morphology, and edge characteristics were observed in accordance with Bergey’s Manual of Determinative Bacteriology. The strains were stained through Gram staining, and the microstructure was observed with an electron microscope.
Ezup column bacterial genomic DNA extraction kit was used, and the bacterial 16S rRNA genes were amplified with the universal primer pairs 27F (5′-AGTTTGATCMTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The amplification conditions were as follows: 95 °C for 4 min; 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 1 min, with a total of 30 cycles; 72 °C at 10 min; and 4 °C insulation. The PCR products were sent to Guangzhou IGE Bio Co., Ltd., for 16S rRNA gene sequence detection. The obtained sequences were recorded in the NCBI database for BLAST comparison, and a phylogenetic evolutionary tree was constructed.
2.5. Test of Antagonism Between Functional Strains
Strains BR-1, BR-16, BR-17, and BR-44 were crossed on NA and cultured in reverse at 30 °C for 24 h. Inhibition bands appeared at the intersection, indicating that the two examined strains had antagonistic effects.
2.6. Measurement of Growth Curves
The activated strains were propagated in BPM and cultured in a vibrating incubator at 30 °C and 170 r/min. The bacterial biomass (OD600) of the suspensions was measured with a UV–VIS spectrophotometer every 4 h, from 0 to 48 h after inoculation. Uninoculated BPM was used as a blank control. Each trial was repeated 3 times. A strain growth curve was drawn.
2.7. Preparation of Liquid Inoculants
2.7.1. Preparation of Seed Liquid
Strains BR-1, BR-16, BR-17, and BR-44 were inoculated in BPM; incubated at 30 °C and 170 r/min for 24 h; and inoculated in new BPM at a 4% inoculation rate for 24 h. The 4 bacterial strain inoculants were mixed in equal parts and centrifuged at 8000 rpm for 10 min; the bacterial sediments were suspended in sterile distilled water with OD600 = 1.50 ± 0.05; and a BR seed solution was prepared.
2.7.2. Single-Factor Optimization of Liquid Inoculant
Six different nitrogen sources were added to aseptic water, namely beef extract powder, peptone, yeast extract, NH4Cl, NH4NO3, and (NH4)2SO4, with the dosages of 5 g/L, 10 g/L, 15 g/L, or 20 g/L. The BR bacterial solution was fermented under the initial culture conditions (initial pH: 7.0; bottled fluid volume: 100 mL/250 mL; inoculum size: 4%, incubation temperature: 30 °C; shaking speed: 170 r/min; incubation period: 24 h—the same applies below). This was repeated 3 times; then, the OD600 of the bacterial inoculant was determined, and the optimal nitrogen source type and additive amount were identified according to the biomass of the bacteria. After the nitrogen source was determined, a carbon source and inorganic salts were added successively, and the optimal carbon source, mineral salt type, and additive amount were determined via the same method. The studied carbon sources included soluble starch, sucrose, glucose, lactose, fructose, and mannose, and the additive amounts were 5 g/L, 10 g/L, 15 g/L, and 20 g/L, respectively. The mineral salts included NaCl, KCl, KH2PO4, K2HPO4, NaH2PO4, and Na2HPO4, and the additive amounts were 2 g/L, 4 g/L, 6 g/L, and 8 g/L, respectively.
After determining the components of the medium, the one-variable-at-a-time rotation approach was used to optimize the environmental fermentation conditions. The initial fermentation conditions were changed, including the initial pH values (5.0, 6.0, 7.0, 8.0, and 9.0), bottled fluid volumes (50 mL/250 mL, 100 mL/250 mL, 150 mL/250 mL, and 200 mL/250 mL), inoculum sizes (2%, 4%, 6%, 8%, and 10% (v/v)), incubation temperatures (26 °C, 28 °C, 32 °C, 34 °C, and 36 °C), and shaking speeds (110 r/min, 130 r/min, 150 r/min, 170 r/min, 190 r/min, and 210 r/min). This was repeated 3 times, and the OD600 of the inoculant was determined.
2.7.3. Plackett–Burman Design
The Design-Expert.v8.0.6 software was used for test design and analysis, with the details shown in Table 1. Based on the single-factor optimization results, the Plackett–Burman test design (n=12) was implemented for 9 factors—namely the amount of peptone added (A), amount of sucrose added (B), amount of KCl added (C), initial pH (D), bottled fluid volume (E), inoculum size (F), incubation temperature (G), shaking speed (H), and incubation time (J)—and the response value was the OD600. The response interval values were transformed into a low level (−1) and a high level (1).

Table 1.
Plackett–Burman design: factor levels and coding values.
2.7.4. Steepest Climb Test
Based on the Plackett–Burman design, the three significant factors were determined by performing the steepest climb test. The climbing direction and step length were determined according to the positive and negative action values of the significant factors, and the optimal action range of each factor was determined. The value of the non-significant factor was determined according to the single-factor optimization results.
2.7.5. Box–Behnken Response Surface Analysis
The Box–Behnken RSM was conducted on the 3 significant factors, namely the bottled fluid volume, incubation temperature, and shaking speed. The optimal treatment in the optimization of the steepest climb test was taken as the central point. The test variables and coding levels were as shown in Table 2, and the OD600 was taken as the response value. The optimal fermentation parameters were obtained.

Table 2.
Variables and levels in the Box–Behnken experiment.
2.8. Screening of Substrate and Preparation of Solid Inoculant
Bran, soya meal, and biogas residue were crushed; sterilized in an autoclave at 121 °C for 60 min; and set aside. The liquid microbial inoculant was prepared according to the optimal fermentation parameters obtained via the RSM. The different substrates, namely bran, corn flour, soya meal, biogas residue, and straw biochar, were weighed to obtain 100 g of each. Then, 50 mL of the liquid microbial inoculant was added, and the samples were mixed evenly and dried at 30 °C for 8 h in a blast drying oven. Based on the “Microbial Inoculants in Agriculture” standard [30], the effective viable counts of the microbial inoculants were determined.
2.9. Experiment on Growth-Promoting Effects of Inoculants in Watermelon
2.9.1. Pot Experiment Design
The pot experiment was conducted in the greenhouse (37°77′ N, 112°58′ E) of the Shanxi Institute of Organic Dryland Farming, Shanxi Agricultural University, from March to June 2023. The test medium consisted of peat–perlite = 2:1. The physical and chemical properties of the matrix were as follows: the pH was 4.74, the electrical conductivity (EC) was 688.83 μS/cm, the organic matter content was 482.49 g/kg, the total nitrogen content was 7.97 g/kg, the available phosphorus content was 32.77 mg/kg, and the available potassium content was 6.5 mg/kg. The experiment consisted of 3 treatments: CK—No inoculant, T1—BR liquid inoculant, and T2—BR solid inoculant. Watermelon was cultivated in plastic flowerpots (diameter—24 cm, depth—26.5 cm, base diameter—19.3 cm) with plant spacing of 30 cm × 60 cm. Hanging vine cultivation and double-stem pruning were adopted, and 1 fruit was kept in each plant for growth. Each treatment was repeated 10 times. Before planting, the inoculants were applied once; then, they were applied every 15 days during the watermelon vine extension period. The inoculant was diluted 500 times (500 mL per pot) and root-irrigated around the watermelon root system. Conventional water and fertilizer management was applied, and the management measures for each treatment were kept consistent.
2.9.2. Determination Index and Method
The plant height and stem diameter of the watermelon plants were measured one week after the first application of the BR inoculants. The fresh weight and dry weight of the watermelon roots were calculated after harvest. The roots were scanned with an EPSON Expression 2400 scanner, and the root length and surface area were analyzed with the WinRHIZO image analysis software. The soil and plant analyzer development (SPAD) 502Plus chlorophyll detector was used to detect the relative content of chlorophyll, known as SPAD value. The transpiration rate (Tr), net photosynthetic rate (Pn), and stomatal conductance (Gs) of the watermelon were measured with a Li-6800 photocopiometer (USA), with one functional leaf at the top and bottom of the fruit node as the test leaves. The watermelon yield was determined after the fruit had ripened and been harvested. The measured transverse diameter (TD) and longitudinal diameter (LD) of the fruit, cut the fruit longitudinally, and measure the thickness of the pericarp thickness. The melon flesh was homogenated, the soluble total sugar content (STS) was determined via the anthrone colorimetric method; the soluble protein content (SP) and vitamin C content (VC) were determined with a kit produced by the Nanjing Jiancheng Bioengineering Institute; the organic acid content was determined via the acid–base titration method; the nitrate content was determined via the salicylic acid–sulfuric acid colorimetric method.
2.10. Statistical Analysis
The data were analyzed using the SPSS 16.0 software for variance analysis. Duncan’s new complex range method was adopted for multiple comparisons, and the MEGA7.0 software was adopted to construct the phylogenetic tree through the neighbor-joining method. The data were processed and plotted using Excel, Design-Expert V8.0.6, and the Origin 2024 software.
3. Results
3.1. Functional Strain Screening
In total, 45 strains of bacteria were isolated from the biogas residue compost, 22 strains of nitrogen-fixing bacteria were screened, and the nitrogen fixation content ranged from 12.3 to 23.91 mg/L. There were 18 strains of phosphate-solubilizing bacteria, and the phosphorus solubilization content ranged from 15.53 to 121.27 mg/L. There were 20 strains of potassium-solubilizing bacteria, and the potassium solubilization content ranged from 42.24 to 90.07 mg/L. The IAA secretion of 18 strains ranged from 20.20 to 45.06 μg/mL. The siderophore synthesis rates of 21 strains ranged from 2.7% to 41.06%. Following the functional analysis, four strains with a strong capacity for comprehensive growth promotion—namely BR-1, BR-16, BR-17, and BR-44—were screened. As shown in Figure 1, strains BR-16, BR-17, and BR-44 had a stronger nitrogen fixation capacity. BR-1 had higher phosphorus solubilization content. BR-1 and BR-44 had higher potassium solubilization content. The levels of IAA produced by BR-16 and BR-17 were higher. The siderophore synthesis rates of BR-1 and BR-17 were higher.
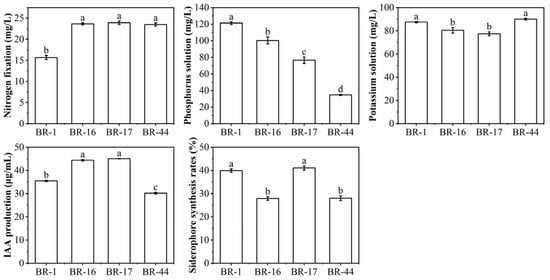
Figure 1.
Growth-promoting abilities of strains. Different lowercase letters indicate a significant difference at p < 0.05 between different treatments.
3.2. Classification and Identification of Functional Strains
As shown in Figure 2, the colony of strain BR-1 was milky white, with folds on the surface and irregular edges. The colonies of strain BR-16 were creamy white, waxy, soft, and slightly shiny. The colony of strain BR-17 was milky white, shiny, opaque, and sticky and did not expand. The colony of strain BR-44 was pink, with rough and opaque surfaces and irregular edges. According to the Gram staining, BR-1, BR-16, BR-17, and BR-44 were all Gram-positive bacteria.
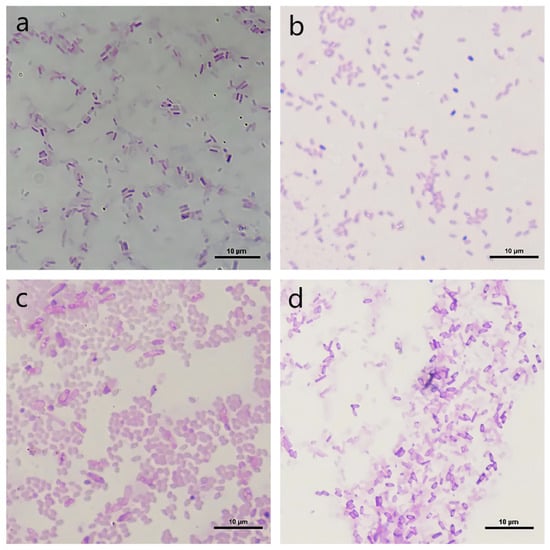
Figure 2.
Gram-staining effects of strains. A Nikon microscope was used to capture the colored pictures of strains. (a): BR-1; (b): BR-16; (c): BR-17; (d): BR-44.
Neighbor joining was applied to construct the phylogenetic tree (Figure 3). The result showed that BR-1 had strong similarity to Bacillus subtilis ZIM3 (MT539995.1). BR-16 was highly homologous to Bacillus cereus AFS071657 (OP986972.1), and BR-17 was highly homologous to Bacillus megaterium YN7 (MK961265.1). BR-44 showed strong similarity to Bacillus subtilis NRCB033 (OR244203.1). Combining this analysis with the morphological observation of the strains, it was determined that BR-1 belonged to Bacillus subtilis, BR-16 belonged to Bacillus cereus, BR-17 belonged to Bacillus megaterium, and BR-44 belonged to Bacillus subtilis.
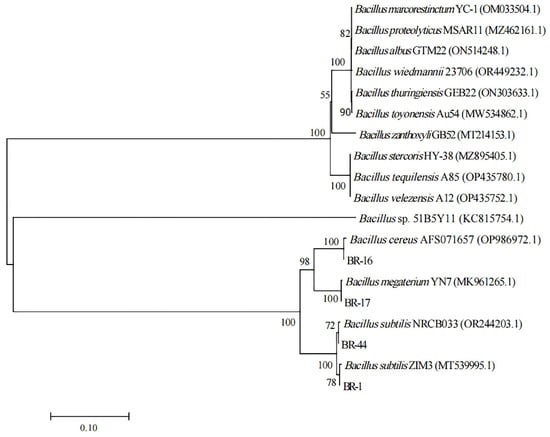
Figure 3.
Phylogenetic tree of functional strains.
3.3. Antagonistic Reactions Between Strains and Their Growth Time Courses
The antagonism between the strains was determined via the cross-line method. The results showed that strains BR-1, BR-16, BR-17, and BR-44 were not antagonistic toward each other; thus, they could be used for the preparation of compound microbial inoculants. The growth time courses of strains BR-1, BR-16, BR-17, and BR-44 are shown in Figure 4. The biomass of strains BR-1, BR-16, and BR-17 increased slowly at the initial stage of fermentation, from 0 to 4 h, while the biomass of strain BR-44 increased rapidly at this stage, with the OD600 reaching 0.442. At 4~12 h, the strains exhibited growth in the exponential phase. With the extension of the fermentation period, the maximum level of biomass was obtained after 20–24 h of incubation. BR-1 and BR-17 obtained their maximum biomass levels at 20 h, amounting to 1.010 and 1.101, respectively. Meanwhile, BR-16 and BR-44 obtained their maximum biomass levels at 24 h, amounting to 1.252 and 0.989, respectively, and then entered a period of stability.
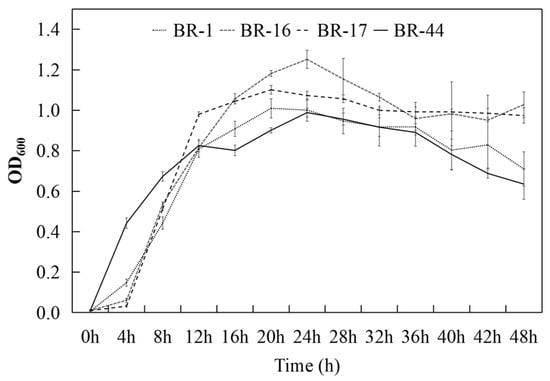
Figure 4.
Time course of growth for isolates BR-1, BR-16, BR-17, and BR-44.
3.4. Single-Factor Optimization of Liquid Fermentation Process
The OD600 of the BR inoculant from the organic nitrogen source was 0.864~1.503, indicating that it was superior to that from the inorganic nitrogen source (less than 0.205). This indicates that the optimum concentrations of peptone that produced the highest OD600 were 15 g/L and 20 g/L, and the highest OD600 values were obtained at 15 g/L (Figure 5a). When the dosage was 5 g/L, the OD600 after fermentation with starch as the carbon source was 1.181; this was significantly higher than the OD600 after fermentation using other carbon sources. When the concentration was 10 g/L, 15 g/L, or 20 g/L, the OD600 after fermentation with sucrose as the carbon source was 1.556, 1.658, and 1.388, respectively; these were significantly higher than the OD600 values seen after fermentation with other carbon sources. Therefore, sucrose was selected as the optimal carbon source. The OD600 of the BR inoculant was higher when the sucrose concentration was 10 g/L or 15 g/L, and the highest OD600 was achieved when the concentration was 15 g/L (Figure 5b). When the concentration was 2 g/L or 8 g/L, the OD600 values of the mineral salts NaCl and KCl after fermentation were significantly higher than those of the other tested mineral salts. When the concentration was 4 g/L or 6 g/L, the OD600 of the BR inoculant with KCl as the mineral salt was significantly higher than those obtained with the other inorganic salts. The OD600 of the BR inoculant reached 1.645~1.670 depending on the KCl concentration, with the maximum achieved at 2 g/L (Figure 5c).
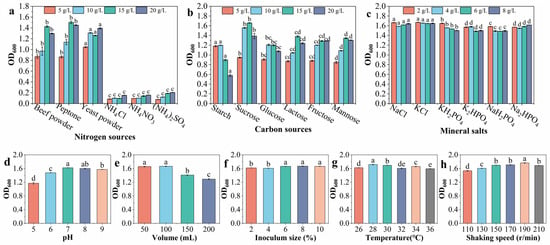
Figure 5.
Effects of different nitrogen sources (a), carbon sources (b), mineral salts (c), pH values (d), volumes (e), inoculum sizes (f), temperatures (g), and shaking speeds (h) on microbial biomass (OD600). Different lowercase letters indicate a significant difference at p < 0.05 between different treatments.
The OD600 of the BR inoculant increased first and then decreased with the increase in the pH. The OD600 was higher at pH 7 and 8, reaching 1.622 and 1.601, respectively. There was no significant difference between them, but they showed a significant difference in the case of the other treatments (Figure 5d). When the volume was 50 mL/250 mL or 100 mL/250 mL, the OD600 of the BR inoculant was higher, reaching 1.658 and 1.666, respectively, with no significant difference between them; however, there was a significant difference compared to the other treatments (Figure 5e). When the inoculum size was 6%, 8%, or 10%, the OD600 of the BR inoculant was higher, showing a significant difference compared with the other treatments. When the inoculum size was 8%, the OD600 reached the maximum value of 1.671; meanwhile, when the inoculum size was 6% or 10%, the OD600 was the same, at 1.664 (Figure 5f). A relatively higher OD600 was obtained at temperatures of 28 °C and 30 °C, reaching 1.717 and 1.694, respectively. The OD600 was the maximum at 28 °C, showing a significant difference compared with the other treatments (Figure 5g). With the increase in the rotation speed of the shaking incubator, the OD600 showed a pattern of first increasing and then decreasing. When the shaking speed was 190 r/min, the OD600 reached the maximum value of 1.765, showing a significant difference compared with the other treatments, followed by 170 r/min (Figure 5h).
3.5. Plackett–Burman Analysis
By analyzing the effects of each component on the response value, the Pareto diagram of the standardization effects of the factors was obtained (Figure 6). This indicated that the standardization effect values of factors E—volume, H—shaking speed, and G—temperature exceeded the tvalue, and they had a significant effect on the OD600 (p < 0.05). The Pvalue of the model was 0.0202 < 0.05, with R2 = 0.9955, indicating that the model was reliable and had a good correlation. Therefore, the three factors E, H, and G were selected for further experiments. Multiple regression fitting was performed on the results, and the following regression equation was obtained:
Y = 1.75 + 0.013A + 0.00542B − 0.00342C + 0.00108D − 0.035E+ 0.00458F + 0.029G + 0.035H + 0.00992J
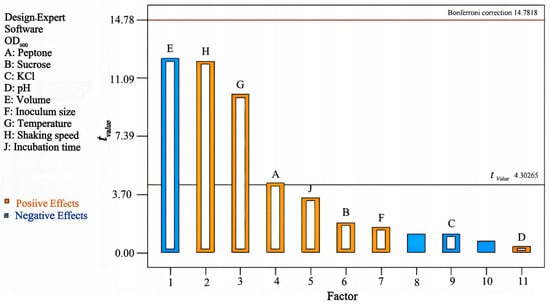
Figure 6.
Standardization effects of Pareto diagram (α= 0.05).
3.6. Steepest Climb Test
Using the regression Equation (1), it was found that significant factor, E, had a negative effect on the OD600, while factors G and H had positive effects. The step length was calculated with the most significant factor, E, as the climbing unit. From an economic perspective, the lowest possible dosage was selected for the non-significant factors, with peptone being set at 15 g/L, sucrose at 10 g/L, KCl at 2 g/L, the initial pH at 7.0, the inoculum size at 6%, and the incubation period at 20 h. The experimental design and results are shown in Table 3. The response values increased first and then decreased, and the response value for treatment 4 was the highest; thus, it was used as the center point of the response surface. Meanwhile, treatments 3, 4, and 5 were selected for the subsequent Box–Behnken response surface experiment.

Table 3.
Test design and results of steepest climb test.
3.7. Box–Behnken Response Surface Methodology
The contour plots and the 3D response surface plot showed that each response surface had a stable point, and the selection ranges of the influencing factors included the area where the maximum value was located, indicating that the liquid volume, temperature, and shaking speed exhibited significant correlations with the OD600. The shapes of the contour lines were all ellipses, indicating that the interaction effects among the factors were significant. Among them, the interaction effect of the temperature and shaking speed on the OD600 was the most significant (Figure 7).
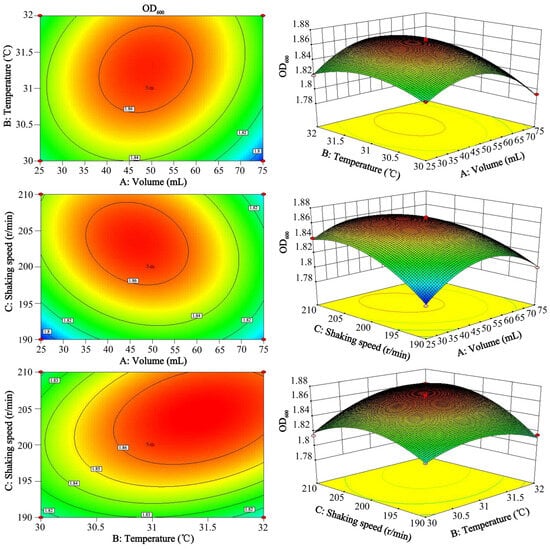
Figure 7.
The 2D contour plot and 3D response surface plot of the temperature and shaking speed with the OD600. The response value on each curve is the same. The color of the graph from blue to red indicates that the response value is from less to more. The faster the color changes, the greater the slope, that is, the more significant the impact on the result.The model was analyzed, and predictions were obtained, and the optimal parameters for each factor were obtained: volume—47.39 mL/250 mL, temperature—31.39 °C, and shaking speed—204.13 r/min. The predicted OD600 was 1.869. In line with the actual operation, the parameters were set to a volume of 47 mL/250 mL, a temperature of 31 °C, and a shaking speed of 205 r/min. The predicted results were verified through experiments. The actual OD600 was 1.866, which was similar to the predicted value of the model, indicating that the optimization results were reliable. The living bacterial count of the inoculant was determined via the plate dilution method, and it reached 8.955 × 109 CFU/mL.
3.8. Effects of Different Substrates on Living Bacterial Counts of BR Inoculants
As shown in Figure 8, the living bacterial count of the solid inoculant prepared by adding various substrates could meet the national standard (2.0 × 108 CFU/g). The influence of each substrate on the living bacterial count of the solid inoculant followed the order of bran > straw biochar > biogas residue > corn flour > soya meal, ranging from large to small, with the highest value of 1.114 × 109 CFU/g achieved with wheat bran; this was significantly different from those of the other treatments. Therefore, bran was chosen as the most suitable substrate for the preparation of the solid inoculant.
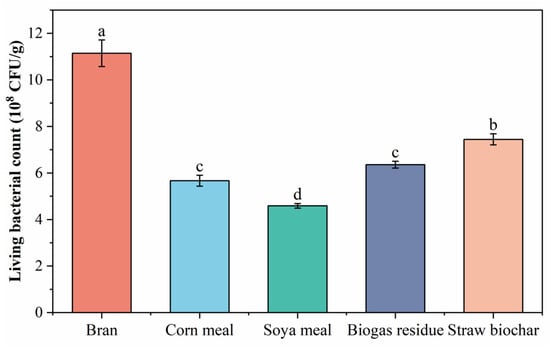
Figure 8.
Effects of different substrates on living bacterial count of solid inoculant. Different lowercase letters indicate a significant difference of 5% between different treatments.
3.9. Effects of Inoculants on Growth Indicators of Watermelon Plants
As shown in Figure 9A, the watermelon height in the seedling stage increased significantly, by 39.28% and 56.94%, in the L and S treatments compared to the CK, respectively. The stem diameter also increased significantly, by 19.62% and 22.14%, in the L and S treatments compared to the CK, respectively. The root length in the L treatment increased significantly, by 33.78%, compared with the CK, and the root fresh weight, root length, and root surface area in the S treatment increased significantly, by 19.33%, 95.95%, and 4.22%, respectively. In terms of the photosynthesis indicators, the Pn, Tr, and Gs in the S treatment increased significantly, by 73.07%, 67.56%, and 102.62%, respectively. The SPAD in the L and S treatments also increased significantly, by 4.69% and 5.09%, compared with the CK, respectively.
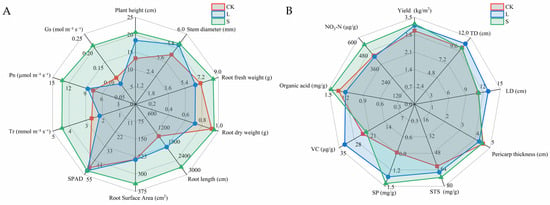
Figure 9.
Effects of BR inoculants on growth, yield, and fruit quality of watermelon. CK: No inoculant, L: BR liquid inoculant, S: BR solid inoculant. A: plant height, stem diameter, root fresh weight, root dry weight, root length, root surface area, relative content of chlorophyll (SPAD), transpiration rate (Tr), net photosynthetic rate (Pn), stomatal conductance (Gs). B: Yield, watermelon transverse diameter (TD), watermelon longitudinal diameter (LD), pericarp thickness, soluble total sugar content (STS), soluble protein content (SP), vitamin C content (VC), organic acid content, Nitrate content.
3.10. Effects of Inoculants on Watermelon Yield and Fruit Quality
As shown in Figure 9B, compared with the CK, the S treatment increased the watermelon yield significantly, by 9.60%. The L treatment increased the fruit diameter (TD) and longitudinal diameter (LD) significantly, by 7.21% and 15.57%, respectively. There was no significant difference in the fruit skin thickness among the different treatments. The soluble protein content (SP) in the L and S treatments increased significantly, by 50.50% and 63.63%, respectively. The vitamin C content (VC) in the L treatment was significantly higher than that in the CK, by 35.08%. The nitrate content in the S treatment was significantly higher than that in the CK, by 22.83%. There was no significant difference in the soluble total sugar content (STS) or organic acid content among the different treatments.
4. Discussion
Bacillus genus has a large number of strains with special functions; due to its variety and strong stress resistance, it is widely used in the production of inoculants. Many studies have shown that the inoculation of Bacillus can improve the microecological environment of crop rhizosphere soil and enable the recruitment of more beneficial microorganisms. Moreover, its metabolic substances can promote the effective transformation of soil mineral elements, thus promoting root growth, improving the crop’s photosynthetic efficiency, increasing the yield, and improving the quality of agricultural products [7,31,32,33]. In this study, four strains with good functional characteristics were screened from biogas residue, an agricultural waste, which had the functions of nitrogen fixation, phosphate and potassium solubilization, and IAA and siderophore production, namely, B. subtilis BR-1 and BR-44, B. cereus BR-16 and B. megaterium BR-17 (Figure 1 and Figure 2). This finding proves that biogas residue contains beneficial microorganisms such as Bacillus spp., which can be effectively developed and recycled into the soil, and the beneficial microbial community can exert its functional mechanism to improve the soil structure and ensure that crops can obtain the nutrients required for growth, providing a suggestion for recycling the biogas residue as an agricultural waste [24]. The combination of different functional strains can give full play to their complementary advantages and realize the superposition of different functions among strains, so as to prepare an efficient composite inoculant [34]. In this study, the enrichment medium and fermentation conditions of Bacillus spp. were determined by single factor test and RSM. Amino acids, nucleic acids, and nitrogen-containing metabolites required for bacterial proliferation were synthesized by using peptone, a protein-rich and nutrient-rich organic nitrogen source [35]. Sucrose is used as the most suitable carbon source to provide carbon skeleton components for microbial synthesis, provide energy for strains, regulate their cell functions, and body metabolism, thus achieving proliferation effects. KCl is used as the optimal mineral salt to provide osmotic balance for cells, thereby maintaining their structural stability and activity [36] (Figure 5). In addition, a liquid inoculant is adsorbed and fixed to the substrate, which can improve the stability of the corresponding product’s quality. Due to the differences in the ability of carriers to transport and release microorganisms, it is necessary to select a suitable substrate when preparing an inoculant. As a by-product of wheat flour processing, wheat bran is rich in carbohydrates, starch, protein, minerals, and fat, and it is widely used as a substrate for the preparation of microbial inoculants [37]. In this study, it was found that the most suitable substrate for the inoculant was bran (Figure 8), this might have been due to the fact that the nutrients in bran were conducive to maintaining the growth and reproduction of the strains. Moreover, the substrate was relatively loose, with suitable porosity and permeability for the growth and metabolism of the studied strains. In this study, two kinds of enrichment media of liquid and solid form were optimized, which could provide a suitable nutrient environment for the rapid propagation of Bacillus spp., and provide a guarantee for the effective colonization of the rhizosphere soil of watermelon. In the future, the industrial application of these two inoculants will be further explored.
The addition of exogenous compound inoculants is conducive to the transformation of nutrient elements in soil and the effective absorption and the utilization of them by plants, thus realizing the mutual benefit and win–win situation among microorganisms, soil, and crops, which is ultimately reflected in the yield and quality of crops. Pot experiment results showed that the application of liquid and solid inoculants had a certain effect on plant growth, and the root growth of watermelon was better after the application of solid inoculants. The application of liquid inoculant effectively increased the SPAD values of watermelon leaves, while the application of BR solid inoculant effectively increased the SPAD, Pn, and Tr values (Figure 9A). BR liquid inoculant significantly increased watermelon soluble protein content and VC, and BR solid inoculant significantly increased watermelon soluble protein content and yield (Figure 9B). The reason why beneficial microorganisms can promote plant growth is mainly manifested in such mechanisms as nitrogen fixation, phosphorus solution, potassium solution, IAA production, and siderophore production. Nitrogen-fixing bacteria can fix nitrogen molecules in the air and eventually release nitrogen into the soil, providing nutrients for plants and increasing nitrogen accumulation in crops [1]. Phosphorus solubilizing microorganisms can produce organic acid-chelated metal cations and convert insoluble phosphorus into available phosphorus that can be absorbed and utilized by plants [38]. The use of phosphorus solubilizing bacteria can not only affect the soil functional microorganisms involved in phosphorus conversion, improve the phosphorus conversion and utilization rate, increase the soil soluble phosphorus content, and achieve crop yield increase, but also reduce the application amount of chemical phosphorus fertilizer and avoid phosphorus pollution [2]. Potassic bacteria can produce tartaric acid, citric acid, and other organic acids chelate with silicon ions to decompose soil mineral potassium and increase soil available potassium, thus promoting plant growth and potassium absorption and increasing yield [3]. Indole-3-acetic acid (IAA) is an important plant growth hormone that can stimulate cell elongation, division, and differentiation. IAA can not only be synthesized from plants, but it also can be metabolized by some microorganisms. The application of IAA-producing bacteria can play an important role in microbe-plant signal transduction, improve fertilizer utilization rate, and thus promote crop root growth. Increasing crop biomass [4]. The normal growth of plants requires iron, and iron solubility in the environment is very low and difficult for organisms to be absorbed. Some beneficial microorganisms can secrete siderophore, which can form soluble complexes by chelating Fe3+ and releasing them into the soil environment, promoting the absorption of iron ions by plants [5,39]. Our results demonstrated that after the multifunctional Bacillus spp. composite inoculants were applied to the watermelon rhizosphere; the functional bacteria colonization gave full play to its functional properties of nitrogen fixation, phosphorus solubilization, potassium solubilization, IAA production, and siderophore production. By secreting active substances, soil nutrients were regulated, the rhizosphere growth environment of watermelon was improved, the effective nutrient absorption capacity of watermelon root was improved, and photosynthetic efficiency was increased. Thus, it provides more material basis and energy source for the growth and development of plants, and makes important contributions to the improvement of crop yield and quality [7,31,40]. It may be concluded that the inoculants have shown effectiveness in terms of microbial activity and watermelon cultivation application. The development potential of Bacillus spp. composite inoculants as a plant growth promotion agent to enhance the growth and development of other crops will be further studied.
5. Conclusions
Four bacterial isolates with the capacity for nitrogen fixation, phosphate and potassium solubilization, and IAA and siderophore production were screened from the biogas residue, namely, Bacillus subtilis BR-1, Bacillus cereus BR-16, Bacillus megaterium BR-17, and Bacillus subtilis BR-44, respectively. The strains were combined, and the optimal fermentation parameters were obtained, including peptone (15 g/L), sucrose (10 g/L), and KCl (2 g/L), an initial pH of 7.0, a volume of 47 mL/250 mL, an inoculum size of 6% v/v, an incubation temperature of 31 °C, a shaking speed of 205 r/min, and an incubation period of 20 h. In the optimized process, a liquid bacterial inoculant with an OD600 of 1.866 was obtained, with an effective viable count of 8.955 × 109 CFU/mL. A solid bacterial inoculant was prepared by using bran as a substrate, and its effective viable count was 1.11 × 109 CFU/g. The application of these inoculants promoted the growth of watermelon, increased its photosynthetic capacity, and improved its yield and quality. Our study provided an idea of the potential of mining high-quality bacterial resources from agricultural waste, such as biogas residue, and the positive impact of the multifunctional Bacillus spp. composite inoculants composed of these strains on plant growth and development, which is good application value for the efficient cultivation of watermelon, and conducive to promoting sustainable agriculture. This study provided a complete preparation and application system of complex Bacillus spp. composite inoculants. In the future, it is expected to explain the plant-growth-promoting mechanism of the strains at the level of functional genes or protein metabolic pathways.
Author Contributions
Conceptualization, L.L.; methodology, L.L. and B.W.; software, J.Z. (Jing Zhou) and Y.W.; validation, J.Z. (Jitao Zhang), Y.W., and X.W.; formal analysis, J.Z. (Jitao Zhang); investigation, L.L., K.L., F.L., and W.X.; resources, B.W., X.W. and X.S.; data curation, L.L. and K.L.; writing—original draft preparation, L.L.; writing—review and editing, L.L.; visualization, J.Z. (Jing Zhou), F.L. and W.X.; supervision, X.W. and X.S.; project administration, X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number 2021YFD1901105; the Science and Technology Major Project of Shanxi Province, China, grant number 202101140601026-7; and the Key R&D Program of Shanxi Province, China, grant number 202102140601012; the Fundamental Research Program of Shanxi Province, China, 202203021221165.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dal Cortivo, C.; Barion, G.; Visioli, G.; Mattarozzi, M.; Mosca, G.; Vamerali, T. Increased root growth and nitrogen accumulation in common wheat following PGPR inoculation: Assessment of plant-microbe interactions by ESEM. Agric. Ecosyst. Environ. 2017, 247, 396–408. [Google Scholar] [CrossRef]
- Arias, R.M.; Heredia Abarca, G.; Carmen Perea Rojas, Y.; Cruz Elizondo, Y.; García Guzman, K.Y. Selection and characterization of phosphate-solubilizing fungi and their effects on coffee plantations. Plants 2023, 12, 3395. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Awad, M.Y.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2021, 44, 411–420. [Google Scholar] [CrossRef]
- Park, S.; Kim, A.L.; Hong, Y.K.; Shin, J.H.; Joo, S.H. A highly efficient auxin-producing bacterial strain and its effect on plant growth. J. Genet. Eng. Biotechnol. 2021, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Nazir, A.; Asghar, H.N.; Zahir, Z.A. Interactive effect of siderophore-producing bacteria and l-tryptophan on physiology, tuber characteristics, yield, and iron concentration of potato. Potato Res. 2022, 65, 1015–1027. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Li, M.; Zhang, K.; Ma, W.; Zheng, L.; Xu, H.; Cui, B.; Liu, R.; Yang, Y.; et al. Functional assembly of root-associated microbial consortia improves nutrient efficiency and yield in soybean. J. Integr. Plant Biol. 2021, 63, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Mehta, P.; Chauhan, A.; Shirkot, C.K. Effect of Bacillus subtilis strain CKT1 as inoculum on growth of tomato seedlings under net house conditions. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 145–155. [Google Scholar] [CrossRef]
- Muthuraja, R.; Muthukumar, T. Isolation and characterization of potassium solubilizing Aspergillus species isolated from saxum habitats and their effect on maize growth in different soil types. Geomicrobiol. J. 2021, 38, 672–685. [Google Scholar] [CrossRef]
- Posada-Uribe, L.F.; Romero-Tabarez, M.; Villegas-Escobar, V. Effect of medium components and culture conditions in Bacillus subtilis EA-CB0575 spore production. Bioprocess Biosyst. Eng. 2015, 38, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Klausmann, P.; Hennemann, K.; Hoffmann, M.; Treinen, C.; Aschern, M.; Lilge, L.; Heravi, K.M.; Henkel, M.; Hausmann, R. Bacillus subtilis high cell density fermentation using a sporulation-deficient strain for the production of surfactin. Appl. Microbiol. Biotechnol. 2021, 105, 4141–4151. [Google Scholar] [CrossRef]
- Chettri, D.; Verma, A.K. Statistical optimization of cellulase production from Bacillus sp. YE16 isolated from yak dung of the Sikkim Himalayas for its application in bioethanol production using pretreated sugarcane bagasse. Microbiol. Res. 2024, 281, 127623. [Google Scholar] [CrossRef]
- Huang, J.; Zhuo, Y.; Lu, J.; Lai, Q.; Zhang, Y. Bacillus cereus liquid fertilizer was produced from Agaricus bisporus industrial wastewater. J. Biotechnol. 2021, 327, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lebrazi, S.; Fadil, M.; Chraibi, M.; Fikri-Benbrahim, K. Screening and optimization of indole-3-acetic acid production by Rhizobium sp. strain using response surface methodology. J. Genet. Eng. Biotechnol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Chen, P.; Yan, L.; Wu, Z.; Li, S.; Bai, Z.; Yan, X.; Wang, N.; Liang, N.; Li, H. A microbial transformation using Bacillus subtilis B7-S to produce natural vanillin from ferulic acid. Sci. Rep. 2016, 6, 20400. [Google Scholar] [CrossRef] [PubMed]
- Slivinski, C.T.; Mallmann, E.; Araújo, J.M.; Mitchell, D.A.; Krieger, N. Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process Biochem. 2012, 47, 1848–1855. [Google Scholar] [CrossRef]
- Berikashvili, V.; Sokhadze, K.; Kachlishvili, E.; Elisashvili, V.; Chikindas, M.L. Bacillus amyloliquefaciens spore production under solid-state fermentation of lignocellulosic residues. Probiotics Antimicrob. Proteins 2018, 10, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Campagnol, R.; Mello, S.D.C.; Barbosa, J.C. Vertical growth of mini watermelon according to the training height and plant density. Hortic. Bras. 2012, 30, 726–732. [Google Scholar] [CrossRef]
- Abbou, M.; Chabbi, M.; Benicha, M. Assessment of pesticide use by determination of environmental indicators: Case study of watermelon from Loukkos (Northwest Morocco). Euro-Mediterr. J. Environ. Integr. 2023, 8, 463–480. [Google Scholar] [CrossRef]
- Wang, Z.; Piao, Y.; Zhang, F.; Hu, Y.; Zeng, J.; Nan, J. Promoting effects on watermelon and fermentation optimization of Plantibacter sp. WZW03. J. Plant Growth Regul. 2020, 39, 970–980. [Google Scholar] [CrossRef]
- Ferchichi, N.; Toukabri, W.; Boularess, M.; Smaoui, A.; Mhamdi, R.; Trabelsi, D. Isolation, identification and plant growth promotion ability of endophytic bacteria associated with lupine root nodule grown in Tunisian soil. Arch. Microbiol. 2019, 201, 1333–1349. [Google Scholar] [CrossRef]
- Goswami, D.; Pithwa, S.; Dhandhukia, P.; Thakker, J.N. Delineating Kocuria turfanensis 2M4 as a credible PGPR: A novel IAA-producing bacteria isolated from saline desert. J. Plant Interact. 2014, 9, 566–576. [Google Scholar] [CrossRef]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic screening and expression analysis of psychrophilic Bacillus spp. reveal their potential to alleviate cold stress and modulate phytohormones in wheat. Microorganisms 2019, 7, 337. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, J.; Cui, Q.; Zhang, W.; Zhu, X. A feasible biochar derived from biogas residue and its application in the efficient adsorption of tetracycline from an aqueous solution. Environ. Res. 2022, 207, 112175. [Google Scholar] [CrossRef] [PubMed]
- Arthurson, V. Closing the global energy and nutrient cycles through application of biogas residue to agricultural land–potential benefits and drawbacks. Energies 2009, 2, 226–242. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.; Alamgir, M.; Kibria, M.G. Growth response of Indian spinach to biogas plant residues. IOSR J. Pharm. Biol. Sci. 2014, 9, 1–6. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers–Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Li, Z.; Luo, Y.; Teng, Y. Type and preparation of medium. In Microbiological Research Methodology for Soil and Environment; Science Press: Beijing, China, 2008; pp. 52–63. [Google Scholar]
- Suliasih; Widawati, S. Isolation of Indole Acetic Acid (IAA) producing Bacillus siamensis from peat and optimization of the culture conditions for maximum IAA production. IOP Conf. Ser. Earth Environ. Sci. 2020, 572, 012025. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef]
- GB 20287-2006; Microbial Inoculants in Agriculture. Standards Press of China: Beijing, China, 2006.
- Zhao, Y.; Mao, X.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. The application of Bacillus Megaterium alters soil microbial community composition, bioavailability of soil phosphorus and potassium, and cucumber growth in the plastic shed system of North China. Agric. Ecosyst. Environ. 2021, 307, 107236. [Google Scholar] [CrossRef]
- Samaniego-Gámez, B.Y.; Garruña, R.; Tun-Suárez, J.M.; Kantun-Can, J.; Reyes-Ramírez, A.; Cervantes-Díaz, L. Bacillus spp. inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, J.; Li, M.; Fang, F.; Hu, J.; Sun, Z.; Zhang, A.; Gao, X.; Li, J. Synergistic effect of Bacillus subtilis and Paecilomyces lilacinus in alleviating soil degradation and improving watermelon yield. Front. Microbiol. 2023, 13, 1101975. [Google Scholar] [CrossRef] [PubMed]
- Denaya, S.; Yulianti, R.; Pambudi, A.; Effendi, Y. Novel microbial consortium formulation as plant growth promoting bacteria (PGPB) agent. IOP Conf. Ser. Earth Environ. Sci. 2021, 637, e012030. [Google Scholar] [CrossRef]
- Vieira, G.H.; Vieira, R.H.; Macrae, A.; Sousa, O.V. Peptone preparation from fishing by-products. J. Sci. Food Agric. 2005, 85, 1235–1237. [Google Scholar] [CrossRef]
- Oren, A. Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front. Microbiol. 2013, 4, 315. [Google Scholar] [CrossRef] [PubMed]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A sight to wheat bran: High value-added products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef]
- Ram, H.; Malik, S.S.; Dhaliwal, S.S.; Kumar, B.; Singh, Y. Growth and productivity of wheat affected by phosphorus-solubilizing fungi and phosphorus levels. Plant Soil Environ. 2015, 61, 122–126. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Shang, X.; Xue, L.; Ji, G.; Chang, S.; Niu, J.; Emaneghemi, B. Screening of siderophore-producing bacteria and their effects on promoting the growth of plants. Curr. Microbiol. 2022, 79, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sang, T.; Tian, M.; Jahan, M.S.; Wang, J.; Li, X.; Guo, S.; Liu, H.; Wang, Y.; Shu, S. Effects of Bacillus cereus on photosynthesis and antioxidant metabolism of cucumber seedlings under salt stress. Horticulturae 2022, 8, 463. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).