Fermented Deproteinized Alfalfa Juice Modified with Fly Ash Filtrate as an Alternative Nutrient Source for Winter Wheat (Triticum aestivum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Treatments
2.2. Preparation and Amount of Solutions for Treatments
2.3. Samplings, Measurements, and Data Evaluation
2.3.1. Measurements at the First Sampling
2.3.2. Measurements at the Second Sampling
2.3.3. Data Evaluation
3. Results
3.1. Results of First Sampling
3.2. Results of Second Sampling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishfaq, M.; Wang, Y.; Xu, J.; Hassan, M.U.; Yuan, H.; Liu, L.; He, B.; Ejaz, I.; White, P.J.; Cakmak, I.; et al. Improvement of Nutritional Quality of Food Crops with Fertilizer: A Global Meta-Analysis. Agron. Sustain. Dev. 2023, 43, 74. [Google Scholar] [CrossRef]
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Walan, P.; Davidsson, S.; Johansson, S.; Höök, M. Phosphate Rock Production and Depletion: Regional Disaggregated Modeling and Global Implications. Resour. Conserv. Recycl. 2014, 93, 178–187. [Google Scholar] [CrossRef]
- Kulhánek, M.; Asrade, D.A.; Suran, P.; Sedlář, O.; Černý, J.; Balík, J. Plant Nutrition—New Methods Based on the Lessons of History: A Review. Plants 2023, 12, 4150. [Google Scholar] [CrossRef]
- Withers, P.; Neal, C.; Jarvie, H.; Doody, D. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Van Raamsdonk, L.W.D.; Meijer, N.; Gerrits, E.W.J.; Appel, M.J. New Approaches for Safe Use of Food By-Products and Biowaste in the Feed Production Chain. J. Clean. Prod. 2023, 388, 135954. [Google Scholar] [CrossRef]
- Santamaría-Fernández, M.; Molinuevo-Salces, B.; Kiel, P.; Steenfeldt, S.; Uellendahl, H.; Lübeck, M. Lactic Acid Fermentation for Refining Proteins from Green Crops and Obtaining a High Quality Feed Product for Monogastric Animals. J. Clean. Prod. 2017, 162, 875–881. [Google Scholar] [CrossRef]
- Pirie, N.W. Leaf Protein: A Beneficiary of Tribulation. Nature 1975, 253, 239–241. [Google Scholar] [CrossRef]
- Pirie, N.W. Leaf Protein and Other Aspects of Fodder Fractionation. In Leaf Protein and Other Aspects of Fodder Fractionation; Cambridge University Press (CUP): Cambridge, UK, 1978. [Google Scholar]
- El-Ramady, H.; Abdalla, N.; Kovacs, S.; Domokos-Szabolcsy, E.; Bákonyi, N.; Fari, M.; Geilfus, C.-M. Sustainable Biorefinery and Production of Alfalfa (Medicago sativa L.). Egypt. J. Bot. 2020, 60, 621–639. [Google Scholar] [CrossRef]
- Rathor, D.B.M. Studies and analysis of growth of aspergillus niger fungi on deproteinized leaf juice (DPJ). Life Sci. Inform. Publ. 2016, 2, 192–197. [Google Scholar]
- Bákonyi, N.; Kisvarga, S.; Barna, D.; O. Tóth, I.; El-Ramady, H.; Abdalla, N.; Kovács, S.; Rozbach, M.; Fehér, C.; Elhawat, N.; et al. Chemical Traits of Fermented Alfalfa Brown Juice: Its Implications on Physiological, Biochemical, Anatomical, and Growth Parameters of Celosia. Agronomy 2020, 10, 247. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Bech, D.; Kiel, P. Manufacturing of Stabilised Brown Juice for L-Lysine Production—From University Lab Scale over Pilot Scale to Industrial Production. Chem. Biochem. Eng. Q. 2004, 18, 37–46. [Google Scholar]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From Yogurt to Yield: Potential Applications of Lactic Acid Bacteria in Plant Production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Bákonyi, N.; Barna, D.; Fári, M.G.; Veres, S.; Alshaal, T.; Domokos-Szabolcsy, E.; Makleit, P. Role of Alfalfa Brown Juice in Plant Growth and Development. In Biostimulants in Plant Protection and Performance; Elsevier: Amsterdam, The Netherlands; pp. 149–163.
- Ferrarezi, R.S.; Lin, X.; Gonzalez Neira, A.C.; Tabay Zambon, F.; Hu, H.; Wang, X.; Huang, J.-H.; Fan, G. Substrate pH Influences the Nutrient Absorption and Rhizosphere Microbiome of Huanglongbing-Affected Grapefruit Plants. Front. Plant Sci. 2022, 13, 856937. [Google Scholar] [CrossRef]
- Johansen, J.L.; Nielsen, M.L.; Vestergård, M.; Mortensen, L.H.; Cruz-Paredes, C.; Rønn, R.; Kjøller, R.; Hovmand, M.; Christensen, S.; Ekelund, F. The Complexity of Wood Ash Fertilization Disentangled: Effects on Soil pH, Nutrient Status, Plant Growth and Cadmium Accumulation. Environ. Exp. Bot. 2021, 185, 104424. [Google Scholar] [CrossRef]
- Treeby, M.; Marschner, H.; Römheld, V. Mobilization of Iron and Other Micronutrient Cations from a Calcareous Soil by Plant-Borne, Microbial, and Synthetic Metal Chelators. Plant Soil 1989, 114, 217–226. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll Determination in Intact Tissues Using N,N -Dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A Standardized Protocol for the Determination of Specific Leaf Area and Leaf Dry Matter Content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Thamm, F.; Krámer, M.; Sarkadi, J. Növények És Trágyanyagok Foszfor-Tartalmaénak Meghatározása Ammónium-Molibdo-Vanadátos Módszerrel. Agrokémia Talajt. 1968, 17, 145–156. [Google Scholar]
- Jadhav, R.K. Effect Of Deproteinised Juice From Eicchornia crassipes L. On Mitotic Cell Division Allium cepa L. and Allium sativum L. Bioinfolet 2009, 6, 245–248. [Google Scholar]
- Jadhav, R.K. Effect of Deproteinised Juice (DPJ) on Root Growth And Nodulation of Leguminous. Plants. J. Bionano Front. 2017, 10, 84–87. [Google Scholar]
- Jadhav, R.K.; Kadam, R.; Joshi, D. Physiology of Gramineae Crops by Deproteinised Foliage Extract and Its Influence on Photosynthetic Chemistry. J. Phytochem. 2019, 110, 149–163. [Google Scholar]
- Ream, H.W.; Smith, D.; Walgenbach, R.P. Effects of Deproteinized Alfalfa Juice Applied to Alfalfa—Bromegrass, Bromegrass, and Corn1. Agron. J. 1977, 69, 685–689. [Google Scholar] [CrossRef]
- Welch, D.A.; Smith, D.; Soberalske, R.M.; Ream, H.W. Growth and Composition of Alfalfa Fertilized in Greenhouse Trials with Deproteinized Juice from Low and High Saponin Alfalfa and from Oat Herbage. J. Plant Nutr. 1979, 1, 151–170. [Google Scholar] [CrossRef]
- Sreenivasan, E.; Pathak, S.S.; Joshi, R.N. Deproteinized Leaf Juica- a Novel Medium for Rhizogenesis in Vitro. Curr. Sci. 1995, 68, 586. [Google Scholar]
- Patil, R. Effect of Potassium Humate and Deproteinised Juice (DPJ) on Seed Germination and Seedling Growth of Wheat and Jowar. Ann. Biol. Res. 2011, 2, 26–29. [Google Scholar]
- Manwatkar, V.G.; Gogle, D.P. The Effect of Deproteinised Juice (DPJ) on Seed Germination and Seedling Growth of Different Plants (by Paper Towel Method). Int. J. Life Sci. 2014, 2, 65–68. [Google Scholar]
- Kisvarga, S.; Barna, D.; Kovács, S.; Csatári, G.; O. Tóth, I.; Fári, M.G.; Makleit, P.; Veres, S.; Alshaal, T.; Bákonyi, N. Fermented Alfalfa Brown Juice Significantly Stimulates the Growth and Development of Sweet Basil (Ocimum basilicum L.) Plants. Agronomy 2020, 10, 657. [Google Scholar] [CrossRef]
- Pushnik, J.C.; Miller, G.W.; Manwaring, J.H. The Role of Iron in Higher Plant Chlorophyll Biosynthesis, Maintenance and Chloroplast Biogenesis. J. Plant Nutr. 1984, 7, 733–758. [Google Scholar] [CrossRef]

| Characteristics | Quantity |
|---|---|
| pH (KCl) | 5.35 |
| KA | <25 |
| Density (kg m−3) | 1400 |
| Water-soluble total salt (m m−1)% | <0.02 |
| Lime (m m−1)% | <0.100 |
| Humus content (m m−1)% | 0.99 |
| Phosphorus pentoxide (mol m−3) (AL-soluble) | 2.21 × 10−7 |
| Potassium oxide (mol m−3) (AL-soluble) | 1.07 × 10−6 |
| Nitrate (mol m−3) (KCl-soluble) | 2.78 × 10−8 |
| Sodium (mol m−3) (AL-soluble) | 8.43 × 10−7 |
| Magnesium (mol m−3) (KCl-soluble) | 7.50 × 10−7 |
| Sulphur (mol m−3) (KCl- soluble) | 4.64 × 10−8 |
| Manganese (mol m−3) (EDTA-soluble) | 3.08 × 10−7 |
| Zinc (mol m−3) (EDTA-soluble) | 1.94 × 10−8 |
| Copper (mol m−3) (EDTA-soluble) | 3.41 × 10−8 |
| Treatments | Marking |
|---|---|
| Irrigation with deionized water | DW |
| Irrigation with nutrient solution | NS |
| Irrigation with a nutrient solution in half concentration | half NS |
| Irrigation with 5% fermented DAJ completed with fly ash solution | 5W |
| Irrigation with 10% fermented DAJ completed with fly ash solution | 10W |
| Spraying with 5% fermented DAJ completed with fly ash solution and irrigation with a nutrient solution in half concentration | half NS+5S |
| Spraying with 10% fermented DAJ completed with fly ash solution and irrigation with a nutrient solution in half concentration | half NS+10S |
| Spraying with 2.5% fermented DAJ solution and irrigation with deionized water | DW+2.5S |
| Autumn treatments |
| Irrigation with deionized water every two days in September |
| Treatments according to Table 2; once a week, in October |
| First sampling at the end of October |
| Spring treatments |
| Treatments according to Table 2; once a week, in March |
| Treatments according to Table 2; twice a week, from April to June |
| Second sampling at the end of June |
| Element | NS | half NS | 5W | 10W | half NS+5S | half NS+10S | DW+2.5S |
|---|---|---|---|---|---|---|---|
| N | 1008.000 | 504.000 | 2581.992 | 4172.731 | 762.199 | 921.274 | 164.445 |
| P | 222.984 | 111.492 | 29.772 | 48.110 | 114.473 | 116.303 | 1.993 |
| K | 1055.653 | 527.827 | 1779.001 | 2907.335 | 705.727 | 818.560 | 45.975 |
| Ca | 1442.808 | 721.404 | 806.562 | 1322.946 | 802.060 | 853.699 | 10.017 |
| Mg | 218.628 | 109.314 | 42.743 | 69.077 | 113.854 | 116.222 | 2.772 |
| S | 693.410 | 346.705 | 445.320 | 731.596 | 391.237 | 419.865 | 3.287 |
| Cu | 0.457 | 0.229 | 0.047 | 0.043 | 0.234 | 0.233 | 0.002 |
| Fe | 2.009 | 1.004 | 0.277 | 0.450 | 1.030 | 1.049 | 0.018 |
| Mn | 1.105 | 0.553 | 0.167 | 0.270 | 0.570 | 0.580 | 0.001 |
| Zn | 1.178 | 0.589 | 0.281 | 0.454 | 0.617 | 0.634 | 0.002 |
| Mo | 0.032 | 0.016 | 0.097 | 0.155 | 0.025 | 0.031 | 0.002 |
| B | 0.039 | 0.019 | 1.094 | 1.789 | 0.128 | 0.198 | 0.003 |
| Na | - | - | 36.180 | 59.472 | 3.618 | 5.947 | 0.186 |
| Cl | 63.817 | 31.909 | - | - | 31.909 | 31.909 | - |
| Al | - | - | 0.068 | 0.112 | 0.007 | 0.011 | 0.005 |
| Ba | - | - | 0.454 | 0.745 | 0.045 | 0.075 | 0004 |
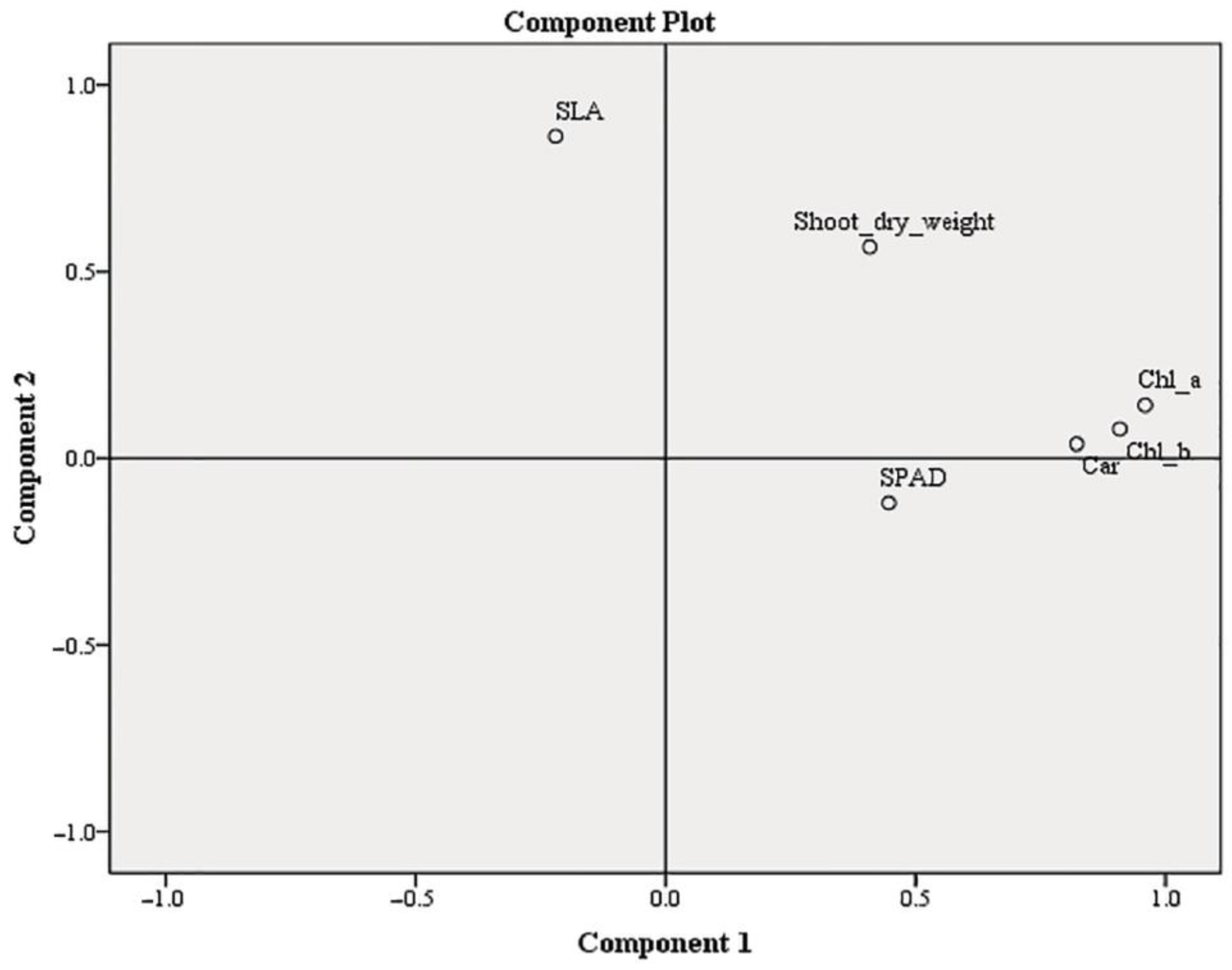
| Treatments | Shoot’s Dry Weight mg Shoot−1 | Specific Leaf Area (SLA) mm2 mg−1 | Relative Chlorophyll Content (SPAD Values) |
|---|---|---|---|
| DW | 260.09 ± 14.92 ab | 5.24 ± 0.20 ab | 30.04 ± 0.84 a |
| NS | 273.46 ± 14.56 ab | 4.80 ± 0.26 a | 35.75 ± 1.49 d |
| half NS | 294.93 ± 27.40 b | 5.17 ± 0.14 ab | 33.84 ± 0.62 bcd |
| DW+2.5S | 233.93 ± 11.70 a | 4.84 ± 0.26 a | 29.55 ± 1.79 a |
| half NS+5S | 272.60 ± 14.22 ab | 5.24 ± 0.20 ab | 32.55 ± 0.97 abcd |
| half NS+10S | 267.54 ± 21.76 ab | 4.64 ± 0.23 a | 35.14 ± 0.59 cd |
| 5W | 243.45 ± 13.67 ab | 5.63 ± 0.29 b | 31.83 ± 0.83 abc |
| 10W | 237.67 ± 22.28 ab | 4.76 ± 0.26 a | 30.61 ± 0.88 ab |
| Treatments | Chlorophyll-a mg g−1 | Chlorophyll-b mg g−1 | Carotenoids mg g−1 |
|---|---|---|---|
| DW | 20.72 ± 0.37 abc | 7.19 ± 0.17 a | 1.71 ± 0.03 bc |
| NS | 22.77 ± 0.49 d | 8.09 ± 0.25 bc | 1.67 ± 0.05 b |
| half NS | 23.04 ± 0.47 d | 8.41 ± 0.28 c | 1.82 ± 0.02 c |
| DW+2.5S | 20.72 ± 0.39 abc | 6.97 ± 0.18 a | 1.78 ± 0.02 bc |
| half NS+5S | 21.48 ± 0.31 bcd | 7.39 ± 0.20 ab | 1.76 ± 0.04 bc |
| half NS+10S | 21.60 ± 0.67 cd | 7.49 ± 0.37 ab | 1.73 ± 0.06 bc |
| 5W | 19.44 ± 0.38 a | 6.67 ± 0.27 a | 1.46 ± 0.02 a |
| 10W | 19.78 ± 0.81 a | 6.79 ± 0.36 a | 1.51 ± 0.04 a |
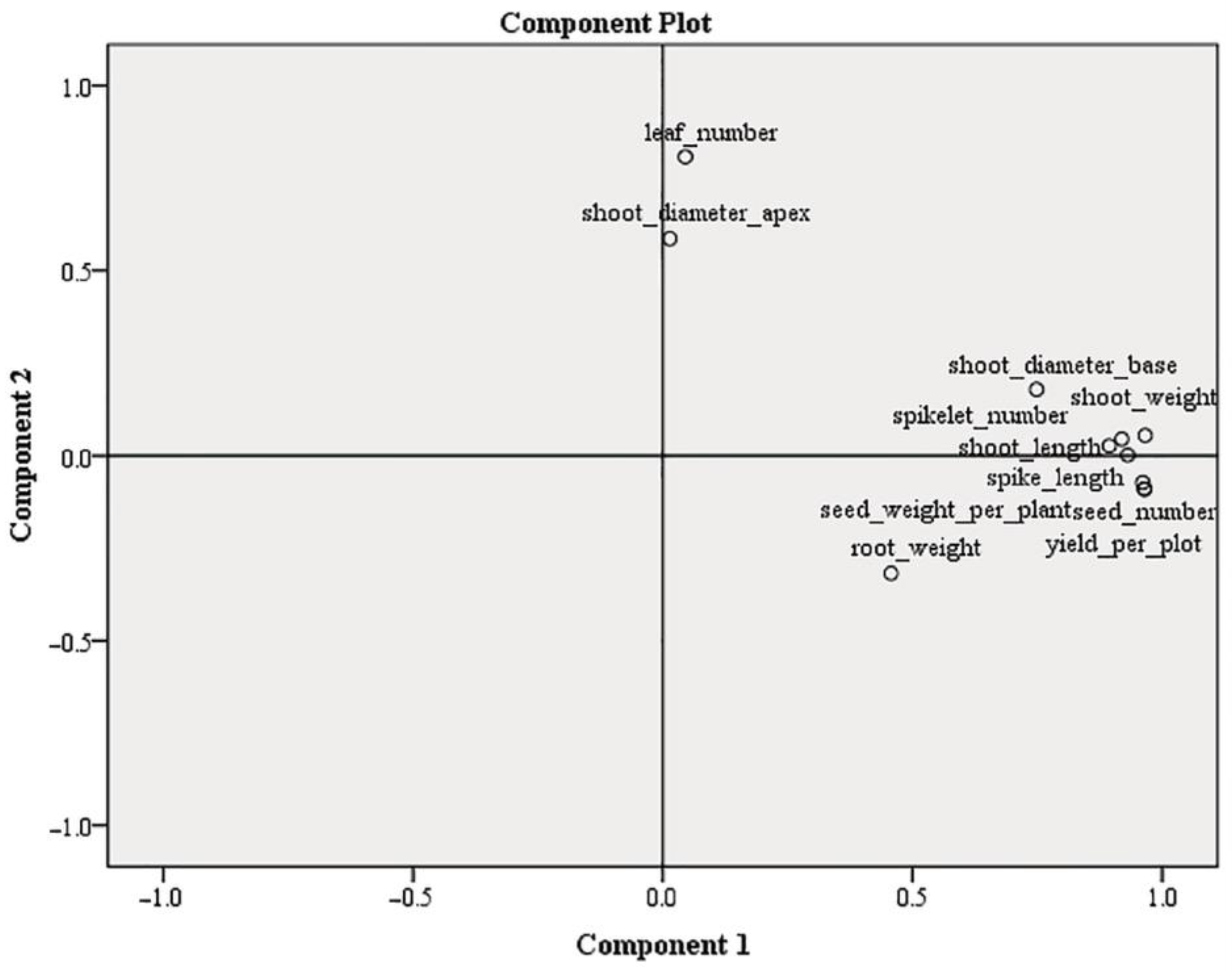
| Treatments | Shoot Length (mm) | Spike Length (mm) |
|---|---|---|
| DW | 442.50 ± 18.09 a | 38.13 ± 9.81 ab |
| NS | 592.81 ± 7.16 d | 58.44 ± 5.70 d |
| half NS | 595.31 ± 12.00 d | 59.06 ± 10.20 d |
| DW+2.5S | 428.44 ± 15.57 a | 35.94 ± 8.00 a |
| half NS+5S | 543.75 ± 8.90 c | 49.06 ± 7.58 c |
| half NS+10S | 529.38 ± 9.00 bc | 42.50 ± 5.77 b |
| 5W | 500.31 ± 14.29 b | 41.25 ± 8.66 ab |
| 10W | 543.75 ± 5.45 c | 49.06 ± 5.54 c |
| Treatments | Leaf Number (Piece) | Shoot Diameter at the Base (mm) | Shoot Diameter at the Top (mm) |
|---|---|---|---|
| DW | 5.00 ± 0.24 ab | 0.18 ± 0.01 a | 0.09 ± 0.01 a |
| NS | 5.13 ± 0.24 ab | 0.22 ± 0.01 c | 0.15 ± 0.00 c |
| half NS | 5.44 ± 0.22 ab | 0.22 ± 0.01 c | 0.14 ± 0.01 c |
| DW+2.5S | 5.88 ± 0.18 b | 0.19 ± 0.01 ab | 0.10 ± 0.00 a |
| half NS+5S | 5.06 ± 0.14 ab | 0.22 ± 0.00 c | 0.12 ± 0.00 ab |
| half NS+10S | 4.75 ± 0.17 a | 0.22 ± 0.00 c | 0.12 ± 0.00 ab |
| 5W | 4.88 ± 0.13 ab | 0.20 ± 0.00 bc | 0.12 ± 0.00 ab |
| 10W | 4.81 ± 0.16 a | 0.22 ± 0.01 c | 0.13 ± 0.00 ab |
| Treatments | Shoot Dry Weight (g Plant−1) | Root Dry Weight (g Plant−1) |
|---|---|---|
| DW | 0.36 ± 0.03 a | 0.11 ± 0.01 a |
| NS | 0.68 ± 0.02 d | 0.27 ± 0.02 d |
| half NS | 0.73 ± 0.05 d | 0.18 ± 0.01 c |
| DW+2.5S | 0.33 ± 0.02 a | 0.13 ± 0.01 ab |
| half NS+5S | 0.54 ± 0.02 c | 0.14 ± 0.01 b |
| half NS+10S | 0.49 ± 0.03 bc | 0.13 ± 0.00 ab |
| 5W | 0.45 ± 0.03 b | 0.12 ± 0.01 ab |
| 10W | 0.53 ± 0.02 bc | 0.12 ± 0.00 ab |
| Treatments | Spikelet Number per spike | Grain Number per Spike | Weight of Grains per Spike (g Spike−1) | Yield per Pot (g) |
|---|---|---|---|---|
| DW | 3.06 ± 0.29 a | 9.50 ± 1.44 a | 0.25 ± 0.05 a | 2.97 ± 0.54 a |
| NS | 5.56 ± 0.13 de | 22.00 ± 0.71 de | 0.88 ± 0.04 d | 10.58 ± 0.43 d |
| half NS | 5.81 ± 0.23 e | 23.88 ± 1.47 e | 0.90 ± 0.07 d | 10.82 ± 0.88 d |
| DW+2.5S | 3.31 ± 0.24 a | 7.81 ± 1.01 a | 0.23 ± 0.03 a | 2.76 ± 0.34 a |
| half NS+5S | 5.00 ± 0.24 cd | 18.63 ± 0.90 bc | 0.72 ± 0.05 c | 8.59 ± 0.54 c |
| half NS+10S | 4.56 ± 0.20 bc | 15.88 ± 0.74 b | 0.58 ± 0.03 b | 6.99 ± 0.40 b |
| 5W | 4.13 ± 0.24 b | 15.63 ± 0.93 b | 0.56 ± 0.04 b | 6.76 ± 0.45 b |
| 10W | 5.13 ± 0.18 cd | 19.50 ± 0.63 cd | 0.64 ± 0.03 bc | 7.74 ± 0.35 bc |
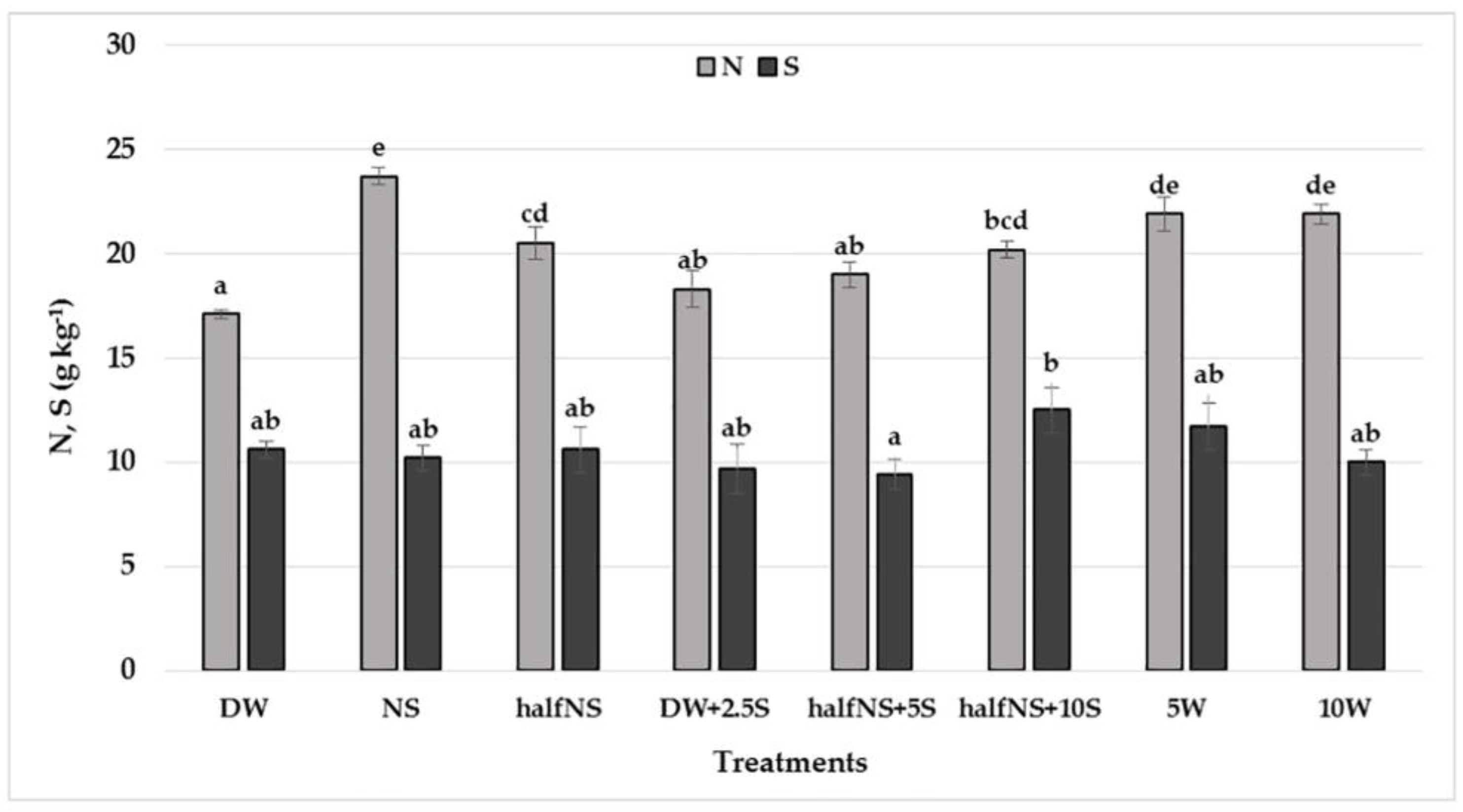
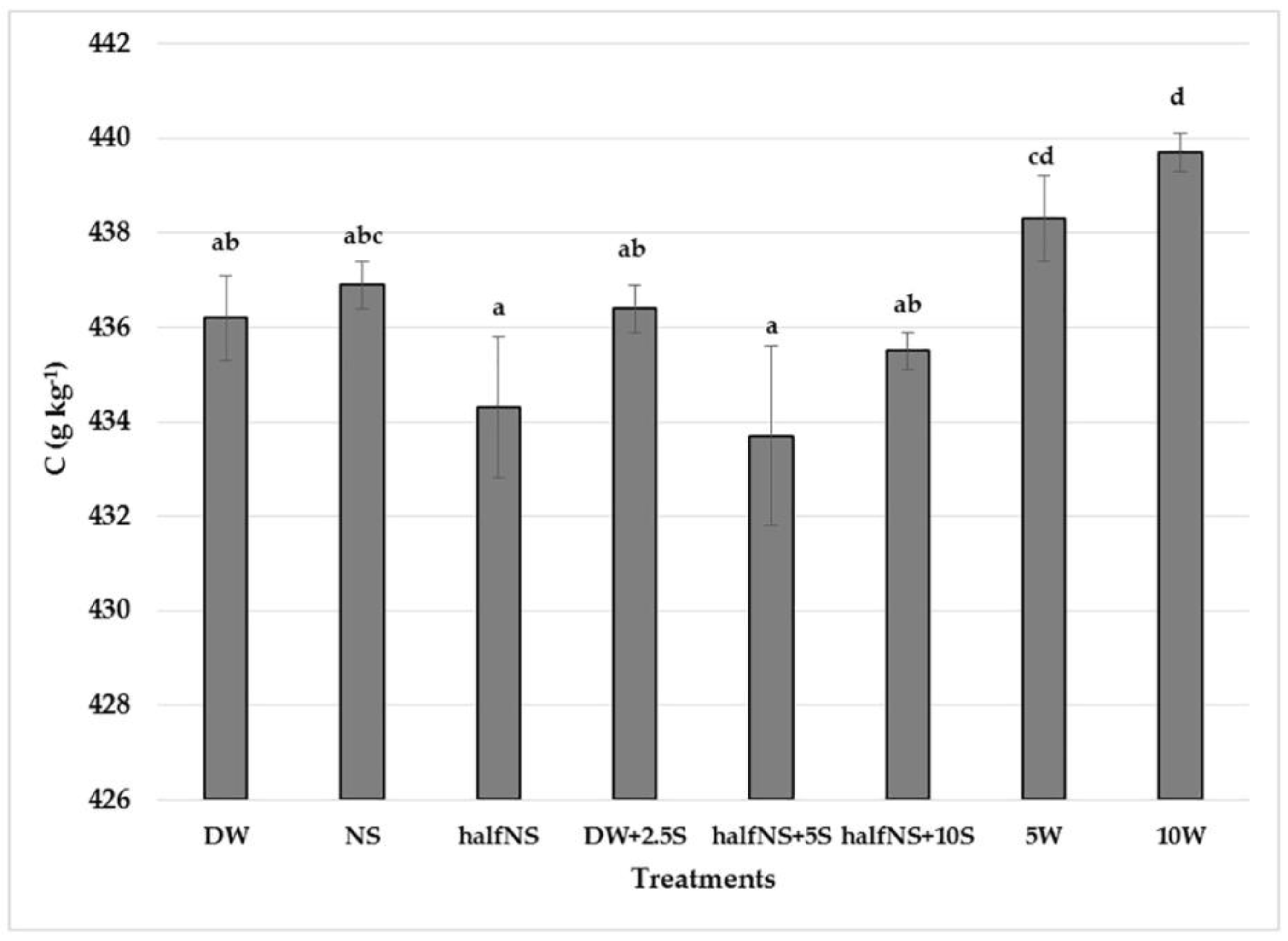
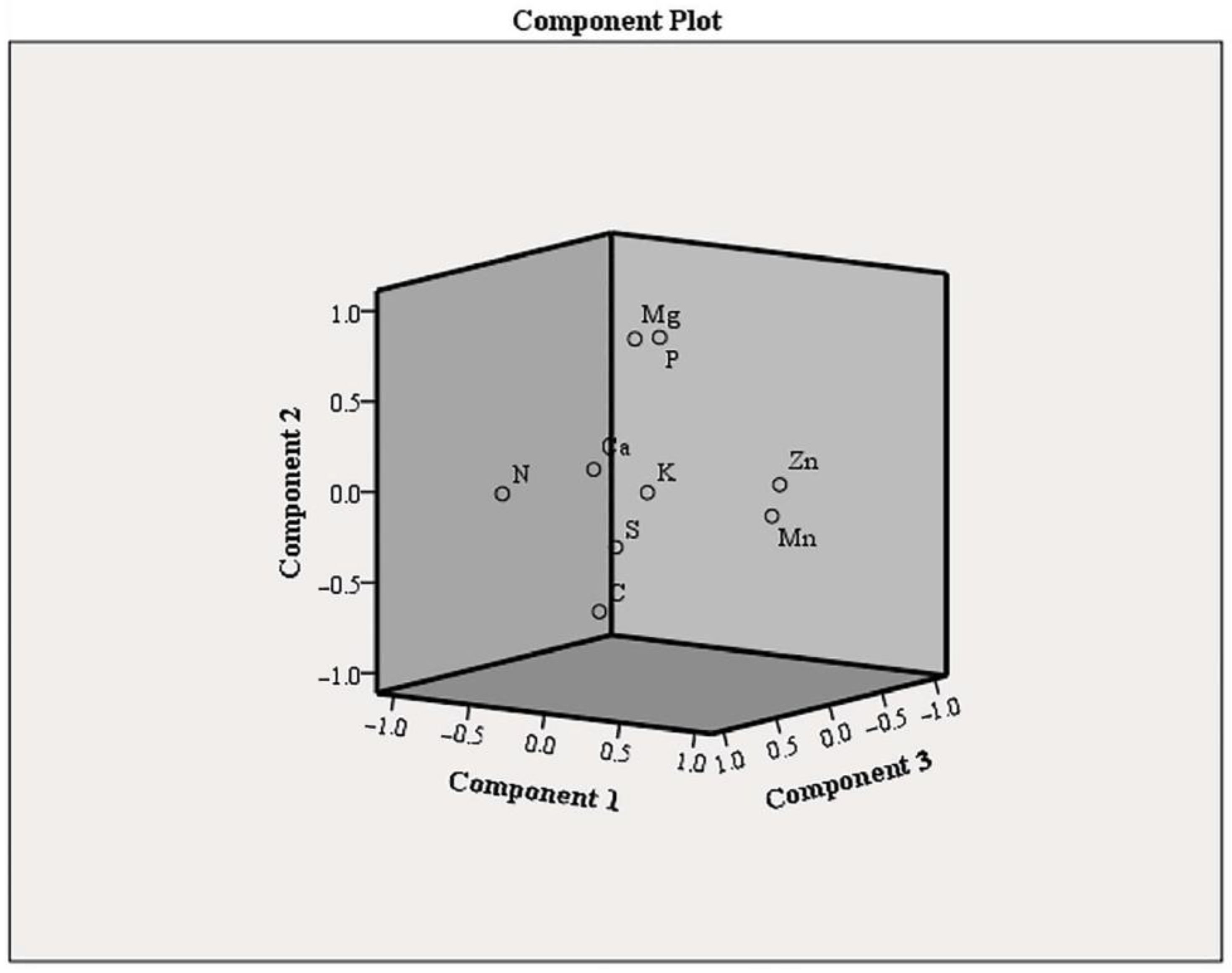
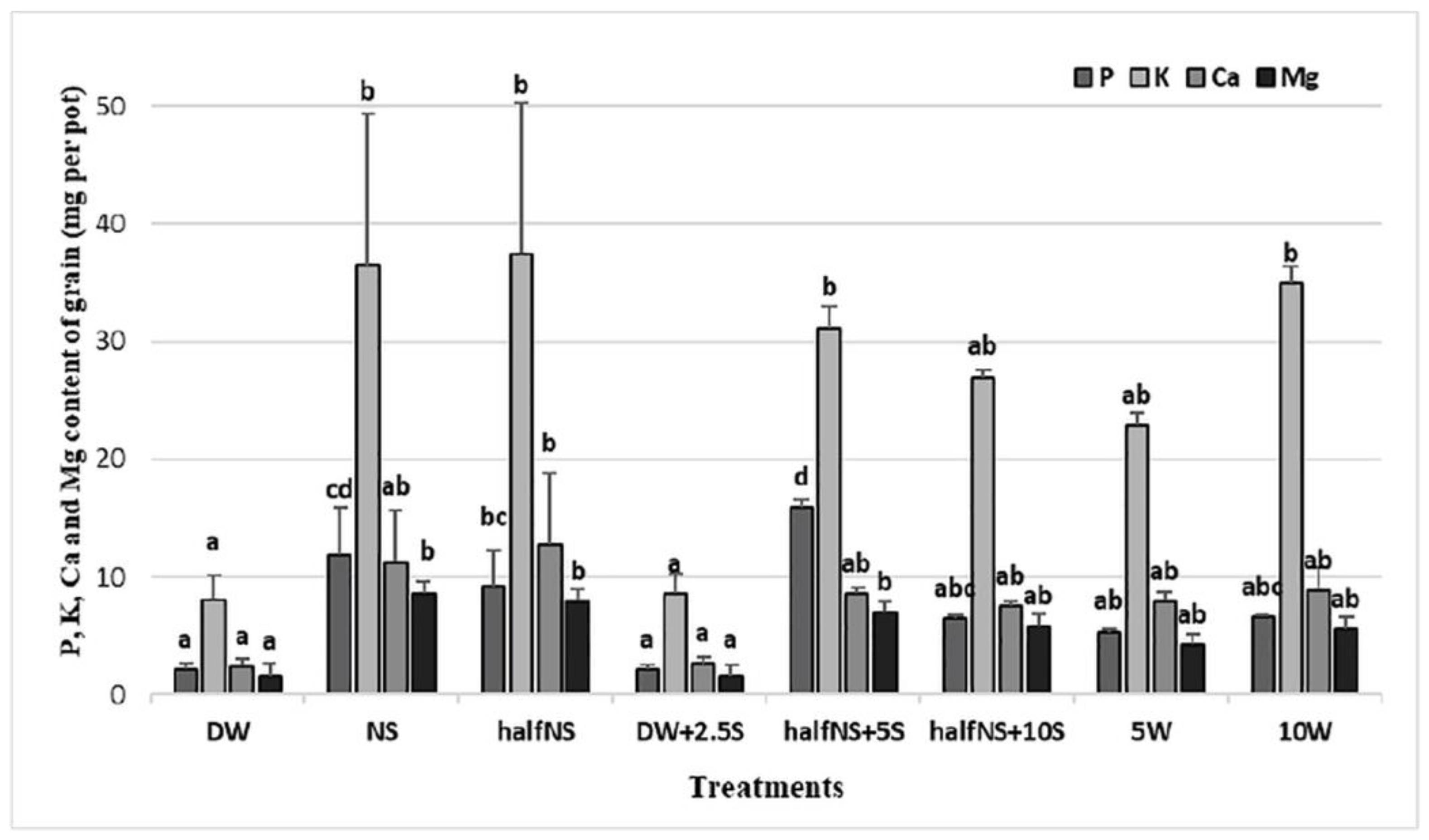
| Treatments/ Elements | P (g kg−1) | K (g kg−1) | Ca (g kg−1) | Mg (g kg−1) | Mn mg kg−1 | Zn mg kg−1 |
|---|---|---|---|---|---|---|
| DW | 1.69 ± 0.12 bc | 6.10 ± 0.13 abc | 1.83 ± 0.01 a | 1.23 ± 0.05 abc | 69.25 ± 3.12 c | 35.49 ± 0.45 cd |
| NS | 1.97 ± 0.09 d | 5.93 ± 0.23 ab | 1.78 ± 0.19 a | 1.35 ± 0.11 bc | 51.90 ± 4.21 b | 28.30 ± 1.90 ab |
| half NS | 1.64 ± 0.09 bc | 6.46 ± 0.17 bcd | 2.10 ± 0.38 a | 1.39 ± 0.05 bc | 30.90 ± 4.89 a | 28.35 ± 1.50 ab |
| DW+2.5S | 1.53 ± 0.09 ab | 5.98 ± 0.13 abc | 1.83 ± 0.04 a | 1.11 ± 0.16 ab | 72.48 ± 5.57 c | 39.82 ± 2.21 d |
| half NS+5S | 1.85 ± 0.09 cd | 5.79 ± 0.07 a | 1.59 ± 0.08 a | 1.29 ± 0.01 abc | 41.58 ± 3.04 ab | 28.55 ± 1.75 ab |
| half NS+10S | 1.55 ± 0.07 ab | 6.51 ± 0.17 cd | 1.81 ± 0.13 a | 1.40 ± 0.10 c | 51.15 ± 5.98 b | 33.47 ± 0.770 bc |
| 5W | 1.36 ± 0.05 a | 5.78 ± 0.15 a | 2.01 ± 0.23 a | 1.07 ± 0.05 a | 53.00 ± 2.65 b | 26.53 ± 2.33 a |
| 10W | 1.33 ± 0.02 a | 7.00 ± 0.29 d | 1.78 ± 0.38 a | 1.11 ± 0.06 ab | 37.48 ± 1.75 a | 29.72 ± 1.30 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makleit, P.; Balláné, A.K.; Bákonyi, N.; Domokos-Szabolcsy, É.; Fári, G.M.; Veres, S. Fermented Deproteinized Alfalfa Juice Modified with Fly Ash Filtrate as an Alternative Nutrient Source for Winter Wheat (Triticum aestivum L.). Agronomy 2025, 15, 339. https://doi.org/10.3390/agronomy15020339
Makleit P, Balláné AK, Bákonyi N, Domokos-Szabolcsy É, Fári GM, Veres S. Fermented Deproteinized Alfalfa Juice Modified with Fly Ash Filtrate as an Alternative Nutrient Source for Winter Wheat (Triticum aestivum L.). Agronomy. 2025; 15(2):339. https://doi.org/10.3390/agronomy15020339
Chicago/Turabian StyleMakleit, Péter, Andrea Kovács Balláné, Nóra Bákonyi, Éva Domokos-Szabolcsy, Gábor Miklós Fári, and Szilvia Veres. 2025. "Fermented Deproteinized Alfalfa Juice Modified with Fly Ash Filtrate as an Alternative Nutrient Source for Winter Wheat (Triticum aestivum L.)" Agronomy 15, no. 2: 339. https://doi.org/10.3390/agronomy15020339
APA StyleMakleit, P., Balláné, A. K., Bákonyi, N., Domokos-Szabolcsy, É., Fári, G. M., & Veres, S. (2025). Fermented Deproteinized Alfalfa Juice Modified with Fly Ash Filtrate as an Alternative Nutrient Source for Winter Wheat (Triticum aestivum L.). Agronomy, 15(2), 339. https://doi.org/10.3390/agronomy15020339







