Genotypic Differences in Maize Root Morphology in Response to Low-Nitrogen Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Procedures
2.3. Shoot Sampling and Determination
2.4. Root Sampling and Determination
2.5. Determination of N Concentration
2.6. Determination of Plant Height, Leaf Area and SPAD
2.7. Statistical Analysis
3. Results
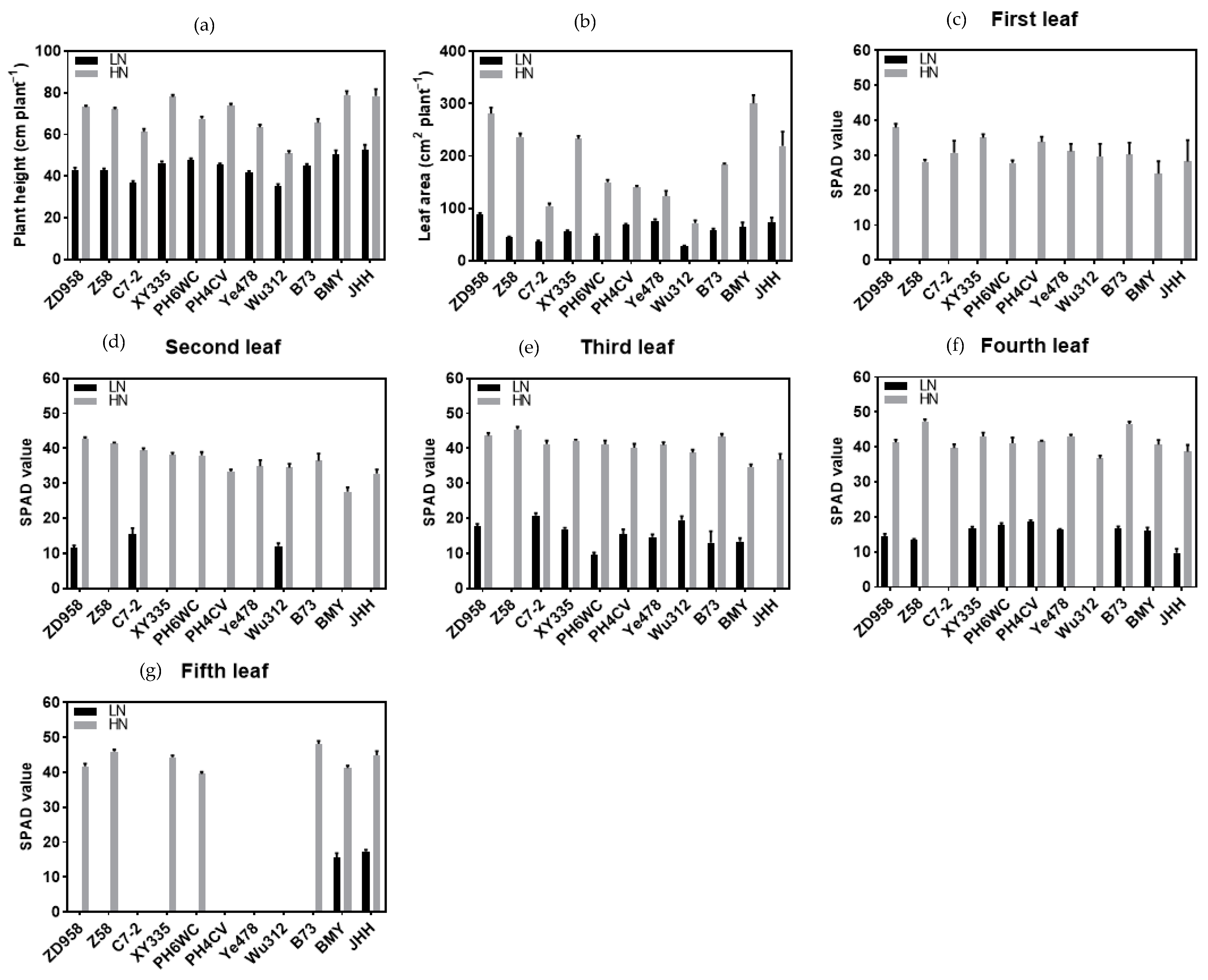
3.1. Plant Height, Leaf Area and SPAD
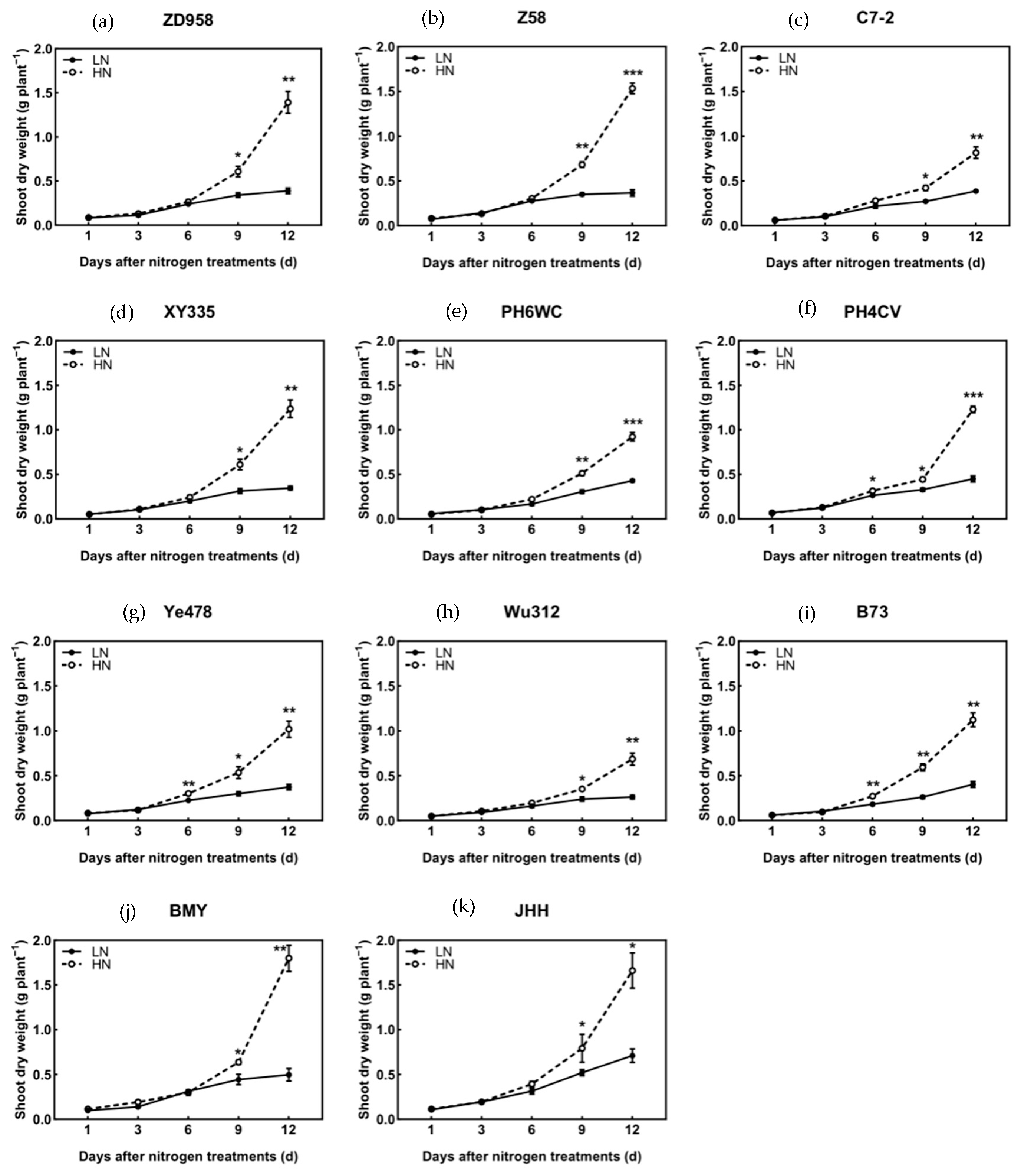
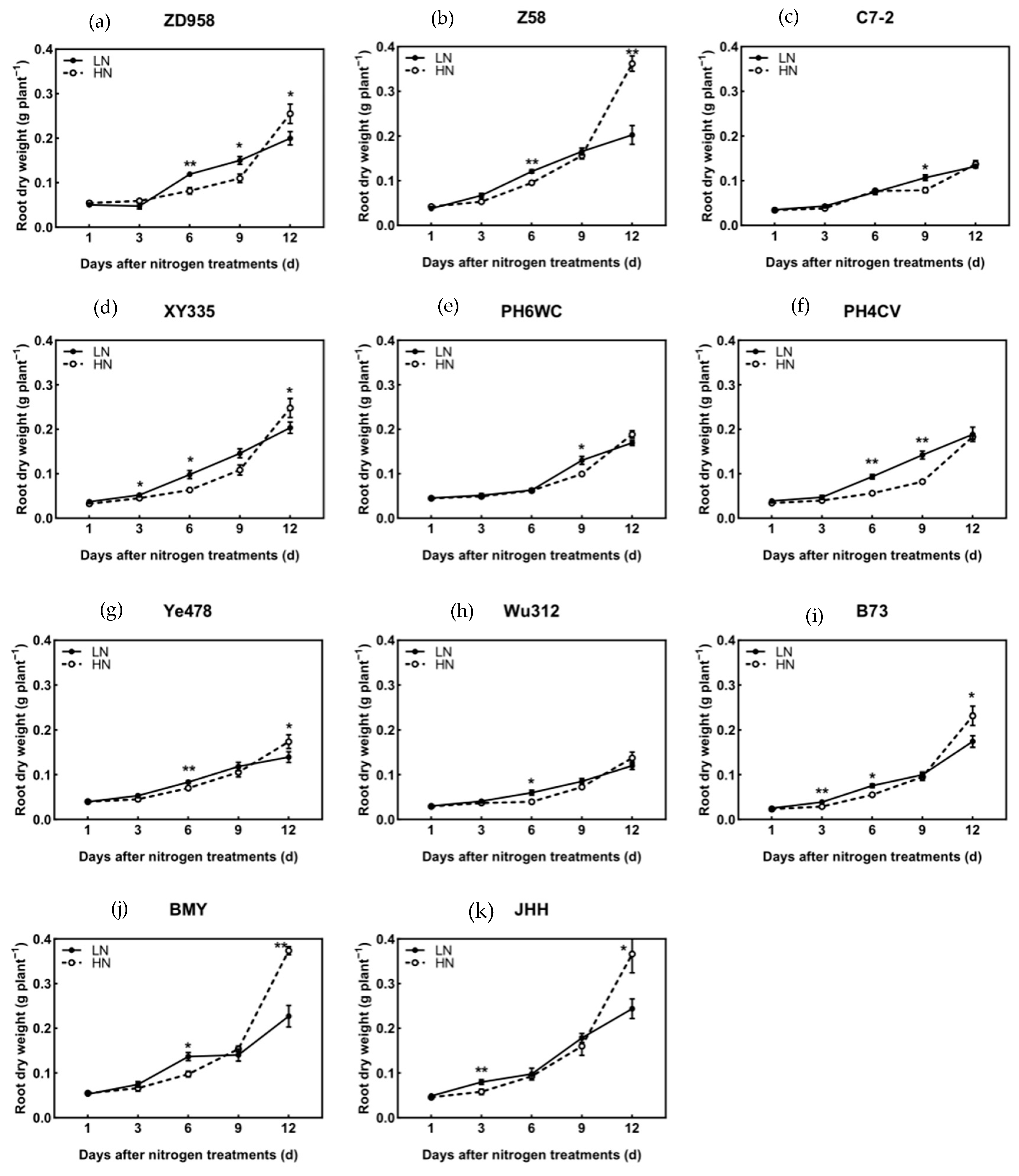
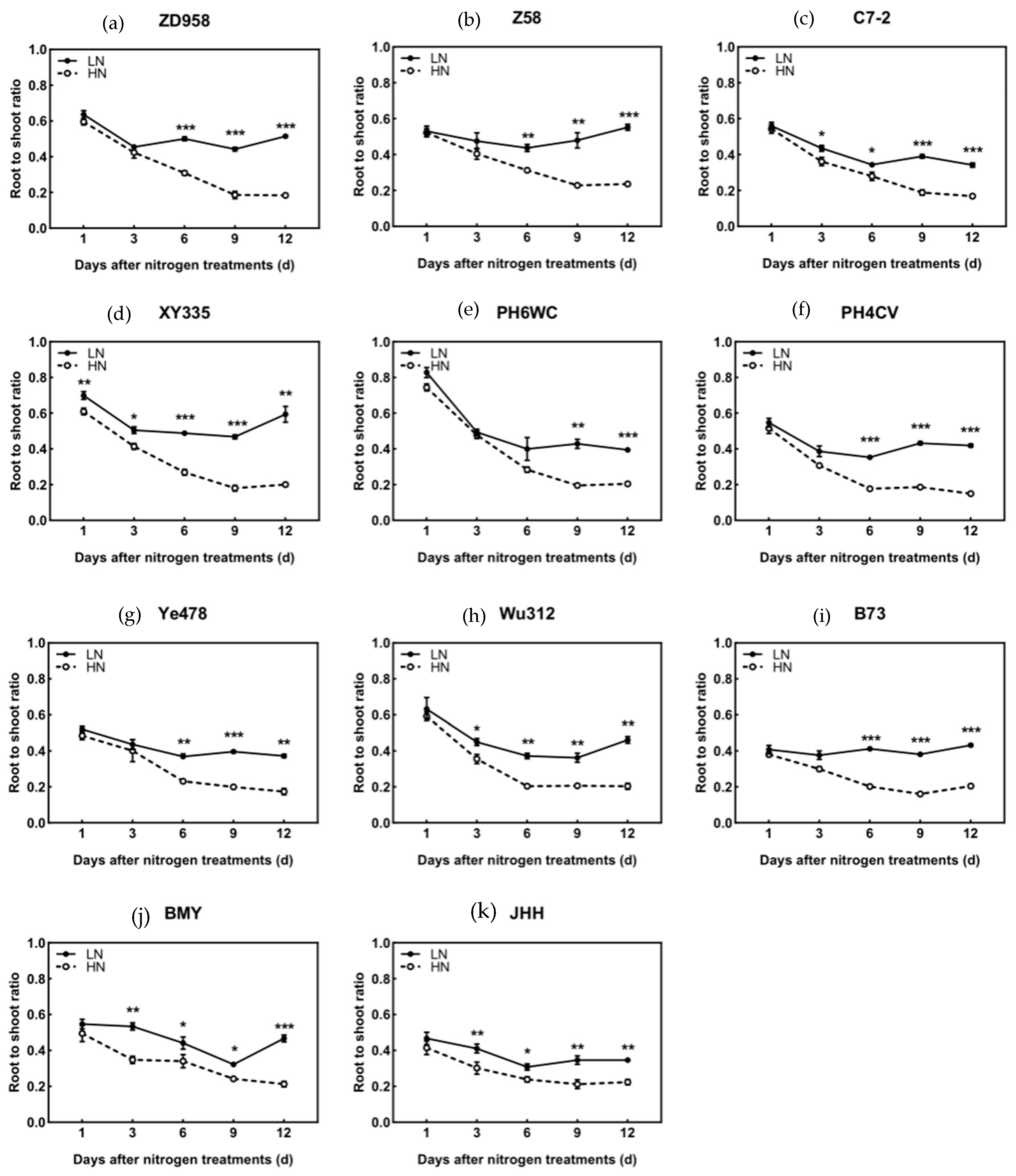
3.2. Biomass
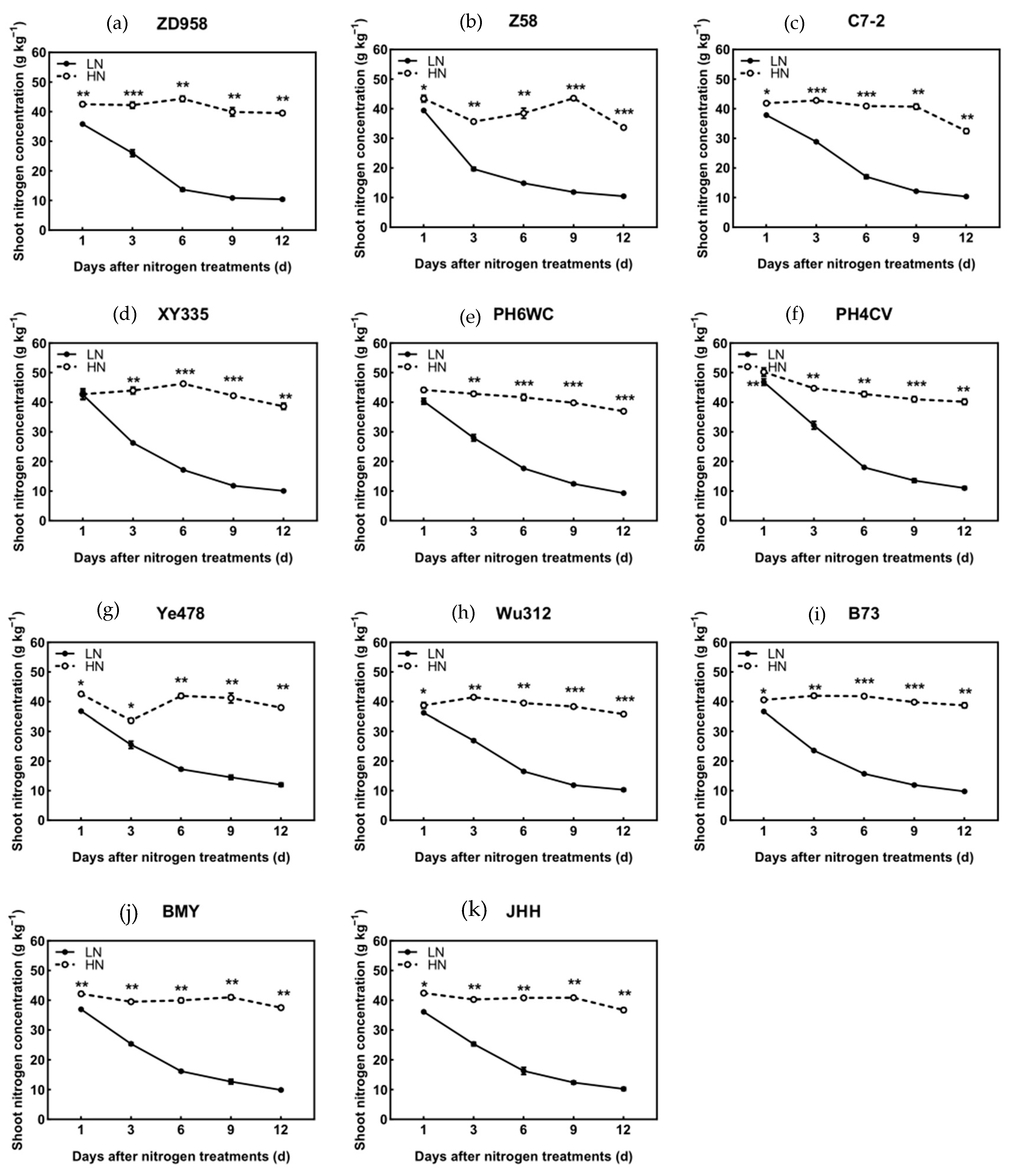
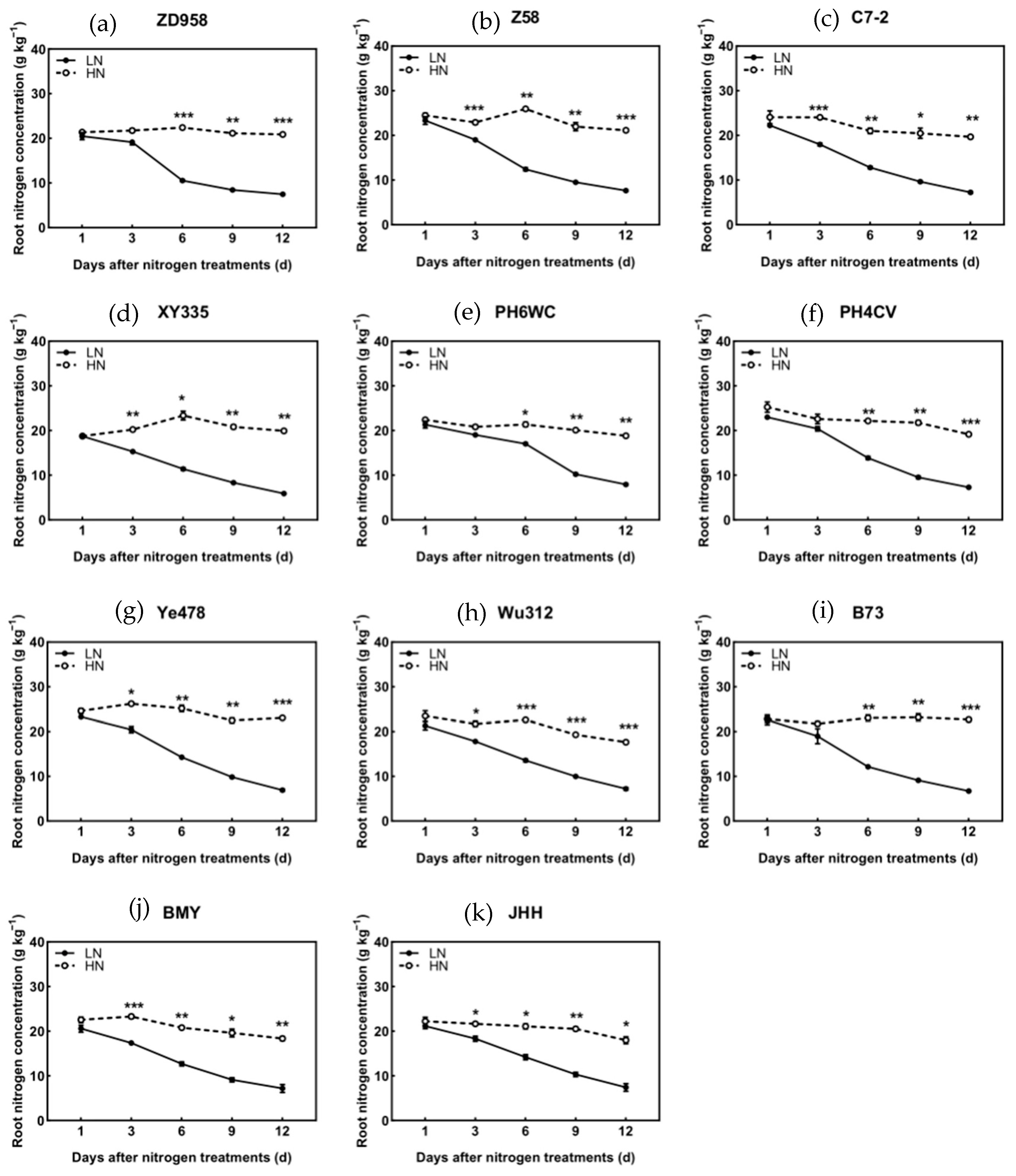
3.3. N Concentration
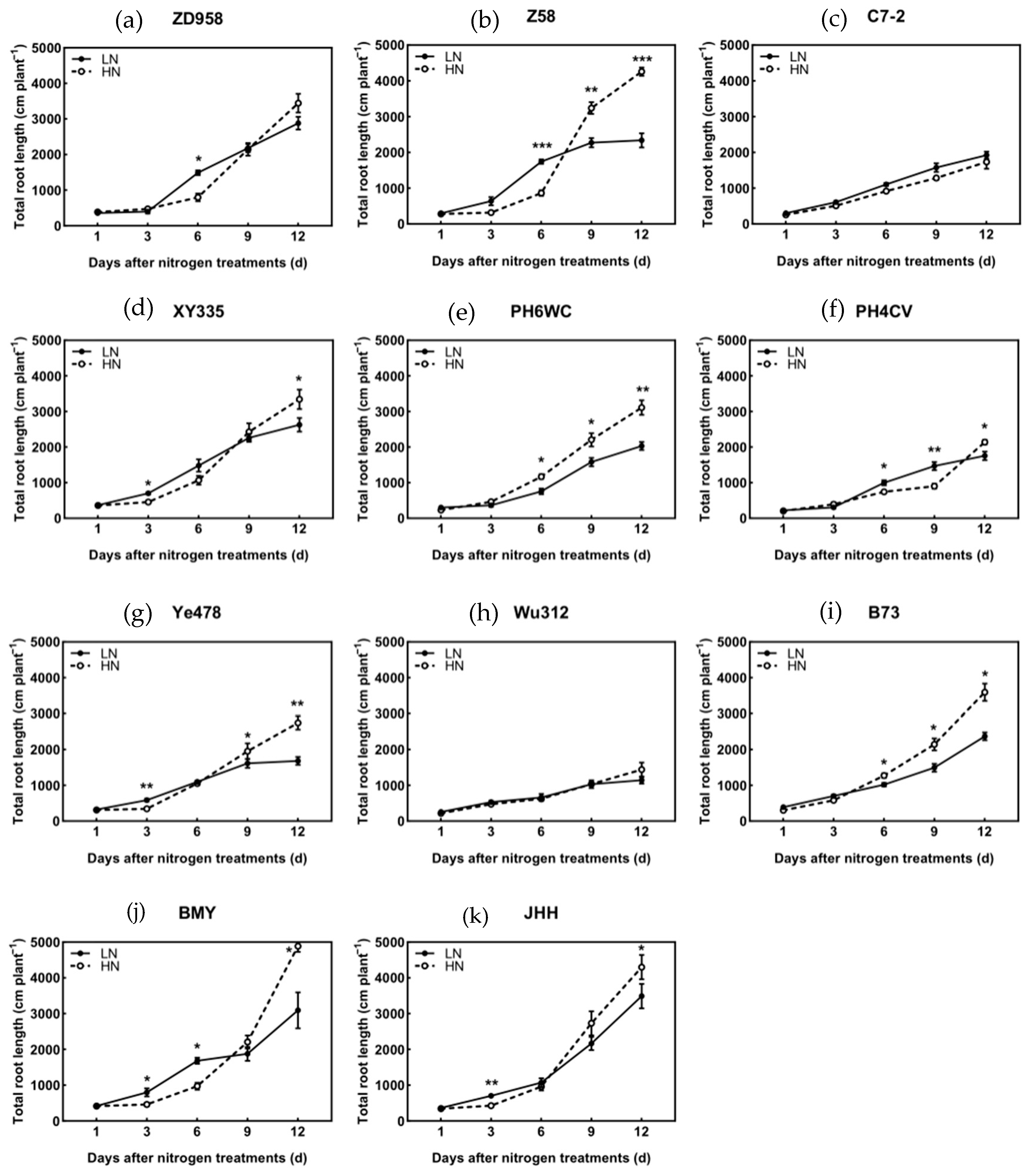
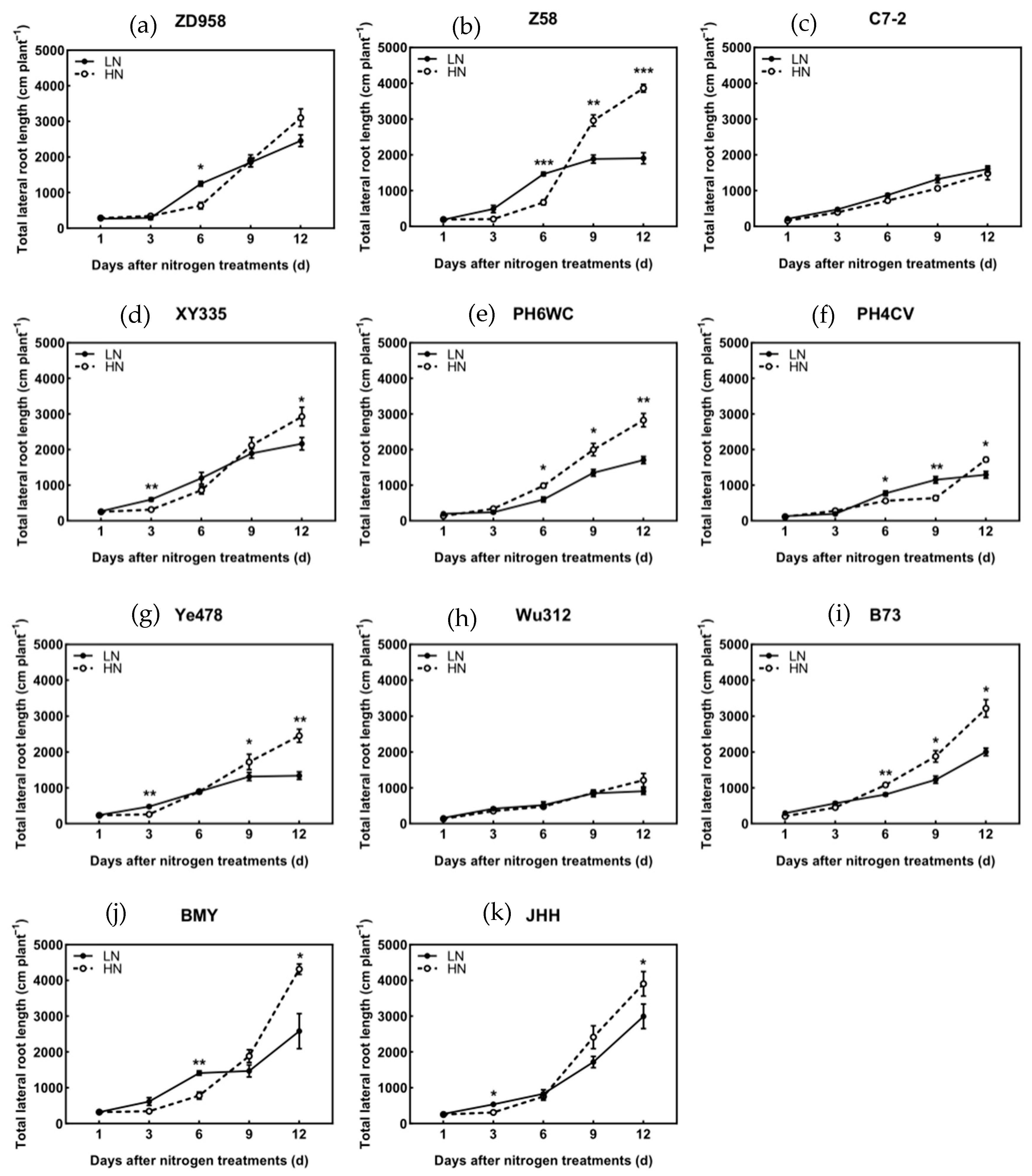
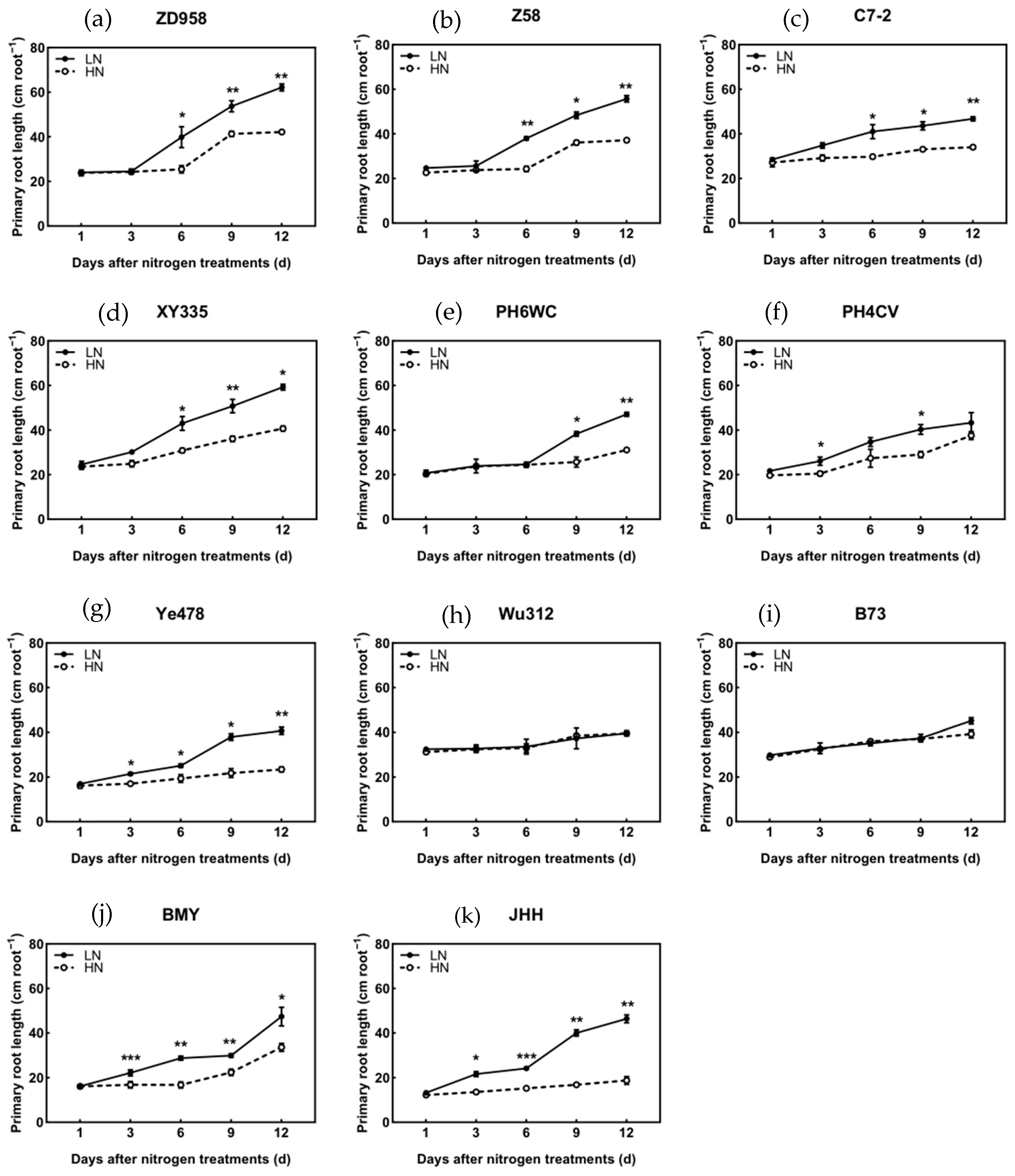
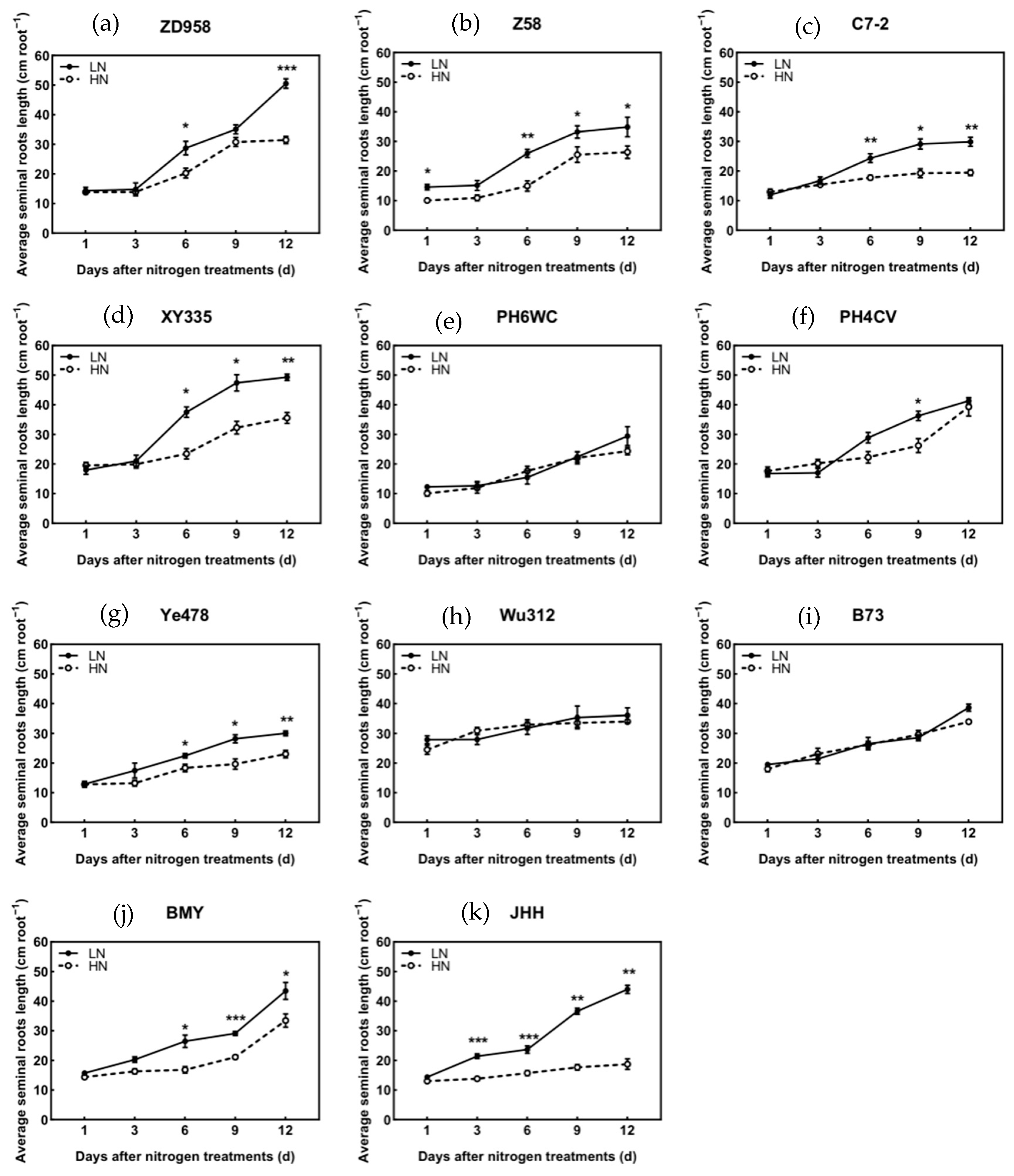
3.4. Root Length
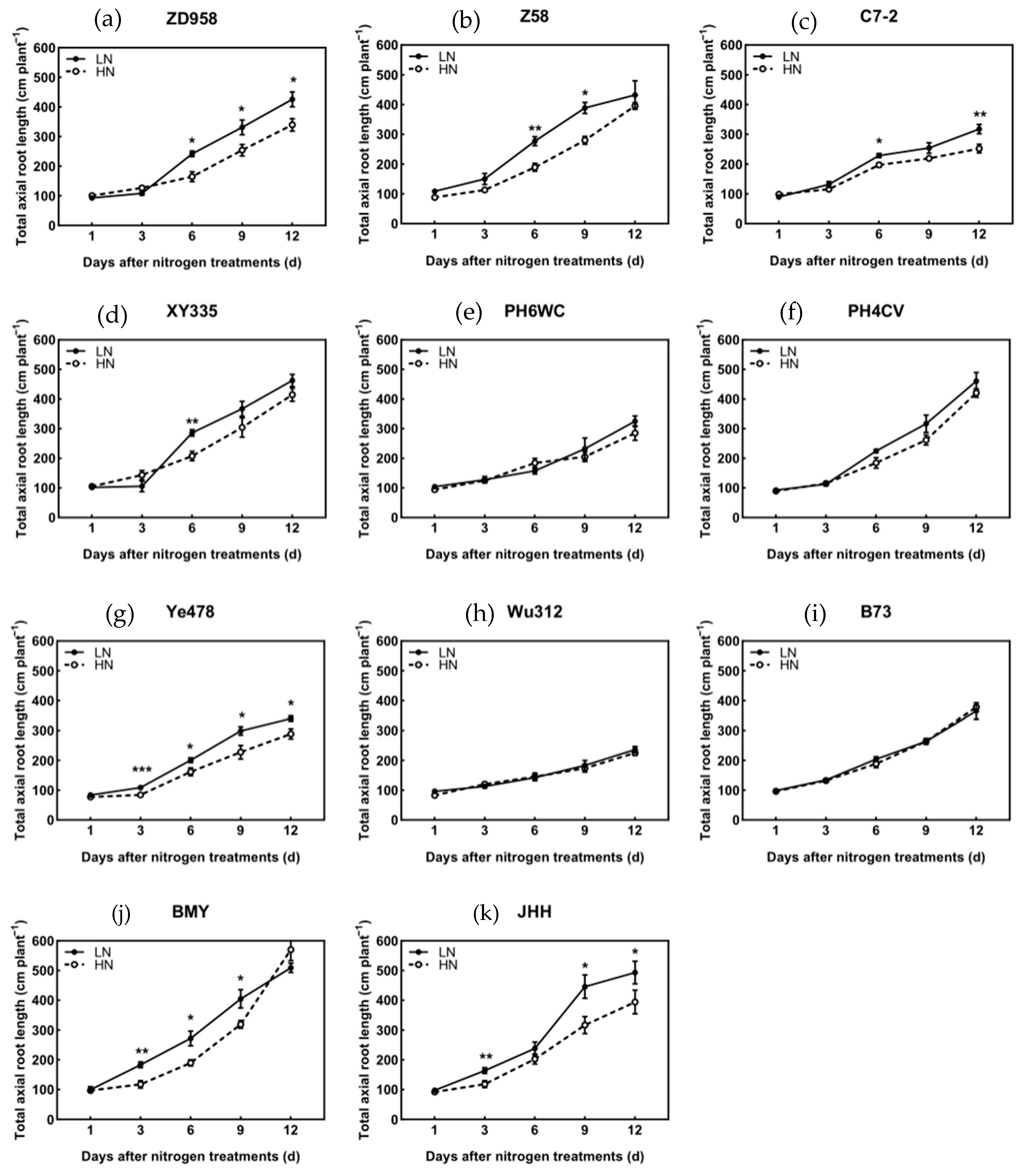
3.5. Axial Root Length of Different Root Types
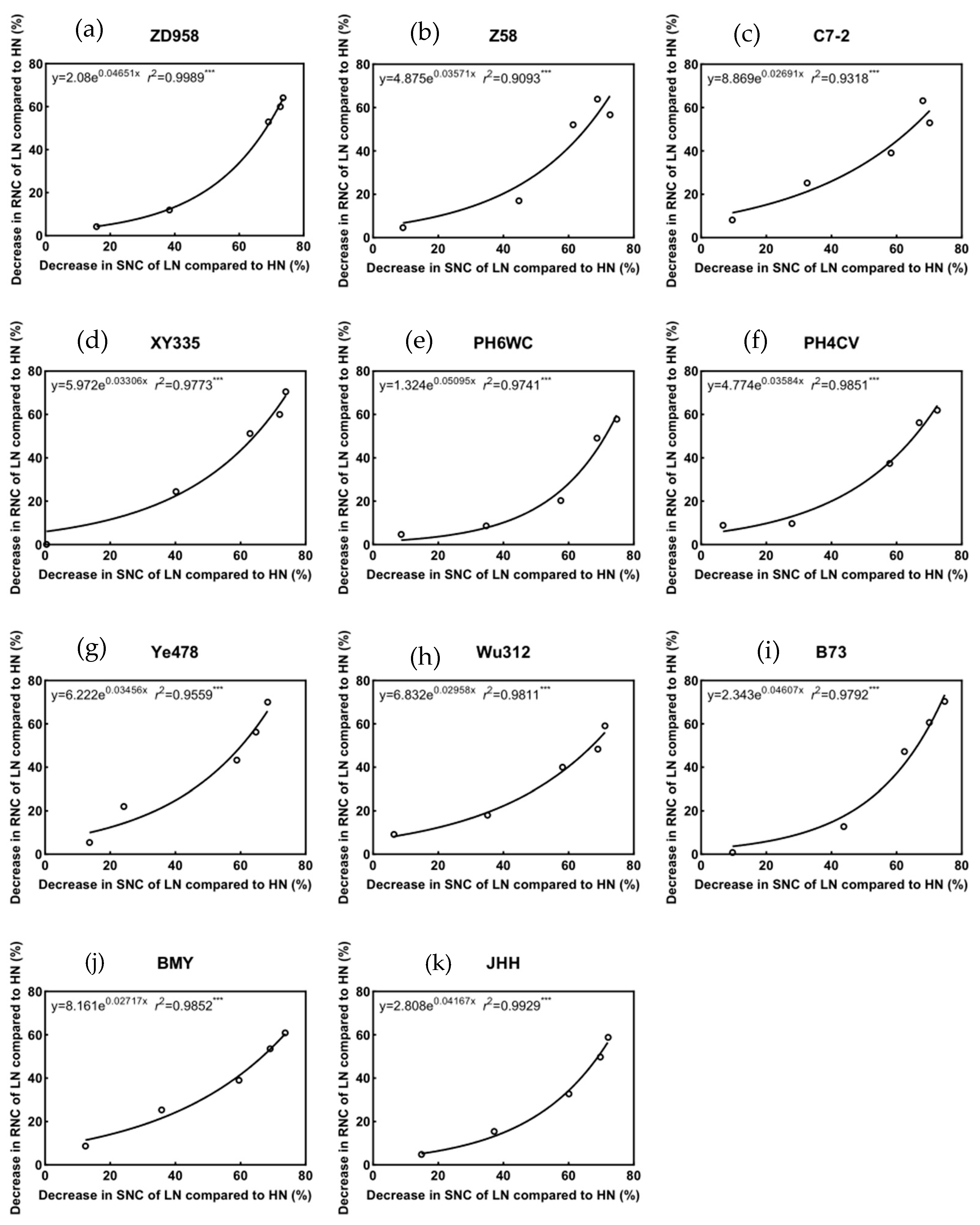
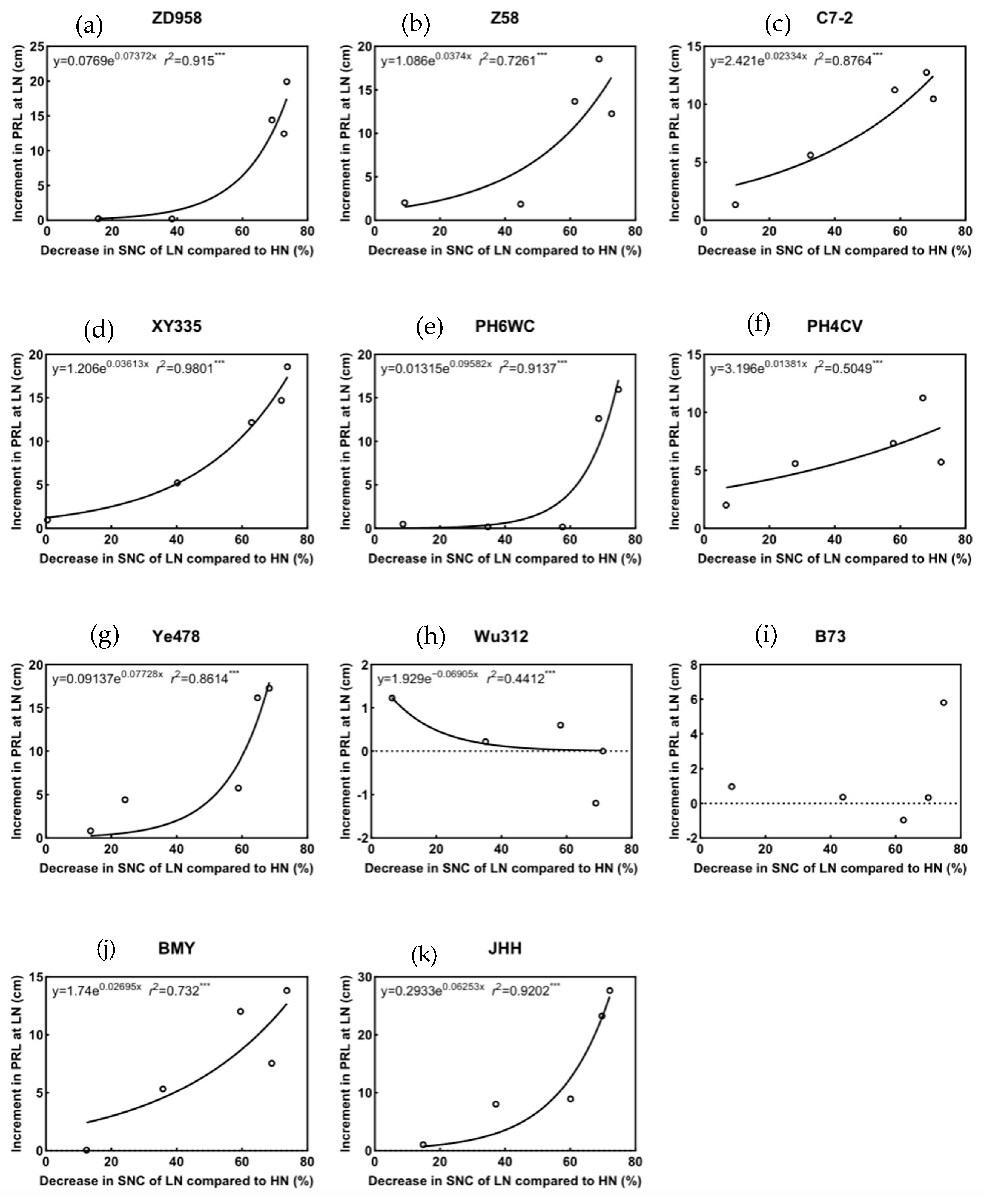
3.6. The Relationship Between Shoot N Status and Partial Phenotypes
4. Discussion
4.1. Low N Enhances Root Growth Earlier than Increases in Shoot-to-Root Dry Matter Allocation
4.2. Regulation of Root Elongation Is Earlier than That of Crown Root Initiation
4.3. The Response of Lateral Root Growth to Low-N Stress Is Genotype-Dependent
4.4. The Roots of Modern Hybrids Become Smaller but More Responsive to Low-N Stress
4.5. The Root Phenotypes of ZD958 and XY335 Come from Different Genetic Models
4.6. Comparison of Root Phenotypes of Different Inbred Lines Under N Stress
4.7. Shoot N Concentration May Play a Regulatory Role in Root Morphogenesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; GK, S.; Vadivel, R.; TK, D.; et al. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef] [PubMed]
- Feyisa, D.S.; Jiao, X.; Mojo, D. Wheat Yield Response to Chemical Nitrogen Fertilizer Application in Africa and China: A Meta-analysis. Soil Sci. Plant Nut. 2024, 24, 102–114. [Google Scholar] [CrossRef]
- Penuelas, J.; Coello, F.; Sardans, J. A better use of fertilizers is needed for global food security and environmental sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- Argento, S.; Garcia, G.; Treccarichi, S. Sustainable and low-input techniques in Mediterranean greenhouse vegetable production. Horticulturae 2024, 10, 997. [Google Scholar] [CrossRef]
- Sapkota, T.B.; Takele, R. Improving nitrogen use efficiency and reducing nitrogen surplus through best fertilizer nitrogen management in cereal production: The case of India and China. Adv. Agron. 2023, 178, 233–294. [Google Scholar]
- Farzadfar, S.; Knight, J.D.; Congreves, K.A. Soil organic nitrogen: An overlooked but potentially significant contribution to crop nutrition. Plant Soil 2021, 462, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.E.; Youngblut, N.D.; Buckley, D.H. Bacterial community dynamics explain carbon mineralization and assimilation in soils of different land-use history. Environ. Microbiol. 2022, 24, 5230–5247. [Google Scholar] [CrossRef] [PubMed]
- Elrys, A.S.; Ali, A.; Zhang, H.; Cheng, Y.; Zhang, J.; Cai, Z.C.; Müller, C.; Chang, S.X. Patterns and drivers of global gross nitrogen mineralization in soils. Global Change Biol. 2021, 27, 5950–5962. [Google Scholar] [CrossRef]
- Lopez, G.; Ahmadi, S.H.; Amelung, W.; Athmann, M.; Ewert, F.; Gaiser, T.; Gocke, M.I.; Kautz, T.; Postma, J.; Rachmilevitch, S.; et al. Nutrient deficiency effects on root architecture and root-to-shoot ratio in arable crops. Front. Plant Sci. 2023, 13, 1067498. [Google Scholar] [CrossRef]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, J.; Moturu, T.R.; Nacry, P.; Ebert, R.; De Gernier, H.; Tillard, P.; Swarup, K.; Wells, D.M.; Haseloff, J.; Murray, S.C.; et al. Root system size and root hair length are key phenes for nitrate acquisition and biomass production across natural variation in Arabidopsis. J. Exp. Bot. 2022, 73, 3569–3583. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, H.; Wang, P.; Chen, F.; Yuan, L.; Mi, G. Low nitrogen induces root elongation via auxin-induced acid growth and auxin-regulated target of rapamycin (TOR) pathway in maize. J. Plant Physiol. 2020, 254, 153281. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chen, F.; Yuan, L.; Zhang, F.; Mi, G. A comprehensive analysis of root morphological changes and nitrogen allocation in maize in response to low nitrogen stress. Plant Cell Environ. 2015, 38, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; An, T.; Chen, Y. Lateral root elongation enhances nitrogen-use efficiency in maize genotypes at the seedling stage. J. Sci. Food Agric. 2022, 102, 5389–5398. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, A.C.; Mcclymont, S.A.; Holmes, B.M.; Lyons, E.; Raizada, M.N. Novel temporal, fine-scale and growth variation phenotypes in roots of adult-stage maize (Zea mays L.) in response to low nitrogen stress. Plant Cell Environ. 2011, 34, 2122–2137. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Lončarić, Z.; Kristek, S.; Kulundžić, A.M.; Rebekić, A.; Antunović, M. Sugar beet root yield and quality with leaf seasonal dynamics in relation to planting densities and nitrogen fertilization. Agriculture 2021, 11, 407. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Al-Ghamdi, A.A.; Khan, A.; Zeeshan, M.; Elshikh, M.S.; Abbasi, A.M.; Zhou, X.B. Nitrogen fertilizer modulates plant growth, chlorophyll pigments and enzymatic activities under different irrigation regimes. Agronomy 2022, 12, 845. [Google Scholar] [CrossRef]
- Pensky, J.; Fisher, A.T.; Gorski, G.; Schrad, N.; Bautista, V.; Saltikov, C. Linking nitrate removal, carbon cycling, and mobilization of geogenic trace metals during infiltration for managed recharge. Water Res. 2023, 239, 120045. [Google Scholar] [CrossRef]
- Lynch, J.P.; Galindo-Castañeda, T.; Schneider, H.M.; Sidhu, J.S.; Rangarajan, H.; York, L.M. Root phenotypes for improved nitrogen capture. Plant Soil 2024, 502, 31–85. [Google Scholar] [CrossRef]
- Wiesler, F.; Horst, W.J. Root growth and nitrate utilization of maize cultivars under field conditions. Plant Soil 1994, 163, 267–277. [Google Scholar] [CrossRef]
- Saengwilai, P.; Tian, X.; Lynch, J.P. Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol. 2014, 166, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, W.; Brodie, G.; Fuentes, S.; Pang, A.; Gupta, D. Silicon improves root system and canopy physiology in wheat under drought stress. Plant Soil 2024, 502, 279–296. [Google Scholar] [CrossRef]
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: A review. Front. Plant Sci. 2021, 12, 657629. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, R.A.; Castellano, M.J.; Danalatos, G.N.; Wright, E.E.; Hatfield, J.L.; Burras, L.; Archontoulis, S.V. Insufficient and excessive N fertilizer input reduces maize root mass across soil types. Field Crop Res. 2021, 267, 108142. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, G.; Chen, F.; Zhang, F. Genotypic differences in nitrogen uptake by maize inbred lines its relation to root morphology. Acta Ecol. Sin. 2003, 23, 79–84. [Google Scholar]
- Wu, Q.; Chen, F.; Chen, Y.; Yuan, L.; Zhang, F.; Mi, G. Root growth in response to nitrogen supply in Chinese maize hybrids released between 1973 and 2009. Sci. China Life Sci. 2011, 54, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, F.; Chen, Y.; Gao, Q.; Yang, X.; Yuan, L.; Zhang, F.; Mi, G. Modern maize hybrids in Northeast China exhibit increased yield potential and resource use efficiency despite adverse climate change. Global Change Biol. 2013, 19, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhao, Z.; Zhang, Y.; Xu, S.; Wang, N.; Wang, W.; Cheng, H.; Xing, G.; Feng, W. The Response and Cluster Analysis of Biomass Accumulation and Root Morphology of Maize Inbred Lines Seedlings to Two Nitrogen Application Levels. Crops 2019, 35, 28–36. [Google Scholar]
- Trentin, H.U.; Krause, M.D.; Zunjare, R.U.; Almeida, V.C.; Peterlini, E.; Rotarenco, V.; Frei, U.K.; Beavis, W.D.; Lübberstedt, T. Genetic basis of maize maternal haploid induction beyond MATRILINEAL and ZmDMP. Front. Plant Sci. 2023, 14, 1218042. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Zheng, H.; Pan, X.; Li, H.; Chen, F.; Li, X. ZD958 is a low-nitrogen-efficient maize hybrid at the seedling stage among five maize and two teosinte lines. Planta 2015, 242, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Jiang, Z.; Zhang, L.; Hussain, L.; Yang, X. Insights on Phytohormonal Crosstalk in Plant Response to Nitrogen Stress: A Focus on Plant Root Growth and Development. Int. J. Mol. Sci. 2023, 24, 3631. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.D.; Nunez-Pascual, V.; Riveras, E.; Ruffel, S.; Gutiérrez, R.A. Recent advances in local and systemic nitrate signaling in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2024, 81, 102605. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, P.; Mi, G. Genotypic Differences in Maize Root Morphology in Response to Low-Nitrogen Stress. Agronomy 2025, 15, 332. https://doi.org/10.3390/agronomy15020332
Sun X, Wang P, Mi G. Genotypic Differences in Maize Root Morphology in Response to Low-Nitrogen Stress. Agronomy. 2025; 15(2):332. https://doi.org/10.3390/agronomy15020332
Chicago/Turabian StyleSun, Xichao, Peng Wang, and Guohua Mi. 2025. "Genotypic Differences in Maize Root Morphology in Response to Low-Nitrogen Stress" Agronomy 15, no. 2: 332. https://doi.org/10.3390/agronomy15020332
APA StyleSun, X., Wang, P., & Mi, G. (2025). Genotypic Differences in Maize Root Morphology in Response to Low-Nitrogen Stress. Agronomy, 15(2), 332. https://doi.org/10.3390/agronomy15020332







